Abstract
The SOS response to DNA damage in bacteria is a well-known component of the complex transcriptional responses to genotoxic environmental stresses such as exposure to reactive oxygen species, alkylating agents, and many of the antibiotics targeting DNA replication. However, bacteria such as Bacillus subtilis also respond to conditions that perturb DNA replication via a transcriptional response mediated by the replication initiation protein DnaA. In addition to regulating the initiation of DNA replication, DnaA directly regulates the transcription of specific genes. Conditions that perturb DNA replication can trigger the accumulation of active DnaA, activating or repressing the transcription of genes in the DnaA regulon. We report here that simply growing B. subtilis in LB medium altered DnaA-dependent gene expression in a manner consistent with the accumulation of active DnaA and that this was part of a general transcriptional response to manganese limitation. The SOS response to DNA damage was not induced under these conditions. One of the genes positively regulated by DnaA in Bacillus subtilis encodes a protein that inhibits the initiation of sporulation, Sda. Sda expression was induced as cells entered stationary phase in LB medium but not in LB medium supplemented with manganese, and the induction of Sda inhibited sporulation-specific gene expression and the onset of spore morphogenesis. In the absence of Sda, manganese-limited cells initiated spore development but failed to form mature spores. These data highlight that DnaA-dependent gene expression may influence the response of bacteria to a range of environmental conditions, including conditions that are not obviously associated with genotoxic stress.
Several species of bacteria, including Bacillus subtilis and Escherichia coli, respond to conditions that perturb DNA replication through at least two independent signaling pathways: the well-known SOS response to DNA damage and an increasingly well-characterized signaling pathway mediated by the replication initiation factor DnaA. DnaA is a site-specific DNA binding protein that recognizes the origin of replication and initiates the assembly of the DNA replication machinery (32, 42). DnaA also functions as a transcription factor, recognizing specific promoters and activating or repressing the transcription of target genes (7, 25, 30, 36, 37, 54). The activity of DnaA is regulated in response to a variety of cell cycle-dependent, developmental, and nutritional signals, only some of which are understood at a mechanistic level for specific organisms (for recent reviews, see references 14, 16, 33, 36, 56, and 57). However, it is clear that some conditions that perturb DNA replication result in the accumulation of active DnaA and the induction or repression of DnaA-dependent gene expression (7, 20, 25, 35). Based on a limited number of studies, some conditions that induce the SOS response also alter DnaA-dependent gene expression, whereas other perturbations affect only one signaling pathway or the other (20, 21).
The SOS response to DNA damage clearly contributes to the transcriptional and adaptive responses of bacteria to environmental stresses such as reactive oxygen species and other DNA-damaging agents. Far less is known about when and how DnaA-dependent gene expression might affect the transcriptional or adaptive responses of bacteria to stressful or changing environments. Here we report that the growth of B. subtilis in LB medium alters DnaA-dependent gene expression as part of a general response to manganese limitation.
The effects of manganese on gene expression have been well characterized for B. subtilis (11, 17, 22, 24). Manganese is transported into cells by at least two different transport systems encoded by the mntH and mntABCD genes (48). The transcription of these genes is repressed at high manganese concentrations by MntR, which binds manganese as a corepressor (19, 22, 48). Manganese also represses the transcription of genes in the PerR and Fur regulons, which encode oxidative stress response proteins and iron acquisition proteins, respectively (11, 17, 22, 24). Although this is likely due in part to the roles of Mn(II) in scavenging reactive oxygen species and promoting the activities of SodA and other manganese-dependent enzymes, Mn(II) also affects PerR-dependent gene expression directly by replacing Fe(II) as a corepressor bound to PerR (24). The PerR-Mn(II) complex is much more resistant to inactivation by H2O2 than is the PerR-Fe(II) complex, limiting the expression of PerR-regulated genes (17, 24).
We realized that manganese limitation might also alter the expression of DnaA-dependent genes after we observed that a gene positively regulated by DnaA, sda, was induced when cells were grown on LB medium but not on LB medium supplemented with manganese. The sda gene encodes an inhibitor of sporulation, a developmental response of B. subtilis to nutrient limitation at a high cell density (9, 50). Cells of B. subtilis initiate spore development with a specialized cell cycle, in which cells switch from dividing at the midcell and instead divide at one pole to create a large cell, termed the mother cell, and a small cell, termed the forespore, each of which inherits a single copy of the chromosome (16, 26). The initiation of sporulation is coordinated with the successful completion of DNA replication in part by the transcriptional regulation of sda (9, 51, 54). The transcription of sda is convergently regulated by DnaA and the SOS response, and sda expression is induced as cells enter the stationary phase if DNA replication initiation or elongation is perturbed and in response to DNA damage (7, 9, 20, 21, 30, 43, 51, 54). In addition, the transcription of sda is transiently induced by DnaA in the absence of replication stress as cells enter the stationary phase, delaying the initiation of sporulation until ongoing rounds of DNA replication are completed (54).
We show here that manganese limitation in LB medium induces sda transcription and alters the expression of other DnaA-dependent genes without inducing the SOS response. Thus, environmental conditions that are not obviously associated with genotoxic stress can affect DnaA-dependent gene expression, perhaps reflecting direct or indirect effects on DNA replication. In addition, DnaA-dependent changes in gene expression can contribute significantly to the transcriptional and adaptive responses of bacteria to stressful or changing environmental conditions.
MATERIALS AND METHODS
Media and strains.
Strains were grown in LB medium (38) supplemented with 100 μM MnCl2 where noted. T-base medium (1× TSS salts [2 g liter−1 ammonium chloride; 0.35 g liter−1 potassium phosphate, dibasic; 6 g liter−1 Tris base, with the pH adjusted to 7.5 with hydrochloric acid] [22a], 1 mM MgSO4) was used to resuspend cell pellets for microscopy. Competent cells were prepared essentially as described previously by Msadek et al. (42a), replacing GE medium with MD medium (100 mM potassium phosphate [pH 7.5], 4 mM trisodium citrate, 2% [wt/vol] glucose, 11 mg liter−1 ferric ammonium citrate, 0.25% [wt/vol] potassium aspartate, 3 mM magnesium sulfate, 50 μg ml−1 l-tryptophan, and 50 μg ml−1 l-phenylalanine). Antibiotic resistance markers were selected by using the following drug concentrations: 5 μg ml−1 chloramphenicol for cat, 0.5 μg ml−1 erythromycin and 12.5 μg ml−1 lincomycin for erm, and 100 μg ml−1 spectinomycin for spc.
The strains used in this study are listed in Table 1, and plasmids and oligonucleotides used in this study are listed in Tables S1 and S2 in the supplemental material. Strains are derivatives of strain JH642 and have the common genotype trpC2 pheA1 (6). The following alleles were described previously: Δsda and amyE::(PspoIIE-lacZ cat) (9) and flgMΔ80 (39). See the supplemental material for details on the construction of strains and plasmids.
TABLE 1.
Strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| JH642 | trpC2 pheA1 | 6 |
| 168 | trpC2 | 8 |
| AM48 | amyE::(PyneA-lacZ cat) trpC2 pheA1 | |
| BB424 | amyE::(PywlC-lacZ cat) trpC2 pheA1 | |
| BB494 | amyE::(Psda-lacZ cat) trpC2 pheA1 | |
| BB668 | Δsda trpC2 pheA1 | 9 |
| BB825 | amyE::(PspoIIE-lacZ cat) trpC2 pheA1 | 51 |
| BB827 | Δsda amyE::(PspoIIE-lacZ cat) trpC2 pheA1 | 51 |
| BB1185 | flgMΔ80 trpC2 pheA1 | 39 |
| KM81 | amyE::(PspoIIE-gfp spc) trpC2 pheA1 | |
| KM96 | Δsda amyE::(PspoIIE-gfp spc) trpC2 pheA1 | |
| SH350 | thrC::(PtagC-lacZ erm) trpC2 pheA1 | |
| SH351 | Δsda thrC::(PtagC-lacZ erm) trpC2 pheA1 | |
| SH430 | flgMΔ80 aroD::erm trpC2 pheA1 | |
| SH455 | flgMΔ80 Δsda trpC2 pheA1 | |
| SH493 | Δsda amyE::(PyneA-lacZ cat) trpC2 pheA1 | |
| SH507 | amyE::(PdnaA-lacZ cat) trpC2 pheA1 | |
| SH517 | amyE::(PkatA-gfp spc) trpC2 pheA1 | |
| SH536 | amyE::(PkatA-lacZ cat) trpC2 pheA1 |
Sporulation and β-galactosidase assays.
Sporulation frequencies were determined as the ratio of heat-resistant (80°C for 20 min) CFU to total CFU. Assays were done 20 to 24 h after the end of exponential growth. β-Galactosidase-specific activity {[ΔA420 per min per ml of culture per optical density at 600 nm (OD600)] × 1,000} was determined as described previously (38). Sample absorbances at 420 nm were measured after pelleting of cell debris.
Immunoblot analysis.
Two-milliliter samples were taken at the indicated times and pipetted into tubes on ice containing 200 μl of 2% (wt/vol) sodium azide-50 mM EDTA (pH 8.0) prior to pelleting of the cells by centrifugation. Cells were washed with phosphate-buffered saline (pH 7.5), resuspended in 50 μl of lysis buffer (50 mM Tris-HCl [pH 7.5], 50 mM NaCl, 5 mM MgCl2, 1 mM dithiothreitol [DTT], 0.5 mg ml−1 lysozyme, 100 U ml−1 DNase I, 20 μg ml−1 RNase A, 0.5% [vol/vol] protease inhibitor cocktail [P-8849; Sigma]), and incubated for 5 min at 37°C. Cell debris was pelleted by centrifugation at 20,000 × g for 10 min, and the protein concentrations of the supernatants were determined by a Bradford assay (Bio-Rad) without transferring the supernatants into fresh tubes. Laemmli SDS loading buffer was then added to a final concentration of 1×, and the cell pellets were resuspended and mixed with the supernatants by vortexing. In this way, the samples consisted of total cellular protein, with the amount of each sample loaded per lane based on the soluble protein concentration. Samples were separated on 13.8% SDS Tris-Tricine polyacrylamide gels and transferred onto polyvinylidene difluoride (PVDF) membranes. After blocking, membranes were probed with polyclonal rabbit antibodies raised against Sda (1:20,000 dilution) or σA (1:25,000 dilution; kindly provided by Masaya Fujita and Richard Losick), followed by incubation with a 1:10,000 dilution of goat anti-rabbit antibodies conjugated to horseradish peroxidase (Bio-Rad). Detection was performed by chemiluminescence using the ECL system (Amersham) and exposure to X-ray film (Kodak BioMax Light film). Images were scanned from film by using a flatbed scanner (Epson) and Adobe Photoshop, and the scanned immunoblots were quantified by using ImageQuant software (Amersham).
Microscopy.
Samples were taken for fluorescence microscopy (0.5 to 1.0 ml) at the times indicated in the figures. Cells were pelleted by centrifugation for 1 min at 5,000 × g and resuspended in a small amount (∼100 μl) of T-base medium. Cell membranes were visualized by using the fluorescent dye FM4-64 (final concentration, 5 μg ml−1; Invitrogen), and DNA was visualized by using DAPI (4′,6-diamidino-2-phenylindole) (1 μg ml−1). Samples were immobilized on 1% agarose pads and visualized by using a Zeiss AxioImager M1 microscope fitted with an Orca-ER charge-coupled-device (CCD) camera (Hamamatsu). The following Zeiss filter sets were used: 43 (FM4-64), 44 (green fluorescent protein [GFP]), and 49 (DAPI). Images were collected and processed by using OpenLAB 5 (Improvision).
Microarray data accession number.
The microarray data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (GEO) (15a) and are accessible through GEO series accession number GSE22296 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE22296).
RESULTS
Induction of the replication checkpoint protein Sda inhibits sporulation when cells are grown in LB medium.
We were led to examine the effects of manganese limitation on the transcriptional regulation of sda and other DnaA-dependent genes after we observed that the transcription of an sda-lacZ transcriptional fusion was strongly induced when cells were plated onto LB agar plates containing X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), whereas expression was off on “rich” sporulation media such as Difco sporulation medium (DSM) (data not shown). The key difference turned out to be the presence or absence of manganese: sda-lacZ expression was off when cells were plated onto LB agar supplemented with 100 μM manganese chloride, the concentration present in our laboratory's inherited recipe for Difco sporulation medium (the original recipe specifies 10 μM MnCl2 [52]). We conjectured that the induction of sda might account in part for the long-standing observation that Bacillus subtilis and other Bacillus spp. sporulate poorly on peptone- or tryptone-containing rich medium unless the medium is supplemented with manganese (10; for a review, see references 31 and 48).
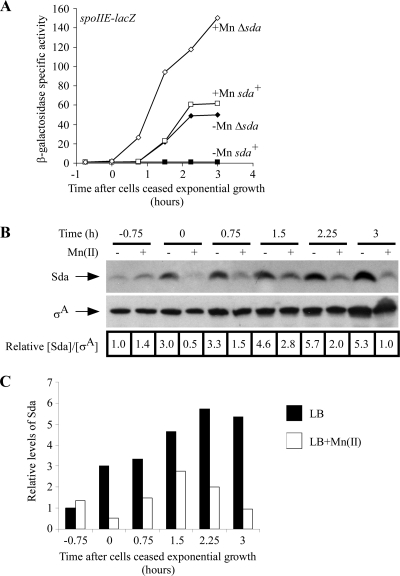
To test this hypothesis, we grew sda+ and Δsda strains in liquid LB medium with or without 100 μM MnCl2, monitoring sporulation-specific gene expression using a lacZ transcriptional fusion to the promoter for a gene that is induced at high levels of phosphorylated Spo0A (Spo0A∼P), spoIIE (18, 46), and monitoring Sda expression levels by quantitative immunoblotting (Fig. 1; data from a representative experiment are shown). We found that the expression of spoIIE-lacZ was induced in sda+ cells shortly after cells ceased exponential growth in LB medium with 100 μM MnCl2 but that expression was inhibited when cells were grown in LB medium lacking manganese (Fig. 1A). In contrast, spoIIE-lacZ expression was induced in Δsda cells in the presence or absence of manganese, suggesting that Sda contributes to the inhibition of sporulation-specific gene expression in LB medium lacking manganese. Concentrations of manganese as low as 1 μM were sufficient to restore spoIIE-lacZ induction in sda+ cells entering stationary phase (see Fig. S1 in the supplemental material). The relative abundance of Sda increased approximately 2- to 5-fold in LB medium compared to that in LB medium plus 100 μM MnCl2 as cells ceased exponential growth (Fig. 1B and C). Sda levels transiently increased to a similar extent following UV irradiation, delaying the initiation of sporulation (51).
FIG. 1.
