Abstract
CXA-101 is a novel, broad-spectrum cephalosporin with excellent antipseudomonal activity. A Phase 1 study was performed to determine the safety, tolerability, and pharmacokinetics of CXA-101 after single- and multiple-dose intravenous administration over 1 h to healthy male and female subjects. In part 1 of the study, five cohorts of eight subjects each (six receiving CXA-101 and two receiving a placebo) received single ascending doses of 250, 500, 1,000, 1,500, and 2,000 mg. In part 2, cohorts 1 and 2 received 500 mg and 1,000 mg, respectively, every 8 h, and cohort 3 received 1,500 mg every 12 h; each cohort received dosing for 10 days. Standard safety and tolerability assessments were performed. Blood and urine pharmacokinetic samples were assayed by a validated bioanalytical method and analyzed using standard noncompartmental methodology. All 64 subjects completed dosing; none withdrew from the study. Drug-related systemic adverse events were infrequent and mild. Mild, non-treatment-limiting infusion site events occurred during multiple-dose administration. No clinically significant laboratory or electrocardiographic finding or dose-limiting toxicity was observed. CXA-101 exhibited dose-linear pharmacokinetics; the mean plasma half-life was ∼2.3 h. More than 90% of the administered dose was eliminated unchanged through renal excretion. In summary, CXA-101 administered as a 1-hour infusion was generally safe and well tolerated in single doses up to 2,000 mg and in multiple doses up to 3 g daily over 10 days. The favorable safety and predictable pharmacokinetic profile of CXA-101 support its continuing clinical development for the treatment of serious bacterial infections.
The medical need for new antibiotics active against multiply drug-resistant Gram-negative pathogens has been clearly established (5). Organisms of concern include Pseudomonas aeruginosa, as well as extended-spectrum β-lactamase-producing Enterobacteriaceae (5, 10, 13). Unfortunately, the current pipeline for such agents is unimpressive (1, 4, 16, 17).
CXA-101 (previously FR264205) is a broad-spectrum cephalosporin with excellent in vitro and in vivo activity against P. aeruginosa, including drug-resistant isolates (3, 12). In general, the anti-Gram-positive and -Gram-negative profile of CXA-101 is similar to that of third-generation cephalosporins, such as ceftazidime, but its antipseudomonal activity is the most potent among all currently available β-lactams, including the cephalosporins and carbapenems (3). Indeed, the minimum concentration of CXA-101 that inhibits 90% of the microbial strains (MIC90) for P. aeruginosa (≤2 μg/ml) is the lowest among all systemically administered antipseudomonal antibiotics (2, 3). Most importantly, CXA-101 has been shown to be active in vitro against strains of P. aeruginosa that are resistant to carbapenems, cephalosporins, fluoroquinolones, and/or aminoglycosides, including the majority of multiple-drug-resistant isolates (3, 8, 9, 14; unpublished data). CXA-101 shares the antibacterial mode of action of other β-lactam antibiotics by targeting penicillin-binding proteins to inhibit the biosynthesis of the bacterial cell wall. The binding affinity of CXA-101 is at least 2-fold higher than that of ceftazidime for each of the essential penicillin-binding proteins in P. aeruginosa (1b, 1c, 2, and 3), and it is particularly potent for penicillin-binding protein 3 (11).
In addition to its clinically useful antibacterial activity, CXA-101 has performed well in standard preclinical toxicological assessments and exhibits modest protein binding in human plasma of approximately 20% (unpublished data). Accordingly, an initial phase 1 study was conducted to determine the safety, tolerability, and pharmacokinetic profile of CXA-101 in humans after single- and multiple-dose intravenous administration.
(Presented in part at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, September 2009 [poster F1-2004].)
MATERIALS AND METHODS
Study design.
Healthy male and female volunteers, 18 to 60 years of age, were eligible for participation in this single-center, prospective, randomized, double-blind, placebo-controlled study of ascending single (part 1) and multiple (part 2) doses of CXA-101 administered intravenously via a peripheral venous catheter. In part 1, five successive cohorts of eight subjects each (six active, two placebo) received a single intravenous dose of the study drug. CXA-101 was given as a 1-hour intravenous infusion at ascending doses of 250, 500, 1,000, 1,500, and 2,000 mg. In part 2, three successive cohorts of eight subjects each (six active, two placebo) received multiple intravenous doses of the study drug for 10 days (in each cohort, a single dose was given on days 1 and 10). Cohorts 1 and 2 received 500 mg and 1,000 mg CXA-101, respectively, infused over 1 h every 8 h; cohort 3 received 1,500 mg infused over 1 h every 12 h.
