Abstract
In contrast to rod-shaped bacteria, little is known about chromosomal maintenance and segregation in the spherical Staphylococcus aureus. The analysis of chromosomal segregation in smc (structural maintenance of chromosomes) and spoIIIE single and double mutants unravels differences in the chromosome dynamics in the spherical staphylococcal cells compared to the model in rods.
In bacteria, studies on chromosome dynamics are mainly focused on model organisms such as Escherichia coli, Bacillus subtilis, and Caulobacter crescentus (8, 28). However, in staphylococci, with their spherical cell shape and their special consecutive perpendicular mode of division (34), little is known about chromosome organization and dynamics so far. Moreover, the important status of Staphylococcus aureus as one of the most prominent human pathogens highlights the significance of studying essential cellular processes that could serve as a basis for antibiotic target development (11, 15).
SMC (structure maintenance of chromosomes) protein is one of the crucial factors involved in various aspects of chromosome dynamics, such as chromosome condensation, packaging, partitioning, and DNA repair (4, 6, 13, 22, 24, 30). B. subtilis SMC and its homolog, MukB, in E. coli are composed of two head regions at N and C termini, setting up an ATPase domain separated by two heptad-rich regions forming a single internal long coiled coil that are connected by a flexible hinge domain in the middle. Homodimerized SMC proteins linked by the hinge domain (17) form a complex with ScpA and ScpB (segregation and condensation proteins A and B) (23), while MukB interacts with MukF and MukE in E. coli correspondingly (27). The B. subtilis smc mutant shows a severe temperature-sensitive lethal phenotype with irregular chromosome organization and chromosome segregation defects (4, 24). It was proposed that the pleiotropic phenotype of the smc mutant was primarily due to its function in chromosome condensation (4). Interestingly, a recent report described that the mycobacterial smc deletion mutant was still proficient in DNA repair and long-term survival (14).
The involvement of SpoIIIE in chromosome segregation was first identified during sporulation in B. subtilis (32). It is required for active translocation of the bulk chromosome into the forespore across the fused septal membranes (5). SpoIIIE consists of an N-terminal transmembrane domain responsible for membrane anchoring and the C-terminal ATPase and DNA translocation domain. The function of SpoIIIE in postseptational chromosome partitioning renders it as a backup mechanism to rescue the nucleoids that have been trapped by the division septum when chromosome segregation was perturbed in vegetative cells (26). Consequently, the smc and spoIIIE double mutant had a synergistic lethal phenotype in B. subtilis (3). The combination of the E. coli mukB null mutation and truncation of ftsK encoding the homolog protein to SpoIIIE resulted in a similar synergistic lethal phenotype (33).
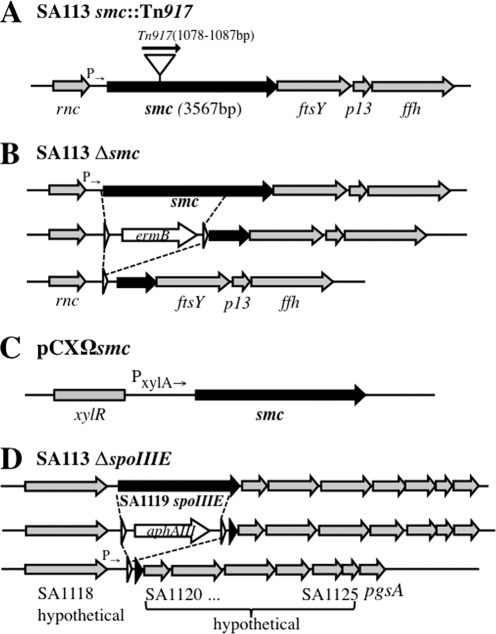
Genome analysis revealed that there was a single smc gene locus present in all of the staphylococcal genomes available so far. S. aureus smc (SAOUHSC_01204) encodes a 1,188-amino-acid polypeptide with a calculated molecular mass of 136.7 kDa that shares 42.8% similarity with B. subtilis SMC and consists of the typical domain structures of the SMC protein family analyzed by ClustalW2 alignment and SMART (Simple Modular Architecture Research Tool). During our previous work, a transposon Tn917 mutagenesis library was constructed in an S. aureus double-knockout strain (DKO1) in which two genes encoding “lipopolysaccharide modification acyltransferase” and “acyltransferase” were deleted (16). One insertion mutant (DKO1.6) was identified that carried Tn917 at nucleotides 1078 to 1087 within the smc gene. In order to study the function of SMC involved in chromosome organization and segregation, smc::Tn917 was phage transduced into wild-type (WT) S. aureus strain SA113, generating a single smc::Tn917 mutant (Fig. 1A). Phage transductions were performed at 23°C to decrease the probability of suppressor mutations. Isolated mutants were confirmed by sequencing. Surprisingly, SA113 smc::Tn917 showed similar growth behavior to WT cells both on tryptic soy agar (TSA) plates and in tryptic soy broth (TSB) liquid medium at all three temperatures (30°C, 37°C, and 42°C) tested (Fig. 2A and B). These observations were in contrast to the previous findings in B. subtilis. The B. subtilis Δsmc mutant was temperature sensitive, the mutation was lethal in rich medium, and the mutant could only grow at 23°C in this medium (4, 24).
