Abstract
We aimed in this study to describe lamivudine concentration-time courses in treatment-naïve children after once-daily administration, to study the effects of body weight and age on lamivudine pharmacokinetics, and to simulate an optimized administration scheme. For this purpose, lamivudine concentrations were measured in 49 children after at least 2 weeks of didanosine-lamivudine-efavirenz treatment. A total of 148 plasma lamivudine concentrations were measured, and a population pharmacokinetic model was developed with NONMEM. The influence of individual characteristics was tested using a likelihood ratio test. Children were divided into two groups, according to their pharmacokinetic parameters, thanks to tree regression analysis. For each patient, the area under the curve was derived from estimated individual pharmacokinetic parameters. Different once-daily doses were simulated in each group, to obtain the same exposure in children as the mean effective exposure in adults (8.9 mg/liter·h). A two-compartment model in which the slope of distribution is assumed to be equal to the absorption rate constant adequately described the data. Parameter estimates were standardized for a mean standard body weight using an allometric model. Children were then divided into 2 groups according to body weight: CL/F was significantly higher in children weighing less than 17 kg (1.12 liters/h/kg) than in children over 17 kg (0.95 liters/h/kg; P = 0.01). The target mean AUC of 8.9 mg/liters·h was obtained with a 10-mg/kg once-daily lamivudine (3TC) dose for children below 17 kg; the recommended dose of 8 mg/kg seems to be sufficient in children weighing more than 17 kg. These assumptions should be prospectively confirmed.
According to the latest UNAIDS estimates, nearly 90% of the 2.5 million children infected by HIV around the world in 2007 were living in sub-Saharan Africa (21). Highly active antiretroviral therapy (HAART) has been shown to be effective in children from industrialized countries (22) and is feasible in developing countries (8, 10). The combination of didanosine (ddI), lamivudine (3TC), and efavirenz (EFV) once daily in adults showed a good antiretroviral efficacy and good long-term tolerability (9, 15). In children, the efficacy and tolerance of this ddI-3TC-EFV combination remain to be shown. The aims of the BURKINAME-ANRS 12103 trial were to estimate the pharmacokinetics of ddI, 3TC, and efavirenz given once daily in children from 30 months to 15 years or age and to evaluate the efficacy and tolerance of this drug combination.
Lamivudine is rapidly absorbed following an oral dose and has a wide distribution due to its relatively low molecular mass (229 Da) and low plasma protein binding (<36%). The majority of lamivudine (approximately 70%) is eliminated unchanged in the urine over 24 h (13). Approximately 5 to 10% is metabolized to the pharmacologically inactive trans-sulfoxide metabolite, the majority of which is also excreted in the urine within 12 h after a single oral dose (13).
The combination of didanosine (ddI), lamivudine (3TC), and efavirenz (EFV) once daily is associated with better compliance (9, 15). In children, maximal concentrations (Cmax) and area under the concentration-time curve (AUC) from 0 to 24 h were not significantly lower with a once-daily administration of lamivudine than with a twice-daily administration (4). However, a few studies showed that younger children had a lower lamivudine exposure than older children and may be exposed to a subtherapeutic concentration, whether in a twice-daily or once-daily regimen (4, 5, 13, 17). We may wonder whether this phenomenon is due to the effect of growth (body weight) and/or maturation (age) and which parameter should be taken into account to perform dose adjustment.
In the present study, lamivudine population pharmacokinetics was investigated in children in order to describe the concentration-time courses, to study the influence of covariates (body weight and age) on pharmacokinetics, and to investigate the relationships between drug concentrations and efficacy.
MATERIALS AND METHODS
Patients.
The BURKINAME-ANRS 12103 study was an open phase II trial evaluating the pharmacokinetics, efficacy, and toxicity of the ddI-3TC-EFV once-daily combination in HIV-infected children. It was conducted in Bobo-Dioulasso, Burkina Faso. The study was approved by the National Ethics Committee on AIDS from Burkina Faso and was registered in the ClinicalTrials.gov database (http://clinicaltrials.gov) with the no. NCT00122538.
The patients enrolled in this study included children from 30 months to 15 years old, weighing at least 10 kg, infected by HIV-1, and naïve to all antiretroviral treatments (except a treatment preventing mother-to-child transmission). They were eligible if their HIV disease was classified, according to the Centers for Disease Control and Prevention (CDC) as meeting one of the following clinical categories: stage C and/or CD4 count of ≤15% for children ≤5 years or CD4 count of ≤200/μl for children >5 years; or stage B, A, or N and CD4 counts of 15% ≤ CD4 ≤ 20% for children ≤5 years or 200/μl ≤ CD4 ≤350/μl for children >5 years and a viral load greater than 100,000 copies/ml.
The following baseline laboratory values were required: hemoglobin concentration of 7 g/dl or greater, a platelet count of at least 50,000/μl, an amylase level of less than 2.5 times the upper limit of normal, and aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT) lower than 5 times the upper limit of normal. The mother or the legal guardian provided informed consent.