Sda is induced and prevents sporulation-specific gene expression when cells are grown in LB medium unless manganese is added. (A) The sda replication checkpoint prevents sporulation-specific gene expression in LB medium in the absence of supplemented manganese. Cells were grown in LB medium at 37°C to an OD600 of 0.3 to 0.5; diluted back to an OD600 of 0.02 into fresh, prewarmed LB medium or LB medium supplemented with 100 μM MnCl2; and then grown until 3 h after exit from exponential growth. The initiation of sporulation was monitored by using a lacZ transcriptional fusion to the sporulation-specific gene spoIIE. Sporulation frequencies were determined 20 to 24 h after cells ceased exponential growth (Table 2). Filled symbols, LB medium without Mn(II); open symbols, LB medium with Mn(II); squares, BB825 (sda+ amyE::PspoIIE-lacZ); diamonds, BB827 (Δsda amyE::PspoIIE-lacZ). (B) Levels of Sda increase as cells are grown into stationary phase in LB medium without additional manganese. Samples were taken at the indicated times from the cultures grown as described above (A), and Sda levels were determined by quantitative immunoblotting as described in Materials and Methods. Membranes were stripped and reprobed to determine σA levels as an internal control for sample preparation and loading. The numbers under each lane indicate the Sda/σA ratio normalized to the ratio of the LB [without Mn(II)] mid-log-phase sample. Five micrograms of total protein was loaded onto each lane, run on a 13.8% SDS Tris-Tricine polyacrylamide gel, transferred onto PVDF membranes, and then probed to determine protein levels. (C) Graph of the data in B.
We incubated the cultures for an additional 20 to 24 h following the cessation of exponential growth and determined the fraction of cells in each culture that had formed heat-resistant spores (Table 2). The sporulation frequency of the sda+ strain was ∼25-fold lower than that of the Δsda strain when cells were grown in LB medium lacking manganese, whereas both strains sporulated at high frequencies in LB medium with Mn(II) (Table 2). Thus, the induction of Sda inhibits sporulation-specific gene expression and spore development when cells are grown in LB lacking added manganese. We note that the sporulation frequency of the Δsda strain was ∼400-fold higher when grown in LB medium with Mn(II) than when grown in LB medium alone and, similarly, that spoIIE-lacZ expression was nearly 2-fold higher when the Δsda strain was grown in LB medium plus Mn(II) than when grown in LB medium alone (Fig. 1A). Based on these data, it appears that manganese limitation inhibits sporulation through both sda-dependent and sda-independent mechanisms.
TABLE 2.
Sda reduces the efficiency of sporulation when cells are grown in LB medium lacking manganesea
| Relevant genotype | Strain | Mn(II) concn (μM) | Total CFU ml−1 | No. of spores ml−1 | Sporulation frequencyb |
|---|---|---|---|---|---|
| Wild type | BB825 | 0 | 1.2 × 109 | 5.9 × 104 | 4.9 × 10−5 |
| Δsda | BB827 | 0 | 6.5 × 108 | 8.4 × 105 | 1.3 × 10−3 |
| Wild type | BB825 | 100 | 9.7 × 108 | 5.3 × 108 | 0.55 |
| Δsda | BB827 | 100 | 8.5 × 108 | 4.6 × 108 | 0.54 |
Data are from the same experiment shown in Fig. 1. Similar sporulation data were obtained in 10 additional independent experiments (data not shown).
The sporulation frequency is the ratio of spores ml−1 to total CFU ml−1.
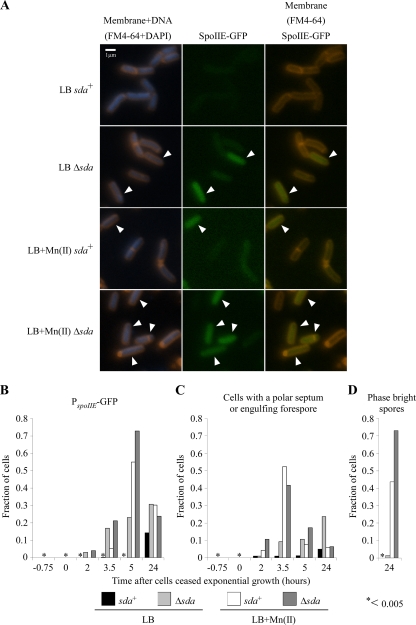
Since spoIIE-lacZ expression in the Δsda strain was reduced only 2-fold when cells were grown in LB medium versus LB medium plus Mn(II), whereas the sporulation frequency was reduced ∼400-fold, we used fluorescence microscopy to determine the fraction of cells in each culture that initiated sporulation-specific gene expression and successfully proceeded through the first major morphological step of spore development, division at the cell pole. We monitored sporulation-specific gene expression using a spoIIE-gfp transcriptional fusion and polar septation using the fluorescent dye FM4-64, which stains the cytoplasmic membrane and the polar division septum prior to the completion of spore engulfment (47) (Fig. 2A to C). After incubating the cultures overnight, we determined the fraction of phase-bright spores that had formed (Fig. 2D).
FIG. 2.
Sda prevents sporulation-specific gene expression in LB medium lacking supplemental manganese. (A) Polar division and sporulation-specific gene expression were assessed by fluorescence microscopy. Cells were grown at 37°C in LB medium alone or LB medium supplemented with 100 μM MnCl2 until 24 h after inoculation. The initiation of sporulation was monitored by using a GFP transcriptional fusion to the sporulation-specific gene spoIIE. At the following time points, samples were collected and examined under a microscope: 45 min prior to the onset of stationary phase, at the onset of stationary phase, 120 min after the onset of stationary phase, 210 min after the onset of stationary phase, 300 min after the onset of stationary phase, and 24 h after sample inoculation. Cells were stained with the fluorescent dyes FM4-64 and DAPI to stain membranes and DNA, respectively. Representative images from the time point at 210 min after the onset of stationary phase are shown, with those cells expressing spoIIE indicated by arrowheads. Strains used were KM81 (sda+ amyE::PspoIIE-gfp) and KM96 (Δsda amyE::PspoIIE-gfp). (B to D) Quantitation of microscopy time course. Cells were grown as described above (A), samples were taken at the indicated times, and microscopy images were scored for spoIIE-gfp expression, polar septa, and phase-bright spores. More than 200 cells were scored for each sample at each time point. At least two independent experiments were performed; the results from one representative experiment are shown. An asterisk indicates that the fraction of cells is less than 0.005. (B) Quantitation of cells expressing spoIIE-gfp. (C) Quantitation of cells that have polar septa. (D) Quantitation of phase-bright spores produced.
Five hours after the onset of stationary phase, spoIIE-gfp expression was induced in approximately 60 to 80% of the population when sda+ and Δsda strains were grown in LB medium with Mn(II) (Fig. 2A and B; arrowheads indicate cells expressing GFP from the spoIIE promoter). When strains were grown in LB medium alone, the Δsda strain showed increasing spoIIE-gfp expression over time, with approximately 25% of the population expressing GFP 5 h after the onset of stationary phase. In contrast, little or no GFP expression was observed in the sda+ strain at the 5-h time point. Thus, the fraction of cells that induced spoIIE-gfp expression directly paralleled the levels of spoIIE-lacZ expression shown in Fig. 1. A similar pattern was observed when we scored the fraction of cells with polar septa or engulfing forespores (Fig. 2C) except that the frequencies of polar septation peaked at 3.5 h rather than 5 h. The subsequent decrease by 5 h reflects the completion of spore engulfment, when FM4-64 no longer stains the forespore membrane. The frequencies of polar septation were somewhat lower for the Δsda strain in both LB medium and LB medium with Mn(II) than for the fraction of cells expressing GFP at 5 h. By 24 h, 40 to 70% of the sda+ and Δsda cells grown in LB medium plus Mn(II) had formed phase-bright spores, whereas when cells were grown in LB medium lacking manganese, fewer than 2% of the Δsda cells formed phase-bright spores, and no phase-bright spores were observed in the sda+ culture (Fig. 2D). These data parallel the sporulation frequencies shown in Table 2. Thus, Δsda cells grown in LB medium proceed through the early stages of sporulation at high frequencies, similar to strains grown in LB medium plus Mn(II), but a large fraction of cells subsequently arrested development prior to the formation of phase-bright or heat-resistant spores. Likewise, a small fraction of sda+ cells expressed spoIIE-gfp and had divided at one cell pole by the 24-h time point (Fig. 2B and C), but the level of production of phase-bright spores was too low for detection (Fig. 2D). These data support the conclusion that manganese is required both for the efficient initiation of sporulation, to prevent induction of Sda, and for efficient progression through one or more stages of development following polar septation.