FIG. 1.
(A) Tn917 insertion site in SA113 smc::Tn917. (B) Construction of SA113 Δsmc. The smc coding sequence, except for 1,294 bp at the 3′ end, was replaced by ermB cassette flanked with lox sites that was further removed by Cre recombinase. (C) Scheme of pCXΩsmc. The smc gene was cloned into pCX15 (31), under the transcriptional control of the xylose promoter/operator system. (D) Construction of SA113 ΔspoIIIE. The spoIIIE coding sequence, except for 68 bp at the 3′ end, was replaced by an aphAIII cassette flanked with lox sites and further removed by Cre recombinase; white arrowheads represent lox sites.
FIG. 2.
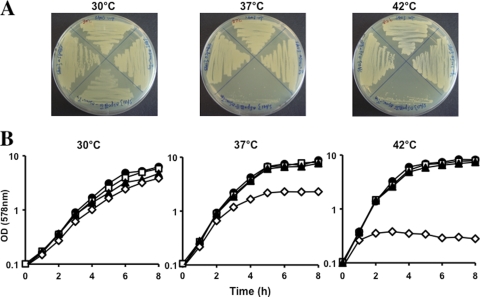
(A) Growth comparison of the SA113 WT (top) and smc::Tn917 (right), ΔspoIIIE (left), and smc::Tn917 ΔspoIIIE (bottom) mutants on TSB agar plates at 30°C, 37°C, and 42°C for 24 h. (B) Growth in TSB rich medium. •, WT; □, smc::Tn917 mutant; □, ΔspoIIIE mutant; ⋄, smc::Tn917 ΔspoIIIE mutant.