Clinical evaluations were carried out each month during the follow-up period. Children were also seen for any intercurrent diseases if necessary. Consultations, hospitalizations, treatments, and tests were done free of charge.
Virological and biochemical measurements were performed, beyond baseline, every trimester until 1 year after the beginning of the treatment. Plasma HIV-RNA viral load was determined using a real-time reverse transcriptase-PCR targeted to the long terminal repeat of HIV-1 (generic HIV viral load assay; Biocentric, Bandol, France). The detection threshold of this assay was 300 copies/ml using 0.2 ml plasma (19). Biochemistry analyses were performed with the Lisa 300 Plus machine (Hycel Diagnostics, Massy, France).
Treatments.
Children were administered a once-daily 8-mg/kg dose of 3TC as tablets (150 mg) or oral solution (10 mg/ml). Children were also given a once-daily 240-mg/m2 dose of ddI and the recommended body-weight-dependent dose of efavirenz (200 mg from 13 to <15 kg, 250 mg from 15 to <20 kg, 300 mg from 20 to <25 kg, 350 mg from 25 to <32.5 kg, 400 mg from 32.5 to <40 kg, and 600 mg above 40 kg).
All parents were instructed to administer all treatment every day at 6:00 p.m. without food.
Sampling.
The pharmacokinetic study was performed after 15 days of treatment in 38 children and between 2 and 5 months of treatment in 11 children. Children underwent blood sampling before as well as 1 and 3 h after 3TC intake (in 39 children) or before as well as 1, 2, 3, 6, 12, and 24 h after 3TC administration (in 10 children). The full pharmacokinetic schedule, initially proposed to determine AUC, could not be applied to all children because it required the presence of the child in a short period of time, beds available for 24 h in the hospital, and children in relative good health to undergo the 7 samplings. The time elapsed between administration and sampling time, age, body weight, and size were carefully recorded. Blood samples were centrifuged at 3,000 × g for 10 min. Plasma samples were aliquoted and stored at −70°C until assayed for drug concentrations. Of the 49 children enrolled, a total of 45 children were available for pharmacokinetic evaluation; 4 children were excluded from the pharmacokinetics analysis because the concentration of their sample was not available.
Analytical method.
Concentrations of lamivudine in plasma were determined using a modified version of a validated reverse-phase high-pressure liquid chromatography (HPLC) assay with detection by UV absorbance as initially described by Harker et al. (11).
The lower limit of quantification was 0.025 mg/liter. The mean recovery of lamivudine from extracted plasma samples was 81% ± 8%. Two control quality samples, prepared in human plasma at concentrations of 0.075 mg/liter and 0.75 mg/liter for lamivudine were included in each study run to monitor assay performance. The intraday precision (coefficient of variation) and accuracy (mean deviation) of the assay were less than 10% and 3%, respectively. The interday precision and accuracy of the assay were less than 14% and 6%, respectively.
Modeling strategy and population pharmacokinetic model.
Data were analyzed using the nonlinear mixed-effect modeling program NONMEM, release 2 (version VI), driven by Wings for NONMEM (http://wfn.sourceforge.net) (3) (ICON Development Solutions, Ellicott City, MD). The first-order conditional estimation (FOCE) was used. To handle the concentration below the limit of quantification (BLQ), three different approaches were tried: (i) replacement of the first BLQ observation with BLQ/2 and deletion of the following concentrations below the BLQ, (ii) use of the built-in Beal M2 method, or (iii) use of the Beal M3 method (1). Data were analyzed according to a one- or two-compartment model. Since in our study, no samples were obtained during the absorption phase, we could not estimate the first-order absorption rate constant (Ka) for the two-compartment model; thus, first, Ka was fixed to a value from the literature (17) and, second, we assumed a model in which Ka and the slope of the distribution phase (alpha) were equal (23). Parameter estimates were standardized for a mean standard body weight using an allometric model, Pi = PSTD × (BWi/BWSTD)PWR, where PSTD is the standard value of parameter for a patient with the standard body weight value and Pi and BWi are the parameter and body weight of the ith individual. The PWR (power) exponents may be estimated from the data. However, from allometric scaling theory, these are typically 0.75 for clearance parameters and 1 for volumes of distribution (2).
