Abstract
We successfully substituted Escherichia coli's origin of replication oriC with the origin region of Vibrio cholerae chromosome I (oriCIVc). Replication from oriCIVc initiated at a similar or slightly reduced cell mass compared to that of normal E. coli oriC. With respect to sequestration-dependent synchrony of initiation and stimulation of initiation by the loss of Hda activity, replication initiation from oriC and oriCIVc were similar. Since Hda is involved in the conversion of DnaAATP (DnaA bound to ATP) to DnaAADP (DnaA bound to ADP), this indicates that DnaA associated with ATP is limiting for V. cholerae chromosome I replication, which similar to what is observed for E. coli. No hda homologue has been identified in V. cholerae yet. In V. cholerae, dam is essential for viability, whereas in E. coli, dam mutants are viable. Replacement of E. coli oriC with oriCIVc allowed us to specifically address the role of the Dam methyltransferase and SeqA in replication initiation from oriCIVc. We show that when E. coli's origin of replication is substituted by oriCIVc, dam, but not seqA, becomes important for growth, arguing that Dam methylation exerts a critical function at the origin of replication itself. We propose that Dam methylation promotes DnaA-assisted successful duplex opening and replisome assembly at oriCIVc in E. coli. In this model, methylation at oriCIVc would ease DNA melting. This is supported by the fact that the requirement for dam can be alleviated by increasing negative supercoiling of the chromosome through oversupply of the DNA gyrase or loss of SeqA activity.
The genomes of Vibrio cholerae and several related Vibrio spp. are distributed between two circular chromosomes. Characterization of the origins of replication of V. cholerae chromosomes I and II (oriCIVc and oriCIIVc, respectively) has shown that oriCIVc is similar to the origin of replication of the Escherichia coli chromosome, oriC, whereas oriCIIVc is completely different (20). Like oriC, oriCIVc has five R-type DnaA boxes (53) as well as boxes conforming to the I and τ types (52, 61), and the DnaA protein is the rate-limiting factor in the initiation of replication in both cases (18). In E. coli, DnaA associates with both ATP and ADP, and the ATP-bound form is absolutely required for initiation to take place (reviewed in reference 60). When reaching a critical level, DnaAATP (DnaA bound to ATP) protein is proposed to form a helical filament, anchored at one or more R-boxes (54, 69), in which origin DNA wraps around the outside of the DnaA core (21) or where the DnaA wraps around oriC (61). In both cases, the topology of the DnaA-oriC nucleoprotein complex leads to formation of compensatory negative supercoiling that facilitates unwinding of the adjacent AT-rich region resulting in initiation. In both models, DnaAATP is absolutely required for initiation, and in agreement with this, DnaAATP was found to be the rate-limiting factor for initiation in vivo (69).
The V. cholerae oriCIVc also resembles oriC in having many potential sites for methylation by DNA adenine methyltransferase (Dam), although the number and position of the GATC sites differ slightly (see Fig. 1). The role of Dam in initiation of chromosome replication has been studied mainly in E. coli. After initiation of DNA replication has occurred on a fully methylated oriC, the newly replicated hemimethylated origins are sequestered from the Dam methyltransferase and from reinitiation for approximately one-third of a doubling time. During this time interval, the activity and amount of DnaA available for initiation are reduced to prevent immediate reinitiation (reviewed in references 57 and 83). The sequestration is carried out by the SeqA protein that binds hemimethylated oriC GATC sequences with high affinity (48). In the absence of Dam methylation or SeqA, the same origin can be reinitiated in the same cell cycle, and initiations become asynchronous (9, 48).
FIG. 1.
Alignment of the E. coli minimal oriC with the corresponding region from V. cholerae chromosome I. The AT-rich sequence and the three 13-mer repeats L, M, and R found in E. coli (5) are indicated above the alignment. The 6-mer (A/T)GATCT boxes (80) are underlined. Other DnaA binding sites, i.e., R-boxes (53), I-boxes (52), and τ-boxes (61), are shown as boxed regions. Dam methylation sites (GATC) are shaded gray. The experimentally defined binding sites for integration host factor (IHF) (22) and factor for inversion stimulation (FIS) (65) in E. coli are indicated, and bases that match the consensus sequence are in boldface type. The single base difference between oriCIVc and oriCIVc* (see Materials and Methods) in the minimal origin region is shown below the two sequences. A gap introduced to maximize alignment of the two sequences is indicated by a dash in the sequence. Nucleotides that are identical in the two sequences are indicated by an asterisk below the two sequences.
Genes encoding a Dam homologue and a SeqA homologue are present on Vibrio genomes, but there appear to be some differences between the functions of the proteins in E. coli and V. cholerae. dam has been found to be an essential gene in V. cholerae (33, 15), which is not the case in E. coli (48, 51). Conflicting data exist concerning the essentiality of seqA in V. cholerae (15, 72). The roles of Dam and SeqA in oriCIVc replication have been studied using minichromosomes, i.e., plasmids replicating exclusively from a cloned copy of oriCIVc (20). oriCIVc-based minichromosomes can replicate in wild-type E. coli cells but were unable to replicate in dam, seqA, and seqA dam mutants (20). The extrachromosomal existence of minichromosomes is dependent on their ability to initiate replication in synchrony with the chromosomal origin (46, 75). In E. coli cells mutated in dam or seqA, incompatibility exists between the oriC carried on minichromosomes and that of the chromosome due to origin competition (13), and when minichromosomes are maintained under selective pressure, they integrate into the origin region of the host chromosome (46, 75). Minichromosomes based on oriCIVc may also compete with the E. coli oriC for initiations in dam or seqA mutant cells. However, due to limited sequence identity, they may not be able to integrate into the E. coli chromosome. This could provide an explanation for the failure to introduce oriCIVc minichromosomes into dam and seqA mutant cells (20). Both dam and seqA genes could therefore be required for viability of V. cholerae for reasons not related to chromosome replication. In addition to its role in DNA replication, roles for Dam methylation in gene regulation and DNA repair have also been demonstrated in a number of bacteria (for reviews, see references 11, 45, 47, and 50). For V. cholerae as well as for Salmonella spp. and Yersinia pseudotuberculosis, Dam plays a role in virulence possibly through regulation of virulence gene expression (33). Less is known about the functions of seqA apart from its role in E. coli replication, but it has been suggested that SeqA functions as a nucleoid-organizing protein (for a review, see reference 83), and the E. coli chromosome has been demonstrated to have increased supercoiling in a seqA strain (85).
Here we describe the first in vivo evidence that Dam plays an important role in the initiation of replication by facilitating the replication initiation at oriCIVc in E. coli. In addition, we show that SeqA does not carry an essential role in the initiation of replication.
MATERIALS AND METHODS
Growth conditions.
Cells were grown in LB medium (8) or AB minimal medium (12) supplemented with 10 μg/ml thiamine and with either 0.2% glucose (Glu) or 0.2% glycerol as a carbon source. Also, 0.5% Casamino Acids (CAA) was added when fast growth conditions were required. Pictures of agarose plates were taken with a Canon digital camera (IXUS 870IS). Some changes to the brightness, contrast, and intensity of photos of agarose plates and microscopy images were made by using CorelDraw v. 9 (Corel Corp.). Antibiotics were used at the following concentrations: tetracycline (10 μg/ml), ampicillin (100 μg/ml), chloramphenicol (10 μg/ml), kanamycin (50 μg/ml), and streptomycin (50 μg/ml).
Bacterial strains and plasmids.