Safety and tolerability evaluations.
Safety was assessed frequently during the study through clinical signs and symptoms; resting vital signs; serial physical examinations; recording of adverse events, including systemic adverse events and those occurring locally at the infusion site; laboratory testing; 12-lead electrocardiogram recordings; and continuous cardiac monitoring.
Pharmacokinetic evaluations.
Plasma samples for pharmacokinetic analysis were collected within 15 min prior to the start of study drug administration; 30 min after the start and at completion of infusion; and 5, 15, and 30 min and 1, 2, 3, 5, 7, 9, 11, 15, and 23 h after completion of infusion. Urine for pharmacokinetic analysis was obtained at 0 to 2, 2 to 4, 4 to 8, 8 to 12, and 12 to 24 h after the start of study drug administration. For subjects enrolled in part 2, sampling was performed on study days 1 and 10.
CXA-101 was quantified using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay, using solid-phase extraction of plasma and urine followed by separation with reverse-phase chromatography and detection by tandem mass spectrometry. The bioanalytical assay was fully validated according to standard Good Laboratory Practice (GLP) procedures, and all pharmacokinetic samples were also tested according to FDA guidelines and GLP. The lower limits of quantification were 100 ng/ml and 5,000 ng/ml for plasma and urine, respectively.
Plasma concentration-time profiles were constructed, and the following pharmacokinetic parameters were estimated for the plasma data using noncompartmental methods: maximum plasma concentration (Cmax), plasma half-life (T1/2), area under the concentration-time curve (AUC), clearance (CL), elimination rate constant, terminal half-life, volume of distribution at steady state (Vss), and mean residence time (MRT). The percentage of the administered dose that was recovered in the urine was determined, and the renal clearance (CLR) was estimated as the percent urinary recovery times CL. The relationships of pharmacokinetic parameters and dose were evaluated by visual/graphical examination and/or linear regression analyses.
RESULTS
Demographic data.
Demographic data for the subjects receiving CXA-101 are shown in Table 1.
TABLE 1.
Demographic data for subjects receiving CXA-101
| Parameter | Value |
|||
|---|---|---|---|---|
| Part 1, single intravenous dosesa |
Part 2, multiple intravenous dosesb |
|||
| Mean | Median; range | Mean | Median; range | |
| Age (yr) | 33.1 | 29.5; 19-59 | 35.2 | 33.0; 22-55 |
| Weight (kg) | 74.1 | 73.3; 58.1-96.5 | 75.3 | 73.2; 60.2-93.5 |
| CLCR (ml/min) | 124.1 | 127.4; 76.4-171.0 | 124.8 | 117.2; 87.1-182.3 |
n (male/female), 30 (17/13).
n (male/female), 18 (13/5).
Safety/tolerability.
All 64 subjects enrolled in parts 1 and 2 of the study completed dosing; none withdrew from the study. Drug-related systemic adverse events were infrequent and mild (Table 2 shows part 1 data, and Table 3 shows part 2 data). The two reports of diarrhea in subjects receiving CXA-101 were brief and self-limited episodes of poorly formed stools; the subject who reported flushing had no objective findings during the event, which resolved during continued administration of the study drug. Mild, non-treatment-limiting infusion site adverse events, most commonly pain or erythema, occurred during multiple-dose CXA-101 administration. Infusion site signs and symptoms also occurred, at a lower frequency, with placebo infusions. No clinically significant untoward trend in biochemistry, hematology, coagulation, or urinalysis laboratory parameters was noted. No clinically significant electrocardiographic finding was reported. No dose-limiting toxicity was observed.
TABLE 2.