Different error models were investigated (i.e., multiplicative and additive error models) to describe residual variability. An exponential model was used for intersubject variability (ISV). Only significant ISVs on pharmacokinetics were finally kept: i.e., a minimum of a 6.63-unit decrease using a likelihood ratio test in a backward elimination procedure. The effect of each patient covariate (age, body weight, size, and creatinine clearance) was systematically tested via generalized additive modeling on the basic model. For example, using CL, 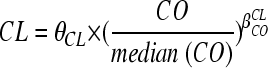 , where θCL is the typical value of clearance for a patient with the median covariate (CO) value and βCOCLis the estimated influential factor for the continuous covariate. The Galenic form was tested as a categorical covariable (CA) on bioavailability in order to point out a difference between the oral tablet and solution according to CL = θCL × βCA. All of the covariates were tested via an upward model building. A covariate was selected if (i) its effect was biologically plausible, (ii) it produced a minimum decrease of 6.63 U (chi-square test, 1 df, P < 0.01) in the objective function value (OFV), and (iii) it produced a reduction in the variability of the pharmacokinetic parameter, assessed by the associated intersubject variability. Among the covariates tested on the base model, the most significant was added in an intermediate model. Then the other covariates were tested on this intermediate model, and the most significant covariate was retained. This process was repeated until no more covariate was significant (i.e., P > 0.01). For evaluation of the goodness of fit, the following graphs were performed for the final model: observed and predicted concentrations versus time, observed concentrations versus population predictions, weighted residuals versus time, and weighted residuals versus predictions. Similar graphs using individual predictive post hoc estimation were displayed. Diagnostic graphics were obtained using RfN (http://wfn.sourceforge.net/) with the R program (12).
, where θCL is the typical value of clearance for a patient with the median covariate (CO) value and βCOCLis the estimated influential factor for the continuous covariate. The Galenic form was tested as a categorical covariable (CA) on bioavailability in order to point out a difference between the oral tablet and solution according to CL = θCL × βCA. All of the covariates were tested via an upward model building. A covariate was selected if (i) its effect was biologically plausible, (ii) it produced a minimum decrease of 6.63 U (chi-square test, 1 df, P < 0.01) in the objective function value (OFV), and (iii) it produced a reduction in the variability of the pharmacokinetic parameter, assessed by the associated intersubject variability. Among the covariates tested on the base model, the most significant was added in an intermediate model. Then the other covariates were tested on this intermediate model, and the most significant covariate was retained. This process was repeated until no more covariate was significant (i.e., P > 0.01). For evaluation of the goodness of fit, the following graphs were performed for the final model: observed and predicted concentrations versus time, observed concentrations versus population predictions, weighted residuals versus time, and weighted residuals versus predictions. Similar graphs using individual predictive post hoc estimation were displayed. Diagnostic graphics were obtained using RfN (http://wfn.sourceforge.net/) with the R program (12).
Evaluation and validation. (i) Bootstrap evaluation.
The accuracy and robustness of the final population model were assessed using a bootstrap method, as previously described in detail (18). Briefly, from the original data set of n individuals, B bootstrap sets (B = 1,000) of n individuals are drawn with replacement (resampling). For each bootstrap sample, the population pharmacokinetic parameters are estimated, and then parameter statistics are obtained from the whole bootstrap set. To validate the model, the parameters estimated from the bootstrap must be close to estimates obtained from the original population set. The entire procedure was performed in an automated fashion using Wings for NONMEM (http://wfn.sourceforge.net/). This procedure also provided statistics of the population parameters.
(ii) Visual predictive check (VPC) validation.
3TC concentration profiles were simulated and compared with the observed data to evaluate the predictive performance of the model. The vector of pharmacokinetic parameters from 1,000 patients was simulated using the final model. Each vector parameter was drawn in a log-normal distribution with a variance corresponding to the ISV previously estimated. A simulated residual error was added to each simulated concentration. All observed and simulated concentrations were standardized for a 150-mg 3TC dose as dose proportionality of lamivudine pharmacokinetics PK has previously been demonstrated (13). The 5th, 50th, and 95th percentiles of the simulated concentrations at each time were then overlaid on the observed concentration data using RfN, and a visual inspection was performed. The variability was reasonably estimated if the 95% confidence interval (95% CI) for the proportion of observed data outside the bounds included the theoretical value of 10%.
In addition, epsilon (ɛ) and eta (η) shrinkage was quantified (20). Shrinkage, when present, adversely affected individual pharmacokinetics parameter estimates.
Concentrations: effect relationships.
For each patient, 3TC maximal (Cmax) and minimal (Cmin) concentrations and area under the curve (AUC) were derived from the estimated individual pharmacokinetic parameters.
The efficacy was studied following the difference in viral loads after 12 months of treatment. The significance of the viral load decrease was tested using a Wilcoxon nonparametric paired test. With respect to efficacy, the link between Cmin, Cmax, AUC, and the difference in HIV-1 RNA levels between inclusion and the time of treatment were evaluated using Spearman's correlation tests. A tree regression analysis for clearance (CL/F [liters/h/kg], where F represents bioavailability) was performed according to body weight, and children were divided into two groups. Different once-daily doses were simulated in each group to obtain the same exposure in children as the mean effective exposure in adults (Epivir: summary of product characteristics [GlaxoSmithKline, Research Triangle Park, NC]).
RESULTS
Demographic data.
