Abstract
Invasive pulmonary aspergillosis is a life-threatening infection in lung transplant recipients; however, no studies of the pharmacokinetics and pharmacodynamics (PKPD) of echinocandins in transplanted lungs have been reported. We conducted a single-dose prospective study of the intrapulmonary and plasma PKPD of 150 mg of micafungin administered intravenously in 20 adult lung transplant recipients. Epithelial lining fluid (ELF) and alveolar cell (AC) samples were obtained via bronchoalveolar lavage performed 3, 5, 8, 18, or 24 h after initiation of infusion. Micafungin concentrations in plasma, ELF, and ACs were determined using high-pressure liquid chromatography. Noncompartmental methods, population analysis, and multiple-dose simulations were used to calculate PKPD parameters. Cmax in plasma, ELF, and ACs was 4.93, 1.38, and 17.41 μg/ml, respectively. The elimination half-life in plasma was 12.1 h. Elevated concentrations in ELF and ACs were sustained during the 24-h sampling period, indicating prolonged compartmental half-lives. The mean micafungin concentration exceeded the MIC90 of Aspergillus fumigatus (0.0156 μg/ml) in plasma (total and free), ELF, and ACs throughout the dosing interval. The area under the time-concentration curve from 0 to 24 h (AUC0-24)/MIC90 ratios in plasma, ELF, and ACs were 5,077, 923.1, and 13,340, respectively. Multiple-dose simulations demonstrated that ELF and AC concentrations of micafungin would continue to increase during 14 days of administration. We conclude that a single 150-mg intravenous dose of micafungin resulted in plasma, ELF, and AC concentrations that exceeded the MIC90 of A. fumigatus for 24 h and that these concentrations would continue to increase during 14 days of administration, supporting its potential activity for prevention and early treatment of pulmonary aspergillosis.
Postoperative invasive pulmonary aspergillosis is a frequent clinical problem among patients who have undergone lung transplantation (13, 23, 38-42). Strategies for management of invasive pulmonary aspergillosis in lung transplant recipients are not well defined. While voriconazole is indicated for the primary treatment of invasive aspergillosis, not all patients are able to tolerate this triazole, and drug interactions may be complicated. The role of echinocandins in treatment and prevention of invasive aspergillosis in lung transplant recipients is unknown. Although most lung transplant centers administer some form of antifungal prophylaxis, these regimens vary widely from center to center, and the optimal strategy for prophylaxis is unknown. Aerosolized amphotericin B, either alone or with systemically administered antifungal agents, may be used for prevention of invasive aspergillosis in lung transplant recipients (13).
Micafungin, a member of the echinocandin class of antifungal agents, has in vitro as well as in vivo activities against Candida spp. and Aspergillus spp. in treatment of experimental disseminated candidiasis (3, 5, 6, 33-35) and invasive pulmonary aspergillosis (33). Micafungin is licensed for the treatment of patients with esophageal candidiasis and candidemia (5, 12, 15, 30, 32, 36, 49). Micafungin also is approved for prevention of candidemia in neutropenic hematopoietic stem cell transplant recipients (44). Micafungin has been studied alone or in combination with other antifungal agents for treatment of invasive aspergillosis in hematopoietic stem cell transplant recipients (22). Although studies of micafungin for treatment and prevention of invasive pulmonary aspergillosis in animal models and in patients have demonstrated activity against this serious infection (8, 26, 33, 45), little is known about its intrapulmonary pharmacokinetics in patients at risk for invasive aspergillosis (28). We therefore studied the simultaneous intrapulmonary and plasma pharmacokinetics and pharmacodynamics of micafungin in adult lung transplant recipients.
MATERIALS AND METHODS
Study design and subjects.
Participation of patients in this prospective study was conducted under a clinical protocol approved by the University of California, San Francisco. Participation was voluntary and expressed through written informed consent. All subjects had undergone lung transplantation at the University of California, San Francisco. The median transplant-to-study time was 14 days, with a range of 5 to 280 days prior to participation in the study. Nineteen subjects received a single 150-mg intravenous dose, administered either in the outpatient infusion center (n = 9) or in the inpatient unit (n = 10), and one received a 150-mg dose once daily for four consecutive days, administered in the intensive care unit. Subjects were assigned to five groups of four subjects each, with bronchoscopy at 3, 5, 8, 18, or 24 h after the single or last dose.
Subjects were 21 years of age or older. Exclusion criteria included known hypersensitivity or intolerance to micafungin or other echinocandin drugs, pregnancy or breast-feeding, or concurrent treatment with nifedipine. If applicable, enrolled subjects confirmed that they were using contraception. Prior to enrollment, clinical laboratory test results (complete blood count with differential, platelet count, blood urea nitrogen, serum creatinine, alkaline phosphatase, total bilirubin, aspartate aminotransferase [AST], alanine aminotransferase [ALT], and albumin), completed in the course of routine posttransplant care, were recorded into the research record.
Subjects were observed during and for 1 h after infusion for adverse effects. Subjects were scheduled to receive bronchoscopy as a part of normal posttransplant care; infusions were scheduled so that the elapsed time between infusion and bronchoscopy corresponded to the time determined by the study schedule. The results of clinical laboratory tests performed after bronchoalveolar lavage (BAL) were recorded in the research record.
Bronchoscopy and bronchoalveolar lavage.
Bronchoscopy and BAL (9-11) were performed at the University of California, San Francisco Medical Center at 3, 5, 8, 18, or 24 h after the administration of the single or the last dose. Local anesthesia with topical lidocaine and systemic sedation with fentanyl and midazolam were used. Pulmonary epithelial lining fluid (ELF) and alveolar cells (AC) were collected by bronchoscopy and BAL in a lobe of the transplanted lung. The duration (mean ± standard deviation [SD]) of the lavage was 7.1 ± 6.0 min. BAL was performed by infusing, and promptly aspirating, 20-ml aliquots of sterile 0.9% saline. In the 19 of 20 patients who underwent BAL, the volume (mean ± SD) of saline infused was 126.3 ± 22.8 ml and the volume (mean ± SD) aspirated was 72.1 ± 10.6 ml. Aspiration without lavage was clinically indicated in one subject; the volume of aspirate was sufficient to include this specimen in our analysis. The last approximately 50 ml of aspirate was mixed by inversion, and 28.3 ± 6.5 ml was allocated for this study. A 1.5-ml aliquot of BAL fluid or aspirate was sent for cell count and differential, and a measured volume of the remaining fluid was spun at 400 × g for 5 min at 4°C. The cell pellet and supernatant were separated and frozen at −80°C until assayed for micafungin concentration. The supernatant was assayed for urea concentration by using a spectrophotometric assay (Infinity BUN reagent, enzymatic determination no. 63-UV; Sigma Diagnostics, St. Louis, MO).
Blood samples.