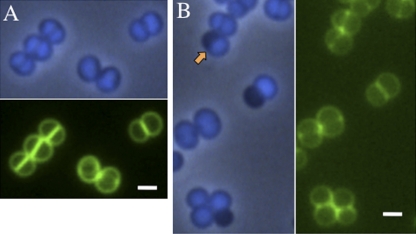
Next, we sought to verify if the loss of functional SMC would affect chromosome segregation in S. aureus. Samples were taken from exponentially growing cultures at three temperatures for fluorescent microscopy examination (Leica DM5500B). DNA was stained with DAPI (4′,6-diamidino-2-phenylindole), the cell membrane was labeled with FM1-43, and the cell wall was labeled with BODIPY (boron-dipyrromethene)-vancomycin (Van-FL) to visualize different cellular structures. While in the rod-shaped cells of E. coli and B. subtilis, the chromosome seems to occupy approximately three-fourths of the cell volume (4, 24), in S. aureus the chromosome almost fills the entire cell compartment, as demonstrated by DAPI staining (Fig. 3A). In SA113 smc::Tn917, about 10% of the cells were devoid of nucleoids; they appeared as anucleate cells (“black cells” stained with DAPI) that indicated defects in chromosome segregation (Fig. 3B). The anucleate cells were also observed in SA113-DKO1.6 that carried smc::Tn917 but not in SA113-DKO1, confirming that the chromosome segregation defect was due to the mutation in the smc gene. In SA113 smc::Tn917, “half-black cells” were observed (2% of 911 cells counted at 30°C) which were composed of one anucleate hemisphere and one normal hemisphere with regular chromosome content and morphology (Fig. 3B, arrow). We assume that this kind of “half-black cell” would be able to further divide into one anucleate daughter cell and one normal daughter cell as the cross wall could correctly form in the middle of the cell (Fig. 3B; staining with Van-FL [green]) and single spherical anucleate cells were observed frequently (Table 1). Furthermore, anucleate diplococci (Fig. 4B, yellow arrow) were observed, although at a low rate of 0.12% (2 pairs of black diplococci out of 1,634 cells counted). It is yet difficult to distinguish whether these anucleate diplococci were divided from one anucleate cell or were divided from two “half-black cells” and afterwards detached from tetrads composing two normal cells and these two anucleate cells. Intriguingly, quantitative analysis revealed that the percentage of anucleate cells decreased by 3-fold at 42°C in the smc::Tn917 strain (Table 1), suggesting that the defect of chromosome segregation caused by SMC mutation could be relieved by higher growth rate, probably due to the accelerated chromosome replication which was assumed to be one of the driving forces for chromosome segregation (7). Notably, no other aberrant chromosome distribution or abnormal cell shape could be found in S. aureus smc::Tn917 mutants, except for the anucleate cells (Table 1). Thus, SMC's function as condensin appeared to be less critical in S. aureus in contrast to B. subtilis SMC or E. coli MukB. Furthermore, the chromosome segregation defect in the S. aureus smc::Tn917 strain could be partially complemented by plasmid-encoded SMC, which is controlled by xylose inducible promoter (pCXΩsmc) (31) (Fig. 1C and Table 1).
FIG. 3.
Chromosome distribution in the SA113 WT and SA113 smc::Tn917 mutant from mid-log-phase TSB cultures at 30°C. (A) SA113 WT. Newly formed equatorial rings could be visualized in two tilted cells. (B) SA113 smc::Tn917. The orange arrow indicates the “half-black cell.” The cell wall was stained with Van-FL (green), and the chromosome was stained with DAPI (blue) and visualized in the phase-contrast-fluorescent merging mode. Scale bar, 1 μm.
TABLE 1.
Quantitive analysis of irregular chromosome appearance from mid-log-phase culturea
| Temp and strain genotype | Total no. of cells counted | % of cells: |
||||
|---|---|---|---|---|---|---|
| Anucleate | With “CUT” phenotype | With uneven chromosome distribution | With increased/decreased DNA content | Big/smallb | ||
| 30°C | ||||||
| WT | 911 | 0.3 | NOc | NO | 0.2/NO | NO |
| Δsmc | 1,258 | 9.4 | NO | 0.3 | 0.3/NO | NO |
| smc::Tn917 | 881 | 10.4 | NO | 0.3 | 0.3/NO | NO |
| smc::Tn917 pCXΩsmc | 1,316 | 2.3 | NO | 0.5 | 0.3/NO | 0.1/NO |
| ΔspoIIIE | 975 | 0.7 | NO | 0.4 | 0.5/NO | NO/0.1 |
| smc::Tn917 ΔspoIIIE | 802 | 4.8 | 1.9 | 10.7 | 3.2/6.1 | 1.6/4.5 |
| 37°C | ||||||
| WT | 1,023 | 0.3 | NO | 0.7 | 0.6/NO | NO |
| Δsmc | 1,144 | 7.9 | NO | 0.7 | 0.5/NO | NO/0.3 |
| smc::Tn917 | 851 | 7.2 | NO | 0.8 | 0.7/0.2 | NO/0.2 |
| smc::Tn917 pCXΩsmc | 1,216 | 1.8 | NO | 0.7 | 0.7/0.3 | NO/0.3 |
| ΔspoIIIE | 732 | 0.4 | NO | 0.7 | 0.8/NO | NO/0.1 |
| smc::Tn917 ΔspoIIIE | 826 | 6.4 | 1.7 | 20.8 | 5.3/6.2 | 2.9/4.5 |
| 42°C | ||||||
| WT | 987 | 0.3 | NO | 1.5 | 1.5/0.8 | NO/0.7 |
| Δsmc | 1,240 | 2.5 | NO | 1.4 | 1.8/1.0 | NO/0.9 |
| smc::Tn917 | 922 | 3.5 | NO | 1.5 | 2.0/0.9 | NO/1.1 |
| smc::Tn917 pCXΩsmc | 1,232 | 1.7 | NO | 1.5 | 1.9/1.2 | 0.3/1.1 |
| ΔspoIIIE | 890 | 0.3 | NO | 1.6 | 2.1/1.0 | NO/0.9 |
| smc::Tn917 ΔspoIIIE | 681 | 6.2 | 2.0 | 28.5 | 5.3/11.2 | 4.0/7.0 |
Data were summarized from three independent experiments.