Forty-five children (17 girls and 28 boys) and 148 plasma concentrations were available for pharmacokinetic evaluation. Table 1 summarizes the patients' characteristics. The median lamivudine dose administered was 150 mg, for doses ranging from 90 to 300 mg.
TABLE 1.
Characteristics of the 45 HIV-infected children enrolled in the pharmacokinetic study of the BURKINAME-ANRS 12103 trial
| Covariate | Median value | Minimum to maximum values |
|---|---|---|
| Age (yr) | 6.75 | 2.5-14 |
| Body wt (kg) | 17 | 11-37 |
| Size (cm) | 112 | 85-150 |
| Serum creatinine concn (μmol/liter) | 44.99 | 9.64-86.19 |
| Alanine aminotransferase concn (U/liter) | 21 | 5-211 |
| Aspartate aminotransferase concn (U/liter) | 54 | 16-457 |
| Total bilirubin concn (μmol/liter) | 6.9 | 1.36-66.35 |
Clinical, immunological, and virological data.
Clinically, 16 children were at stage A, 28 at stage B, and 5 at stage C of HIV infection, according to CDC clinical category (6). Before treatment, the mean percentage of CD4 was 8.62% (median, 8%; range, 0.4 to >26%), and the average CD4 lymphocyte count was 336/mm3 (median, 260/mm3; range, 2 to 1,510 mm3). The median viral load was 5.5 log10 copies/ml (range, 4.6 to 6.7 copies/ml). Patients with this high degree of infection severity were included in this trial, because these were the inclusion criteria for HAART in Burkina Faso.
Population pharmacokinetics.
Seven concentrations were below the BLQ, corresponding to less than 5% of the total observations. The M3 method and the built-in M2 method did not modify the parameter estimates; thus, we kept the method of setting those concentrations to half of the BLQ (1). A two-compartment model in which the distribution rate constant (alpha) is assumed to be equal to Ka adequately described the data. Thus, the apparent parameters of the model were the clearance (CL/F), the central volume of distribution (Vc/F), the peripheral volume of distribution (Vp/F), and the intercompartmental clearance (Q/F), where F is the unknown bioavailability. Residual variability was best described by a multiplicative error model. Intersubject variability (ISV) was retained only for apparent clearance and described by the exponential error model. The allometric scaling of clearance (CL and Q) and volume terms (Vc and Vp) resulted in a 9-U decrease in the objective function value. After the inclusion of the allometric scaling, age had no significant effect on clearance. Neither the biological parameters (basal creatinine, amylase, ALAT, ASAT, and total bilirubin) nor the Galenic form had a significant effect or reduced OFV by more than 6.63 U.
Figure 1 displays observed and predicted plasma 3TC concentrations as a function of time for the children. Table 2 summarizes the final population pharmacokinetic estimates. All of the parameters were well estimated, given their relative standard error (RSE %). There was no significant shrinkage: the η shrinkage for CL/F was 0.07, and the ɛ shrinkage was 0.44, indicating that the empirical Bayesian estimates for individual CL/F values are reliable.
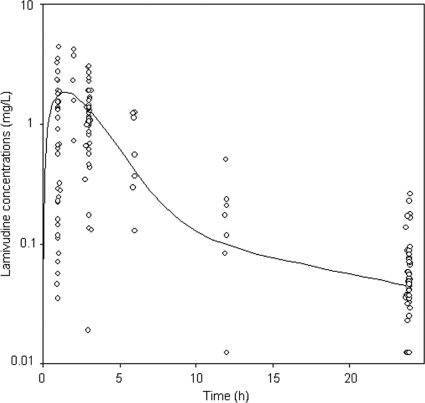
FIG. 1.
Observed lamivudine concentrations (○) and population-predicted lamivudine concentrations (curve) in log scale for a child with median body weight (16.8 kg) as a function of time.
TABLE 2.
Population pharmacokinetic parameters of lamivudine standardized for a median weight of 16.8 kg using an allometric modela
| Model type and parameterb | Mean (RSE %)c value for final model with original data set | Median (95% CI) bootstrap valued |
|---|---|---|
| Structural models | ||
| CL/F (liters/h) | 16.9 (8.7) | 16.7 (13.5-19.8) |
| Vc/F (liters) | 30.8 (9.3) | 31.2 (26-38.3) |
| Vp/F (liters) | 58.6 (23.5) | 57.1 (35-145) |
| Q/F (liters/h) | 4.48 (18.1) | 4.60 (2.87-9.06) |
| Ka (h−1) | 0.71 (NA)e | NA |
| Statistical models | ||
| σ | 0.60 (14.8) | 0.59 (0.51-0.68) |
| ω (CL/F) | 0.30 (30.6) | 0.28 (0.15-0.37) |
Shown are the results from the final model and bootstrap evaluation for 45 HIV-1-infected children enrolled in the BURKINAME-ANRS 12103 study.