Blood was obtained for assay prior to administration of micafungin and at the approximate time of bronchoscopy and BAL. Blood samples were collected into sodium heparin-containing tubes and placed on ice until centrifugation. Plasma was frozen at −80°C until assay.
Micafungin assay.
Concentrations of micafungin were determined by reversed-phase high-pressure liquid chromatography (HPLC) using a Waters 2695 Alliance system as described previously (14). The mobile phase consisted of acetonitrile-ammonium acetate (ACN-AA; 50 mM; pH 4.0), delivered at a gradient flow rate of 0.4 ml/min from a 65/35% buffer/acetonitrile ratio in 12 min. The injection volume was 25 μl. The retention times of micafungin and anidulafungin (AFG; as internal standard [IS]; 10 μg/ml) were 7.9 min and 10.2 min, respectively, using a C8, 5-μm GL Sciences Inc. Inertsil analytical column (150 by 4.6 mm; GL Sciences, Inc., Tokyo, Japan) maintained at 50°C in conjunction with a precolumn C8 5-μm Inertsil (7.5 by 4.6 mm; GL Sciences) and ultraviolet detection (with a Waters 2996 photodiode array detector at 271.5 nm for micafungin and 305 nm for AFG).
Micafungin was extracted from heparinized plasma or BAL fluid by solid-phase extraction. C8 bond elute cartridges (1 ml; 100 mg; Varian, Palo Alto, CA) were conditioned by applying 500 μl of HPLC-grade acetonitrile and drained at 2 in. of Hg (as indicated by a Supelco gauge) followed by 1,000 μl of (10:90) ACN-AA (10:90) 50 mM pH 4.0 buffer with a vacuum of 2 in. of Hg. Three hundred microliters of plasma sample was spiked with 15 μl of the IS and added to the cartridges followed by 300 μl of the same (10:90) reagent. The samples were drained slowly with only the house vacuum pulling on the vacuum manifold (0 in. of Hg; Vac-Elut vacuum; Analytichem International, Harbor City, CA). C8 cartridges were washed, and elution was accomplished using 1.0 ml of a 90:10 mixture of methanol-AA. Eluent was evaporated under air by utilizing a Zymark Turbo Vap LV evaporator (American Laboratory Trading LLC, Niantic, CT) for 40 min (or until dry) at 40°C. Volumes of 100 or 50 μl of 50:50 methanol-AA (50 mM, pH 4.0) solution were used for reconstitution for plasma samples, and 100 μl was used for BAL samples.
Quantification was based on the ratio of the peak area of micafungin and IS. Standard curves that encompassed the expected experimental range of micafungin concentrations were constructed in their respective matrices: 100% plasma for plasma samples, and normal saline (1:5) with human BAL fluid for BAL samples. For plasma, the lower limit of quantification (LLQ) was 0.1 mg/liter with a coefficient of determination (r2) of ≥0.9992; for BAL, the LLQ was 0.075 mg/liter with an r2 of ≥0.9999. The intra- and interday coefficients of variation were ≤9.4% for both plasma and BAL fluid.
Monocyte enumeration.
Human monocytes (MNCs) were obtained from healthy adult volunteers by elutriation from the Transfusion Medicine Department of the National Institutes of Health Warren Grant Magnuson Clinical Center. Cells were initially washed in Hanks' balanced salt solution (HBSS−) without Ca2+ and Mg2+. The cells were then centrifuged at 470 relative centrifugal force (RCF) for 10 min at 4°C and resuspended to a final concentration of 1 × 107 cells/ml in normal saline. Viability was assessed by the trypan blue exclusion assay (2, 4).
Pellet extraction.
Micafungin was extracted from the BAL fluid pellet by solid-phase extraction as described earlier. Monocyte cells at a concentration of 107 cells/ml, spiked with micafungin, were used as the matrix for standards and quality controls (QCs), which were stored at −80°C for 24 h prior to processing. A 1,100-μl aliquot of ACN was added to 300 μl of thawed standard, QC, or pellet samples, vortexed, and incubated for 10 min at 4°C. Afterwards the samples were centrifuged at 800 RCF for 10 min. One milliliter of supernatant was mixed with 9 ml of AA pH 4 buffer and extracted employing the same assay as for plasma or BAL samples, except that 10-ml-capacity LRC Varian C8 SPE cartridges were used instead of 1-ml-capacity Varian C8 cartridges.
The concentration of unbound drug in plasma was calculated from the following formula: unbound fraction = 0.01 × total concentration. Unbound drug concentrations in ELF and AC were not calculated because the extent of protein binding in these compartments is unknown. The volume of ELF in bronchoalveolar lavage fluid, the concentration of antibiotic in the ELF, and the concentration of antibiotic in alveolar cells were derived using methods and calculations that we have previously reported (9-11).
Pharmacokinetic analysis.
For all compartments, the individual subject concentrations at each collection time point were averaged, and the mean data were used to calculate the Cmax and Tmax. Cmax was the maximum mean concentration from among the five time groups, and Tmax was the mean time from the last dose to the time of BAL for the time group in which the Cmax occurred. The terminal-phase half-life in plasma was calculated using model-independent analysis with Kinetica, version 4.4.1 (Adept Scientific). The half-life was not calculated for ELF or AC because the concentration-time profile was flat in those compartments. The AUC over the dosing interval (AUC0-24) was calculated using the population methods described below.
Pharmacodynamic analysis.
The Cmax, AUC0-24, and the concentration-time data were used to calculate the following pharmacodynamic parameters: Cmax/MIC90 ratio, AUC0-24/MIC90 ratio, and time above MIC90 in plasma, ELF, and AC. The pharmacodynamic parameters in plasma were derived from the free (i.e., unbound) drug concentrations as well as total (i.e., unbound plus bound) drug concentrations. Although the presence of protein increases the MIC of micafungin (29), the effect of protein binding on clinical outcomes is unknown (1, 29, 31). The MIC90 value for A. fumigatus was obtained from the recent literature (43).
Population analysis and multiple-dose simulations.
A population PK analysis using the exact concentration-time data for all 20 patients was carried out using the nonparametric adaptive grid (NPAG) algorithm implemented in the MM-USCPack software (24). In the first step, various compartmental models were fit to plasma drug concentrations only. In the second step, all plasma, ELF, and AC drug concentrations were modeled simultaneously. The final model was a linear four-compartment model with first-order processes for all transfers. The model was described by the following system of ordinary differential equations: dX1/dt = R1 − (CL10/V1)X1 − K12X1 + K21X2; dX2/dt = K12X1 − K21X2 + (CL23/V3)X3 + (CL24/V4)X4 − [(CL23+CL24)/V2]X2; dX3/dt = (CL23/V2)X2 + (CL34/V4)X4 − [(CL23 + CL34)/V3]X3; dX4/dt = (CL24/V2)X2 + (CL34/V3)X3 − [(CL24 + CL34)/V4]X4 (where X1, X2, X3, and X4 are the amounts of drug in the central [plasma], peripheral, AC, and ELF compartments, respectively [all in mg]). R1 is the infusion rate constant in the central compartment (in mg/h). The 10 parameters of the model were two transfer rate constants between the central and the peripheral compartments (K12 and K21, in h−1), the elimination clearance from the central compartment (CL10, in liters/h), three intercompartmental clearances (CL23, CL24, and CL34, in liters/h), and four volumes of distribution (V1, V2, V3, and V4, all in liters).