Previously, Freese and coworkers established that the activity of the glycolytic enzyme phosphoglycerate mutase was strictly dependent on Mn(II) and that cells of B. subtilis were unable to sporulate in LB medium with glucose due to the accumulation of the enzyme's substrate during glycolysis, 3-phosphoglycerate (3-phosphyglyceric acid [3-PGA]) (45, 53). Supplementation of the medium with manganese prevents the accumulation of 3-PGA and restores efficient sporulation (45, 53). Supplementation of the medium with l-malate also prevents 3-PGA accumulation and restores efficient sporulation by providing a route for metabolizing 3-PGA via the tricarboxylic acid (TCA) cycle independently of phosphoglycerate mutase (53). To see if a similar mechanism of sporulation inhibition and 3-PGA accumulation due to insufficient manganese was involved in the block to sporulation in LB medium, we looked at spoIIE-lacZ expression in cells grown in LB medium that had been supplemented with l-malate and compared that pattern of expression to those of cells grown in LB medium and LB medium with additional manganese. We found no difference in the patterns of sporulation-specific gene expression or the production of heat-resistant spores with the addition of l-malate (data not shown, and see Fig. S2 in the supplemental material), indicating that this mechanism is not likely to be involved in the block to sporulation that we observed with LB medium without additional manganese.
Changes in DnaA-dependent gene expression suggest that the levels of active DnaA are higher when cells are grown in LB medium lacking supplemental manganese.
A prolonged expression of Sda is induced by conditions that perturb replication initiation or elongation or that induce DNA damage (4, 7, 9, 20, 30, 43, 51, 54). Thus, the data presented above suggest that cells grown in LB medium without supplemental manganese are experiencing some kind of replication stress.
Several conditions that block replication elongation activate sda transcription by activating the DnaA-dependent pathway and by inducing the SOS response. However, sda is also induced by a variety of conditions that activate DnaA alone without inducing the SOS response (9, 20, 30, 43, 54). We sought to determine if one or both of these pathways were activated when cells were grown in LB medium lacking manganese.
We used lacZ transcriptional fusions to the promoters of two SOS-inducible genes, tagC (dinC) and yneA, to determine if DNA damage could be responsible for the activation of sda expression (12, 34). Neither reporter was detectably induced on LB medium or LB medium plus Mn(II) indicator plates in either the sda+ or Δsda strain background (strains AM48 [sda+ amyE::PyneA-lacZ], SH493 [Δsda amyE::PyneA-lacZ], SH350 [sda+ thrC::PtagC-lacZ], and SH351 [Δsda thrC::PtagC-lacZ] [data not shown]). yneA is a particularly sensitive marker, since it is induced at concentrations of DNA-damaging agents that do not induce tagC (4; A. Mo and W. F. Burkholder, unpublished data). Therefore, since no expression was seen from either reporter, we concluded that DNA damage resulting in the activation of the SOS response was not a major factor in the activation of sda expression in LB medium.
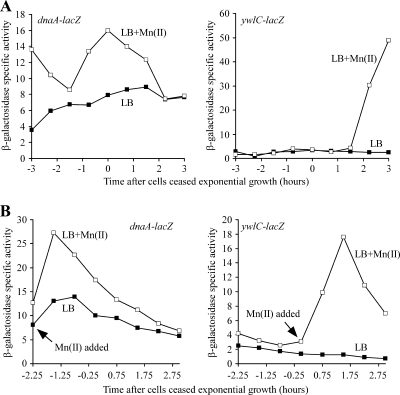
In order to determine if sda transcription was activated via the DnaA-dependent pathway, we used lacZ transcriptional fusions to monitor the expression of two genes that are repressed when active DnaA accumulates, dnaA itself and ywlC (7, 20, 30, 44). The expression of each reporter gene was monitored over time as samples were grown in LB medium and LB medium with additional manganese until 3 h after the cessation of exponential growth (Fig. 3A). Looking at levels of active DnaA during exponential growth using the dnaA reporter, we found that the reporter gene expression level was higher in LB medium with manganese added than in LB medium until approximately 2 h after the onset of stationary phase, when the levels were indistinguishable. This pattern was consistent with higher levels of active DnaA being present in LB medium without additional manganese than in LB medium with additional manganese. The ywlC reporter was used to examine the levels of active DnaA during stationary phase, and again, expression was seen only starting 2 h after cells had ceased exponential growth when manganese was added to the medium, indicating that levels of active DnaA were higher in LB medium without manganese than in LB medium with manganese.
FIG. 3.
The expression patterns of DnaA-dependent reporter genes suggests that the levels of active DnaA are elevated in LB medium lacking supplemental manganese. (A) dnaA-dependent reporter genes show higher levels of expression when grown in LB medium with supplemented manganese than when grown in LB medium without additional manganese. Cells were grown in LB medium to an OD600 of 0.3 to 0.5, diluted back into fresh LB medium alone or LB medium supplemented with 100 μM MnCl2, and then grown at 37°C until 3 h after exit from exponential growth. To monitor levels of active DnaA during exponential growth, a lacZ transcriptional fusion to dnaA was used. Levels of active DnaA during stationary phase were monitored by using a lacZ transcriptional fusion to ywlC (see the text for details). Three independent experiments were performed; the results from one representative experiment are shown. Strains used were SH507 (sda+ amyE::PdnaA-lacZ) and BB424 (sda+ amyE::PywlC-lacZ). (B) dnaA-dependent reporters show higher expression levels after manganese is added to cultures growing in LB medium than in control cultures of LB medium without additional manganese. Cells were grown in LB medium at 37°C. At the indicated times, 100 μM MnCl2 was added to half of each culture, while the other half received no supplementation. The same reporters as those detailed above (A) were monitored for expression until 3 h after cells had ceased exponential growth. Three independent experiments were performed; the results from one representative experiment are shown. Strains used were SH507 (sda+ amyE::PdnaA-lacZ) and BB424 (sda+ amyE::PywlC-lacZ).
To rule out the possibility that the differences in reporter gene expression were due to long-term effects of growing cells in the presence or absence of manganese, we measured the acute effect of adding manganese to cultures grown initially in LB medium alone on gene expression (Fig. 3B). Strains were grown in LB medium to mid-exponential phase (for the dnaA reporter strain) and the onset of stationary phase (for the ywlC reporter strain). The cultures were then split, adding 100 μM manganese to one of the two flasks for each strain. Consistent with our previous results, the addition of manganese induced the expression of both the dnaA-lacZ and the ywlC-lacZ transcriptional fusions. These data indicate that levels of active DnaA are higher in LB medium lacking supplemental manganese.