Big cells are ≥1.5 μm in diameter, and small cells are ≤0.5 μm in diameter.
NO, not observed within the total number of cells counted.
FIG. 4.
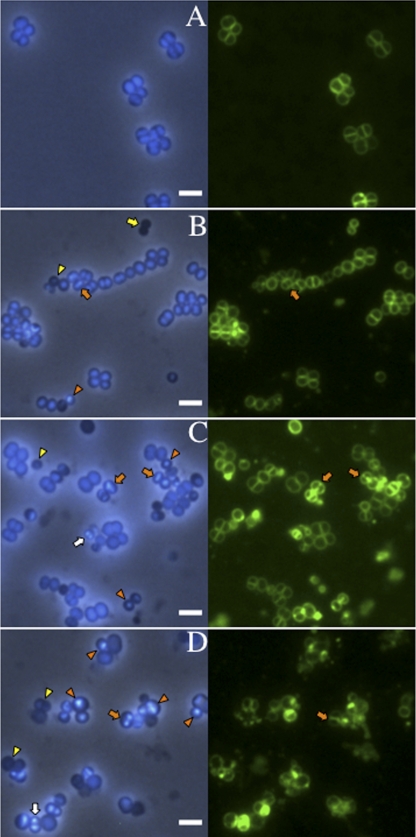
Altered chromosome morphology aggravated at higher temperatures in SA113 smc::Tn917 ΔspoIIIE. (A) SA113 WT grown for 1.5 h at 42°C. (B to D) SA113 smc::Tn917 ΔspoIIIE grown for 1.5 h after dilution of the overnight culture into fresh TSB medium at 30°C, 37°C, and 42°C respectively. DNA was stained with DAPI (blue), and the cell membrane was labeled with FM1-43 (green). Orange arrows indicate examples of the “guillotine effect,” or ‘CUT’ phenotype; yellow arrows indicate anucleate diplococci; white arrows indicate increased DNA content; yellow arrowheads indicate decreased DNA content; and orange arrowheads show the unevenly distributed nucleoid. Scale bar, 2 μm.
In the genome of S. aureus, smc is located directly upstream of ftsY, p13, and ffh, the genes encoding components of the signal recognition particle protein translocation system. In order to rule out the possible polar effect of Tn917 on the downstream genes’ expression, a markerless partial deletion smc mutant was constructed (Fig. 1B). A 1.3-kb fragment of smc gene at its 3′ was left intact due to the consideration that it contains a potential promoter controlling the downstream genes’ expression. SA113 Δsmc exhibited the same growth curve as SA113 smc::Tn917 (data not shown) and a similar rate of chromosome segregation deficiency (Table 1).