In addition, there were three output equations, defined as follows: Y1 = X1/V1; Y2 = X3/V3; Y3 = X4/V4 (where Y1, Y2, and Y3 are the micafungin concentrations [in mg/liter] in the central [plasma], AC, and ELF compartments, respectively).
The goodness of fit was assessed using the log-likelihood criterion during the model building. A graphical analysis of predicted versus observed concentration plots and individual residual plots was performed for model assessment. Predictive performance of the final model was assessed using the mean error and mean weighted error as measures of bias; the mean squared error and mean weighted squared error were used as measures of precision.
A statistical analysis of covariate influence on the individual PK parameter values was carried out. It included the random PK parameters of the final model (i.e., CL23, CL24, CL23, V2, V3, and V4) and the following factors: age, gender (male or female), race (Caucasian or other races), height, weight, BAL location (lingula or other sites), and BAL duration.
Drug exposure was explored by simulation using the individual PK parameter values estimated by the use of the NPAG algorithm. Those values were imported into Matlab software (version 6.5; MathWorks, Natick, MA) to calculate individual time-concentration profiles in plasma, AC, and ELF. The drug regimen incorporated into the model was 150 mg of micafungin administered intravenously once daily for 14 days. The infusion time was set at 1 h. The individual PK parameter values from the plasma drug concentration model were used to calculate drug concentration profiles in plasma, while the individual parameter values from the final model were used to calculate profiles in AC and ELF. For each compartment, the AUC0-24 was calculated at day 1, day 7, and day 14. The cumulative AUC from day 1 to day 14 (AUCday 1-14) also was computed. For each individual, the ratios AUCAC/AUCplasma and AUCELF/AUCplasma were computed at the various end points to quantify micafungin penetration in those media.
RESULTS
Twenty subjects were enrolled in the study. The primary pulmonary diagnoses included idiopathic pulmonary fibrosis (13 patients), chronic obstructive pulmonary disease (3 patients), bronchiolitis obliterans (1 patient), pulmonary agenesis (1 patient), right heart failure (1 patient), and unspecified end-stage lung disease (1 patient). Eighteen of the 20 underwent bilateral and 2 underwent unilateral lung transplantation. In all cases, the BAL was performed in the transplanted lung, and all 20 subjects who were enrolled in the study completed the bronchoscopy procedure. BAL was performed in the lingula in 10, the right middle lobe in 5, the right upper lobe in 2, and the left upper lobe in 3 of the 20 patients.
The mean age (± SD) of the patients was 59.0 ± 9.8 years; 10 were men and 10 were women; 15 were Caucasian, 1 was Hispanic, 2 were African-American, and 2 were multiracial. The weight (mean ± SD) of the subjects was 69.6 ± 12.3 kg. Prior to drug administration, serum creatinine, AST, ALT, alkaline phosphatase, and total bilirubin were 0.95 ± 0.28 mg/dl, 36.1 ± 27.1 IU/liter, 34.1 ± 37.4 IU/liter, 89.9 ± 47.6 IU/liter, and 0.75 ± 0.34 mg/dl, respectively.
Seventeen of the 20 subjects reported no adverse effects of the study drug. One subject experienced burning at the site of the intravenous infusion, which resolved with a change of the site. Two subjects experienced episodes of altered mental status, one of which was self-limited and required no intervention and the other for which the altered status lasted for 3 days and was considered unrelated to the study drug.
Poststudy laboratory testing was performed at 10.7 ± 17.6 days following infusion. There was no significant difference between the pre- and postdrug serum creatinine levels (1.11 ± 0.38 mg/dl; P > 0.05). The AST following drug administration (28.6 ± 22.4 IU/liter) was less than (P < 0.05) the predrug AST determination. The mean ALT and total bilirubin levels stayed within the normal ranges (41.6 ± 62.3 IU/liter and 0.74 ± 0.39 mg/dl, respectively). The poststudy alkaline phosphatase exceeded the normal range (160.0 ± 258 IU/liter). This elevated poststudy mean value was due to a single patient with underlying cirrhosis whose alkaline phosphatase was not measured until 2.5 months after the end of the study.
AC and ELF recovery.
The number (mean ± SD) of AC recovered from BAL fluid ranged from 3.8 × 108 ± 1.3 × 108 to 7.7 × 108 ± 6.5 × 108 for the five time groups (Table 1). Alveolar cell recovery was not significantly different among the groups (P > 0.05). The majority of the cells in all time groups consisted of monocytes and macrophages (range, 74.0 ± 41.4% to 85.8 ± 20.2%). The calculated volume (mean ± SD) of ELF recovered was 1.2 ± 1.0 ml. ELF recovery was not significantly different among the time groups (P > 0.05) (Table 1). The cell counts and differential cell counts obtained in these patients were similar to those previously reported in normal subjects (10, 11).
TABLE 1.
Recovery of cells and ELF from BAL fluid
| Parameter | Mean ± SD valuea at indicated time point (n) |
||||
|---|---|---|---|---|---|
| 3 h (4) | 5 h (4) | 8 h (4) | 18 h (4) | 24 h (4) | |
| No. of cells/liter | 4.2 (±2.4) × 108 | 7.7 (±6.5) × 108 | 3.6 (±3.0) × 108 | 4.2 (±1.1) × 108 | 3.8 (±1.3) × 108 |
| PMNs (%) | 5.5 ± 6.4 | 18.2 ± 12.1 | 14.8 ± 20.3 | 12.0 ± 20.0 | 7.0 ± 10.8 |
| Lymphocytes (%) | 3.0 ± 2.2 | 2.0 ± 2.3 | 1.2 ± 1.5 | 2.0 ± 2.2 | 6.8 ± 5.1 |
| Monocytes/macrophages (%) | 74.0 ± 41.4 | 78.8 ± 12.3 | 82.5 ± 19.9 | 85.8 ± 20.2 | 84.2 ± 17.3 |
| Eosinophils (%) | 0.0 ± 0.0 | 0.5 ± 1.0 | 0.2 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Other cells (%) | 0.0 ± 0.0 | 0.5 ± 1.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Degenerated cells (%) | 17.5 ± 35.0 | 0.0 ± 0.0 | 1.2 ± 2.5 | 0.2 ± 0.5 | 2.0 ± 4.0 |
| ELF volume (ml) | 1.1 ± 1.0 | 2.0 ± 1.5 | 1.3 ± 1.2 | 1.0 ± 0.6 | 0.8 ± 0.1 |
No significant differences were found between time groups for cell recovery or ELF volume. PMNs, polymorphonuclear leukocytes; other cells, other unrecognizable cells.