All bacterial strains and plasmids used in this study are listed in Table 1. The pOriCImini plasmid has the 256-bp V. cholerae strain Bah-2 region corresponding to the E. coli minimal origin (see Fig. 1) cloned into pSW29TsacB. Plasmid pOriCI has the entire region between the mioC and gidA genes and a small part of the mioC and gidA genes from V. cholerae Bah-2, i.e., 555 bp cloned into pSW29TsacB. For construction of pOriCI and pOriCImini, the oriCI region equipped with NotI and SacI restriction sites were PCR amplified from V. cholerae Bah-2 (64) using the primers AAAGGCCAGAGCTCATTAAATATATATAAAGATCTATATAGAGATCTTTTTATTAG and TAGGAAAAAAGCGGCCGCTGTGGATAACTATACGATTATCCG for pOriCImini and primers AAAGGCCAGAGCTCTCATCAATCGCTTCTAAATAATGACC and TAGGAAAAAAGCGGCCGCGGCTTCGTTCCTGCGTACCG for pOriCI. The NotI/SacI-digested PCR products were cloned into NotI/SacI-digested pSW29TsacB (16), giving pOriCImini and pOriCI. Compared to the published sequence for V. cholerae El Tor N16961 (28), one sequenced pOriCI clone (pOriCI-1) had a C→T base shift in a GATC site 11 bp before the start of the minimal oriCI region as defined in Fig. 1. This mutation was not found in another pOriCI clone, pOriCI-2, or in the V. cholerae El Tor derivative Bah-2 (B. Koch and A. Løbner-Olesen, unpublished observations).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and/or characteristic(s)a | Reference or source |
|---|---|---|
| Vibrio cholerae Bah-2 | Derivative of El Tor strain E7946 | 64 |
| E. coli strains | ||
| ALO863 | LJ24 damX::mini-Tn10; Tcr | 42 |
| ALO1639 | MG1655 seqAΔ10 | 78 |
| ALO1917 | MG1655 hda::Cmr | 69 |
| ALO2031 | MG1655 dam16::Kmr | 63 |
| ALO3150 | MG1655 oriC replaced by oriCIVc*; Smr | This study |
| ALO3152 | MG1655 seqAΔ10 oriC replaced by oriCIVc*; Smr | This study |
| ALO3189 | MG1655 oriC replaced by oriCIVc*; hda::Cmr; Smr | This study |
| ALO3199 | MG1655 damX::mini-Tn10; Tcr | This study |
| ALO3203 | MG1655 oriC replaced by oriCIVc*; damX::mini-Tn10; Tcr Smr | This study |
| ALO3270 | MG1655 I2 G3→T I3 G3→T | 68 |
| ALO3470 | MC1000 PA1lacO1gyrA gyrB zhe-3084::Tn10; Tcr | This study |
| ALO3481 | MC1000 PA1lacO1gyrA gyrB zhe-3084::Tn10; oriC replaced by oriCIVc* | This study |
| ALO3509 | MC1000 PA1lacO1gyrA gyrB zhe-3084::Tn10 seqAΔ10 | This study |
| ALO3521 | MC1000 PA1lacO1gyrA gyrB zhe-3084::Tn10; seqAΔ10 oriC replaced by oriCIVc* | This study |
| ALO3689 | MG1655 dam13::Tn9 argE86::Tn10; Tcr Cmr | This study |
| ALO3699 | MG1655 ΩSm cassette inserted between gidA and oriC; Smr | This study |
| ALO3702 | MG1655 oriC replaced by oriCIVc; Smr | This study |
| ALO3705 | MC1000 PlacO1gyrA gyrB zhe-3084::Tn10ΩSm; Smr Tcr | This study |
| ALO3733 | MC1000 PlacO1gyrA gyrB zhe-3084::Tn10 seqAΔ10ΩSm; Smr Tcr | This study |
| ALO3736 | MG1655 oriC replaced by oriCIVc*; damX::mini-Tn10; Tcr Smr | This study |
| ALO3745 | MG1655 seqAΔ10 oriC replaced by oriCIVc; Smr | This study |
| ALO3749 | MG1655 ΩSm cassette inserted between gidA and oriC; hda::Cmr; Smr | This study |
| ALO3751 | MG1655 oriC replaced by oriCIVc; hda::Cmr; Smr | This study |
| ALO3753 | MG1655 seqAΔ10ΩSm cassette inserted between gidA and oriC; Smr | This study |
| ALO3756 | MC1000 PlacO1gyrA gyrB zhe-3084::Tn10; oriC replaced by oriCIVc | This study |
| BW25113 | ΔaraBAD hsdR | 14 |
| CAG18456 | MG1655 zhe-3084::Tn10; Tcr | Martin Martinus |
| CAG 12185 | MG1655 argE86::Tn10; Tcr | Martin Martinus |
| GM2927 | dam13::Tn9; Cmr | 49 |
| DH5αλpir | Pir+ | Rasmus Bugge Jensen |
| EH3827 | ΔdnaA zia::pKN500 | 25 |
| PJ4240 | MC1000 PA1lacO1gyrA gyrB | 31 |
| MG1655 | Wild type | 24 |
| SS211 | MG1655 pBIP- seqAΔ10 cointegrant | Kirsten Skarstad |
| WM2482 | MG1655 | 84 |
| WM2759 | MG1655 oriC160 asnA::km; Kmr | 84 |
| WM2762 | MG1655 oriC13 asnA::km; Kmr | 84 |
| WM2764 | MG1655 oriC15 asnA::km; Kmr | 84 |
| WM2844 | MG1655 oriC17 asnA::km; Kmr | 84 |
| WM28 45 | MG1655 oriC162 asnA::km; Kmr | 84 |
| XL1-Blue | Tcr | Stratagene |
| Plasmids | ||
| pFHC539 | DnaA (wild type); Apr | 27 |
| pRUC672 | DnaAK178T; Apr | 76 |
| pLR40 | pBR322 derivative with E. coli dnaA exclusively under lac promoter control | 69 |
| pHRP315 | pUCBM20 with ΩSmr/Spr cassette; Apr Smr | 62 |
| pSW29T | R6K replicon-based cloning vector; Kmr | 16 |
| pSW29TsacB | pSW29T with sacB from Bacillus subtilis cloned in BglII site; Kmr | Ole Skovgaard |
| pKD46 | ParaB γ β exo; Apr | 14 |
| pOriCImini | pSW29TsacB with a 256-bp oriCI region from V. cholerae Bah-2 | This study |
| pOriCI-1 | pSW29TsacB with a 555-bp oriCI region from V. cholerae Bah-2 with C→T point mutation | This study |
| pOriCI-2 | pSW29TsacB with a 555-bp oriCI region from V. cholerae Bah-2 | This study |
| pOriCISmB | pOriCI-1 with ΩSmr/Spr cassette from pHRP315; Kmr Smr | This study |
| pOriCISmF | poriCI-2 with ΩSmr/Spr cassette from pHRP315; Kmr Smr | This study |
| pKG339 | Plac::copA; Tcr Apr | 32 |
| pALO160 | RepA, contains a BglII fragment carrying the E. coli dam operon; Apr | 42 |
| pRUC1443 | pBR322 derivative with V. cholerae dnaA under PA10403 promoter control | Ole Skovgaard |
Kmr, kanamycin resistance; Apr, ampicillin resistance; Smr, streptomycin resistance; Tcr, tetracyclin resistance; Cmr, chlorampenicol resistance.
For replacement of E. coli oriC with oriCI from Vibrio cholerae, a ΩSmr cassette was cut out from pHRP315 (62) with BamHI and cloned into the BamHI site of pOriCI-1, giving pOriCISmB, or cloned into the BamHI site of pOriCI-2, giving pOriCISmF. Recombinational replacement of E. coli oriC with oriCI from Vibrio cholerae (oriCIVc) was achieved by using a PCR-based method (14). Using pOriCISmB or pOriCISmF as a template, the ΩSm cassette in combination with oriCIVc (product A) or only the ΩSm cassette (product B) was PCR amplified with primer gidA-Sm (GATTGAAGCCCGGGCCGTGGATTCTACTCAACTTTGTCGGCTTGAGAAAGTGATATCGAATTCCTGCAGC and primer for product A (AAGATCCGGCAGAAGAATGGCTGGGATCGT GGGTTAATTTACTCAAATAAATAATGACCTATTCCATGCAG) or primer gidA-Sm in combination with primer for product B (TCACAATAGAACAGATCTCTAAATAAATAGATCTTCTTTTTAATACCCAGGATCCCAGGTCGCTCTAGAACTAGTGGATC). The parts of the primers homologous to sequences in the oriC region in E. coli are shown in boldface type. The PCR products were introduced into E. coli BW25113 rnhA(pKD46) or E. coli BW25113(pKD46) by electroporation. Streptomycin-resistant recombinants were analyzed by PCR using primers recognizing genes flanking oriC, i.e., gidA (primer gidA-conf [CACGGCCACCGCTGTAATTAT]) and mioC (primer mioC-conf [ATCCCATACTTTTCCACAGG]) in combination with primers recognizing the streptomycin resistance cassette (primer Sm-conf [GAAGAAGATCGCTTGGCCTC]) and oriCIVc (primer oriCI-conf [GTGATAAAGCATGAACGACCT]). Sequence analyses of three mutants that passed the PCR test showed that in two mutants, E. coli oriC was replaced by oriCIVc and the oriCIVc sequence matched the corresponding sequence from V. cholerae N16961 (28). The third mutant carried two mutations compared to the sequences for V. cholerae N16961 (28), the C→T base shift in a GATC site 11 bp before the start of the minimal oriCI region originating from pOriCISmB and a T→C base shift near R1 (see Fig. 1) at a nonconserved position (87). This mutant and derivatives of this mutant will be referred to as oriCIVc*, while the strain carrying oriCIVc without point mutations will be referred to as oriCIVc. oriCIVc::Smr, oriCIVc*::Smr, and ΩSmr were transferred to E. coli MG1655 (24) and combined with various mutations by P1 phage-mediated transduction (55).
PCR verification of the E. coli MG1655 dam16::Kmr P1 transductants were carried with the primer pair dam-forward (CGCTTTTTTGAAGTGGGCAG) and dam-reverse (TTTCGCGGGTGAAACGAC) and primer pair dam-forward/kan1 (AATTGCAGTTTCATTTGATGCTC) and dam-reverse/kan2 (GAGCAAGACGTTTCCCGTTG). PCR verification of the hda::cat mutation was done as described previously (69).
Genomic blot.
To determine the copy numbers of pOriCI-1, pOriCI-2, and pOriCImini, cellular DNAs were isolated from cells growing exponentially at 37°C in AB minimal medium supplemented with glucose, Casamino Acids, and kanamycin. The DNA was double digested with BamHI and XhoI. A 33P-labeled probe recognizing the chromosomal terC region located on a 1.6-kb fragment was made essentially as described previously (56). Similarly, a probe specific for the Tn5-derived kanamycin resistance gene on the minichromosome was made using the primers k-m1 (GCGATACCGTAAAGCACGAG) and k-m2 (GGCTATTCGGCTATGACTGG). Hybridization was carried out as previously described (46).
Dam depletion.
The cells were grown at 42°C for at least 6 generations in AB minimal medium supplemented with glucose and Casamino Acids containing tetracycline to select for plasmid pKG339 and in AB minimal medium supplemented with glucose and Casamino Acids containing ampicillin to select for plasmid pALO160, before the cultures were diluted in the same medium supplemented with 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) but without ampicillin. To maintain the cells in exponential growth, the cultures were diluted in fresh prewarmed medium, and samples were taken at appropriate time intervals for flow cytometry.
Changing the intracellular gyrase activity.
In order to study the effects of variations in the cellular gyrase level, we used derivatives of E. coli strain PJ4240, in which gyrase expression is IPTG dependent (31). To obtain a streptomycin-sensitive derivative of strain PJ4240, rpsL+ was P1 transduced from E. coli strain CAG18456 zhe-3084::Tn10 to PJ4240, resulting in strain ALO3470. A seqAΔ10 deletion derivative of strain ALO3470 (strain ALO3509) was obtained by the method described in reference 77, and the presence of the seqAΔ10 deletion was confirmed by PCR using the primer pair ybf (TTTACCAGATCGCGAGCCAG)/pgm (CCCTGCTTCTGGTTTCAGTAC). oriCIVc, oriCIVc*, and ΩSm derivatives of strains ALO3470 and ALO3509 were obtained by P1 transduction.
Sequence analysis.
The WEB-THERMODYN sequence analysis software for profiling DNA helical stability (30) was used to analyze the helical stability of the E. coli oriC region and the V. cholerae oriCIVc region.
Flow cytometry.
Flow cytometry was performed as described previously (44) using an Apogee A10 flow cytometer.
RESULTS
Structure of V. cholerae oriCI.