The typical parameters represent a patient weighing 16.8 kg according to an allometric model: (typical value) = (typical parameter) × (body weight/16.8)PWR, where PWR = 0.75 for the CL and Q terms and 1 for the Vc and Vp terms. Ka, absorption rate constant (Ka equals the rapid distribution rate constant derived from the estimated pharmacokinetics parameters); CL/F, apparent elimination clearance, Vc/F apparent central volume of distribution; Vp/F, apparent peripheral volume of distribution; Q/F, intercompartmental clearance; σ, residual variability estimates; and ω, interindividual variability estimates.
RSE %, relative standard error (standard error of estimate/estimate × 100).
Statistics from 1,000 bootstrap analyses.
NA, not applicable.
Evaluation and validation.
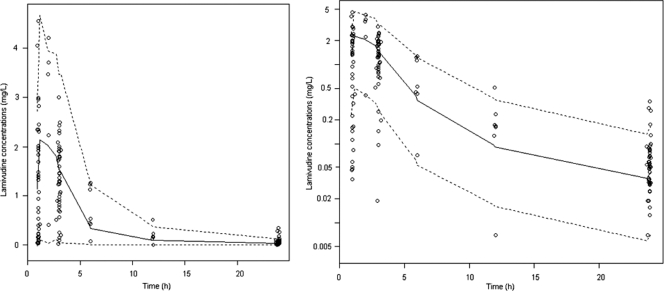
As shown in Table 2, the medians and 95% confidence intervals (95% CIs) of parameter estimates obtained from the bootstrap process (1,000 runs), were close to the estimates previously obtained with the original data set (Table 2). Figure 2 (VPC) shows that the average prediction matches the observed concentration time-courses and that the variability is reasonably estimated. The number (percentage) of observed points within the 90% prediction interval was 133/148 (90%).
FIG. 2.
Evaluation of the final model: comparison between the 5th (dash line), 50th (full line), and 95th (dash line) percentiles obtained from 1,000 simulations, standardized to a 150-mg dose for all children, and the observed data (○) for lamivudine concentrations in cartesian and logarithm scales.
Drug concentrations in children and efficacy.
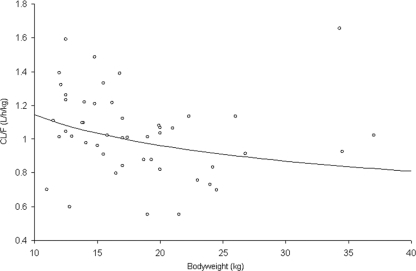
Table 3 summarizes lamivudine Cmax, Cmin AUC, and CL/F in the present and previous studies. Viral loads at baseline and after 12 months of treatment were both available in 48 out of the 49 children included in the study; The viral load significantly decreased after 12 months of treatment from median (minimum to maximum) values of 5.5 (3.6 to 6.7) to 2.5 (2.5 to 6.0) log10 copies/ml (P < 10−4). The concentration-efficacy analysis was performed using the 45 children for which both pharmacokinetics and pharmacodynamics data were available. The Cmin, Cmax, and AUC could not be correlated to the significant viral load decrease. As we could not find a significant concentration-effect relationship, a target AUC of 8.9 mg·h/liter associated with its 95% CI (5.2 to 12.6) obtained in adults was used (Epivir: summary of product characteristics [GlaxoSmithKline, Research Triangle Park, NC]). Since only body weight was included in our model, different body weight groups were separated thanks to tree regression analysis. CL/F (liters/h/kg) was significantly higher in children weighing less than 17 kg (n = 22) than that in children over 17 kg (n = 23): 1.12 liters/h/kg against 0.95 liter/h/kg (P = 0.01) (Fig. 3).
TABLE 3.
Comparison of 3TC-derived pharmacokinetic parameters between our study and the latest publications of data from HIV-1-infected children
| Study (reference) | Group | Dose | Value for parametera: |
|||
|---|---|---|---|---|---|---|
| Cmin (mg/liter) | Cmax (mg/liter) | AUC0→τ (mg/liter·h) | CL/F (liters/h/kg) | |||
| l'Homme et al. (14) | Mean age, 6.9 yr (n = 65) | 4 mg/kg q12h | 0.09 | 1.33 [0.69] | 5.42 [2.26] | |
| Burger et al. (5) | Median age, 3.8 yr (IQR, 2.5-4.8 yr) (n = 17) | 4 mg/kg q12h | 0.07 (0.06-0.08) | 0.98 (0.82-1.43) | 3.71 (3.44-6.17) | 1.03 (0.67-1.22) |
| Burger et al. (5) | Median age, 10.1 yr (IQR 8.5-12.3 yr) (n = 34) | 4 mg/kg q12h | 0.09 (0.07-0.11) | 1.84 (1.21-2.28) | 6.54 (5.21-8.58) | 0.57 (0.45-0.74) |
| Bergshoeff et al. (4) | Age, 2.1-12.8 yr (n = 19) | 4 mg/kg q12h | 0.067 | 1.11 (0.96-1.29) | 4.44 (3.83-5.14) | 0.90 (0.78-1.04) |
| Bergshoeff et al. (4) | Age, 2.1-12.8 yr (n = 19) | 8 mg/kg q24h | 0.05 | 2.09 (1.80-2.42) | 9.80 (8.64-11.12) | 0.80 (0.70-0.92) |
| This study | Age, 2.5-14 yr (n = 45) | 8 mg/kg q24h | 0.04 (0.01-0.14) | 1.7 (1.34-2.01) | 7.8 (4.72-14.27) | 1.03 (0.55-1.62) |
Values for the study by l'Homme et al. (14) are given as means [standard deviation]. All other values are median values, with interquartile ranges (IQR) shown in parentheses.