Micafungin concentrations and pharmacokinetics.
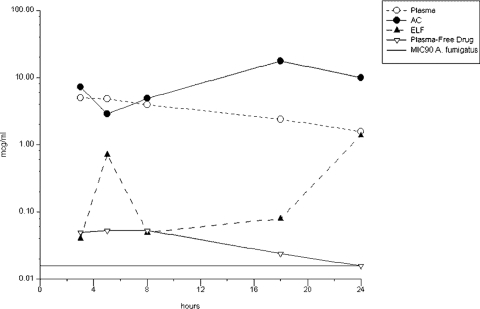
The concentrations of micafungin in plasma, AC, and ELF and the AC/plasma and ELF/plasma ratios at the time of BAL are summarized in Fig. 1 and Table 2. The Cmax in plasma, ELF, and AC was 4.93 ± 0.97, 1.38 ± 1.93, and 17.41 ± 24.25 μg/ml, respectively. The Tmax in plasma, ELF, and AC was 4.0 ± 0.5, 24.2 ± 1.8, and 17.9 ± 1.4 h, respectively. The elimination half-life in plasma was 12.1 h.
FIG. 1.
Concentrations of micafungin in plasma, AC, and ELF.
TABLE 2.
Concentrations and ratios of micafungin in plasma, ELF, and AC
| Compartment | Drug concn or ratio between compartments at indicated sampling point (n) |
||||
|---|---|---|---|---|---|
| 3 h (4) | 5 h (3a) | 8 h (4) | 18 h (4) | 24 h (4) | |
| Plasma (μg/ml) | 4.93 ± 0.97 | 4.81 ± 0.38 | 3.93 ± 0.39 | 2.38 ± 0.56 | 1.57 ± 0.33 |
| ELF (μg/ml) | 0.04 ± 0.07 | 0.71 ± 0.20 | 0.05 ± 0.09 | 0.08 ± 0.14 | 1.38 ± 1.93 |
| ELF/plasma ratio | 0.01 | 0.15 | 0.01 | 0.04 | 1.10 |
| AC (μg/ml) | 7.17 ± 13.21 | 2.84 ± 1.32 | 4.86 ± 5.62 | 17.41 ± 24.25 | 9.99 ± 10.68 |
| AC/plasma ratio | 1.53 | 0.61 | 1.23 | 7.62 | 6.22 |
The subject who received four doses was excluded from the time-group analysis. His plasma, ELF, and AC drug concentrations were 5.38 μg/ml, 0.69 μg/ml, and 53.31 μg/ml, respectively.
Population analysis and multiple-dose simulations. (i) Plasma concentration modeling.
A linear two-compartment model best fit the plasma concentration data. The four model parameters were the clearance from the central compartment (CL10, in liters per hour), the volume of distribution in the central compartment (V1, in liters), and two intercompartmental transfer rate constants (K12 and K21, in h−1). The NPAG algorithm provided a grid of nine support points as a population parameter distribution. The means, medians, and standard deviations of the probability distribution are presented in Table 3. Population predictions calculated using these means correlated well with observed concentrations, and predictive performance was good (Fig. 2A). The goodness of fit for Bayesian posterior predictions was also very good (Fig. 2B). Body weight was tested as a covariate on clearance and also on volume of distribution. However, this did not result in any significant increase in the log likelihood, which indicated no improvement in the fit. Thus, body weight was not included in the model.
TABLE 3.
Population PK parameter values of the plasma micafungin concentration model
| Statistic | CL10 (liters/h) | K12 (h−1) | K21 (h−1) | V1 (liters) |
|---|---|---|---|---|
| Mean | 1.309 | 3.068 | 6.795 | 17.617 |
| Median | 1.411 | 1.625 | 7.096 | 16.750 |
| SD | 0.262 | 2.766 | 2.034 | 5.109 |
FIG. 2.
Plot of predicted versus observed concentrations for the plasma drug concentration model. (Top) Population predictions; (bottom) individual predictions (calculated using the means of the individual Bayesian posterior parameter joint densities).
(ii) Final model.
A 10-parameter, four-compartment linear model best fit the data. The model structure assumed a linear diffusion of micafungin from the peripheral compartment into both the ELF and the AC compartments. The model also included a possible transfer between ELF and AC compartments.
A 14-support point grid was provided by the NPAG algorithm as a population parameter distribution. Parameter values of the final model are summarized in Table 4. Pharmacokinetic variability, especially concerning intercompartmental clearances, was high. Based on median clearance values, micafungin penetration into the ELF was approximately eight times slower than in AC.
TABLE 4.
Population PK parameter valuesa of the final model
| Statistic | Random parameters |
Fixed parameters |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CL23 | CL24 | CL34 | V2 | V3 | V4 | CL10 | K12 | K21 | V1 | |
| Mean | 0.279 | 0.00967 | 0.362 | 5.407 | 1.607 | 12.024 | 1.309 | 3.068 | 6.795 | 17.617 |
| Median | 0.0903 | 0.0118 | 0.0143 | 3.519 | 0.851 | 15.361 | ||||
| SD | 0.772 | 0.0236 | 0.708 | 4.992 | 1.455 | 5.712 | ||||
Subscript numeral correspond to compartments as follows: 1, central (plasma) compartment; 2, peripheral compartment; 3, AC compartment; 4, ELF compartment. K12 and K21 (in h−1) are transfer rate constants between the central and the peripheral compartments. CL10 (in liters/h) is the elimination clearance from the central compartment; CL23, CL24, CL34 (in liters/h) are the intercompartmental clearances. V1, V2, V3, and V4 (in liters) are the volumes of distribution in the indicated compartment.
Overall, population predictions correlated quite well with observed concentrations except for the four highest micafungin levels measured in AC. As a result, the predictive performance of the population model was heavily biased (data not shown). However, individual predictions obtained after the Bayesian step showed better agreement with observed concentrations (Table 5).
TABLE 5.
Bias, precision and correlation to observed data for the individual predictions of the final model
| Plasma concentrations (n = 20) | AC concentrations (n = 20) | ELF concentrations (n = 20) | All concentrations (n = 60) | |
|---|---|---|---|---|
| Measures of bias | ||||
| ME (mg/L) | −0.316 | −0.732 | +0.153 | −0.298 |
| MWE (mg/L) | −0.641 | −0.024 | +4.605 | +1.313 |
| Measures of precision | ||||
| MSE (mg2/L2) | 0.614 | 12.982 | 0.139 | 4.579 |
| MWSE (mg2/L2) | 2.647 | 1.873 | 77.975 | 27.498 |
| Regression equation | y = 1.08x + 0.063 | y = 1.08x−0.136 | y = 1.04x−0.178 | y = 1.09x-0.102 |
| R2 | 0.79 | 0.96 | 0.87 | 0.96 |
Abbreviations: ME = mean error, MWE = mean weighted error, MSE = Mean squared error, MWSE = mean weighted squared error.