A 447-bp DNA fragment situated between the mioC and gidA genes on Vibrio cholerae chromosome I can function as an autonomous replicating sequence (ARS) in both V. cholerae and surrogate host E. coli (20). By sequence alignment, a 256-bp region corresponding to the 257-bp minimal E. coli origin (5) can be identified within the 447-bp DNA fragment in V. cholerae (Fig. 1). To examine whether this 256-bp region could also replicate in E. coli, it was cloned into the oriVR6Kγ-based plasmid pSW29TsacB, giving pOriCImini. Replication from oriVR6Kγ depends on the pir-encoded protein. pOriCImini was able to replicate as a minichromosome in a Pir-deficient E. coli strain (strain XL1-Blue), demonstrating the ARS activity of the cloned fragment. We also cloned a longer 555-bp region covering the intergenic region between the gidA and mioC genes into pSW29TsacB, giving pOriCI. Compared to the published sequence for V. cholerae El Tor N16961 (28) and to the V. cholerae Bah-2 chromosomal sequence, one sequenced pOriCI clone (pOriCI-1) had a C→T base shift in a GATC site 11 bp before the start of the minimal oriCI region as defined in Fig. 1 in a nonconserved region (71). This mutation was not found in another pOriCI clone pOriCI-2 or in V. cholerae El Tor derivative Bah-2 (64). A genomic blot was performed to compare the minichromosome copy numbers for pOriCI-1 (4.4 kb), pOriCI-2 (4.4 kb), and pOriCImini (4.1 kb) (Fig. 2) (see Materials and Methods). The genomic blot was hybridized with two probes, one recognizing the terC region on the chromosome (1.6 kb) and one recognizing the kanamycin resistance gene on the linearized minichromosomes. No major difference in copy number for the three minichromosomes was observed (Fig. 2).
FIG. 2.
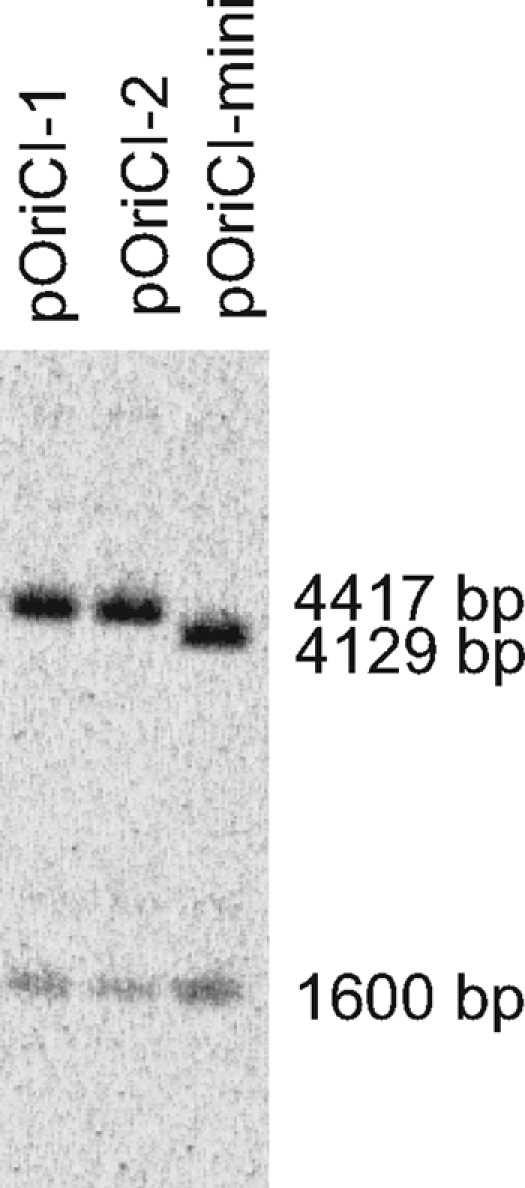
Copy number of oriCI-based minichromosomes. Total DNA was isolated from E. coli strain XL1-Blue containing the indicated minichromosomes. Cells were grown exponentially at 37°C in AB minimal medium supplemented with glucose and Casamino Acids and containing 50 μg/ml of kanamycin. Individual DNA samples were digested with BamHI plus XhoI before Southern blot hybridization was performed. Two probes were used simultaneously; the two probes were homologous to sequences specific for terC on the chromosome and the kanamycin resistance gene carried by the minichromosomes.
The E. coli replication origin can be replaced with oriCIVc.
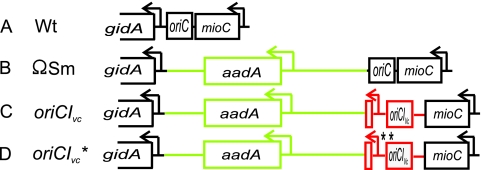
The oriC region of the E. coli chromosome was replaced by oriCIVc by lambda red-mediated homologous recombination (14) as described in Materials and Methods. Data from E. coli indicate that origin activity is stimulated by transcription from the gidA promoter located immediately adjacent to oriC and transcribing away from oriC (5, 59), probably due to increased negative supercoiling behind transcribing RNA polymerases (40).The constructed strain has aadA inserted between oriCIVc and the gidA promoter (Fig. 3 C), i.e., the native E. coli gidA promoter is moved about 2 kb away from the origin. We were uncertain whether the promoter would still be able to exert its stimulatory effect on oriCIVc at this new location, since it was previously shown that the presence of a GC-rich element between the stimulatory D-loop and the origin can be alleviated if a GC-rich element is present in the intervening sequence (73). Therefore, we decided to include the native V. cholerae gidA promoter on the replacement cassette. The aadA promoter directs transcription away from oriCIVc and is situated more than 500 bp away from the V. cholerae gidA (gidAVc) promoter (66). Whereas the downstream aadA gene may very well be affected by transcription from the gidAVc promoter (4), the opposite should not be the case. To look for possible effects of the insertion of the resistance cassette, we constructed a control strain that retained the E. coli oriC but had the ΩSm cassette inserted (Fig. 3B). Following replacement, oriCIVc was amplified from the E. coli chromosome by PCR, and the nucleotide sequence was determined. One mutant strain had a oriCIVc sequence identical to the V. cholerae Bah-2 chromosomal sequence and to the published sequence for V. cholerae El Tor N16961 (28). A second mutant strain carried two mutations, the described C→T base shift in a GATC site 11 bp before the start of the minimal oriCI region and a T→C base shift at a nonconserved position (87) immediately to the right of DnaA box R1 (Fig. 1). The oriCIVc region with these two mutations will be referred to as oriCIVc*, while the oriCIVc region without mutations will be referred to as oriCIVc (Fig. 3). The oriCIVc, oriCIVc*, and the ΩSm cassette were transduced into a wild-type E. coli strain (MG1655) with equally high frequency (not shown), demonstrating that the V. cholerae oriCI is capable of directing replication of the E. coli chromosome. Most analyses were carried with all four strains, i.e., MG1655 (referred to as the wild type), MG1655 oriCIVc (referred to as oriCIVc), MG1655 oriCIVc* (referred to as oriCIVc*), and MG1655 ΩSm (referred to as ΩSm) strains (Fig. 3).
FIG. 3.
Structure of genes in the origin regions of the strains used in this study. (A) E. coli wild type (Wt). (B) The aadA gene (GenBank accession no. AAC33912) encoding streptomycin adenylyltransferase flanked by transcription and translation termination signals (the ΩSm cassette [66]) is inserted upstream of the E. coli gidA promoter as defined in reference 37. (C) The E. coli oriC was replaced by the oriCIVc region comprising the intergenic region between the V. cholerae mioC and gidA gene homologues and the sequence encoding the first 20 amino acids of the V. cholerae gidA gene homologue in combination with the ΩSm cassette. (D) Same as panel C but with two point mutations. The precise locations of the point mutations are described in the text.
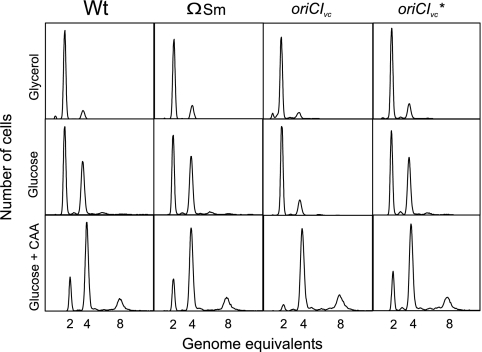
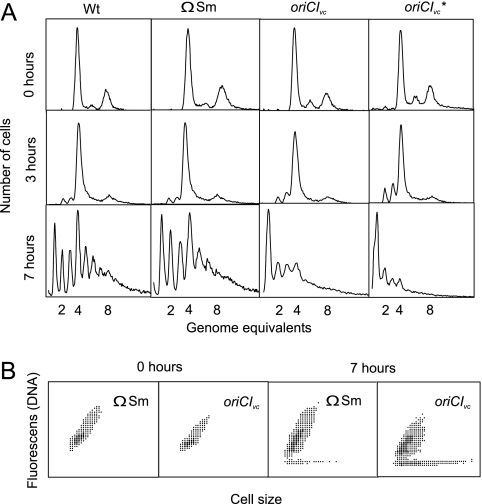
When the wild-type, ΩSm, and oriCIVc* strains were grown in minimal medium supplemented with either glycerol, glucose, or glucose and Casamino Acids, the cells grew with similar doubling times (Table 2). In contrast, the doubling time of oriCIVc cells were 10 to 20% longer in all three media (Table 2). When analyzed by flow cytometry, all four strains contained mainly two and four or four and eight origins of replication, depending on the culture doubling time, indicating that the origins were initiated in synchrony (Fig. 4) (74).
TABLE 2.
Growth rate, cell mass, and origin content of wild-type and mutant strains
| Medium supplementa | Strainb | Doubling time (min) | No. of origins/cellc | Cell mass (LS)d | Mass/origin ratioe |
|---|---|---|---|---|---|
| Glycerol | wt | 84 | 2.3 | 1.0 | 1.0 |
| ΩSm | 84 | 2.4 | 1.0 | 1.0 | |
| oriCI* | 84 | 2.5 | 1.1 | 1.0 | |
| oriCI | 95 | 2.2 | 0.7 | 0.7 | |
| Glucose | wt | 57 | 3.2 | 1.7 | 1.2 |
| ΩSm | 57 | 3.3 | 1.8 | 1.2 | |
| oriCI* | 57 | 3.2 | 1.8 | 1.3 | |
| oriCI | 70 | 2.6 | 1.2 | 1.1 | |
| Glucose + Casamino Acids | wt | 36 | 4.7 | 2.3 | 1.1 |
| ΩSm | 36 | 4.7 | 2.4 | 1.2 | |
| oriCI* | 36 | 4.7 | 2.3 | 1.1 | |
| oriCI | 40 | 5.1 | 2.3 | 1.0 |
The cells were grown in minimal medium supplemented with glycerol, glucose, or glucose plus Casamino Acids.