Systemic adverse events judged by the investigator as related to the study drug in part 1 of the studya
| Event | No. of occurrences in cohort |
|||||
|---|---|---|---|---|---|---|
| Total placebo (n = 10) | 250 mg (n = 6) | 500 mg (n = 6) | 1,000 mg (n = 6) | 1,500 mg (n = 6) | 2,000 mg (n = 6) | |
| Abdominal pain | 1 | |||||
| Nausea | 1 | |||||
| Headache | 1 | 1 | 1 | |||
| Paresthesia | 1 | |||||
| Somnolence | 1 | |||||
| Vulvo-vaginal pruritus | 1 | |||||
Single doses of CXA-101 or placebo (6 CXA-101, 2 placebo per cohort).
TABLE 3.
Systemic adverse events judged by the investigator as related to the study drug in part 2 of the studya
| Event | No. of occurrences in cohort |
|||
|---|---|---|---|---|
| Total placebo (n = 6) | 500 mg q8h (n = 6) | 1,000 mg q8h (n = 6) | 1,500 mg q12h (n = 6) | |
| Diarrhea | 2 | |||
| Hypoaesthesia | 1 | |||
| Paraesthesia | 1 | |||
| Flushing | 1 | |||
Multiple doses of CXA-101 or placebo (6 CXA-101, 2 placebo per cohort).
Major pharmacokinetic observations.
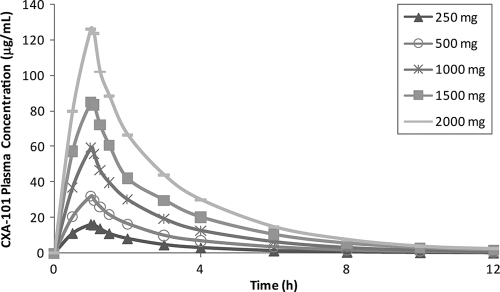
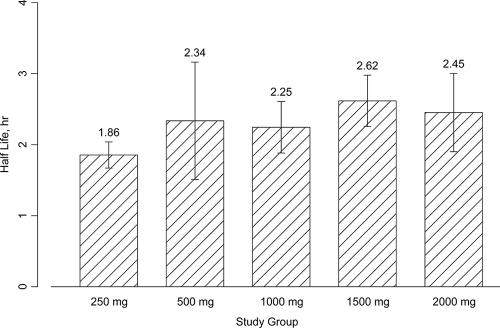
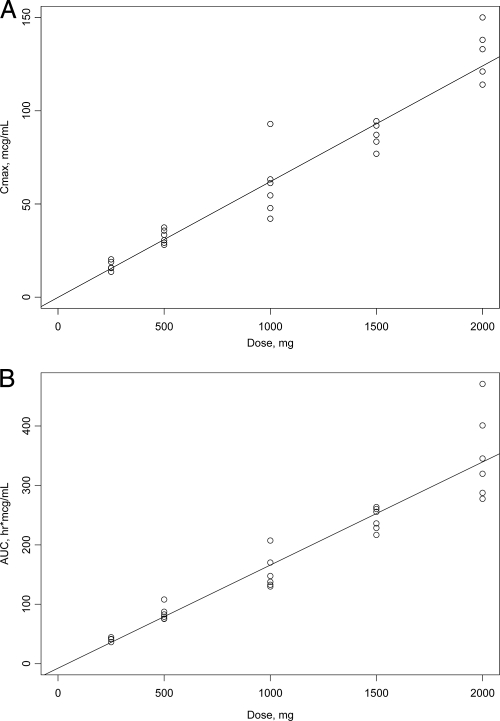
Pharmacokinetic parameters after single- and multiple-dose administration are shown in Table 4 and Table 5, respectively. Concentration-time profiles of CXA-101 after single-dose administration are shown in Fig. 1. CXA-101 Cmax and AUC were linear over the dose range (Fig. 2). Plasma T1/2 was independent of the dose and dosing duration, averaging 2.3 h (range, 1.86 to 2.64 h) (Fig. 3). Negligible drug accumulation occurred with the multiple-dose regimens, as evidenced by the minimal change in AUC after 10 days of repeated dosing (Table 5). Clearance, which was primarily renal and independent of dose and dosing duration, averaged 102.4 ml/min (±15.2%) after a single intravenous dose and 112.2 ml/min (±18.7%) after the last of the multiple doses; CL correlated well with CL of creatinine (CLCR). The large majority of CXA-101 (92.5% following a single intravenous dose and 95% following multiple intravenous doses) was excreted in the urine as unchanged CXA-101.
TABLE 4.