Predictive performance of the final model is presented in Table 5 (individual predictions). Overall results were acceptable but varied according to the type of concentration and also according to the measure considered. Weighted measures were better than nonweighted measures for AC drug concentrations because of the lower credibility given to the higher concentrations by the assay error pattern. The opposite tendency was observed for ELF drug concentrations, due to a large number of undetectable and low micafungin concentrations observed, which were given a high credibility in the modeling framework.
The statistical analysis did not find any significant relationship between the tested covariates and the pulmonary diffusion PK parameter values of the final model except for BAL location and the volume of distribution in the ELF compartment (V4). V4 was significantly higher (P = 0.0084) in individuals sampled in the lingula (mean volume, 15.69 ± 0.33 liters; n = 11) than in those sampled in other sites (mean volume, 7.47 ± 6.25 liters; n = 9).
Drug exposure simulation.
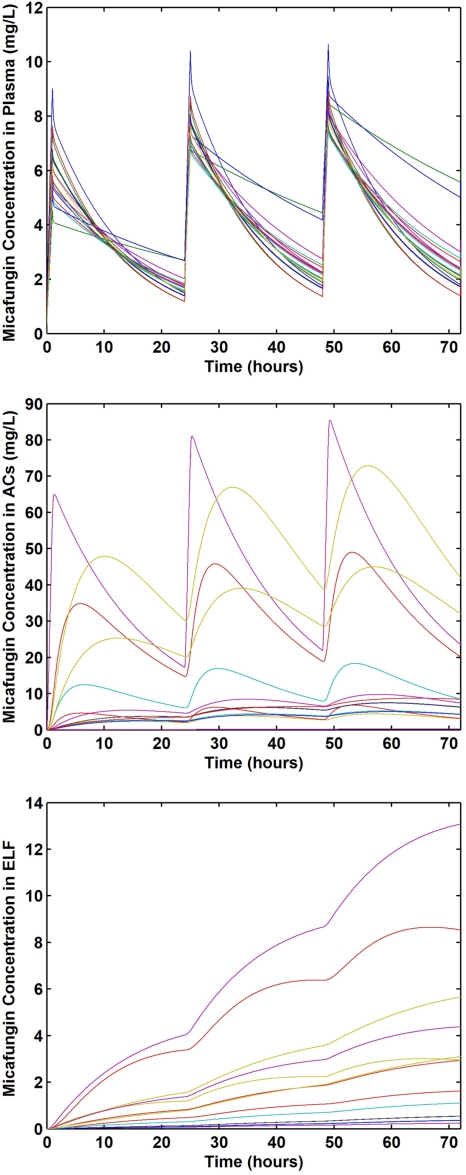
The simulated time-concentration profiles in plasma, ELF, and AC of the 20 individuals over the first 72 h of micafungin therapy (150 mg intravenously once daily; 1-h infusion) are depicted in Fig. 3. AUC and AUC ratios predicted in the 20 individuals during 14-day micafungin therapy administered once daily (150 mg) are presented in Table 6. The simulation indicated that micafungin exposure in plasma increased from day 1 to day 7 but not significantly from day 7 to day 14. Significant drug accumulation over the 14 days was predicted in the two lung compartments, especially in the ELF compartment, but interindividual variability in drug exposures was high. The median value of AUCAC/AUCplasma increased from 0.98 on day 1 to 2.13 on day 7 and 2.26 on day 14. Over the 14-day therapy course, the median value was about 2. The median AUCELF/AUCplasma value was very low on day 1 (0.07) but greatly increased to 0.66 and then 1.12 on day 7 and day 14, respectively. The median value of this ratio was 0.68 over the 14-day period.
FIG. 3.
Simulated concentration-time profiles of micafungin in plasma (top), AC (middle), and ELF (bottom) from 20 lung transplant patients.
TABLE 6.
Drug exposures predicted in lung transplant patients receiving micafungin administered intravenously once daily for 14 days
| Plasma | ACs | ELF | |
|---|---|---|---|
| Day 1 | |||
| AUC0-24h (mg.h.L−1) | 79.2 ± 4.4* | 208.1 ± 275.6 | 14.4 ± 18.4 |
| AUC ratios** | NA | 2.65 ± 3.51 | 0.18 ± 0.23 |
| Day 7 | |||
| AUC0-24h (mg.h.L−1) | 117.4 ± 31.4 | 387.7 ± 437.6 | 137.6 ± 154.1 |
| AUC ratios | NA | 3.59 ± 4.08 | 1.28 ± 1.41 |
| Day 14 | |||
| AUC0-24h (mg.h.L−1) | 118.1 ± 33.6 | 409.9 ± 467.2 | 214.4 ± 240.2 |
| AUC ratios | NA | 3.80 ± 4.36 | 1.98 ± 2.16 |
| Day 1 to 14 | |||
| AUC0-14d (mg.h.L−1) | 1584.6 ± 376.9 | 5190.6 ± 5958.7 | 1864.6 ± 2070.8 |
| AUC ratios | NA | 3.53 ± 4.07 | 1.27 ± 1.38 |
*All data are given as mean ± SD.
**AUC ratio = AC AUC0-24h/Plasma AUC0-24h and ELF AUC0-24h/ Plasma AUC0-24h.
Micafungin pharmacodynamics.
The plasma drug free and total Cmax/MIC90 ratios were 3.2 and 316.0, respectively. Cmax/MIC90 ratios were 88.5 and 1,115.4 in ELF and AC, respectively. The mean time above MIC90 was 24 h in all compartments (Fig. 1). AUC0-24/MIC90 ratios were 5,077, 923.1, and 13,340 in plasma, ELF, and AC, respectively.
DISCUSSION
This single-dose prospective study of the intrapulmonary and plasma PKPD of 150 mg intravenous micafungin in adult lung transplant recipients found distribution into the ELF and AC of this echinocandin that substantially exceeded the reported MIC90 of A. fumigatus. At concentrations near the Cmax, the ELF/plasma and AC/plasma ratios were approximately 0.3 and 3.5. Consistent with these observations, the total AUC0-24/MIC90 ratios in ELF and AC were 923 and 13,340, respectively. Concentrations of micafungin in ELF and AC were sustained throughout the 24-h dosing interval. These PKPD findings indicate that micafungin achieves sufficiently elevated and sustained intrapulmonary concentrations in ELF and AC to support activity for prevention and early treatment of invasive aspergillosis.