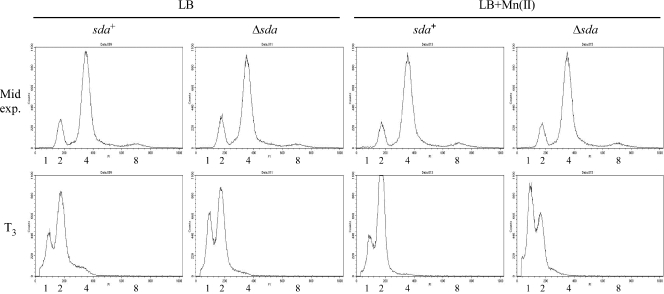
Since the SOS response to DNA damage is not induced, activated DnaA might accumulate due to the misregulation of replication initiation or to changes in replication fork processivity that are independent of significant DNA damage, helicase uncoupling, or replication fork collapse. Conditions that perturb replication initiation and induce sda expression affect the chromosome copy number by causing asynchronous initiation (so that cells have an odd number of origins of replication rather than 1, 2, 4, and 8, etc.), by increasing the number of origins per cell (overinitiation), or by inhibiting replication initiation (9, 40, 43). We asked if the presence or absence of manganese affected replication synchrony or chromosome copy numbers by growing sda+ and Δsda strains in LB medium and LB medium plus 100 μM Mn(II) at 26°C and taking samples during exponential growth and 3 h after cells had ceased exponential growth (T3). Cells were then incubated in the presence of chloramphenicol to block replication initiation and cell division for several hours to allow ongoing rounds of DNA replication to be completed, and the relative DNA content per cell was determined by flow cytometry (see the supplemental material). Since cells of B. subtilis normally grow in chains during exponential growth, we used strains that lack the flgM gene (BB1185 [sda+ flgMΔ80] and SH455 [Δsda flgMΔ80]), which rapidly separate into single cells during exponential growth due to the overexpression of the autolysins that promote cell separation (39). As expected for cells entering the stationary phase, the chromosome copy number in both strains decreased from predominantly 2 to 4 chromosome equivalents to 1 to 2 chromosome equivalents between the mid-exponential time point and T3 (Fig. 4). There were slight differences in the chromosome copy number profiles between the sda+ and Δsda strains grown in the presence of manganese at T3, but none of the profiles suggested that there were significant differences in replication synchrony and chromosome copy number between strains grown in the presence or absence of manganese (Fig. 4). Although we cannot rule out the possibility that sda expression is induced due to a subtle perturbation in replication initiation, it seems most likely that the level of DnaA activity is higher in LB medium lacking manganese due to effects on replication fork progression that do not significantly activate the SOS response.
FIG. 4.
The presence or absence of manganese in LB medium has little or no effect on chromosome copy number or the synchrony of replication initiation. Cells were grown in LB medium or LB medium plus 100 μM MnCl2 at 26°C to an OD600 of 0.3 to 0.5 for mid-exponential-phase samples or 37°C until 3 h after the onset of stationary phase for T3 samples. At the indicated times, a 5-ml aliquot was removed and incubated with 200 μg/ml of chloramphenicol for 6 h to inhibit further rounds of replication from initiating but to allow current rounds to finish. Cells were then fixed in ethanol, washed twice with 200 mM Tris-HCl at pH 7.5, and subsequently incubated with 100 μg/ml RNase A for 1 h at 60°C. Cells were then stained with propidium iodide to a final concentration of 5 μg/ml and diluted (if necessary), and 10,000 cells were analyzed for DNA content with a flow cytometer. The numbers under the flow cytometry profiles correspond to chromosomal equivalents. Strains used were BB1185 (sda+ flgMΔ80) and SH455 (Δsda flgMΔ80).
Microarray analysis.
To better understand how manganese limitation might increase the levels of active DnaA, we performed whole-genome expression profiling using 65-mer oligonucleotide microarrays (Genosys). sda+ and Δsda strains were grown in LB medium and LB medium supplemented with 100 μM manganese, and RNA was purified from samples collected at three time points: mid-exponential phase (OD600 of 0.3 to 0.5; “Tm”), when cells ceased exponential growth (T0), and 3 h after cells ceased exponential growth (T3) (GEO accession number for microarray data, GSE22296; see the supplemental material for experimental details).
The results from the microarray analysis indicated that the number of differentially expressed genes increased over time under these conditions (see Table S8 in the supplemental material for the fold differences in relative gene expression organized by regulon and Table S9 for complete microarray data and statistics). At the first two time points (Tm and T0), the differences in gene expression were due mainly to the presence or absence of manganese. At T3, when cells initiated spore development in LB medium supplemented with manganese, a large number of genes were differentially expressed, depending on the presence or absence of manganese, the presence or absence of sda, or both.
The microarray data supported our conclusions that the induction of sda was due to the activation of the DnaA-dependent signaling pathway but not to the SOS response to DNA damage. Little or no induction of SOS-inducible genes (LexA-repressed genes and prophage genes) was observed at any time point when cells were grown in the absence of manganese (see Tables S8 and S9 in the supplemental material). dnaA expression was significantly reduced at T0 in both strains when cells were grown in the absence of manganese (or, as shown in Table S3, expressed at significantly higher levels in the presence of manganese), paralleling our data obtained using the dnaA-lacZ transcriptional fusion (Fig. 3). Similarly, one of the operons positively regulated by DnaA, yurY-yurX-csd-nifU-yurU, encoding orthologs of the E. coli Fe-S assembly complex subunits SufB, SufC, and SufD, was expressed at significantly higher levels in the absence of manganese at T0 (Table S3). No significant differences in expression were observed for ywlC at T3, which probably reflects the lower sensitivity of the array data under these growth conditions.
Several DnaA-regulated operons were differentially expressed at T0 in the direction opposite of that expected if changes in expression were due solely to activated DnaA, including ykuNOP, encoding two flavodoxins, and dhbACEBF, encoding enzymes required for the biosynthesis of the iron siderophore bacillibactin (see Table S3 in the supplemental material). The ykuNOP and dhbACEBF operons are repressed by Fur and were expressed at significantly higher levels in both strains in the absence of manganese. Several other Fur-repressed operons were also expressed at significantly higher levels in the absence of manganese (Table S4). Many, like dhbACEBF, are involved in siderophore production and iron-siderophore uptake. These data are consistent with previous observations that high concentrations of Mn(II) repress the Fur regulon via PerR (17, 22).
The expressions of many sporulation-specific genes were inhibited at T3 when sda+ cells were grown in the absence of manganese (see Table S5 in the supplemental material). These data parallel the results described above, obtained using the spoIIE-lacZ and spoIIE-gfp transcriptional fusions. In addition to spoIIE, several other genes are induced by high levels of Spo0A∼P during the initiation of sporulation (18): spoIIA (spoIIAA, spoIIAB, or sigF), spoIIG (spoIIGA, sigE, or sigG), racA, yttP, sinI, and sirA (yneE), which encodes a protein that inhibits further rounds of replication in sporulating cells by inhibiting DnaA (49, 55). The levels of expression of all of these genes were significantly lower (q ≤ 0.005) at T3 in sda+ strains grown in the absence of manganese based on the following ratios of relative gene expression from the four growth conditions: [sda+ strain in LB medium plus Mn(II)]/(sda+ strain in LB medium) ratio of ≥1.5-fold (log10) and (sda+ strain in LB medium)/(Δsda strain in LB medium) ratio of ≤1.5-fold (Table S5). As observed with the spoIIE transcriptional fusions, the levels of expression of many sporulation genes were also significantly higher in the Δsda strain grown in LB medium plus Mn(II) than in LB medium.
Only 13 genes were found to have significant changes in gene expression at the first time point (Tm) (see Table S6 in the supplemental material). Of these genes, 11 were significantly downregulated in both strains at all three time points when cells were grown in LB medium plus Mn(II) (Table S6). Consistent with the growth conditions, these included two operons encoding manganese transport systems (mntABCD and mntH), regulated by MntR. The gene encoding vegetative catalase (katA), a member of the PerR regulon, was also expressed at significantly lower levels in LB medium with Mn(II) at all three time points. The PerR regulon is most closely associated with the response to inorganic peroxides and is induced during exposure to peroxide and superoxide stress. Several other PerR-regulated operons were expressed at significantly lower levels in LB medium plus Mn(II) at T0 and T3: ahpCF, encoding alkyl hydroperoxide reductase; hemAXCDBL, required for heme biosynthesis; and perR itself (Table S7). The manganese-dependent regulation of MntR- and PerR-dependent gene expression has been well characterized (11, 17, 22, 24). Interestingly, but for reasons that are not clear, the PerR-regulated gene encoding the iron-sequestering protein MrgA was expressed at lower levels at T3 but only for the Δsda strain (Table S7). These data suggest that cells may be undergoing oxidative stress when grown in the absence of manganese.