FIG. 3.
Apparent elimination clearance expressed in liters/h/kg (○) as a function of body weight. The line was drawn after the equation used in the allometric model.
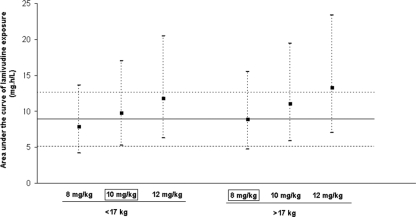
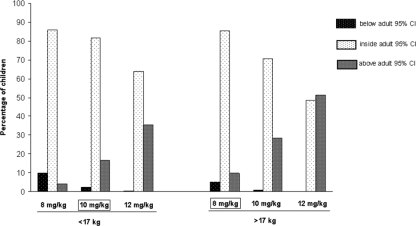
If the 45 children were really administered 8 mg/kg every 24 h (q24h), the mean AUC would be 8.1 mg·h/liter for children weighing less than 17 kg and 9.1 mg/h/liter for children over 17 kg. This suggests that a higher dose (in mg/kg) should be needed in children <17 kg to reach the target AUC of 8.9 mg·h/liter. A dose adjustment was then evaluated by simulating for 45,000 children (1,000 replicates of the database) the mean AUC after 3TC doses of 8, 10, and 12 mg/kg (Fig. 4). The percentage of children below, inside, and above the adult 95% CI for AUC was then evaluated for each dose (Fig. 5). The 8-mg/kg dose once a day (q.d.) for the children with a body weight of more than 17 kg (producing a mean AUC of 8.9 mg/liter) and the 10-mg/kg dose q.d. for the children with a body weight of less than 17 kg (producing a mean AUC of 9.8 mg/liter) seems to be an appropriate dosage for the sake of efficacy. Indeed as shown in Fig. 5, these doses appear to reduce the percentage of children below the adult 95% CI thus potentially being exposed to subtherapeutic concentrations without improving the percentage of children overexposed.
FIG. 4.
Mean area under the curve (▪) of lamivudine and 95% CI (dashed line) following different doses based on simulations of 45,000 children divided in two body weight groups. The solid line represents the target mean AUC of 8.9 mg·h/liter, and the dotted lines represent the 95% CI seen in an adult.
FIG. 5.
Percentage of children below, inside, and above the adult 95% CI for AUC for each simulated dose for the two body weight groups.
DISCUSSION
This paper describes pharmacokinetics for lamivudine combined with efavirenz and didanosine in 45 children from 2.5 to 14 years of age. No pharmacokinetic interaction between these drugs has previously been shown (16). 3TC concentrations were satisfactorily described by a two-compartment model with first order elimination in which the fast distribution rate constant is assumed to be equal to the absorption rate constant. Lamivudine freely penetrates tissue beyond the systemic circulation and is able to distribute through a peripheral compartment (13, 17). Thanks to the infants with a full pharmacokinetics profile, the two-compartment model parameters could be used, as previously described in children (17). The following observations support this model: (i) minimal and maximal concentrations and area under the curve were consistent with previous studies (4, 5, 14) (Table 3), and (ii) the apparent elimination clearance (CL/F = 1.03 liters/h/kg) was consistent with previous studies (4, 5). The population model was also used to investigate the effect of growth (body weight) and maturation (age) on pharmacokinetic parameters. In our model, no effect of age on clearance was observed after allometric scaling of the parameters. As only body weight was significant in our model, children were divided using tree regression analysis: the clearance was significantly lower in the group with body weights less than 17 kg. This is in agreement with Burger et al. (5), who showed that the youngest children—and thus the lightest—had lower exposure and would be exposed to subtherapeutic concentrations due to a higher apparent elimination clearance.
The increase in apparent clearance (per kg) of lamivudine previously reported in the youngest children (4, 5, 13) could reflect the physiological evolution of the clearance with body weight. Thus, the allometric scale required that clearance increases with body weight to the 0.75th power, but lamivudine is usually given on a proportional mg/kg basis, which could explain the inaccurate dosing in children with the lowest body weight. On the other hand, increased renal clearance in young children can also be explained by an important value of their kidney weight as a percentage of total body weight, and this value tends to decline as body weight increases (7).