Why is the effect of smc mutation in staphylococci less severe than that in rod-shaped bacteria? First, the discrepancy could be explained partially by the morphological difference between rods and cocci. Given that E. coli or B. subtilis cells are 1.1 to 1.5 μm wide and 2.0 to 6.0 μm long, the staphylococcal cell is only half the size (0.5 to 1.5 μm in diameter) of a bacillus cell (12). Besides, S. aureus has a much smaller cell volume of 0.15 μm3 compared to E. coli with 0.5 to 0.7 μm3 (19, 25). Despite different genome sizes between B. subtilis and S. aureus (4.1 Mbp versus 2.9 Mbp, respectively) (20, 29), the small staphylococcal cell size becomes spatially limiting for the nucleoids; therefore, decondensed chromosomes were not observed in S. aureus smc mutant cells. Second, as chromosome segregation is one of the most important cellular processes, bacteria must have employed redundant mechanisms. Other factors may play a more important role during chromosome segregation in the spherical cells.
Earlier studies showed that the DNA translocase SpoIIIE could rescue the trapped chromosome from septum membrane during vegetative growth in B. subtilis (26). In order to evaluate the function of SpoIIIE in S. aureus, an SA113 spoIIIE deletion mutant was constructed via double-cross homologous recombination by using a counterselection vector, pKOR1 (1). A majority of the spoIIIE reading frame, except for 68 bp at the 3′ end (which contains the Shine-Dalgarno sequence of the downstream gene), was replaced with an aphAIII cassette that renders kanamycin resistance (Fig. 1D). The aphAIII cassette was flanked with lox sites and was further removed by Cre recombinase (21). Afterwards, smc::Tn917 was transduced into SA113ΔspoIIIE, resulting in the SA113 smc spoIIIE double mutant. Consistent with previous findings in B. subtilis (26), S. aureus spoIIIE single mutant cells had neither a significant growth disadvantage (Fig. 2A and B) nor an obvious defect in chromosome distribution compared to the WT (data not shown).
However, distinct from B. subtilis, in S. aureus a viable smc spoIIIE double mutant could be isolated. Growth curves showed that the SA113 smc spoIIIE double mutants were temperature sensitive (Fig. 2A and B). Colonies can only be formed at low temperatures on TSA plates. In liquid medium, the SA113 smc spoIIIE double mutant could grow to an optical density at 578 nm (OD578) of 2.2 after 8 h at 30°C, whereas the growth ceased after only two generations (growing for 2 h to an OD578 of 0.4) at 42°C. Shifting the culture from 24°C to 42°C resulted in rapid growth cessation. To examine the morphological changes in the SA113 smc spoIIIE double mutant, samples were taken at early (1.5 h) and late (5 h) growth phases after dilution of overnight cultures into fresh TSB medium and incubated at various temperatures. At the early growth phase, the SA113 smc spoIIIE double mutant had distorted chromosome organization and heterogeneous cell sizes, as depicted in Fig. 4B to D and quantified in Table 1. Approximately 2% of the cells showed the so-called “guillotine effect” or “CUT” phenotype that occurred when the chromosome was bisected by the septal membrane (Fig. 4, orange arrows; Table 1), which was not observed in WT cells or smc single mutants. The percentage could not be exactly counted due to the limitations of the two-dimensional microscopy technique. Four to 6% of the double mutant cells were anucleate, which did not increase significantly compared to the smc single mutant (Table 1). While the same staining method was always applied, 3-fold more mutant cells appeared to contain an increased amount of DNA compared to WT (bright blue cells in Fig. 4C and D [white arrows]). More significantly, about 11% of the mutant cells showed decreased content of DNA (Fig. 4, yellow arrowheads), which was 10 fold higher than WT or the single mutants. The most severe irregular morphology is the unevenly distributed chromosomes that were no more homogeneous but rather accumulated punctately in nearly 30% of the mutant cells (Fig. 4, orange arrowheads). These aberrant chromosomal morphological changes suggested that the smc spoIIIE double mutant was greatly deficient not only in chromosome segregation but also in chromosome structure maintenance, and the defect in chromosome structure appeared even more severe than the segregation defect. The SA113 smc spoIIIE double mutant also had heterogeneous cell sizes; cells appeared as big (≥1.5 μm in diameter) as well as small (≤0.5 μm in diameter) (Table 1). The heterogeneous cell sizes are probably an indirect consequence of the smc spoIIIE mutations, since (i) there is no significant correlation between cell size change and the degree of chromosomal disorder (Table 1) and (ii) the defect in chromosome structure and segregation may interfere with many other cellular processes that affect cell size. At the late growth stage, the chromosome structure was severely disrupted, the cells eventually lysed, and more cell debris was produced at 42°C (data not shown). However, at 30°C, although the irregular chromosome disorder was aggravated and the cell size differentiated more obviously than during the earlier growth phase, there were still considerable amounts of normal cells with regular chromosome structure (data not shown).