Four strains, i.e., E. coli MG1655 (wild type [wt]), MG1655 oriCIVc (oriCIVc), MG1655 oriCIVc* (oriCIVc*), and MG1655 ΩSm (ΩSm) strains, were analyzed.
Based on flow cytometric analysis of rifampin- and cephalexin-treated cells.
Based on flow cytometric analysis of exponentially growing cells. The cell mass of wt cells growing in medium supplemented with glycerol is set at 1.0. LS, light scatter.
The ratio of cell mass to the number of origins per cell for wt cells growing in minimal medium supplemented with glycerol is set at 1.0.
FIG. 4.
Cell cycle parameters for wild-type (Wt) (E. coli MG1655), ΩSm, oriCIVc, and oriCIVc* strains. Wt, ΩSm, oriCIVc, and oriCIVc* cells were grown at 37°C in minimal medium supplemented with glycerol, glucose, or glucose plus Casamino Acids (CAA). Cells were treated with rifampin and cephalexin (Materials and Methods) prior to flow cytometric analysis.
The sizes and origin content for ΩSm and oriCIVc* strains were similar to wild-type cells in all three media. On the other hand, oriCIVc cells were smaller than wild-type cells in the two slow growing cultures (Table 2). The oriCIVc cells also had a reduced origin content at slow growth, but not to the same extent, and the average cell mass/origin was lower for oriCIVc cells relative to wild-type cells. This indicates that the initiation mass (17) was reduced during slow growth (Table 2) and that oriCIVc is slightly more efficient than oriC. One or both of the point mutations in oriCIVc* influence it in such a way that it becomes similar to oriC with respect to growth and initiation parameters (Table 2). Insertion of an ΩSm cassette between gidA and oriC (Fig. 3) did not influence growth or initiation of replication (Table 2).
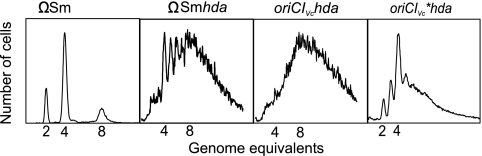
Initiation from oriCIVc is stimulated by the loss of Hda activity.
V. cholerae does not contain an hda homologue (18). Since Hda is essential for RIDA (regulatory inactivation of DnaA), a process described for E. coli where active DnaA protein associated with ATP is converted to the inactive ADP-bound form (34), it was unclear whether replication initiation from oriCIVc is affected by the nucleotide-bound form of DnaA. Deletion of hda (69) from oriCIVc cells led to asynchrony of initiation and an elevated number of origins per cell (Fig. 5). Hda deficiency in oriCIVc, oriCIVc*, and ΩSm strains led to different degrees of overinitiation, which is not surprising, since such cells are known to accumulate secondary mutations (69). Because loss of Hda and thereby RIDA activity was previously shown to increase the DnaAATP/DnaAADP ratio (DnaAADP is DnaA bound to ADP) (35), these data suggest that DnaAATP is also the active form of the initiator protein for oriCIVc-dependent initiation. In agreement with this, we observed that oriCIVc minichromosomes could be introduced into a ΔdnaA E. coli strain EH3827 (25) when it contained the dnaA plasmid pFH539 (27) but not when it contained the dnaAK178T plasmid pRUC672 (76). The latter directs synthesis of the DnaAK178T protein that is mutated in the ATP binding site (data not shown).
FIG. 5.
Loss of Hda activity stimulates replication initiation from oriCIVc. ΩSm cells and hda::cat derivatives of ΩSm, oriCIVc, and oriCIVc* cells were grown at 37°C in minimal medium supplemented with glucose (Glu) plus Casamino Acids (CAA). The cells were treated with rifampin and cephalexin (Materials and Methods) prior to flow cytometric analysis.
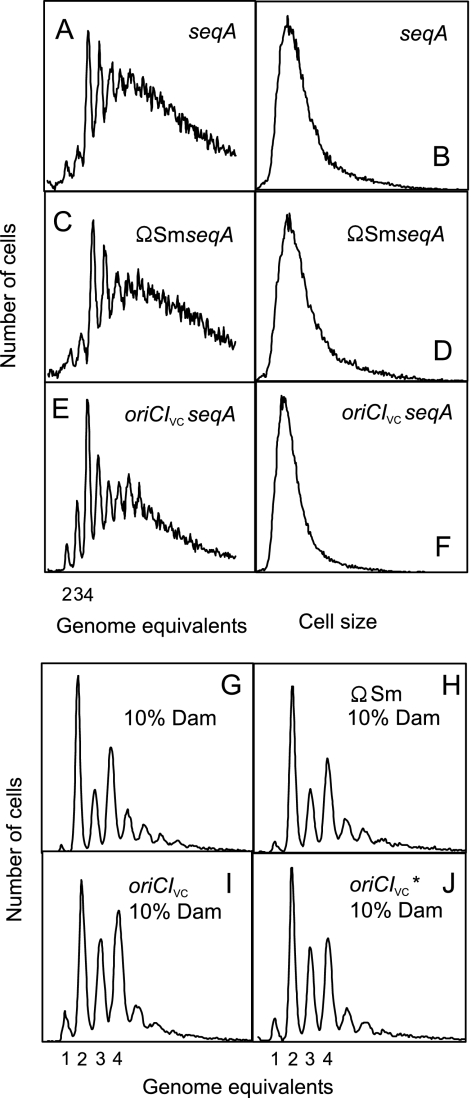
SeqA is required for synchronous replication from oriCIVc.
The ΩSm, oriCIVc*, and oriCIVc genetic elements (Fig. 3) were transferred into seqAΔ10 mutant cells (78) by P1 transduction. Transductants were obtained at similar frequencies, indicating that the SeqA protein is dispensable for function of oriCIVc. Flow cytometric analysis revealed initiation asynchrony and an increased number of origins for seqA cells initiating from both oriC and oriCIVc cells relative to SeqA+ cells (Fig. 6). This is in agreement with previous data for E. coli (48). Both cell mass and number of origins per cell of seqA oriCIVc cells were somewhat lower than for their oriC counterparts (compare Fig. 6E and F with Fig. 6A, B, C, and D), giving similar origin/mass ratios (not shown).
FIG. 6.
Synchronous replication initiation from oriCIVc is dependent on seqA and dam gene products. Cells were grown at 37°C in minimal medium supplemented with Glu plus CAA. Panels A, C, E, G, H, I, and J show cells treated with rifampin and cephalexin prior to flow cytometric analysis, whereas the cells in panels B, D, and F were in the exponential growth phase. seqA (A and B), ΩSm seqA (C and D), oriCIVc seqA (E and F), damX::mini-Tn10 (G), ΩSm damX::mini-Tn10 (H), oriCIVcdamX::mini-Tn10 (I), and oriCIVc* damX::mini-Tn10 (J) strains were studied. Insertion of mini-Tn10 in damX reduces the transcription of the dam gene to approximately 10% of the level in wild-type cells (42).
E. coli cells carrying oriCIVc requires Dam methylation for viability.
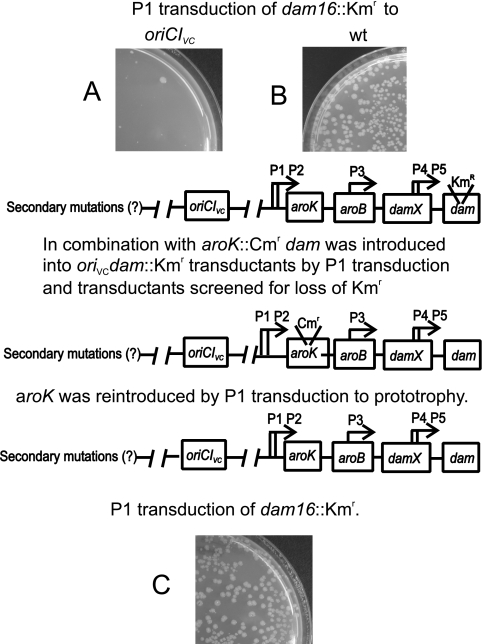
The oriCIVc* region could not be transduced into dam16::Kmr (63) cells. In contrast, the dam16::Kmr mutation could be transduced into cells replicating from oriCIVc and oriCIVc* with low frequency and resulting in transductants that were heterogeneous in colony size and generally appeared slow growing (Fig. 7 A). Approximately 25% of the transductants contained both a mutated dam gene and a wild-type dam gene (not shown). Such cells were never observed for transductants with the dam16::Kmr allele introduced into wild-type cells (not shown). The low number of dam16::Kmr transductants obtained in oriCIVc and oriCIVc* cells and the fact that different phenotypes were observed for the transductants indicate that the attempt to combine these origins with a null mutation in dam led to the selection of strains with secondary dam suppressor mutations (dsm mutations). To test this possibility, we constructed four independent isolates of oriCIVc* dam16::Kmr cells and transduced these back to Dam+ by a two-step procedure as described previously (Fig. 7) (46). The resultant cells were Dam+ but contained putative hsm mutations. When these cells were subsequently transduced to dam16::Kmr, this occurred with the same frequency as the frequency observed for wild-type cells (Fig. 7C). This demonstrates that all four initial oriCIVc* dam clones tested carried secondary mutations. The nature of the dsm mutations is not known at present, although transductant data indicate that neither of the dsm mutations were linked to oriCIVc.
FIG. 7.
Dam-deficient oriCIVc* cells contain suppressor mutations. The dam16::Kmr allele was P1 transduced into oriCIVc* (A) and wild-type (wt) (B) cells. Four independent oriCIVc* dam cells (from panel A) were transduced back to Dam+ by a two-step procedure (details in Materials and Methods) (46). By this procedure, putative dam suppressor mutations (dsm mutations) will be present in an otherwise Dam+ background. The dam16::Kmr allele was subsequently transduced back into the oriCIVc* (hsm?) cells. This transduction resulted in a high number of transductants, indicating that all four oriCIVc* dam clones tested contained secondary mutations to compensate for loss of Dam activity. The same dam16::Kmr P1 lysate was used for all strains, and the same number of cells was plated on selective media and incubated for 24 h at 37°C prior to inspection.