Oxidative stress response genes are induced in cells grown to stationary phase in LB medium lacking supplemental manganese.
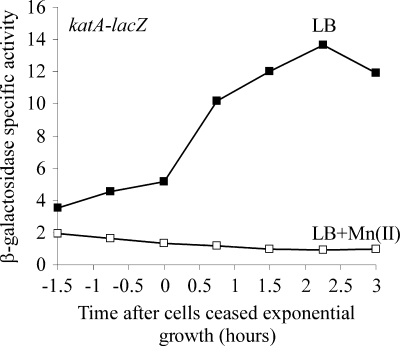
To corroborate the finding that katA was induced when cells were grown in LB medium lacking manganese, we monitored katA expression using a lacZ transcriptional fusion and grew cells in LB medium in the presence or absence of 100 μM manganese (Fig. 5). Consistent with the microarray data, the level of expression of katA increased over time, particularly after the onset of stationary phase, when cells were grown in LB medium. Little or no expression of katA was observed when cells were grown in LB medium with Mn(II). The stationary-phase induction of katA and the strong repression of katA transcription by manganese have been extensively characterized (11, 17, 22, 24). Microscopy experiments using a katA-gfp transcriptional fusion revealed that katA-gfp expression was induced in the entire population when cells were grown in LB medium, whereas little or no GFP fluorescence was observed when cells were grown in LB medium with Mn(II) (strain SH517 [amyE::PkatA-gfp] [data not shown]). Taken together with the microarray results, the expression pattern for katA indicates that cells may be experiencing oxidative stress when grown in LB medium lacking supplemented manganese, which in turn might be responsible for perturbing DNA replication.
FIG. 5.
katA expression is induced when cells are grown in LB medium lacking supplemental manganese. Cells were grown in LB medium at 37°C to an OD600 of 0.3 to 0.5; diluted back to an OD600 of 0.02 into fresh, prewarmed LB medium or LB medium supplemented with 100 μM MnCl2; and then grown until 3 h after exit from exponential growth. Catalase levels were monitored by using a lacZ transcriptional fusion to katA. Filled symbols, LB medium without Mn(II); open symbols, LB medium with Mn(II). Strain SH536 (sda+ amyE::PkatA-lacZ) was used.
DISCUSSION
Based on our results, we conclude that changes in DnaA-dependent gene expression contribute to the transcriptional and developmental responses of B. subtilis to manganese-limited growth in LB medium. Cells of B. subtilis sporulate poorly in LB medium in part because of the activation of the Sda checkpoint, which delays or inhibits sporulation until DNA replication has been completed. Sda expression is induced and prevents sporulation-specific gene expression in LB medium unless the medium is supplemented with additional manganese (Fig. 1B and C). Cells lacking sda initiate spore development at a high frequency, inducing sporulation-specific gene expression and dividing at the cell pole (Fig. 1A and 2, and see Table S4 in the supplemental material). However, following polar septation, Δsda cells frequently fail to form stress-resistant spores due to a requirement for manganese at some later stage of development (Table 2 and Fig. 2D). The transcription of sda appears to be activated solely via the DnaA-dependent pathway, which responds to perturbations of replication initiation and replication elongation, based on the following results: (i) the levels of expression of several other DnaA-dependent genes differ significantly in LB medium compared to that in LB medium plus Mn(II), consistent with the model that levels of active DnaA are higher in LB medium lacking manganese (Fig. 3 and Table S3), and (ii) the SOS response to DNA damage is not induced (based on data from lacZ transcriptional fusions and microarray data discussed in the text) (Tables S8 and S9). Based on flow cytometry data, it seems that replication initiation is not significantly perturbed (Fig. 4), suggesting that DnaA may activate sda transcription due to a perturbation of replication elongation that does not induce the SOS response. DNA replication might be perturbed due to secondary effects of reactive oxygen species, since the gene encoding vegetative catalase, katA, and other PerR-regulated genes involved in the oxidative stress response are expressed at significantly higher levels when cells are grown in LB medium lacking manganese (Fig. 5 and Tables S6 and S7). DNA replication might also be perturbed due to the effects of manganese limitation on one or more manganese-dependent enzymes. Regardless of the source, these data demonstrate that small perturbations in DNA replication can have large effects on development, effectively blocking endospore formation in B. subtilis.
The data presented here provide new insight into the long-standing observation that Bacillus subtilis sporulates poorly in peptone- or tryptone-containing rich medium unless the medium is supplemented with manganese (10; for a review, see references 31 and 48). However, we have only pushed the questions back by a step: how does manganese limitation in LB medium affect DNA replication or metabolism, and how do those effects lead to the activation of DnaA and DnaA-dependent changes in gene expression?
Our data indicate that manganese limitation also inhibits sporulation by sda-independent mechanisms (Fig. 2 and Table 2). The induction of Sda thus prevents cells from initiating sporulation under conditions that preclude the successful completion of spore morphogenesis, as observed previously (9, 51). Although Sda is required to fully inhibit the transcription of spoIIE during manganese limitation, we note that manganese limitation has a small effect on the levels of spoIIE expression and polar septation in cells lacking sda, reducing the levels of spoIIE-lacZ expression and the fraction of cells that express spoIIE-gfp or that have undergone polar septation 2- to 5-fold (Fig. 1 and 2). The largest effects of manganese limitation likely occur at later stages of spore development, since cells lacking sda sporulate at ∼400-fold-lower frequencies in LB medium than in LB medium supplemented with manganese (Table 2). Any number of mechanisms might account for the sda-independent effects of manganese limitation on sporulation.
Manganese is a cofactor for enzymes involved in diverse metabolic pathways, including glycolysis, the TCA cycle, amino acid utilization, and nucleotide metabolism. Some of the manganese-dependent changes in gene expression inferred from our whole-genome expression data (see Tables S8 and S9 in the supplemental material) are likely responses to the reduced activity of particular manganese-dependent enzymes in LB medium compared to LB medium with Mn(II). This same general observation has also been made regarding the whole-genome transcriptional response of B. subtilis grown in a defined minimal medium in the presence or absence of manganese (22). Thus, changes in energy status, deoxyribonucleotide pools, amino acid or carbohydrate utilization, or other metabolic effects may contribute alone or in combination to impede DNA replication.
Manganese also plays a major role in protecting cells from reactive oxygen species, both as a cofactor for enzymes such as manganese-dependent superoxide dismutase (SodA in B. subtilis) and, perhaps in complex with cellular metabolites, as a direct scavenger of reactive oxygen species (27). For instance, the high-level resistance of organisms such as Deinococcus radiodurans and Lactobacillus plantarum to radiation or oxidative stress may result in part from their exceptionally high concentrations of intracellular manganese and the direct scavenging of reactive oxygen species by manganese in complex with small molecules (2, 3, 13, 15). In cells of B. subtilis lacking sodA, manganese still confers resistance to oxidative stress in growing and sporulating cells and stimulates a hydrogen peroxide-scavenging activity that may otherwise require one or more low-molecular-weight metabolites (29).