Thus, compared to SA113 smc and spoIIIE single mutants, the highly distorted chromosome arrangement in S. aureus smc spoIIIE double mutants indicated that SpoIIIE compensated for SMC's function in chromosome segregation to a certain extent. The aggravated chromosome segregation defect in the smc spoIIIE double mutant disclosed the chromosome maintenance deficiency in the smc single mutant, since it is unlikely that SpoIIIE had a direct role in chromosome organization. In other words, the optimal chromosome segregation facilitates proper chromosome organization in S. aureus. While the number of anucleate cells decreased with increased temperature in the smc single mutant, the effect was reversed in the smc spoIIIE double mutant, where the number of anuclate cells was slightly increased at higher temperature. Interestingly, earlier transcriptomic data for the heat shock response in S. aureus showed that spoIIIE was transcribed 2.15-fold higher at 48°C (9). This finding could explain that the defect in the smc single mutant is in part compensated for by overexpression of SpoIIIE. Distinct from B. subtilis or E. coli, S. aureus smc spoIIIE double mutants are viable at low temperature, although possible suppressor mutations could not be excluded. The distinction underlies a different mechanism for guaranteeing maximum chromosome segregation in staphylococci. Presumably, besides the difference in cell shape, other factors that play more significant roles in chromosome partitioning in cocci than in rods need to be identified.
Recently it has been found in B. subtilis that a second FtsK/SpoIIIE-like protein, SftA (septum-associated FtsK-like translocase of DNA), coordinates chromosome translocation to ensure maximum chromosome segregation, however, at a different stage of cell division from SpoIIIE (2, 18). SftA contains the C-terminal DNA binding and ATPase domain, but instead of the N-terminal transmembrane domain in SpoIIIE, it has a soluble domain. SftA translocates chromosomes before septation, while SpoIIIE comes into play postseptationally when chromosomes are trapped by the septal membrane. Like B. subtilis spoIIIE mutants, the sftA mutants had a synergistic lethal defect with an smc deletion (18). The B. subtilis sftA spoIIIE double mutant undergoes normal growth, while the defect in chromosome segregation is exacerbated significantly compared to that in both single mutants. A protein homologous to B. subtilis SftA with conserved domain structures is encoded in all staphylococcal genomes, and the homologs share 33.3% identity. It is highly interesting to evaluate the function of SftA in staphylococci for future investigation.
Another aspect that should be considered about staphylococcal chromosome segregation is the specific cell division mode of staphylococci. In contrast to B. subtilis or E. coli, where division always takes place in the middle of the longitudinal cell, in S. aureus the division plane shifts by 90° to the previous one (10). Accordingly, the chromosome replication and segregation that occur prior to cell division must occur perpendicularly as well. Theoretically, in the spherical cell, there are infinite numbers of future division planes that are perpendicular to the previous division disc. The flexibility in shifting the orientation of chromosome replication and segregation might allow staphylococci to cope with the deficiency of chromosome segregation in smc spoIIIE double mutants. The regulation of setting the division plane and chromosome segregation direction in staphylococci is totally unknown yet.
Taken together, we have obtained a first insight into chromosome segregation in Staphylococcus and presented intriguing results regarding the genetic interaction of SMC and SpoIIIE, two critical factors involved in chromosomal dynamics. These findings shed light on different mechanisms of chromosome dynamics in the spherical staphylococcal cells, in contrast to the model in rods, which definitely deserves future investigation.
Acknowledgments
We thank Karl Forchhammer, Department of Organismic Interactions, University of Tübingen, for assistance with the microscopy studies.