Pharmacokinetic parameters of CXA-101 after single ascending doses
| Parametera | Statisticb | Value in cohort |
||||
|---|---|---|---|---|---|---|
| 250 mg (n = 6) | 500 mg (n = 6) | 1,000 mg (n = 6) | 1,500 mg (n = 6) | 2,000 mg (n = 6) | ||
| Cmax (μg/ml) | Mean (% CV) | 16.5 (15.6) | 32.2 (12.1) | 58.4 (31.5) | 87.4 (8) | 127.7 (11.8) |
| Median (range) | 15.8 (13.6-20.3) | 31.9 (28.1-37.4) | 57.8 (42.1-92.9) | 89.5 (76.9-94.4) | 126.9 (114-150) | |
| Tmax (h) | Mean (% CV) | 1.03 (4.1) | 1.03 (2.7) | 1.05 (3.8) | 1.03 (3.8) | 1.06 (7) |
| Median (range) | 1.01 (1-1.09) | 1.02 (1.01-1.08) | 1.05 (1.01-1.09) | 1.02 (1-1.08) | 1.04 (1-1.16) | |
| AUC0-∞ (h·μg/ml) | Mean (% CV) | 40.1 (8.5) | 84.1 (14.4) | 152.1 (19.7) | 242.8 (8.3) | 344.2 (22.5) |
| Median (range) | 41.3 (36.2-44.4) | 80.9 (75.4-108) | 142.5 (129.9-207.2) | 245.7 (216.7-263.6) | 332.2 (277.6-470.8) | |
| CL (ml/min) | Mean (% CV) | 103.9 (8.5) | 99.1 (14.4) | 109.6 (19.7) | 103 (8.3) | 96.9 (22.5) |
| Median (range) | 100.9 (93.8-115) | 103.1 (77.2-110.6) | 116.9 (80.5-128.3) | 101.8 (94.9-115.4) | 100.4 (70.8-120.1) | |
| CLR (ml/min) | Mean (% CV) | 100.9 (11) | 90.4 (16.8) | 93.4 (47.6) | 96.2 (6.6) | 92.9 (29.9) |
| Median (range) | 100.4 (87.1-120.1) | 96.6 (69.5-105.2) | 103.4 (43.5-130.6) | 98.6 (86-102.2) | 94 (68.3-123.6) | |
| Vss (liter) | Mean (% CV) | 13.1 (14.9) | 14.8 (22) | 16.3 (25.5) | 17.6 (12.3) | 14.8 (9.2) |
| Median (range) | 13.7 (10.5-15) | 15.5 (11.1-18.6) | 16.5 (11-20.8) | 17.8 (15.4-20.8) | 14.4 (13.1-16.7) | |
| T1/2 (h) | Mean (% CV) | 1.86 (9.9) | 2.34 (35.4) | 2.25 (16.2) | 2.62 (13.8) | 2.45 (22.5) |
| Median (range) | 1.86 (1.63-2.06) | 2.35 (1.71-3.88) | 2.27 (1.82-2.68) | 2.5 (2.29-3.14) | 2.54 (1.85-2.98) | |
| MRT (h) | Mean (% CV) | 2.09 (11.2) | 2.5 (31) | 2.48 (13.3) | 2.85 (9.1) | 2.54 (22.9) |
| Median (range) | 2.05 (1.87-2.37) | 2.48 (1.91-4.01) | 2.53 (2.03-2.86) | 2.83 (2.57-3.25) | 2.59 (1.97-3.42) | |
| Urinary recovery in 24 h (% of dose) | Mean (% CV) | 97.1 (7.9) | 91.2 (4.5) | 85.2 (26.4) | 93.4 (6.1) | 97.7 (5.2) |
| Median (range) | 98.9 (85.8-104.8) | 90.6 (85.3-96.3) | 91 (54.1-104) | 93.9 (85.6-102.5) | 98.3 (91.5-102.9) | |
Cmax, maximum plasma concentration; Tmax, time of maximum plasma concentration; AUC0-∞, area under the concentration-time curve from zero hour to infinity; CL, clearance; CLR, renal clearance; Vss, volume of distribution at steady state; T1/2, half-life in plasma.
CV, coefficient of variation.
TABLE 5.