Three of the predominant reactive oxygen species encountered by aerobically growing cells are superoxide, hydrogen peroxide, and hydroxyl radicals generated by the reaction of hydrogen peroxide with Fe(II) in the Fenton reaction (27). Superoxide and hydrogen peroxide both induce the perR and fur regulons in B. subtilis as well as in E. coli (1, 5, 17, 23, 41, 58), so the accumulation of either regulon would be consistent with the induction of katA and other genes in the PerR and Fur regulons that we observed (Fig. 5, and see Table S6 and S7 in the supplemental material). Hydroxyl radicals generated by the Fenton reaction are particularly reactive and cause damage to cellular proteins, lipids, stable RNA, and DNA (28). We consider it unlikely, however, that significant oxidative damage to DNA is responsible for the induction of sda, since we did not observe a significant induction of either SOS response genes repressed by LexA or prophage genes. We cannot rule out the possibility, however, that low levels of oxidative damage insufficient to induce the SOS response nonetheless perturb replication fork progression sufficiently to activate DnaA-dependent gene expression. The main targets of superoxide in vivo are enzymes with iron-sulfur clusters (most of which are key players in central metabolism) and branched-chain, aromatic, and sulfur-containing amino acids (28). Thus, oxidative stress might affect DNA replication by perturbing metabolic pathways in much the same way that manganese limitation could impair the activities of manganese-dependent enzymes.
If superoxide contributes to the oxidative stress response, it seems surprising that expression levels of the principal superoxide dismutase, SodA, were not significantly different in the presence or absence of manganese. In contrast, expression levels of a gene encoding a putative sporulation-specific Fe(II)-dependent superoxide dismutase, sodF, were significantly higher in the presence of manganese in both strains at T0 and in the Δsda strain at T3. In a study characterizing the transcriptional response of B. subtilis to the superoxide-generating compound paraquat, the expression of sodA was not significantly different in the presence or absence of paraquat (41).
The results presented here underscore the point that DNA replication can be affected by a variety of environmental conditions not obviously associated with genotoxic stress and that small perturbations in DNA replication can have large developmental consequences. Consequently, mechanisms for coordinating cell cycle progression with chromosome replication and segregation, such as the Sda checkpoint pathway, are likely to play broad roles in promoting competitive fitness in natural environments.
Supplementary Material
Acknowledgments
We thank Masaya Fujita and Richard Losick for anti-σA antiserum; Steven Biller, Kathleen Mach, and Allison Mo in the Burkholder laboratory for plasmids and strains; Robert Britton for microarray protocols and assistance with microarray printing; and Steven Biller for assistance with microarray experiments. We thank members of the Burkholder laboratory and other generous colleagues for many helpful discussions.
S.E.H. was supported by an NIH predoctoral training grant (5T32GM007276). This work was supported by National Science Foundation award ID 0744872.
Footnotes
Published ahead of print on 28 May 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Antelmann, H., J. Bernhardt, R. Schmid, H. Mach, U. Volker, and M. Hecker. 1997. First steps from a two-dimensional protein index towards a response-regulation map for Bacillus subtilis. Electrophoresis 18:1451-1463. [DOI] [PubMed] [Google Scholar]
- 2.Archibald, F. S., and I. Fridovich. 1981. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J. Bacteriol. 145:442-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archibald, F. S., and I. Fridovich. 1982. The scavenging of superoxide radical by manganous complexes: in vitro. Arch. Biochem. Biophys. 214:452-463. [DOI] [PubMed] [Google Scholar]
- 4.Au, N., E. Kuester-Schoeck, V. Mandava, L. E. Bothwell, S. P. Canny, K. Chachu, S. A. Colavito, S. N. Fuller, E. S. Groban, L. A. Hensley, T. C. O'Brien, A. Shah, J. T. Tierney, L. L. Tomm, T. M. O'Gara, A. I. Goranov, A. D. Grossman, and C. M. Lovett. 2005. Genetic composition of the Bacillus subtilis SOS system. J. Bacteriol. 187:7655-7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernhardt, J., K. Buttner, C. Scharf, and M. Hecker. 1999. Dual channel imaging of two-dimensional electropherograms in Bacillus subtilis. Electrophoresis 20:2225-2240. [DOI] [PubMed] [Google Scholar]
- 6.Brehm, S. P., S. P. Staal, and J. A. Hoch. 1973. Phenotypes of pleiotropic-negative sporulation mutants of Bacillus subtilis. J. Bacteriol. 115:1063-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breier, A. M., and A. D. Grossman. 2009. Dynamic association of the replication initiator and transcription factor DnaA with the Bacillus subtilis chromosome during replication stress. J. Bacteriol. 191:486-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkholder, P. R., and N. H. Giles, Jr. 1947. Induced biochemical mutations in Bacillus subtilis. Am. J. Bot. 34:345-348. [PubMed] [Google Scholar]
- 9.Burkholder, W. F., I. Kurtser, and A. D. Grossman. 2001. Replication initiation proteins regulate a developmental checkpoint in Bacillus subtilis. Cell 104:269-279. [DOI] [PubMed] [Google Scholar]
- 10.Charney, J., W. P. Fisher, and C. P. Hegarty. 1951. Managanese as an essential element for sporulation in the genus Bacillus. J. Bacteriol. 62:145-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, L., L. Keramati, and J. D. Helmann. 1995. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. U. S. A. 92:8190-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheo, D. L., K. W. Bayles, and R. E. Yasbin. 1991. Cloning and characterization of DNA damage-inducible promoter regions from Bacillus subtilis. J. Bacteriol. 173:1696-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox, M. M., and J. R. Battista. 2005. Deinococcus radiodurans—the consummate survivor. Nat. Rev. Microbiol. 3:882-892. [DOI] [PubMed] [Google Scholar]
- 14.Curtis, P. D., and Y. V. Brun. 2010. Getting in the loop: regulation of development in Caulobacter crescentus. Microbiol. Mol. Biol. Rev. 74:13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daly, M. J. 2009. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat. Rev. Microbiol. 7:237-245. [DOI] [PubMed] [Google Scholar]
- 15a.Edgar, R., M. Domrachev, and A. E. Lash. 2002. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30:207-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Errington, J. 2010. From spores to antibiotics via the cell cycle. Microbiology 156:1-13. [DOI] [PubMed] [Google Scholar]
- 17.Fuangthong, M., A. F. Herbig, N. Bsat, and J. D. Helmann. 2002. Regulation of the Bacillus subtilis fur and perR genes by PerR: not all members of the PerR regulon are peroxide inducible. J. Bacteriol. 184:3276-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita, M., J. E. Gonzalez-Pastor, and R. Losick. 2005. High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J. Bacteriol. 187:1357-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glasfeld, A., E. Guedon, J. D. Helmann, and R. G. Brennan. 2003. Structure of the manganese-bound manganese transport regulator of Bacillus subtilis. Nat. Struct. Biol. 10:652-657. [DOI] [PubMed] [Google Scholar]
- 20.Goranov, A. I., L. Katz, A. M. Breier, C. B. Burge, and A. D. Grossman. 2005. A transcriptional response to replication status mediated by the conserved bacterial replication protein DnaA. Proc. Natl. Acad. Sci. U. S. A. 102:12932-12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goranov, A. I., E. Kuester-Schoeck, J. D. Wang, and A. D. Grossman. 2006. Characterization of the global transcriptional responses to different types of DNA damage and disruption of replication in Bacillus subtilis. J. Bacteriol. 188:5595-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guedon, E., C. M. Moore, Q. Que, T. Wang, R. W. Ye, and J. D. Helmann. 2003. The global transcriptional response of Bacillus subtilis to manganese involves the MntR, Fur, TnrA and sigmaB regulons. Mol. Microbiol. 49:1477-1491. [DOI] [PubMed] [Google Scholar]
- 22a.Harwood, C. R., and S. M. Cutting (ed.). 1990. Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, England.