To quantify the transfer of a dam mutation into cells with various mutations in oriC, we used a strain carrying both the dam13::Tn9 and argE::Tn10 mutations (strain ALO3689) as a donor. The efficiency of dam13::Tn9 transduction could thus be determined relative to the unlinked argE::Tn10 mutation carried in the same P1 lysate (Table 3). The dam13::Tn9 and argE::Tn10 mutations were transduced into wild-type and ΩSm cells with the same frequency, whereas the efficiency of dam13::Tn9 transduction into oriCIVc or oriCIVc* cells was reduced approximately 10,000-fold (Table 3). Transduction of dam13::Tn9 into oriCIVc at 37°C resulted in microcolonies that could not be restreaked, while incubation of plates at 42°C resulted in very few heterogeneous colonies similar to what we observed for oriCIVc*. Because replication initiation is facilitated by negative supercoiling, which in turn decreases with temperature (3), these observations suggest that the events leading to duplex opening and productive initiation at oriCIVc are severely compromised in the absence of Dam methylation.
TABLE 3.
Relative P1 transduction of argE::Tn10 and dam13::Tn9 into strains with mutated oriC regionsa
| Recipient | Mnemonic | Minichromosome phenotypeb | Cmr/Tcr ratioc |
|---|---|---|---|
| WM2482 | Wild-type | ++ | 0.9 |
| WM2759 (oriC160) | Deletion of 77 bp to the right of R4 | + | 1.0 |
| WM2762 (oriC13) | Scrambled R2 | + | 0.9 |
| WM2764 (oriC15) | Scrambled R4 | − | 9.0 × 10−4 |
| WM2844 (oriC17) | Scrambled R5 | − | 3.3 × 10−4 |
| WM2845 (oriC162) | Addition of 14 bp between R3 and R4 | − | 2.6 × 10−4 |
| ALO3270 (oriC-I3,I6) | G3T in I2 and G3→T in I3 | +++ | 1.4 |
| ALO3699 (ΩSm) | ΩSm inserted next to oriC | ND | 1.2 |
| ALO3150 (oriCIVc*) | oriC replaced by oriCIVc* | ND | 2.2 × 10−4 |
| ALO3702 (oriCIVc) | oriC replaced by oriCIVc | ||
| Incubated at 37°Cd | ND | 1.0 × 10−3 | |
| Incubated at 42°Cd | ND | 1.3 × 10−4 |
Plates were inspected after overnight incubation at 37°C. Strains with oriC mutations oriC14, oriC21, oriC131, oriC132, and oriC136 (84) behaved similar to wild-type (wt), oriC160, and oriC13 strains.
Data from reference 84, except for the oriC-I3,I6 mutant (data from reference 23). Symbols: +++, increased oriC function; ++, normal oriC function; +, reduced oriC function; −, inactive oriC. ND, not determined.
Transduction efficiency of argE::Tn9 into the recipient was 1 × 10−6 to 2 × 10−6 in all cases except for oriC15 and oriC162 strains, where it was approximately 5-fold lower.
There were about as many microcolonies as seen on the plates containing tetracycline. These microcolonies could not really restreak and did not appear when the plates were incubated at 42°C.
Next, we wanted to determine whether oriCIVc and oriCIVc* cells were viable when the amount of Dam was significantly reduced. We used the damX::mini-Tn10 mutation resulting in dam gene transcription from promoters P4 and P5 only (out of a total of five) at approximately 10% of the wild-type level (42). The damX::mini-Tn10 mutation was transduced into wild-type, ΩSm, oriCIVc, and oriCIVc* cells with similar frequencies, showing that only small amounts of Dam are needed for viability of oriCIVc and oriCIVc* cells. The reduction in dam gene expression led to initiation asynchrony in all cells (Fig. 6G to J).
Only severely compromised E. coli origins require Dam methylation for viability.
In order to examine whether changes in initiation efficiency from the E. coli replication origin itself could result in a requirement for Dam methylation, we transduced dam13::Tn9 into a previously characterized set of oriC mutants (69, 84), again using the argE::Tn10 mutation as an internal control (Table 3).
For oriC mutants (oriC13, oriC14, oriC21, oriC131, oriC132, oriC136, and oriC160 mutants) that all show reduced oriC function when carried on a minichromosome (84), the two markers were transduced with the same frequency, indicating that Dam methylation is dispensable for the function of these origins (Table 3). For an oriC mutation (MG1655 oriC-I3,I6) that increases origin function when present on the chromosome (68), Dam methylation was also dispensable (Table 3). We found methylation to be essential only for the viability of the oriC15, oriC17, and oriC162 mutants that carry severely truncated origins that cannot sustain minichromosome replication (i.e., with an average replication rate well below that of oriC) and that result in severe asynchrony when carried on the chromosome (84).
Together, these data indicate that dam is dispensable for replication initiation from mutant oriCs, provided that origin function is increased or only somewhat reduced relative to wild type. A severe reduction in oriC function, on the other hand, results in a requirement for Dam methylation. In contrast, oriCIVc does not seem to be so severely defective as to warrant a Dam requirement, suggesting a different role for Dam in initiation of replication from oriCIVc compared to oriC.
Overexpression of DnaA does not restore the viability of Dam-deficient oriCIVc cells.
E. coli cells deficient in Dam methylation have decreased expression of dnaA (10, 36). A possibility for the inviability of Dam-deficient oriCIVc cells could be that their reduced DnaA content was insufficient for initiation of replication. We therefore transduced the dam16::Kmr mutation into oriCIVc and oriCIVc* cells carrying either plasmid pRUC1443, which carries the V. cholerae dnaA gene under the control of the strong lac PA10403 promoter (O. Skovgaard, unpublished observation), or plasmid pLR40, which carries the E. coli dnaA gene under the control of the indigenous lac promoter (69). Plasmid pRUC1443 complemented an E. coli dnaA46 mutant at low IPTG levels, whereas full induction was deleterious to cells, suggesting that this plasmid directs high-level V. cholerae DnaA production. Plasmid pLR40 was previously shown to overproduce E. coli DnaA about 3-fold when fully induced (69).
We did not obtain an increased transduction frequency at any IPTG concentration for either plasmid (not shown). Therefore, additional DnaA protein cannot compensate for loss of Dam methylation in the process of oriCIVc-specific initiation.
Dam methylation is required for initiation from oriCIVc.
To further study the effect of Dam on replication initiation from oriCIVc, we used a conditional replication system based on pKG339 that carries copA under lac promoter control (18, 32) for Dam depletion. This system relies on the replication control system of plasmid R1, where the copy number is negatively regulated by the CopA antisense RNA. Hence, if CopA is overproduced from a coresident plasmid, R1 replication is blocked within a few minutes (39). We introduced the dam16::Kmr mutation into wt, ΩSm, oriCIVc, and oriCIVc* cells containing pALO160, a R1-based plasmid carrying the entire dam operon under the control of its indigenous promoters (42). A further introduction of plasmid pKG339 that carries copA under lac promoter control (39) allowed Dam depletion in both strains after the addition of IPTG (Materials and Methods).
Dam depletion in wild-type cells gradually led to decreased initiation synchrony, and 7 h after the addition of IPTG, the cells were completely asynchronous (Fig. 8 A). The cells appeared relatively homogeneous with a good correlation between cell size and DNA content, although a small subpopulation of cells with variable size containing one fully replicated chromosome was observed after 7 h (Fig. 8B). For oriCIVc and oriCIVc* cells, the situation was aggravated. Approximately 7 h after the addition of IPTG, cells containing one origin of replication started to dominate the population (Fig. 8A). For these cells, there was poor correlation between size and DNA content and cells appeared “trapped” with only one fully replicated chromosome (Fig. 8B). The accumulation of large cells containing only one fully replicated oriCIVc chromosome suggests that these cells were able to grow but were unable to initiate replication from oriCIVc. Therefore, Dam methylation was required for one or more processes leading to initiation from oriCIVc. After longer times of incubation, oriCIVc and oriCIVc* cells regained the ability to initiate replication, most likely due to the accumulation of secondary mutations.
FIG. 8.
Depletion of Dam methylase leads to oriCIVc initiation arrest. Dam methylase was depleted from wt, ΩSm, oriCIVc, and oriCIVc* cells as described in Materials and Methods. Samples were incubated with rifampin and cephalexin prior to flow cytometric analysis. (A) DNA histograms of oriC and oriCIVc after incubation in the presence of IPTG for the indicated time period. Cells were treated with rifampin and cephalexin prior to flow cytometric analysis. (B) Two-parameter histograms showing DNA content versus cell size after incubation with IPTG for 0 and 7 h.
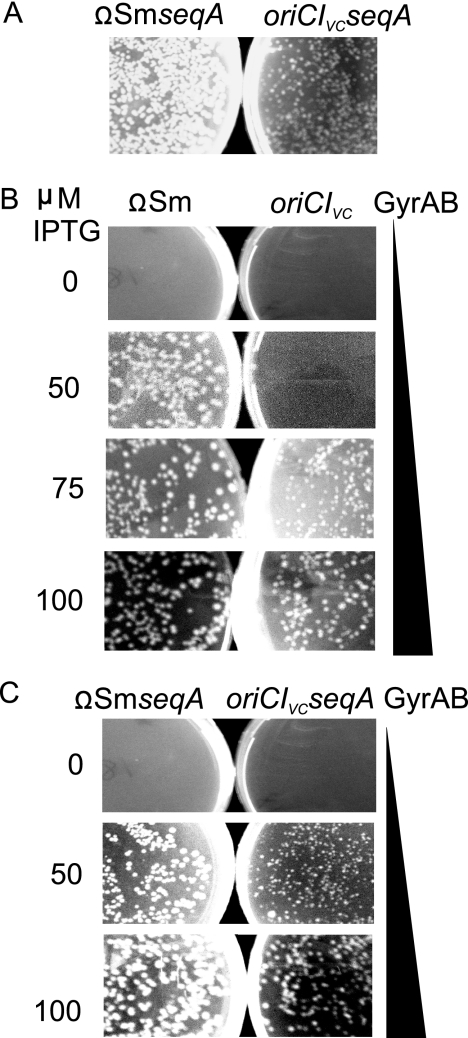
Increased negative supercoiling restores viability of Dam-deficient E. coli cells carrying oriCIVc.