Pharmacokinetic parameters of CXA-101 after multiple intravenous infusions
| Parametera | Statisticb | Value in cohort |
|||||
|---|---|---|---|---|---|---|---|
| 500 mg q8h (n = 6) |
1,000 mg q8h (n = 6) |
1,500 mg q12h (n = 6) |
|||||
| Day 1 | Day 10 | Day 1 | Day 10 | Day 1 | Day 10 | ||
| Cmax (μg/ml) | Mean (% CV) | 34.3 (9) | 33.3 (15.9) | 52.8 (23.7) | 58 (10.3) | 85.4 (15.2) | 82.6 (15.9) |
| Median (range) | 33.3 (31.8-38.5) | 34.8 (25.6-37.8) | 53.3 (39.8-65.2) | 58.8 (48.1-64) | 89.1 (67.3-102) | 87.7 (67.1-93.8) | |
| Tmax (h) | Mean (% CV) | 1.03 (2.6) | 0.93 (24.1) | 1.03 (4.1) | 1.05 (9.1) | 1.03 (4) | 1 (0.2) |
| Median (range) | 1.03 (1-1.06) | 1.01 (0.6-1.08) | 1 (1-1.08) | 1.02 (1-1.25) | 1 (1-1.08) | 1 (1-1.01) | |
| AUC0-∞ (h·μg/ml) | Mean (% CV) | 88.1 (14.1) | 82.9 (20.6) | 148.6 (18.2) | 143.3 (15.4) | 219 (20) | 207 (15.9) |
| Median (range) | 84.7 (79.7-110.9) | 82.5 (61.5-104.7) | 155.3 (108.9-178.2) | 145.8 (115.5-173) | 243.1 (163.2-250) | 220.1 (166.5-240.6) | |
| CL (ml/min) | Mean (% CV) | 94.6 (14.1) | 100.6 (20.6) | 112.1 (18.2) | 116.4 (15.4) | 114.2 (20) | 120.8 (15.9) |
| Median (range) | 98.4 (75.2-104.5) | 101.1 (79.6-135.6) | 107.3 (93.6-153.1) | 114.3 (96.3-144.3) | 102.8 (100-153.2) | 113.6 (103.9-150.2) | |
| CLR (ml/min) | Mean (% CV) | 78.4 (34.2) | 87.6 (21.8) | 111.5 (13.4) | 111.2 (16.4) | 118.9 (26.9) | 127.2 (17.4) |
| Median (range) | 84.6 (57.7-113) | 84.6 (69.1-109.8) | 113.4 (92.4-133.2) | 110.6 (89-131) | 109.6 (91.3-176.9) | 125.7 (99.6-162.1) | |
| Vss (liter) | Mean (% CV) | 13.8 (7.2) | 14 (19.6) | 17.8 (21.5) | 17.1 (13.7) | 18.1 (13.8) | 18.1 (14.2) |
| Median (range) | 13.6 (13-15.5) | 13.4 (11.2-18.8) | 17.6 (14.5-23.4) | 17.6 (14.3-19.7) | 17.6 (15.3-21.6) | 17.5 (15.7-21.7) | |
| T1/2 (h) | Mean (% CV) | 2.21 (14.8) | 2.2 (17.7) | 2.38 (15.1) | 2.69 (24) | 2.72 (12.9) | 2.34 (4.9) |
| Median (range) | 2.22 (1.76-2.52) | 2.3 (1.78-2.6) | 2.36 (1.97-2.86) | 2.5 (2.12-3.71) | 2.76 (2.25-3.16) | 2.32 (2.22-2.52) | |
| MRT (h) | Mean (% CV) | 2.42 (15.9) | 2.32 (13.4) | 2.64 (13.4) | 2.45 (14) | 2.64 (11.7) | 2.5 (7.2) |
| Median (range) | 2.25 (2.1-3.02) | 2.36 (1.94-2.72) | 2.58 (2.27-3.3) | 2.39 (2.07-2.96) | 2.7 (2.2-3.03) | 2.45 (2.38-2.87) | |
| Urinary recovery (% of dose) | Mean (% CV) | 82.9 (25) | 84.6 (21.7) | 99.4 (8.1) | 94.1 (4) | 104.1 (8.9) | 105.3 (7.6) |
| Median (range) | 87.3 (59.4-108.1) | 81 (64.1-106.3) | 100.7 (87-107.2) | 92.4 (90.8-98.5) | 105.5 (89.4-115.5) | 106.5 (95.8-117) | |
| Fluctuation % | Mean (% CV) | NAc | 304.3 (9) | NA | 304.6 (12.2) | NA | 471.3 (5.9) |
| Median (range) | NA | 309.8 (264.4-334.6) | NA | 310.6 (253-341.6) | NA | 479.4 (422.9-497.1) | |
| Accumulation index | Mean (% CV) | NA | 1.092 (3.5) | NA | 1.155 (7.2) | NA | 1.03 (0.5) |
| Median (range) | NA | 1.099 (1.046-1.134) | NA | 1.123 (1.079-1.289) | NA | 1.029 (1.024-1.038) | |
Cmax, maximum plasma concentration; Tmax, time of maximum plasma concentration; AUC0-∞, area under the concentration-time curve from zero hour to infinity; CL, clearance; CLR, renal clearance; Vss, volume of distribution at steady state; T1/2, half-life in plasma.