This work was supported by DFG, graduate college GK 685, and SFB 766.
Footnotes
Published ahead of print on 4 June 2010.
REFERENCES
- 1.Bae, T., and O. Schneewind. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58-63. [DOI] [PubMed] [Google Scholar]
- 2.Biller, S. J., and W. F. Burkholder. 2009. The Bacillus subtilis SftA (YtpS) and SpoIIIE DNA translocases play distinct roles in growing cells to ensure faithful chromosome partitioning. Mol. Microbiol. 74:790-809. [DOI] [PubMed] [Google Scholar]
- 3.Britton, R. A., and A. D. Grossman. 1999. Synthetic lethal phenotypes caused by mutations affecting chromosome partitioning in Bacillus subtilis. J. Bacteriol. 181:5860-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britton, R. A., D. C. Lin, and A. D. Grossman. 1998. Characterization of a prokaryotic SMC protein involved in chromosome partitioning. Genes Dev. 12:1254-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton, B. M., K. A. Marquis, N. L. Sullivan, T. A. Rapoport, and D. Z. Rudner. 2007. The ATPase SpoIIIE transports DNA across fused septal membranes during sporulation in Bacillus subtilis. Cell 131:1301-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dervyn, E., M. F. Noirot-Gros, P. Mervelet, S. McGovern, S. D. Ehrlich, P. Polard, and P. Noirot. 2004. The bacterial condensin/cohesin-like protein complex acts in DNA repair and regulation of gene expression. Mol. Microbiol. 51:1629-1640. [DOI] [PubMed] [Google Scholar]
- 7.Draper, G. C., and J. W. Gober. 2002. Bacterial chromosome segregation. Annu. Rev. Microbiol. 56:567-597. [DOI] [PubMed] [Google Scholar]
- 8.Errington, J., R. A. Daniel, and D. J. Scheffers. 2003. Cytokinesis in bacteria. Microbiol. Mol. Biol. Rev. 67:52-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleury, B., W. L. Kelley, D. Lew, F. Gotz, R. A. Proctor, and P. Vaudaux. 2009. Transcriptomic and metabolic responses of Staphylococcus aureus exposed to supra-physiological temperatures. BMC Microbiol. 9:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giesbrecht, P., T. Kersten, H. Maidhof, and J. Wecke. 1998. Staphylococcal cell wall: morphogenesis and fatal variations in the presence of penicillin. Microbiol. Mol. Biol. Rev. 62:1371-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Götz, F. 2004. Staphylococci in colonization and disease: prospective targets for drugs and vaccines. Curr. Opin. Microbiol. 7:477-487. [DOI] [PubMed] [Google Scholar]
- 12.Götz, F., T. Bannerman, and K. H. Schleifer. 2006. The genera Staphylococcus and Macrococcus, p. 5-75. In M. Dworkin (ed.), The procaryotes, vol. 4. Springer, New York, NY. [Google Scholar]
- 13.Graumann, P. L. 2000. Bacillus subtilis SMC is required for proper arrangement of the chromosome and for efficient segregation of replication termini but not for bipolar movement of newly duplicated origin regions. J. Bacteriol. 182:6463-6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Güthlein, C., R. M. Wanner, P. Sander, E. C. Böttger, and B. Springer. 2008. A mycobacterial smc null mutant is proficient in DNA repair and long-term survival. J. Bacteriol. 190:452-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haydon, D. J., N. R. Stokes, R. Ure, G. Galbraith, J. M. Bennett, D. R. Brown, P. J. Baker, V. V. Barynin, D. W. Rice, S. E. Sedelnikova, J. R. Heal, J. M. Sheridan, S. T. Aiwale, P. K. Chauhan, A. Srivastava, A. Taneja, I. Collins, J. Errington, and L. G. Czaplewski. 2008. An inhibitor of FtsZ with potent and selective anti-staphylococcal activity. Science 321:1673-1675. [DOI] [PubMed] [Google Scholar]