No relationship between concentration and efficacy of lamivudine in children had been successfully demonstrated in this study. Thus, we considered an AUC for lamivudine of 8.9 mg·h/liter, corresponding to the level that adults would reach if they were perfectly adherent to the standard dose regimen and had average values for bioavailability and total body clearance. The once-daily dose of 8 mg/kg was not sufficient to reach this mean AUC in children weighing less than 17 kg. Different once-daily doses were simulated in the <17-kg and >17-kg groups (1,000 simulations per group). The target mean AUC was obtained with a 10-mg/kg once-daily dose of 3TC for children below 17 kg; the recommended dose of 8 mg/kg seems to be sufficient in children weighing more than 17 kg. These dose recommendations found in this study should be prospectively confirmed, as conclusions were drawn from only 45 children, and no data on toxicity are available with these increased doses. The limit of 17 kg does not correspond to the weight group recommended by the WHO; however, it was the statistical limit obtained with tree regression analyses which discriminate the clearances. On the other hand, post hoc analyses using the weight groups recommended showed us that the 15- to 17-kg children were a part of the 10- to 15-kg group and not the >17-kg group for dose adjustment.
No difference in virologic responses was noted in patients with suboptimal versus optimal exposures; however, the question of low lamivudine exposure resulting in development of lamivudine resistance over the long term should be addressed.
In conclusion, this study reports 3TC pharmacokinetics in children after once-daily administration. The pharmacokinetic parameter was consistent with previous study. The lamivudine elimination clearance (in liters/h/kg) decreases with body weight. According to simulations, to reach the target adult AUC of 8.9 mg/liter·h, children weighing less than 17 kg should receive 10 mg/kg/day, whereas the actual recommended dose of 8 mg/kg seems to be sufficient for the >17-kg children. These assumptions should be prospectively confirmed.
Acknowledgments
We acknowledge the French “Agence Nationale de Recherche contre le VIH/SIDA et les Hepatitis Virales” (ANRS) for sponsoring the trial.
We thank the children of the study and their parents. We also thank Naïma Tafzi and Martine Babiard for the plasma quantification of lamivudine.
Footnotes
Published ahead of print on 1 June 2010.
REFERENCES
- 1.Ahn, J. E., M. O. Karlson, A. Dunne, and T. M. Ludden. 2008. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J. Pharmacokinet Pharmacodyn. 35:401-421. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, B. J., and N. H. G. Holford. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 48:303-332. [DOI] [PubMed] [Google Scholar]
- 3.Beal, S. L., and L. B. Sheiner. 1998. NONMEM user's guide. NONMEM Project Group, University of California, San Francisco.
- 4.Bergshoeff, A., D. Burger, C. Verweij, L. Farrelly, J. Flynn, M. Le Prevost, S. Walker, V. Novelli, H. Lyall, S. Khoo, and D. Gibb. 2005. Plasma pharmacokinetics of once- versus twice-daily lamivudine and abacavir: simplification of combination treatment in HIV-1-infected children (PENTA-13). Antivir. Ther. 10:239-246. [PubMed] [Google Scholar]
- 5.Burger, D. M., G. Verweel, N. Rakhamanina, C. P. Verwey-Van Wissen, C. J. La Porte, A. S. Bergshoeff, H. Lyall, N. G. Hartwig, H. Green, S. Soldin, D. M. Gibb, and R. de Groot. 2007. Age-dependent pharmacokinetics of lamivudine in HIV-infected children. Clin. Pharmacol. Ther. 81:517-520. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1994. Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Morb. Mortal. Wkly. Rep. 43:No-RR12. [Google Scholar]
- 7.Chen, N., K. Aleska, C. Woodland, M. Rieder, and G. Koren. 2006. Ontogeny of drug elimination by the human kidney. Pediatr. Nephrol. 21:160-168. [DOI] [PubMed] [Google Scholar]
- 8.Djomand, G., T. Roels, T. Ellerbrock, D. Hanson, F. Diomande, B. Monga, C. Maurice, J. Nkengasong, R. Konan-Koko, A. Kadio, S. Wiktor, E. Lackritz, J. Saba, and T. Chorba. 2003. Virologic and immunologic outcomes and programmatic challenges of an antiretroviral treatment pilot project in Abidjan, Cote d'Ivoire. AIDS 17(Suppl. 3):S5-S15. [DOI] [PubMed] [Google Scholar]