Micafungin is not absorbed orally and can only be given by intravenous administration. Plasma pharmacokinetic studies with micafungin have been performed in healthy and hospitalized adults (16-20). In healthy subjects receiving a 150-mg intravenous dose, the Cmax, half-life (t1/2), clearance, and AUC are 8.7 ± 2.9 μg/ml, 14.8 ± 1.7 h, 10 ± 1.6 ml/h/kg, and 120.9 ± 16.7 μg·h/ml. Steady state is achieved in approximately 4 to 5 days. Moderately reduced liver function and moderate to severe impaired renal function have no significant effects on the pharmacokinetics of micafungin and therefore require no dosage adjustment. A study of the intrapulmonary PKPD in healthy subjects found that micafungin concentrates in AC; the AUC0-24 in ELF and AC were 10.2 and 233.6 μg·h/ml, respectively (28). The half-life, Cmax, and Tmax have not been reported for pulmonary compartments. Protein binding of micafungin in humans is 99%, predominantly to albumin and independent of circulating drug concentrations over the range of 10 to 100 μg/ml.
Three of 20 subjects (15%) had treatment-emergent adverse events during the course of the study. Among these adverse events, the episode of altered mental status was considered to be unrelated to the study drug. This relatively well-tolerated safety profile is consistent with the observations of the randomized trial of micafungin in prevention of invasive fungal infections in neutropenic hematopoietic stem cell transplant recipients, in which its safety profile was comparable to that of fluconazole (44).
The intrapulmonary distribution of micafungin into AC and ELF has important implications for lung transplant recipients and other immunocompromised host populations. As pulmonary alveolar macrophages ingest conidia of Aspergillus spp. as the first line of phagocytic host defense (46, 47), the high intracellular concentrations of micafungin may further augment its properties when used as prophylaxis. The effects of denervation and lack of bronchial circulation on the pharmacology of echinocandins in the transplanted lung have not been studied. Laboratory and clinical observation studies of immunocompromised hosts have demonstrated impairment of pulmonary alveolar macrophages and monocyte-derived macrophages (7, 37). Given the intrinsic and pharmacological immunosuppression associated with lung transplantation and other transplant conditioning regimens, the high intracellular drug concentrations may be particularly advantageous in this population. These findings also warrant several questions as to the mechanism of intracellular drug uptake and the possible immunomodulatory effects on the host response.
Distribution of micafungin into the ELF may be particularly beneficial in lung transplant recipients, where bronchial anastamotic aspergillosis can be a catastrophic complication (40). Such devastating complications include loss of the transplanted lung, bronchopleural fistula, and bronchial-pulmonary artery fistula. Sustained drug concentrations in the ELF, as demonstrated in the study reported herein, may reduce the development of anastamotic aspergillosis. A prospective study investigating micafungin in prevention of invasive aspergillosis in lung transplant recipients is warranted to confirm this hypothesis.
The pharmacodynamic parameters that best predict outcomes for treatment of invasive pulmonary aspergillosis with micafungin have not been defined (25). The Cmax/minimally effective concentration (MEC) ratio has been reported to be the parameter best associated with efficacy for caspofungin (48), with an optimal range of 10 to 20 against experimental murine aspergillosis. However, Louie and colleagues found in a study of the pharmacodynamics of caspofungin in a murine model of disseminated candidiasis that the AUC/MIC ratio was a better predictor, possibly as a result of the echinocandin's slow elimination from tissues (27). Similarly, Andes et al. found that the in vivo pharmacodynamic target for micafungin against Candida albicans and Candida glabrata in a neutropenic murine model of disseminated candidiasis were free-drug AUC/MIC ratios for stasis and killing of approximately 10 and 20, respectively (3). Although the parameter that best predicts efficacy is not known, our results showed that even when estimated free drug levels are taken into account, the Cmax and total exposure (AUC) were both high relative to the MIC90, indicating a strong likelihood of favorable outcomes for treatment of Aspergillus infections in lung transplant patients.
The intrapulmonary PKPD of micafungin in normal volunteers has been reported elsewhere (28). Those results, which are similar to our findings, demonstrate that micafungin achieves levels that are sustained above the MEC in all compartments, with significant concentration in AC. In that multiple-dose study (three doses), the AUC0-24 for plasma (219.7 ± 37.6 μg·h/ml) was approximately three times greater than that which we observed (79.2 μg·h/ml) in this single-dose study. The AUC0-24 for ELF and AC were 10.2 μg·h/ml and 233.6 μg·h/ml, respectively, which were similar to those we observed (14.4 μg·h/ml and 208.1 μg·h/ml). These differences are relatively small and likely due in part to differences in study design (three doses versus one dose), lavage technique, specimen collection, and the populations studied.
Our population modeling suggests that steady state would be achieved in plasma by day 7 at an AUC0-24 of approximately 117 μg·h/ml, which is consistent with reports that plasma drug steady state is achieved within 3 to 6 days (21, 28). However, 24-h exposure in AC and especially ELF continued to increase from days 7 to 14. This suggests the possibility that the attainment of steady state may be delayed in pulmonary compartments and that the half-lives of micafungin in ELF and AC are greater than that in plasma. The model also predicts that exposure in ELF, which had the lowest drug concentrations of any compartment after a single dose, would continue to increase with repeated dosing and would exceed exposure in plasma over a 2-week dosing period. The drug accumulation, prolonged residence time, and high inhibitory ratios in pulmonary compartments further suggest that micafungin would be effective in prevention and early treatment of invasive pulmonary aspergillosis.
Once deeply invasive aspergillosis is well established as a pneumonic process, distribution into the AC and ELF compartments may not necessarily be predictive of response. Other physiological and compartmental factors affecting penetration of micafungin would then be relevant. These factors include penetration of micafungin into an infarcted lung in neutropenic hosts, local tissue hypoxia, and concentration of drug in circulating neutrophils in nonneutropenic hosts. The effects of other routes of delivery, such as aerosol administration, on these PKPD parameters have not been studied.
We conclude the following: (i) a single intravenous dose of 150 mg resulted in sustained plasma, ELF, and AC drug concentrations that were above the MIC90 for Aspergillus fumigatus during the entire 24-hour dosing interval; (ii) multiple-dose simulations indicate that ELF and AC concentrations of micafungin will continue to increase during 14 days of administration; (iii) the high intrapulmonary Cmax/MIC90 and AUC0-24/MIC90 ratios and time above MIC90 observed in this study are favorable for the treatment or prevention of aspergillosis; (iv) cell counts, differential cell counts, and ELF recovered from transplanted lungs are similar to those observed in normal subjects; (v) intravenous micafungin was well tolerated in adult lung transplant recipients.
Acknowledgments
This study was supported in part by Astellas Pharma, Inc., and by the intramural program of the National Cancer Institute.
We thank Laurent Bourguignon of the Hospices Civils de Lyon, Hôpital P. Garraud, Service Pharmaceutique, Lyon, France, for his assistance with the development of the population model and multiple-dose simulations.
Footnotes
Published ahead of print on 3 May 2010.