- 16.Herbert, S., A. Bera, C. Nerz, D. Kraus, A. Peschel, C. Goerke, M. Meehl, A. Cheung, and F. Götz. 2007. Molecular basis of resistance to muramidase and cationic antimicrobial peptide activity of lysozyme in staphylococci. PLoS Pathog. 3:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano, M., and T. Hirano. 2002. Hinge-mediated dimerization of SMC protein is essential for its dynamic interaction with DNA. EMBO J. 21:5733-5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaimer, C., J. E. González-Pastor, and P. L. Graumann. 2009. SpoIIIE and a novel type of DNA translocase, SftA, couple chromosome segregation with cell division in Bacillus subtilis. Mol. Microbiol. 74:810-825. [DOI] [PubMed] [Google Scholar]
- 19.Kubitschek, H. E. 1990. Cell volume increase in Escherichia coli after shifts to richer media. J. Bacteriol. 172:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 21.Leibig, M., B. Krismer, M. Kolb, A. Friede, F. Götz, and R. Bertram. 2008. Marker removal in staphylococci via Cre recombinase and different lox sites. Appl. Environ. Microbiol. 74:1316-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindow, J. C., R. A. Britton, and A. D. Grossman. 2002. Structural maintenance of chromosomes protein of Bacillus subtilis affects supercoiling in vivo. J. Bacteriol. 184:5317-5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascarenhas, J., J. Soppa, A. V. Strunnikov, and P. L. Graumann. 2002. Cell cycle-dependent localization of two novel prokaryotic chromosome segregation and condensation proteins in Bacillus subtilis that interact with SMC protein. EMBO J. 21:3108-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriya, S., E. Tsujikawa, A. K. Hassan, K. Asai, T. Kodama, and N. Ogasawara. 1998. A Bacillus subtilis gene-encoding protein homologous to eukaryotic SMC motor protein is necessary for chromosome partition. Mol. Microbiol. 29:179-187. [DOI] [PubMed] [Google Scholar]
- 25.Pilavtepe-Çelik, M., M. O. Balaban, H. Alpas, and A. E. Yousef. 2008. Image analysis based quantification of bacterial volume change with high hydrostatic pressure. J. Food Sci. 73:M423-M429. [DOI] [PubMed] [Google Scholar]
- 26.Sharpe, M. E., and J. Errington. 1995. Postseptational chromosome partitioning in bacteria. Proc. Natl. Acad. Sci. U. S. A. 92:8630-8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.She, W., Q. Wang, E. A. Mordukhova, and V. V. Rybenkov. 2007. MukEF Is required for stable association of MukB with the chromosome. J. Bacteriol. 189:7062-7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thanbichler, M., and L. Shapiro. 2006. MipZ, a spatial regulator coordinating chromosome segregation with cell division in Caulobacter. Cell 126:147-162. [DOI] [PubMed] [Google Scholar]
- 29.Trevors, J. T. 1996. Genome size in bacteria. Antonie Van Leeuwenhoek 69:293-303. [DOI] [PubMed] [Google Scholar]
- 30.Volkov, A., J. Mascarenhas, C. Andrei-Selmer, H. D. Ulrich, and P. L. Graumann. 2003. A prokaryotic condensin/cohesin-like complex can actively compact chromosomes from a single position on the nucleoid and binds to DNA as a ring-like structure. Mol. Cell. Biol. 23:5638-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wieland, K. P., B. Wieland, and F. Götz. 1995. A promoter-screening plasmid and xylose-inducible, glucose-repressible expression vectors for Staphylococcus carnosus. Gene 158:91-96. [DOI] [PubMed] [Google Scholar]
- 32.Wu, L. J., and J. Errington. 1994. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science 264:572-575. [DOI] [PubMed] [Google Scholar]
- 33.Yu, X. C., E. K. Weihe, and W. Margolin. 1998. Role of the C terminus of FtsK in Escherichia coli chromosome segregation. J. Bacteriol. 180:6424-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zapun, A., T. Vernet, and M. G. Pinho. 2008. The different shapes of cocci. FEMS Microbiol. Rev. 32:345-360. [DOI] [PubMed] [Google Scholar]