- 9.Dybul, M., E. Nies-Kraske, R. Dewar, F. Maldarelli, C. W. Hallahan, M. Daucher, S. C. Piscitelli, L. Ehler, A. Weigand, S. Palmer, J. A. Metcalf, R. T. Davey, D. M. Rock Kress, A. Powers, I. Beck, L. Frenkel, M. Baseler, J. Coffin, and A. S. Fauci. 2004. A proof-of-concept study of short-cycle intermittent antiretroviral therapy with a once-daily regimen of didanosine, lamivudine, and efavirenz for the treatment of chronic HIV infection. J. Infect. Dis. 189:1974-1982. [DOI] [PubMed] [Google Scholar]
- 10.Fassinou, P., N. Elenga, F. Rouet, R. Laguide, K. A. Kouakoussui, M. Timite, S. Blanche, and P. Msellati. 2004. Highly active antiretroviral therapies among HIV-1-infected children in Abidjan, Cote d'Ivoire. AIDS 18:1905-1913. [DOI] [PubMed] [Google Scholar]
- 11.Harker, A. J., G. L. Evans, A. E. Hawley, and D. M. Morris. 1994. High-performance liquid chromatography assay for 2′-deoxy-3′-thiacytidine in human serum. J. Chromatogr. B Biomed. Appl. 657:227-232. [DOI] [PubMed] [Google Scholar]
- 12.Ihaka, R., and R. Gentleman. 1996. R: a language for data analysis and graphics. J. Comput. Graph. Stat. 5:299. [Google Scholar]
- 13.Johnson, M. A., K. H. Moore, G. J. Yuen, A. Bye, and G. E. Pakes. 1999. Clinical pharmacokinetics of lamivudine. Clin. Pharmacokinet. 36:41-66. [DOI] [PubMed] [Google Scholar]
- 14.l'Homme, R. F. A., D. Kabamba, F. M. Ewings, V. Mulenga, C. Kankasa, M. J. Thomason, A. S. Walker, C. Chintu, D. M. Burger, and D. M. Gibb. 2008. Nevirapine, stavudine and lamivudine pharmacokinetics in African children on pediatric fixed-dose combination tablets. AIDS 22:557-565. [DOI] [PubMed] [Google Scholar]
- 15.Maggiolo, F., D. Ripamonti, G. Gregis, G. Quinzan, A. Callegaro, C. Arici, L. Ravasio, and F. Suter. 2003. Once-a-day therapy for HIV infection: a controlled, randomized study in antiretroviral-naive HIV-1-infected patients. Antivir. Ther. 8:339-346. [PubMed] [Google Scholar]
- 16.Moreno, S., B. Hernandez, and F. Dronda. 2007. Didanosine enteric-coated capsule: current role in patients with HIV-1 infection. Drugs 67:1441-1462. [DOI] [PubMed] [Google Scholar]
- 17.Mueller, B. U., L. Lewis, G. Yuen, M. Farley, A. Keller, J. Church, J. Goldsmith, D. Venzon, M. Rubin, P. Pizzo, and F. Balis. 1998. Serum and cerebrospinal fluid pharmacokinetics of intravenous and oral lamivudine in human immunodeficiency virus-infected children. Antimicrob. Agents Chemother. 42:3187-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parke, J., N. H. Holford, and B. G. Charles. 1999. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput. Methods Programs Biomed. 59:19-29. [DOI] [PubMed] [Google Scholar]
- 19.Rouet, F., M. L. Chaix, E. Nerrienet, N. Ngo-Giang-Huong, J. C. Plantier, M. Burgard, M. Peeters, F. Damond, D. K. Ekouevi, P. Msellati, L. Ferradini, S. Rukobo, V. Marechal, N. Schvachsa, L. Wakrim, C. Rafalimanana, B. Rakotoambinina, J. P. Viard, J. M. Seigneurin, and C. Rouzioux. 2007. Impact of HIV-1 genetic diversity on plasma HIV-1 RNA quantification: usefulness of the Agence Nationale de Recherches sur le SIDA second-generation long terminal repeat-based real-time reverse transcriptase polymerase chain reaction test. J. Acquir. Immune. Defic. Syndr. 45:380-388. [DOI] [PubMed] [Google Scholar]
- 20.Savic, R. M., and M. O. Karlson for Population Approach Group Europe. 2007. Shrinkage in empirical Bayes estimates for diagnostics and estimation. Division of Pharmacokinetics and Drug Therapy, Department of Pharmaceutical Biosciences, Uppsala University, Uppsala, Sweden.
- 21.United Nations Programme on HIV/AIDS and World Health Organization. Accessed 27 February 2009. AIDS epidemic update: December 2007. http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf2007.
- 22.Van Rossum, A. M., P. L. Fraaij, and R. de Groot. 2002. Efficacy of highly active antiretroviral therapy in HIV-1 infected children. Lancet Infect. Dis. 2:93-102. [DOI] [PubMed] [Google Scholar]
- 23.Wijnand, H. P. 1988. Pharmacokinetic model equations for the one and two-compartment models with first-order processes in which the absorption and exponential elimination or distribution rate constants are equal. J. Pharmacokinet. Pharmacodyn. 16:109-128. [DOI] [PubMed] [Google Scholar]