The dam16::Kmr mutation was transduced into ΩSm seqA and oriCIVc seqA cells with the same frequency although colonies of the latter were smaller (Fig. 9 A). The absence of SeqA therefore rendered oriCIVc dam mutants viable although somewhat growth compromised. Because loss of SeqA activity leads to increased negative supercoiling of the chromosome (85), that in turn facilitates duplex opening, we speculated that this was a way of reactivating an otherwise inactive oriCIVc in dam mutant cells. This could provide the explanation for the ability of a seqA mutation to suppress the initiation defect of oriCIVc dam cells.
FIG. 9.
Dam methylation is dispensable for oriCIVc function in cells with increased negative supercoiling. (A) The dam16::Kmr allele was transduced into ΩSm seqA and oriCIVc seqA cells derived from E. coli MG1655. (B) The dam16::Kmr allele was transduced into ΩSm and oriCIVc cells carrying the gyrAB genes under the control of the IPTG-inducible PA1lacO1 promoter. (C) The dam16::Kmr allele was transduced into ΩSm seqA and oriCIVc seqA cells carrying the gyrAB genes under the control of the IPTG-inducible PA1lacO1 promoter. Transductants were plated on LB agar plates containing kanamycin (A) or kanamycin, tetracycline, and the indicated IPTG concentration (B and C) and were inspected after 22 h of incubation at 42°C. The same dam16::Kmr P1 lysate was used for all strains, and the same number of cells was plated.
In order to test this hypothesis further, we used strain ALO3470, a derivative of PJ4240 where the level of supercoiling could be exogenously controlled (31). In ALO3470, DNA gyrase (gyrAB) is under the control of the IPTG-inducible PA1lacO1 promoter (48a). Since DNA gyrase is an essential gene, the ALO3470 strain grows only in the presence of IPTG. Approximately 70 μM IPTG has been determined to give a wild-type expression level of DNA gyrase, whereas higher IPTG levels result in more negatively supercoiled DNA (31). The dam16::Kmr mutation was transduced into oriCIVc, ΩSm, ΩSm seqA, and oriCIVc seqA mutant derivatives of ALO3470, and transductants were plated in the presence of various IPTG concentrations. The dam16::Kmr mutation could be efficiently transferred into ΩSm cells at IPTG concentrations of 50 μM or higher (Fig. 9B). On the other hand, the dam16::Kmr mutation could be efficiently transduced into oriCIVc cells only when IPTG concentrations were 75 μM or above, and growth appeared to be best at the highest concentration tested (Fig. 9) (similar data were observed for oriCIVc* cells). The absence of SeqA reduced the IPTG requirement for growth of dam16::Kmr transductants into oriCIVc cells to approximately 50 mM (Fig. 9C), but better growth was observed at a higher IPTG concentration (Fig. 9C).
These data strongly suggest that unmethylated oriCIVc is initiated poorly, but the origin can gain activity by loss of SeqA activity or if the level of negative superhelicity is increased by increased expression of the DNA gyrase.
DISCUSSION
Studies of in vivo replication initiation in V. cholerae are complicated by the presence of two chromosomes in this bacterial species; it is often difficult to attribute certain replication defects or phenotypes to a specific chromosome (19, 67). In order to study replication initiation from oriCIVc, the origin of the larger chromosome, chromosome I, we replaced the E. coli oriC region with the corresponding oriCIVc region. When the cells were growing slowly, replication initiation at oriCIVc took place at a similar or slightly reduced initiation mass relative to initiation from oriC. Otherwise, replication initiation at oriCIVc resembles oriC-dependent initiation with respect to cell cycle parameters, such as initiation frequency, SeqA-dependent initiation synchrony, and stimulation of initiation by loss of Hda activity, but differ with respect to the requirement for Dam methylation.
The minimal oriCIVc region.
The minimal replication origins from the E. coli chromosome and chromosome I of V. cholerae are quite similar. The left-hand side of E. coli oriC contains an AT-rich cluster followed by three 13-mer repeats (L, M, and R) each starting with (A/T)GATCT, a 6-mer sequence that binds DnaAATP in both single- and double-stranded DNA (Fig. 1) (80). The corresponding region in oriCIVc contains two 13-mer regions corresponding to M and R and three 6-mer sequences, and the rightmost two 6-mer sequences are found within M and R 13-mer regions (Fig. 1). The DnaA R boxes R1, R2, and R4 are completely conserved between E. coli and V. cholerae, while R3, R5, I2, and τ1 are highly conserved (52, 53, 61) (Fig. 1).
We found no significant difference in copy number of oriCIVc minichromosomes carrying the entire region between the mioC and gidA genes and of those carrying a minimal origin only (Fig. 2). This is different from the situation in E. coli where the presence of the gidA and mioC promoters stimulates replication initiation (5, 41, 81).
Replication initiation from oriC and oriCIVc.
The DnaA protein serves as the initiator protein for both E. coli oriC and oriCIVc (18). The facts that oriCIVc minichromosomes can replicate in E. coli and that oriCIVc can replace oriC show that E. coli DnaA can functionally replace V. cholerae DnaA. Similarly, the DnaA proteins from Vibrio harveyi and V. cholerae can replace the E. coli DnaA protein (7). In V. cholerae, a homologue of the hda gene has not been identified (18). Because Hda is essential for RIDA (regulatory inactivation of DnaA), this process may be absent or operate differently in this organism. The construction of the oriCIVc strain allowed us to address the roles of the different nucleotide-bound forms of DnaA (i.e., DnaAATP and DnaAADP) in regulation of V. cholerae chromosome I replication. Deletion of hda from the oriCIVc strain, which presumably raised the DnaAATP/DnaAADP ratio, stimulated replication initiation (Fig. 5) and oriCIVc minichromosomes could not be introduced into E. coli cells having only a DnaA protein mutated in the ATP binding site. We believe that initiation from oriCIVc is similar to initiation from oriC with an absolute requirement for DnaAATP.
In wild-type E. coli cells, regulation of the initiation frequency at oriC depends primarily on sequences outside the origin (13). This is because formation of the prereplication complex (pre-RC) is a low-affinity process that takes place at a critical level of DnaAATP that is reached only when all DnaA binding sites outside the origin are filled (6, 26, 29). Therefore, differences in initiation efficiency between oriC and oriCIVc that can be observed by the analyses done here are expected to be small. In agreement with this, we observed only a 10% reduction in growth rate and no difference in cell cycle parameters, such as the number of origins per cell, origin concentration, or single cell synchrony between E. coli cells initiating from their normal oriC sequence and those initiating from oriCIVc when the cells were grown in rich medium. At lower growth rates, cells replicating from oriCIVc initiated replication in synchrony with a reduced cell mass, suggesting that the pre-RC complex forms more efficiently at this origin than at oriC. Therefore, oriCIVc seems more efficient than oriC in initiation of replication in E. coli. Despite this, oriCIVc-based minichromosomes did not compete with the chromosomal origin when present in E. coli and were maintained as extrachromosomal plasmids. Therefore, the cascade of initiations triggered by firing of the first origin, whether the origin is located on the chromosome or minichromosome, has the potential to initiate all remaining origins in the cell, suggesting that the differences between oriC and oriCIVc are small (75).
Roles of dam and seqA genes in replication from oriCIVc.
In V. cholerae, the dam gene is essential, while there is conflicting data on whether seqA is essential in V. cholerae (15, 72). We found that the loss of SeqA function led to overinitiation from oriCIVc and asynchrony, but the cells remained viable. A reduction in the level of Dam methylase also led to initiation asynchrony. It is therefore likely that sequestration of hemimethylated oriCIVc is involved in prevention of immediate reinitiation, similar to the case for oriC-dependent replication. In addition to this postinitiation role of Dam methylation, our data also show that dam is required for efficient initiation from oriCIVc in E. coli. This requirement was not a result of limited DnaA availability or quality, because overproduction of neither the E. coli or V. cholerae DnaA protein could compensate for loss of Dam activity. Our data are in agreement with the observation that overexpression of SeqA in V. cholerae led to loss of viability and replication arrest (72); because Dam and SeqA proteins compete for the same GATC sites on DNA, overexpression of SeqA leads to the phenotype associated with the lack of the Dam protein (43).
Dam methylation may be required for duplex opening at oriCIVc.
On the basis of our data, Dam methylation seems to play dual roles for replication from V. cholerae oriCI in E. coli. One of these is the well-characterized role in SeqA-dependent sequestration of newly initiated and hemimethylated origins that is required to prevent rereplication within the same cell cycle. The second role of Dam methylation is the requirement for methylation of oriCIVc prior to initiation to allow for efficient duplex opening either directly or indirectly. Similar roles for Dam methylation have previously been reported for replication of plasmid P1 (1, 2). The first step in initiation of chromosome replication in E. coli is unwinding of the origin in the region containing the AT-rich cluster and 13-mer repeats in a process assisted by DnaA as described above. This part of the E. coli origin exhibits helical instability and can unwind even in the absence of DnaA when present on a negatively supercoiled plasmid (38). Therefore, it is not surprising that mutations in gyrase and topoisomerase I, key players in controlling DNA supercoiling in E. coli (79), influence initiation of replication in E. coli, leading to asynchrony and changes in origin concentration (82). Thermal melting determinations of a oriC-carrying plasmid by differential scanning calorimetry indicated that Dam methylation can lower the oriC melting point and thereby facilitate strand separation in the oriC region (86). The inability to delete dam from cells with oriC15, oriC17, and oriC162 mutations suggest that DnaA fails to promote strand separation at these truncated origins when unmethylated. The lower intrinsic melting point of the fully methylated mutant origins may, however, augment DnaA in the duplex opening process to allow for initiation, albeit at reduced frequency compared to that of wild-type oriC. A similar explanation for the inability to initiate replication from unmethylated oriCIVc is therefore that an increase in thermodynamic stability of DNA, associated with the absence of N6 methylation, renders the AT-rich region of oriCIVc unable to unwind in response to DnaA binding. In agreement with this hypothesis, conditions that decrease helical stability, such as increased expression of gyrAB genes (31) or deletion of seqA (85), can compensate for the loss of Dam activity. It is not clear why methylation is required for initiation from oriCIVc but not oriC. The 256-bp oriCIVc minimal origin region has a lower GC content than the corresponding 257-bp oriC region (87 and 102 bp, respectively). The helical stability of the oriC and oriCIVc minimal origin regions was analyzed using WEB-THERMODYNE sequence analysis software (30). As expected, the lowest helical stability for both minimal origins was found in the left part of the sequence where the initial unwinding of the E. coli origin occurs (38). The helical stability in this region as well as the overall helical stability is lower for the oriCIVc minimal origin than for the corresponding oriC regions, indicating that the increased negative supercoiling needed to suppress the Dam requirement of oriCIVc does not result from differences in the DNA sequence between the minimal origin regions. Another possible explanation is that the formation of the orisome is perturbed on unmethylated origins. The Dam/SeqA system normally ensures a highly organized orisome assembly at the origin (58). In its absence, one might imagine that less active complexes are formed and productive initiations from these complexes could be augmented by increased negative superhelicity. The difference between oriC and oriCIVc could then be due to the slightly different positions of the GATC sites and/or the extra GATC sites in the minimal oriCIVc region compared to the minimal oriC region (Fig. 1). oriCIVc contains two extra GATC sequences relative to oriC. One is located between τ1 and R5 and could, in the absence of methylation, promote untimely DnaA binding to these sites and hence interfere with orisome formation. The other is located within the region corresponding to the E. coli FIS (factor for inversion stimulation) binding site (Fig. 1). Methylation of this site could influence FIS binding and thereby pre-RC assembly (70). We have not yet tested this possibility.