CV, coefficient of variation.
NA, not applicable.
FIG. 1.
Plots of time-concentration profiles for single doses of CXA-101.
FIG. 2.
Plots of geometric mean (± standard deviation [SD]) half-life of CXA-101 after a single intravenous dose.
FIG. 3.
Linear regression analyses of CXA-101 after a single intravenous dose. (A) Cmax (R2, 0.9352); (B) AUC0-∞ (R2, 0.910).
DISCUSSION
In the current phase 1 study, CXA-101 was generally safe and well tolerated at the dose regimens studied. Drug-related systemic adverse events were infrequent and mild. Indeed, no dose-limiting toxicity was observed, suggesting the potential to evaluate higher doses. The most common adverse events occurred at the peripheral catheter infusion site, but these events were mild and not treatment limiting; such events also occurred, at a lower frequency, with placebo infusions. These adverse events are unlikely to be clinically important, especially in patients with severe infection treated in hospital, for whom this antibiotic will often be administered by a centrally inserted peripheral catheter or other central venous line.
The pharmacokinetic profile of CXA-101 after single- and multiple-dose administration was predictable and dose proportional within the dose range evaluated. The mean plasma half-life of CXA-101 was ∼2.3 h. Negligible accumulation of CXA-101 was observed after multiple dosing.
CXA-101 was eliminated almost entirely through urinary excretion; this observation suggests a need for dosage adjustment in patients with the most severe degrees of renal impairment. This consideration will be addressed in a future study. Regardless, the high urinary excretion of unchanged drug supports the hypothesis that CXA-101 could be efficacious in the treatment of urinary tract infection caused by P. aeruginosa and other susceptible pathogens. Furthermore, the absence of any known interaction with hepatic metabolic pathways provides confidence in a low likelihood of cytochrome P450-dependent drug-drug interactions.
The favorable safety and predictable pharmacokinetic profile of CXA-101 in this study and the excellent predicted probability of target attainment at the likely clinical dose regimen of 1,000 mg every 8 h (6) support further clinical development in humans for the treatment of bacterial infections, including in combination with the β-lactamase inhibitor tazobactam (7).
Acknowledgments
We appreciate the participation of MDS Pharma Services (Tempe, AZ); Axistat (San Francisco, CA); MicroConstants, Inc. (San Diego, CA); and InClin, Inc., Clinical Research Consultants (Los Altos Hills, CA).
I.F. is an employee of Calixa Therapeutics, Inc. (now Cubist Pharmaceuticals); Y.G. was an employee of Calixa Therapeutics, Inc.; M.J.W. was a consultant to Calixa Therapeutics, Inc., at the time the study was performed; and G.H.T. was and is a consultant to Calixa Therapeutics, Inc. We all hold equity in the company.
Footnotes
Published ahead of print on 10 May 2010.
REFERENCES
- 1.Boucher, H. W., G. H. Talbot, J. S. Bradley, J. E. Edwards, Jr., D. Gilbert, L. B. Rice, M. Scheld, B. Spellberg, and J. Bartlett. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1-12. [DOI] [PubMed] [Google Scholar]
- 2.Brown, N. P., C. M. Pillar, D. C. Draghi, M. K. Torres, C. Thornsberry, D. F. Sahm, and Y. Ge. 2008. Activity profile of CXA-101 against gram-positive and gram-negative pathogens by broth and agar dilution, abstr. F1-354. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. Washington, DC, 25 to 28 October 2008.