- 23.Helmann, J. D., M. F. Wu, A. Gaballa, P. A. Kobel, M. M. Morshedi, P. Fawcett, and C. Paddon. 2003. The global transcriptional response of Bacillus subtilis to peroxide stress is coordinated by three transcription factors. J. Bacteriol. 185:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbig, A. F., and J. D. Helmann. 2001. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Mol. Microbiol. 41:849-859. [DOI] [PubMed] [Google Scholar]
- 25.Herrick, J., and B. Sclavi. 2007. Ribonucleotide reductase and the regulation of DNA replication: an old story and an ancient heritage. Mol. Microbiol. 63:22-34. [DOI] [PubMed] [Google Scholar]
- 26.Hilbert, D. W., and P. J. Piggot. 2004. Compartmentalization of gene expression during Bacillus subtilis spore formation Microbiol. Mol. Biol. Rev. 68:234-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imlay, J. A. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77:4.1-4.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imlay, J. A. 2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57:395-418. [DOI] [PubMed] [Google Scholar]
- 29.Inaoka, T., Y. Matsumura, and T. Tsuchido. 1999. SodA and manganese are essential for resistance to oxidative stress in growing and sporulating cells of Bacillus subtilis. J. Bacteriol. 181:1939-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishikawa, S., Y. Ogura, M. Yoshimura, H. Okumura, E. Cho, Y. Kawai, K. Kurokawa, T. Oshima, and N. Ogasawara. 2007. Distribution of stable DnaA-binding sites on the Bacillus subtilis genome detected using a modified ChIP-chip method. DNA Res. 14:155-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakubovics, N. S., and H. F. Jenkinson. 2001. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology 147:1709-1718. [DOI] [PubMed] [Google Scholar]
- 32.Kaguni, J. M. 2006. DnaA: controlling the initiation of bacterial DNA replication and more. Annu. Rev. Microbiol. 60:351-375. [DOI] [PubMed] [Google Scholar]
- 33.Katayama, T., S. Ozaki, K. Keyamura, and K. Fujimitsu. 2010. Regulation of the replication cycle: conserved and diverse regulatory systems for DnaA and oriC. Nat. Rev. Microbiol. 8:163-170. [DOI] [PubMed] [Google Scholar]
- 34.Kawai, Y., S. Moriya, and N. Ogasawara. 2003. Identification of a protein, YneA, responsible for cell division suppression during the SOS response in Bacillus subtilis. Mol. Microbiol. 47:1113-1122. [DOI] [PubMed] [Google Scholar]
- 35.Kurokawa, K., S. Nishida, A. Emoto, K. Sekimizu, and T. Katayama. 1999. Replication cycle-coordinated change of the adenine nucleotide-bound forms of DnaA protein in Escherichia coli. EMBO J. 18:6642-6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAdams, H. H., and L. Shapiro. 2009. System-level design of bacterial cell cycle control. FEBS Lett. 583:3984-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Messer, W., and C. Weigel. 2003. DnaA as a transcription regulator. Methods Enzymol. 370:338-349. [DOI] [PubMed] [Google Scholar]
- 38.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 39.Mirel, D. B., P. Lauer, and M. J. Chamberlin. 1994. Identification of flagellar synthesis regulatory and structural genes in a sigma D-dependent operon of Bacillus subtilis. J. Bacteriol. 176:4492-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moriya, S., K. Kato, H. Yoshikawa, and N. Ogasawara. 1990. Isolation of a dnaA mutant of Bacillus subtilis defective in initiation of replication: amount of DnaA protein determines cells' initiation potential. EMBO J. 9:2905-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mostertz, J., C. Scharf, M. Hecker, and G. Homuth. 2004. Transcriptome and proteome analysis of Bacillus subtilis gene expression in response to superoxide and peroxide stress. Microbiology 150:497-512. [DOI] [PubMed] [Google Scholar]
- 42.Mott, M. L., and J. M. Berger. 2007. DNA replication initiation: mechanisms and regulation in bacteria. Nat. Rev. Microbiol. 5:343-354. [DOI] [PubMed] [Google Scholar]
- 42a.Msadek, T., V. Dartois, F. Kunst, M.-L. Herbaud, F. Denizot, and G. Rapoport. 1998. ClpP of Bacillus subtilis is required for competence development, motility, degradative enzyme synthesis, growth at high temperature and sporulation. Mol. Microbiol. 27:899-914. [DOI] [PubMed] [Google Scholar]
- 43.Murray, H., and J. Errington. 2008. Dynamic control of the DNA replication initiation protein DnaA by Soj/ParA. Cell 135:74-84. [DOI] [PubMed] [Google Scholar]
- 44.Ogura, Y., Y. Imai, N. Ogasawara, and S. Moriya. 2001. Autoregulation of the dnaA-dnaN operon and effects of DnaA protein levels on replication initiation in Bacillus subtilis. J. Bacteriol. 183:3833-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh, Y. K., and E. Freese. 1976. Manganese requirement of phosphoglycerate phosphomutase and its consequences for growth and sporulation of Bacillus subtilis. J. Bacteriol. 127:739-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piggot, P. J., and D. W. Hilbert. 2004. Sporulation of Bacillus subtilis. Curr. Opin. Microbiol. 7:579-586. [DOI] [PubMed] [Google Scholar]
- 47.Pogliano, J., N. Osborne, M. D. Sharp, A. Abanes-De Mello, A. Perez, Y. L. Sun, and K. Pogliano. 1999. A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol. Microbiol. 31:1149-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Que, Q., and J. D. Helmann. 2000. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol. Microbiol. 35:1454-1468. [DOI] [PubMed] [Google Scholar]
- 49.Rahn-Lee, L., B. Gorbatyuk, O. Skovgaard, and R. Losick. 2009. The conserved sporulation protein YneE inhibits DNA replication in Bacillus subtilis. J. Bacteriol. 191:3736-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rowland, S. L., W. F. Burkholder, K. A. Cunningham, M. W. Maciejewski, A. D. Grossman, and G. F. King. 2004. Structure and mechanism of action of Sda, an inhibitor of the histidine kinases that regulate initiation of sporulation in Bacillus subtilis. Mol. Cell 13:689-701. [DOI] [PubMed] [Google Scholar]
- 51.Ruvolo, M. V., K. E. Mach, and W. F. Burkholder. 2006. Proteolysis of the replication checkpoint protein Sda is necessary for the efficient initiation of sporulation after transient replication stress in Bacillus subtilis. Mol. Microbiol. 60:1490-1508. [DOI] [PubMed] [Google Scholar]
- 52.Schaeffer, P., J. Millet, and J. P. Aubert. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. U. S. A. 54:704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vasantha, N., and E. Freese. 1979. The role of manganese in growth and sporulation of Bacillus subtilis. J. Gen. Microbiol. 112:329-336. [DOI] [PubMed] [Google Scholar]
- 54.Veening, J. W., H. Murray, and J. Errington. 2009. A mechanism for cell cycle regulation of sporulation initiation in Bacillus subtilis. Genes Dev. 23:1959-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner, J. K., K. A. Marquis, and D. Z. Rudner. 2009. SirA enforces diploidy by inhibiting the replication initiator DnaA during spore formation in Bacillus subtilis. Mol. Microbiol. 73:963-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, J. D., and P. A. Levin. 2009. Metabolism, cell growth and the bacterial cell cycle. Nat. Rev. Microbiol. 7:822-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zakrzewska-Czerwinska, J., D. Jakimowicz, A. Zawilak-Pawlik, and W. Messer. 2007. Regulation of the initiation of chromosomal replication in bacteria. FEMS Microbiol. Rev. 31:378-387. [DOI] [PubMed] [Google Scholar]
- 58.Zheng, M., B. Doan, T. D. Schneider, and G. Storz. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol. 181:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.