REFERENCES
- 1.Abe, F., J. Ueyama, N. Kawasumi, M. Nadai, T. Hayashi, M. Kato, M. Ohnishi, H. Saito, N. Takeyama, and T. Hasegawa. 2008. Role of plasma proteins in pharmacokinetics of micafungin, an antifungal antibiotic, in analbuminemic rats. Antimicrob. Agents Chemother. 52:3454-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrahamsen, T. G., C. S. Carter, E. J. Read, M. Rubin, H. G. Goetzman, E. F. Lizzio, Y. L. Lee, M. Hanson, P. A. Pizzo, and T. Hoffman. 1991. Stimulatory effect of counterflow centrifugal elutriation in large-scale separation of peripheral blood monocytes can be reversed by storing the cells at 37 degrees C. J. Clin. Apher. 6:48-53. [DOI] [PubMed] [Google Scholar]
- 3.Andes, D. R., D. J. Diekema, M. A. Pfaller, K. Marchillo, and J. Bohrmueller. 2008. In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 52:3497-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 97:77-89. [PubMed] [Google Scholar]
- 5.Carver, P. L. 2004. Micafungin. Ann. Pharmacother. 38:1707-1721. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekar, P. H., and J. D. Sobel. 2006. Micafungin: a new echinocandin. Clin. Infect. Dis. 42:1171-1178. [DOI] [PubMed] [Google Scholar]
- 7.Chilvers, E. R., C. L. Spreadbury, and J. Cohen. 1989. Bronchoalveolar lavage in an immunosuppressed rabbit model of invasive pulmonary aspergillosis. Mycopathologia 108:163-171. [DOI] [PubMed] [Google Scholar]
- 8.Clemons, K. V., and D. A. Stevens. 2006. Efficacy of micafungin alone or in combination against experimental pulmonary aspergillosis. Med. Mycol. 44:69-73. [DOI] [PubMed] [Google Scholar]
- 9.Conte, J. E., Jr., J. A. Golden, J. Kipps, M. McIver, and E. Zurlinden. 2004. Intrapulmonary pharmacokinetics and pharmacodynamics of itraconazole and 14-hydroxyitraconazole at steady state. Antimicrob. Agents Chemother. 48:3823-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conte, J. E., Jr., J. A. Golden, G. Krishna, M. McIver, E. Little, and E. Zurlinden. 2009. Intrapulmonary pharmacokinetics and pharmacodynamics of posaconazole at steady state in healthy subjects. Antimicrob. Agents Chemother. 53:703-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conte, J. E., Jr., J. A. Golden, M. McIver, and E. Zurlinden. 2006. Intrapulmonary pharmacokinetics and pharmacodynamics of high-dose levofloxacin in healthy volunteer subjects. Int. J. Antimicrob. Agents 28:114-121. [DOI] [PubMed] [Google Scholar]
- 12.de Wet, N., A. Llanos-Cuentas, J. Suleiman, E. Baraldi, E. F. Krantz, N. M. Della, and H. Diekmann-Berndt. 2004. A randomized, double-blind, parallel-group, dose-response study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV-positive patients. Clin. Infect. Dis. 39:842-849. [DOI] [PubMed] [Google Scholar]
- 13.Dummer, J. S., N. Lazariashvilli, J. Barnes, M. Ninan, and A. P. Milstone. 2004. A survey of anti-fungal management in lung transplantation. J. Heart Lung Transplant. 23:1376-1381. [DOI] [PubMed] [Google Scholar]
- 14.Groll, A. H., D. Mickiene, R. Petraitiene, V. Petraitis, C. A. Lyman, J. S. Bacher, S. C. Piscitelli, and T. J. Walsh. 2001. Pharmacokinetic and pharmacodynamic modeling of anidulafungin (LY303366): reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling. Antimicrob. Agents Chemother. 45:2845-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groll, A. H., T. Stergiopoulou, E. Roilides, and T. J. Walsh. 2005. Micafungin: pharmacology, experimental therapeutics and clinical applications. Expert Opin. Investig. Drugs 14:489-509. [DOI] [PubMed] [Google Scholar]
- 16.Gumbo, T., J. Hiemenz, L. Ma, J. J. Keirns, D. N. Buell, and G. L. Drusano. 2008. Population pharmacokinetics of micafungin in adult patients. Diagn. Microbiol. Infect. Dis. 60:329-331. [DOI] [PubMed] [Google Scholar]
- 17.Hebert, M. F., D. K. Blough, R. W. Townsend, M. Allison, D. Buell, J. Keirns, and I. Bekersky. 2005. Concomitant tacrolimus and micafungin pharmacokinetics in healthy volunteers. J. Clin. Pharmacol. 45:1018-1024. [DOI] [PubMed] [Google Scholar]
- 18.Hebert, M. F., H. E. Smith, T. C. Marbury, S. K. Swan, W. B. Smith, R. W. Townsend, D. Buell, J. Keirns, and I. Bekersky. 2005. Pharmacokinetics of micafungin in healthy volunteers, volunteers with moderate liver disease, and volunteers with renal dysfunction. J. Clin. Pharmacol. 45:1145-1152. [DOI] [PubMed] [Google Scholar]
- 19.Hebert, M. F., R. W. Townsend, S. Austin, G. Balan, D. K. Blough, D. Buell, J. Keirns, and I. Bekersky. 2005. Concomitant cyclosporine and micafungin pharmacokinetics in healthy volunteers. J. Clin. Pharmacol. 45:954-960. [DOI] [PubMed] [Google Scholar]
- 20.Hiemenz, J., P. Cagnoni, D. Simpson, S. Devine, N. Chao, J. Keirns, W. Lau, D. Facklam, and D. Buell. 2005. Pharmacokinetic and maximum tolerated dose study of micafungin in combination with fluconazole versus fluconazole alone for prophylaxis of fungal infections in adult patients undergoing a bone marrow or peripheral stem cell transplant. Antimicrob. Agents Chemother. 49:1331-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keirns, J., T. Sawamoto, M. Holum, D. Buell, W. Wisemandle, and A. Alak. 2007. Steady-state pharmacokinetics of micafungin and voriconazole after separate and concomitant dosing in healthy adults. Antimicrob. Agents Chemother. 51:787-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kontoyiannis, D. P., V. Ratanatharathorn, J. A. Young, J. Raymond, M. Laverdiere, D. W. Denning, T. F. Patterson, D. Facklam, L. Kovanda, L. Arnold, W. Lau, D. Buell, and K. A. Marr. 2009. Micafungin alone or in combination with other systemic antifungal therapies in hematopoietic stem cell transplant recipients with invasive aspergillosis. Transpl. Infect. Dis. 11:89-93. [DOI] [PubMed] [Google Scholar]
- 23.Kubak, B. M. 2002. Fungal infection in lung transplantation. Transpl. Infect. Dis. 4(Suppl. 3):24-31. [DOI] [PubMed] [Google Scholar]
- 24.Leary, R., R. Jelliffe, A. Schumitzky, and M. Van Guilder. 2001. An adaptive grid nonparametric approach to pharmacokinetic and dynamic (PK/PD) population models, p. 389-394. Proc. 14th IEEE Symp. Comput. Based Med. Syst. IEEE, Los Alamitos, CA.