On the basis of minichromosome data, it has been reported that in E. coli, origin activity is stimulated by transcription from the mioC promoter traversing oriC and from the gidA promoter located immediately adjacent to oriC and transcribing away from oriC (5, 59). In the case of gidA, the stimulation is likely to result from increased negative supercoiling behind transcribing RNA polymerases (40) that have initiated at PgidA. It seemed reasonable that the same role could apply to the V. cholerae PgidA and oriCIVc. However, our minichromosome data (Fig. 2) indicate that initiation from oriCIVc is not stimulated significantly by the presence of the V. cholerae gidA promoter, which would could contribute to the apparent methylation requirement.
The data reported here are in conflict with a recent study (15) where an essential role for dam was not observed when oriC was replaced with oriCIVc. Although in both studies oriCIVc was found to be more active than the E. coli origin, differences do exist. In this work, the oriCIVc strain contained an intact E. coli mioC gene followed by the V. cholerae region between the mioC and gidA genes and the 20 N-terminal codons of the V. cholerae gidA gene. The aadA (Smr) cassette was located 1 kb further downstream of the gidA promoter (Fig. 3). Demarre and Chattoraj (15) used a minimal oriCIVc region with an attached zeocin resistance gene to replace the minimal E. coli oriC sequence. In the final construct, the zeo gene was inserted between the E. coli mioC gene and oriCIVc in such a way that transcription from the zeo promoter was directed toward oriCIVc. The E. coli gidA and the mioC genes remained intact (G. Demarre and D. K. Chattoraj, personal communication). This oriCI strain seems somewhat more active than our oriCIVc strain. It is not clear to us why the Dam requirement for these two oriCIVc strains should differ. The observed differences may, however, stress the contribution of transcriptional events around the origin to the initiation process and indicate that Dam methylation could modulate (i.e., lower) the requirement for transcriptional activation of oriCIVc. The two different sets of data further stress that extreme care should be taken when extrapolating data obtained in E. coli to the situation in V. cholerae. The precise role for dam in initiation of replication from oriCIVc in V. cholerae under different conditions will require studies carried out in the native host.
Acknowledgments
This work was supported by the Danish Natural Sciences Research Council and the Novo Nordisk Foundation.
We thank Ole Skovgaard for providing plasmids pSW29TsacB, and pRUC1443, Kenn Gerdes for providing plasmid pKG339, Martin Martinus for providing strain CAG18456, Kirsten Skarstad for providing strain SS211, Rasmus Bugge Jensen for providing strain DH5αλpir, and Peter Rudahl Jensen for providing strain PJ4240. Christa P. Nielsen is thanked for excellent technical assistance. We thank Godefroid Charbon for critically reading the manuscript.
Footnotes
Published ahead of print on 28 May 2010.
REFERENCES
- 1.Abeles, A., T. Brendler, and S. Austin. 1993. Evidence of two levels of control of P1 oriR and host oriC replication origins by DNA adenine methylation. J. Bacteriol. 175:7801-7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abeles, A. L., and S. J. Austin. 1987. P1 plasmid replication requires methylated DNA. EMBO J. 6:3185-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamcik, J., V. Viglasky, F. Valle, M. Antalik, D. Podhradsky, and G. Dietler. 2002. Effect of bacteria growth temperature on the distribution of supercoiled DNA and its thermal stability. Electrophoresis 23:3300-3309. [DOI] [PubMed] [Google Scholar]
- 4.Adhya, S., and M. Gottesman. 1982. Promoter occlusion: transcription through a promoter may inhibit its activity. Cell 29:939-944. [DOI] [PubMed] [Google Scholar]
- 5.Asai, T., M. Takanami, and M. Imai. 1990. The AT richness and gid transcription determine the left border of the replication origin of the E. coli chromosome. EMBO J. 9:4065-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atlas, J. C., E. V. Nikolaev, S. T. Browning, and M. L. Shuler. 2008. Incorporating genome-wide DNA sequence information into a dynamic whole-cell model of Escherichia coli: application to DNA replication. IET Syst. Biol. 2:369-382. [DOI] [PubMed] [Google Scholar]
- 7.Berenstein, D., K. Olesen, C. Speck, and O. Skovgaard. 2002. Genetic organization of the Vibrio harveyi DnaA gene region and analysis of the function of the V. harveyi DnaA protein in Escherichia coli. J. Bacteriol. 184:2533-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boye, E., and A. Løbner-Olesen. 1990. The role of dam methyltransferase in the control of DNA replication in E. coli. Cell 62:981-989. [DOI] [PubMed] [Google Scholar]
- 10.Braun, R. E., and A. Wright. 1986. DNA methylation differentially enhances the expression of one of the two E. coli dnaA promoters in vivo and in vitro. Mol. Gen. Genet. 202:246-250. [DOI] [PubMed] [Google Scholar]
- 11.Casadesus, J., and D. Low. 2006. Epigenetic gene regulation in the bacterial world. Microbiol. Mol. Biol. Rev. 70:830-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark, D. J., and O. Maaløe. 1967. DNA replication and the division cycle in Escherichia coli. J. Mol. Biol. 23:99-112. [Google Scholar]
- 13.Dasgupta, S., and A. Løbner-Olesen. 2004. Host controlled plasmid replication: Escherichia coli minichromosomes. Plasmid 52:151-168. [DOI] [PubMed] [Google Scholar]
- 14.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demarre, G., and D. K. Chattoraj. 2010. DNA adenine methylation is required to replicate both Vibrio cholerae chromosomes once per cell cycle. PLoS Genet. 6:e1000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demarre, G., A. M. Guerout, C. Matsumoto-Mashimo, D. A. Rowe-Magnus, P. Marliere, and D. Mazel. 2005. A new family of mobilizable suicide plasmids based on broad host range R388 plasmid (IncW) and RP4 plasmid (IncPalpha) conjugative machineries and their cognate Escherichia coli host strains. Res. Microbiol. 156:245-255. [DOI] [PubMed] [Google Scholar]
- 17.Donachie, W. D. 1968. Relationship between cell size and time of initiation of DNA replication. Nature 219:1077-1079. [DOI] [PubMed] [Google Scholar]
- 18.Duigou, S., K. G. Knudsen, O. Skovgaard, E. S. Egan, A. Løbner-Olesen, and M. K. Waldor. 2006. Independent control of replication initiation of the two Vibrio cholerae chromosomes by DnaA and RctB. J. Bacteriol. 188:6419-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egan, E. S., A. Løbner-Olesen, and M. K. Waldor. 2004. Synchronous replication initiation of the two Vibrio cholerae chromosomes. Curr. Biol. 14:R501-R502. [DOI] [PubMed] [Google Scholar]
- 20.Egan, E. S., and M. K. Waldor. 2003. Distinct replication requirements for the two Vibrio cholerae chromosomes. Cell 114:521-530. [DOI] [PubMed] [Google Scholar]
- 21.Erzberger, J. P., M. L. Mott, and J. M. Berger. 2006. Structural basis for ATP-dependent DnaA assembly and replication-origin remodeling. Nat. Struct. Mol. Biol. 13:676-683. [DOI] [PubMed] [Google Scholar]
- 22.Gille, H., J. B. Egan, A. Roth, and W. Messer. 1991. The FIS protein binds and bends the origin of chromosomal DNA replication, oriC, of Escherichia coli. Nucleic Acids Res. 19:4167-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimwade, J. E., J. J. Torgue, K. C. McGarry, T. Rozgaja, S. T. Enloe, and A. C. Leonard. 2007. Mutational analysis reveals Escherichia coli oriC interacts with both DnaA-ATP and DnaA-ADP during pre-RC assembly. Mol. Microbiol. 66:428-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyer, M. S., R. R. Reed, J. A. Steitz, and K. B. Low. 1981. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harb. Symp. Quant. Biol. 45:135-140. [DOI] [PubMed] [Google Scholar]
- 25.Hansen, E. B., and M. B. Yarmolinsky. 1986. Host participation in plasmid maintenance: dependence upon dnaA of replicons derived from P1 and F. Proc. Natl. Acad. Sci. U. S. A. 83:4423-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen, F. G., B. B. Christensen, and T. Atlung. 1991. The initiator titration model: computer simulation of chromosome and minichromosome control. Res. Microbiol. 142:161-167. [DOI] [PubMed] [Google Scholar]
- 27.Hansen, F. G., S. Koefoed, and T. Atlung. 1992. Cloning and nucleotide sequence determination of twelve mutant dnaA genes of Escherichia coli. Mol. Gen. Genet. 234:14-21. [DOI] [PubMed] [Google Scholar]
- 28.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, O. White, S. L. Salzberg, H. O. Smith, R. R. Colwell, J. J. Mekalanos, J. C. Venter, and C. M. Fraser. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrick, J., M. Kohiyama, T. Atlung, and F. G. Hansen. 1996. The initiation mess? Mol. Microbiol. 19:659-666. [DOI] [PubMed] [Google Scholar]
- 30.Huang, Y., and D. Kowalski. 2003. WEB-THERMODYN: sequence analysis software for profiling DNA helical stability. Nucleic Acids Res. 31:3819-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen, P. R., C. C. van der Weijden, L. B. Jensen, H. V. Westerhoff, and J. L. Snoep. 1999. Extensive regulation compromises the extent to which DNA gyrase controls DNA supercoiling and growth rate of Escherichia coli. Eur. J. Biochem. 266:865-877. [DOI] [PubMed] [Google Scholar]