- 3.Brown, N. P., C. M. Pillar, D. F. Sahm, and Y. Ge. 2009. Activity profile of CXA-101 and CXA-101/tazobactam against target gram-positive and gram-negative pathogens, abstr. F1-1986. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 12 to 15 September 2009.
- 4.European Medicines Agency. 2009. The bacterial challenge: time to react. A call to narrow the gap between multidrug-resistant bacteria in the EU and the development of new antibacterial agents. ECDC/EMEA joint technical report. http://www.emea.europa.eu/pdfs/human/antimicrobial_resistance/EMEA-576176-2009.pdf.
- 5.Falagas, M. E., and I. A. Bliziotis. 2007. Pandrug-resistant gram-negative bacteria: the dawn of the post-antibiotic era? Int. J. Antimicrob. Agents 29:630-636. [DOI] [PubMed] [Google Scholar]
- 6.Ge, Y., and S. Liao. 2009. CXA-101 Population PK analysis and Monte Carlo simulation for PK/PD target attainment and dose regimen selection, abstr. F1-2003. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 12 to 15 September 2009.
- 7.Gin, A., L. Dilay, J. A. Karlowsky, A. Walkty, E. Rubinstein, and G. G. Zhanel. 2007. Piperacillin-tazobactam: a beta-lactam/beta-lactamase inhibitor combination. Expert Rev. Anti Infect. Ther. 5:365-383. [DOI] [PubMed] [Google Scholar]
- 8.Giske, C. G., I. Karlsson, and Y. Ge. 2009. CXA-101 (CXA) has high activity against clinical isolates of Pseudomonas aeruginosa including ceftazidime-resistant isolates, abstr. F1-1988. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 12 to 15 September 2009.
- 9.Juan, C., L. Zamorano, J. L. Pérez, Y. Ge, and A. Oliver. 2010. Activity of a new cephalosporin CXA-101 (FR264205) against carbapenem-resistant and multidrug-resistant Pseudomonas aeruginosa clinical strains. Antimicrob. Agents Chemother. 54:846-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livermore, D. M., and N. Woodford. 2006. The β-lactamase threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol. 14:413-420. [DOI] [PubMed] [Google Scholar]
- 11.Moya, B., L. Zamorano, J. L. Pérez, Y. Ge, and A. Oliver. 2009. Affinity of the new cephalosporin CXA-101 (CXA) to penicillin-binding proteins (PBPs) of Pseudomonas aeruginosa, abstr. F1-1985. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 12 to 15 September 2009.
- 12.Moya, B., L. Zamorano, C. Juan, J. L. Pérez, Y. Ge, and A. Oliver. 2010. Activity of a new cephalosporin, CXA-101 (FR264205), against {beta}-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patients. Antimicrob. Agents Chemother. 54:1213-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice, L. B. 2008. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 197:1079-1081. [DOI] [PubMed] [Google Scholar]
- 14.Riera, E., M. D. Macia, A. Mena, X. Mulet, J. L. Pérez, Y. Ge, and A. Oliver. 2009. Activity of the new cephalosporin CXA-101 against biofilms of relevant P. aeruginosa (PA) phenotypes in cystic fibrosis chronic respiratory infection: mucoid and hypermutable strains, abstr. F1-1990. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 12 to 15 September 2009.
- 15.Spellberg, B., J. H. Powers, E. P. Brass, L. G. Miller, and J. E. Edwards, Jr. 2004. Trends in antimicrobial drug development: implications for the future. Clin. Infect. Dis. 38:1279-1286. [DOI] [PubMed] [Google Scholar]
- 16.Spellberg, B., R. Guidos, D. Gilbert, J. Bradley, H. W. Boucher, W. M. Scheld, J. G. Bartlett, and J. Edwards, Jr. 2008. The epidemic of antibiotic resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 46:155-164. [DOI] [PubMed] [Google Scholar]
- 17.Talbot, G. H. 2008. What is in the pipeline for gram-negative pathogens? Expert Rev. Anti Infect. Ther. 6:39-49. [DOI] [PubMed] [Google Scholar]