- 25.Lewis, J. S. 2009. Echinocandin activity against Aspergillus spp. and the importance of pharmacodynamics. Med. Mycol. 47(Suppl. 1):S376-S381. [DOI] [PubMed] [Google Scholar]
- 26.Lewis, R. E., N. D. Albert, and D. P. Kontoyiannis. 2008. Efficacy of single-dose liposomal amphotericin B or micafungin prophylaxis in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 52:4178-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louie, A., M. Deziel, W. Liu, M. F. Drusano, T. Gumbo, and G. L. Drusano. 2005. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob. Agents Chemother. 49:5058-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicasio, A. M., P. R. Tessier, D. P. Nicolau, R. F. Knauft, J. Russomanno, E. Shore, and J. L. Kuti. 2009. Bronchopulmonary disposition of micafungin in healthy adult volunteers. Antimicrob. Agents Chemother. 53:1218-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odabasi, Z., V. Paetznick, J. H. Rex, and L. Ostrosky-Zeichner. 2007. Effects of serum on in vitro susceptibility testing of echinocandins. Antimicrob. Agents Chemother. 51:4214-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostrosky-Zeichner, L., D. Kontoyiannis, J. Raffalli, K. M. Mullane, J. Vazquez, E. J. Anaissie, J. Lipton, P. Jacobs, J. H. van Rensburg, J. H. Rex, W. Lau, D. Facklam, and D. N. Buell. 2005. International, open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. Eur. J. Clin. Microbiol. Infect. Dis. 24:654-661. [DOI] [PubMed] [Google Scholar]
- 31.Paderu, P., G. Garcia-Effron, S. Balashov, G. Delmas, S. Park, and D. S. Perlin. 2007. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrob. Agents Chemother. 51:2253-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pappas, P. G., C. M. Rotstein, R. F. Betts, M. Nucci, D. Talwar, J. J. De Waele, J. A. Vazquez, B. F. Dupont, D. L. Horn, L. Ostrosky-Zeichner, A. C. Reboli, B. Suh, R. Digumarti, C. Wu, L. L. Kovanda, L. J. Arnold, and D. N. Buell. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 45:883-893. [DOI] [PubMed] [Google Scholar]
- 33.Petraitis, V., R. Petraitiene, A. H. Groll, K. Roussillon, M. Hemmings, C. A. Lyman, T. Sein, J. Bacher, I. Bekersky, and T. J. Walsh. 2002. Comparative antifungal activities and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 46:1857-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaller, M. A., L. Boyken, R. J. Hollis, J. Kroeger, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2009. In vitro susceptibility of clinical isolates of Aspergillus spp. to anidulafungin, caspofungin, and micafungin: a head-to-head comparison using the CLSI M38-A2 broth microdilution method. J. Clin. Microbiol. 47:3323-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfaller, M. A., D. J. Diekema, L. Ostrosky-Zeichner, J. H. Rex, B. D. Alexander, D. Andes, S. D. Brown, V. Chaturvedi, M. A. Ghannoum, C. C. Knapp, D. J. Sheehan, and T. J. Walsh. 2008. Correlation of MIC with outcome for Candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interpretive MIC breakpoints. J. Clin. Microbiol. 46:2620-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Queiroz-Telles, F., E. Berezin, G. Leverger, A. Freire, A. van der Vyver, T. Chotpitayasunondh, J. Konja, H. Ekmann-Berndt, S. Koblinger, A. H. Groll, and A. Arrieta. 2008. Micafungin versus liposomal amphotericin B for pediatric patients with invasive candidiasis: substudy of a randomized double-blind trial. Pediatr. Infect. Dis. J. 27:820-826. [DOI] [PubMed] [Google Scholar]
- 37.Roilides, E., A. Holmes, C. Blake, P. A. Pizzo, and T. J. Walsh. 1993. Defective antifungal activity of monocyte-derived macrophages from human immunodeficiency virus-infected children against Aspergillus fumigatus. J. Infect. Dis. 168:1562-1565. [DOI] [PubMed] [Google Scholar]
- 38.Singh, N. 2003. Fungal infections in the recipients of solid organ transplantation. Infect. Dis. Clin. North Am. 17:113-134. [DOI] [PubMed] [Google Scholar]
- 39.Singh, N. 2003. Impact of current transplantation practices on the changing epidemiology of infections in transplant recipients. Lancet Infect. Dis. 3:156-161. [DOI] [PubMed] [Google Scholar]
- 40.Singh, N., and S. Husain. 2003. Aspergillus infections after lung transplantation: clinical differences in type of transplant and implications for management. J. Heart Lung Transplant. 22:258-266. [DOI] [PubMed] [Google Scholar]
- 41.Singh, N., and D. L. Paterson. 2005. Aspergillus infections in transplant recipients. Clin. Microbiol. Rev. 18:44-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sole, A., P. Morant, M. Salavert, J. Peman, and P. Morales. 2005. Aspergillus infections in lung transplant recipients: risk factors and outcome. Clin. Microbiol. Infect. 11:359-365. [DOI] [PubMed] [Google Scholar]
- 43.Tawara, S., F. Ikeda, K. Maki, Y. Morishita, K. Otomo, N. Teratani, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, K. Sakane, H. Tanaka, F. Matsumoto, and S. Kuwahara. 2000. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob. Agents Chemother. 44:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Burik, J. A., V. Ratanatharathorn, D. E. Stepan, C. B. Miller, J. H. Lipton, D. H. Vesole, N. Bunin, D. A. Wall, J. W. Hiemenz, Y. Satoi, J. M. Lee, and T. J. Walsh. 2004. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin. Infect. Dis. 39:1407-1416. [DOI] [PubMed] [Google Scholar]
- 45.Vehreschild, J. J., and O. A. Cornely. 2006. Micafungin sodium, the second of the echinocandin class of antifungals: theory and practice. Future Microbiol. 1:161-170. [DOI] [PubMed] [Google Scholar]
- 46.Waldorf, A. R. 1989. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol. Ser. 47:243-271. [PubMed] [Google Scholar]
- 47.Waldorf, A. R., S. M. Levitz, and R. D. Diamond. 1984. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J. Infect. Dis. 150:752-760. [DOI] [PubMed] [Google Scholar]
- 48.Wiederhold, N. P., D. P. Kontoyiannis, J. Chi, R. A. Prince, V. H. Tam, and R. E. Lewis. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 190:1464-1471. [DOI] [PubMed] [Google Scholar]
- 49.Yokote, T., T. Akioka, S. Oka, T. Fujisaka, T. Yamano, S. Hara, M. Tsuji, and T. Hanafusa. 2004. Successful treatment with micafungin of invasive pulmonary aspergillosis in acute myeloid leukemia, with renal failure due to amphotericin B therapy. Ann. Hematol. 83:64-66. [DOI] [PubMed] [Google Scholar]