- 32.Jensen, R. B., E. Grohmann, H. Schwab, R. Diaz-Orejas, and K. Gerdes. 1995. Comparison of ccd of F, parDE of RP4, and parD of R1 using a novel conditional replication control system of plasmid R1. Mol. Microbiol. 17:211-220. [DOI] [PubMed] [Google Scholar]
- 33.Julio, S. M., D. M. Heithoff, D. Provenzano, K. E. Klose, R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 2001. DNA adenine methylase is essential for viability and plays a role in the pathogenesis of Yersinia pseudotuberculosis and Vibrio cholerae. Infect. Immun. 69:7610-7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katayama, T., T. Kubota, K. Kurokawa, E. Crooke, and K. Sekimizu. 1998. The initiator function of DnaA protein is negatively regulated by the sliding clamp of the E. coli chromosomal replicase. Cell 94:61-71. [DOI] [PubMed] [Google Scholar]
- 35.Kato, J., and T. Katayama. 2001. Hda, a novel DnaA-related protein, regulates the replication cycle in Escherichia coli. EMBO J. 20:4253-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kedar, G. C., F. Ozcan, E. C. Guzman, D. W. Smith, V. G. Newman, and J. W. Zyskind. 2000. Role of DNA methylation at GATC sites in the dnaA promoter, dnaAp2. J. Mol. Microbiol. Biotechnol. 2:301-310. [PubMed] [Google Scholar]
- 37.Kolling, R., A. Gielow, W. Seufert, C. Kucherer, and W. Messer. 1988. AsnC, a multifunctional regulator of genes located around the replication origin of Escherichia coli, oriC. Mol. Gen. Genet. 212:99-104. [DOI] [PubMed] [Google Scholar]
- 38.Kowalski, D., and M. J. Eddy. 1989. The DNA unwinding element: a novel, cis-acting component that facilitates opening of the Escherichia coli replication origin. EMBO J. 8:4335-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kruse, T., J. Bork-Jensen, and K. Gerdes. 2005. The morphogenetic MreBCD proteins of Escherichia coli form an essential membrane-bound complex. Mol. Microbiol. 55:78-89. [DOI] [PubMed] [Google Scholar]
- 40.Liu, L. F., and J. C. Wang. 1987. Supercoiling of the DNA template during transcription. Proc. Natl. Acad. Sci. U. S. A. 84:7024-7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Løbner-Olesen, A., T. Atlung, and K. V. Rasmussen. 1987. Stability and replication control of Escherichia coli minichromosomes. J. Bacteriol. 169:2835-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Løbner-Olesen, A., E. Boye, and M. G. Marinus. 1992. Expression of the Escherichia coli dam gene. Mol. Microbiol. 6:1841-1851. [DOI] [PubMed] [Google Scholar]
- 43.Løbner-Olesen, A., M. G. Marinus, and F. G. Hansen. 2003. Role of SeqA and Dam in Escherichia coli gene expression: a global/microarray analysis. Proc. Natl. Acad. Sci. U. S. A. 100:4672-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Løbner-Olesen, A., K. Skarstad, F. G. Hansen, K. von Meyenburg, and E. Boye. 1989. The DnaA protein determines the initiation mass of Escherichia coli K-12. Cell 57:881-889. [DOI] [PubMed] [Google Scholar]
- 45.Løbner-Olesen, A., O. Skovgaard, and M. G. Marinus. 2005. Dam methylation: coordinating cellular processes. Curr. Opin. Microbiol. 8:154-160. [DOI] [PubMed] [Google Scholar]
- 46.Løbner-Olesen, A., and U. von Freiesleben. 1996. Chromosomal replication incompatibility in Dam methyltransferase deficient Escherichia coli cells. EMBO J. 15:5999-6008. [PMC free article] [PubMed] [Google Scholar]
- 47.Low, D. A., and J. Casadesus. 2008. Clocks and switches: bacterial gene regulation by DNA adenine methylation. Curr. Opin. Microbiol. 11:106-112. [DOI] [PubMed] [Google Scholar]
- 48.Lu, M., J. L. Campbell, E. Boye, and N. Kleckner. 1994. SeqA: a negative modulator of replication initiation in E. coli. Cell 77:413-426. [DOI] [PubMed] [Google Scholar]
- 48a.Lutz, R., and H. Bujard. 1997. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 25:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marinus, M. G., M. Carraway, A. Z. Frey, L. Brown, and J. A. Arraj. 1983. Insertion mutations in the dam gene of Escherichia coli K-12. Mol. Gen. Genet. 192:288-289. [DOI] [PubMed] [Google Scholar]
- 50.Marinus, M. G., and J. Casadesus. 2009. Roles of DNA adenine methylation in host-pathogen interactions: mismatch repair, transcriptional regulation, and more. FEMS Microbiol. Rev. 33:488-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marinus, M. G., and N. R. Morris. 1973. Isolation of deoxyribonucleic acid methylase mutants of Escherichia coli K-12. J. Bacteriol. 114:1143-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGarry, K. C., V. T. Ryan, J. E. Grimwade, and A. C. Leonard. 2004. Two discriminatory binding sites in the Escherichia coli replication origin are required for DNA strand opening by initiator DnaA-ATP. Proc. Natl. Acad. Sci. U. S. A. 101:2811-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Messer, W. 2002. The bacterial replication initiator DnaA. DnaA and oriC, the bacterial mode to initiate DNA replication. FEMS Microbiol. Rev. 26:355-374. [DOI] [PubMed] [Google Scholar]
- 54.Miller, D. T., J. E. Grimwade, T. Betteridge, T. Rozgaja, J. J. Torgue, and A. C. Leonard. 2009. Bacterial origin recognition complexes: direct assembly of higher-order DnaA oligomeric structures. Proc. Natl. Acad. Sci. U. S. A. 106:18479-18484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller, J. S. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 56.Morigen, E. Boye, K. Skarstad, and A. Løbner-Olesen. 2001. Regulation of chromosomal replication by DnaA protein availability in Escherichia coli: effects of the datA region. Biochim. Biophys. Acta 1521:73-80. [DOI] [PubMed] [Google Scholar]
- 57.Nielsen, O., and A. Løbner-Olesen. 2008. Once in a lifetime: strategies for preventing re-replication in prokaryotic and eukaryotic cells. EMBO Rep. 9:151-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nievera, C., J. J. Torgue, J. E. Grimwade, and A. C. Leonard. 2006. SeqA blocking of DnaA-oriC interactions ensures staged assembly of the E. coli pre-RC. Mol. Cell 24:581-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogawa, T., and T. Okazaki. 1991. Concurrent transcription from the gid and mioC promoters activates replication of an Escherichia coli minichromosome. Mol. Gen. Genet. 230:193-200. [DOI] [PubMed] [Google Scholar]
- 60.Ozaki, S., and T. Katayama. 2009. DnaA structure, function, and dynamics in the initiation at the chromosomal origin. Plasmid 62:71-82. [DOI] [PubMed] [Google Scholar]
- 61.Ozaki, S., H. Kawakami, K. Nakamura, N. Fujikawa, W. Kagawa, S. Y. Park, S. Yokoyama, H. Kurumizaka, and T. Katayama. 2008. A common mechanism for the ATP-DnaA-dependent formation of open complexes at the replication origin. J. Biol. Chem. 283:8351-8362. [DOI] [PubMed] [Google Scholar]
- 62.Parales, R. E., and C. S. Harwood. 1993. Construction and use of a new broad-host-range lacZ transcriptional fusion vector, pHRP309, for gram− bacteria. Gene 133:23-30. [DOI] [PubMed] [Google Scholar]
- 63.Parker, B., and M. G. Marinus. 1988. A simple and rapid method to obtain substitution mutations in Escherichia coli: isolation of a dam deletion/insertion mutation. Gene 73:531-535. [DOI] [PubMed] [Google Scholar]
- 64.Pearson, G. D., A. Woods, S. L. Chiang, and J. J. Mekalanos. 1993. CTX genetic element encodes a site-specific recombination system and an intestinal colonization factor. Proc. Natl. Acad. Sci. U. S. A. 90:3750-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Polaczek, P., K. Kwan, D. A. Liberies, and J. L. Campbell. 1997. Role of architectural elements in combinatorial regulation of initiation of DNA replication in Escherichia coli. Mol. Microbiol. 26:261-275. [DOI] [PubMed] [Google Scholar]
- 66.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 67.Rasmussen, T., R. B. Jensen, and O. Skovgaard. 2007. The two chromosomes of Vibrio cholerae are initiated at different time points in the cell cycle. EMBO J. 26:3124-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riber, L., K. Fujimitsu, T. Katayama, and A. Løbner-Olesen. 2009. Loss of Hda activity stimulates replication initiation from I-box, but not R4 mutant origins in Escherichia coli. Mol. Microbiol. 71:107-122. [DOI] [PubMed] [Google Scholar]
- 69.Riber, L., J. A. Olsson, R. B. Jensen, O. Skovgaard, S. Dasgupta, M. G. Marinus, and A. Løbner-Olesen. 2006. Hda-mediated inactivation of the DnaA protein and dnaA gene autoregulation act in concert to ensure homeostatic maintenance of the Escherichia coli chromosome. Genes Dev. 20:2121-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ryan, V. T., J. E. Grimwade, J. E. Camara, E. Crooke, and A. C. Leonard. 2004. Escherichia coli prereplication complex assembly is regulated by dynamic interplay among Fis, IHF and DnaA. Mol. Microbiol. 51:1347-1359. [DOI] [PubMed] [Google Scholar]
- 71.Saha, A., S. Haralalka, and R. K. Bhadra. 2004. A naturally occurring point mutation in the 13-mer R repeat affects the oriC function of the large chromosome of Vibrio cholerae O1 classical biotype. Arch. Microbiol. 182:421-427. [DOI] [PubMed] [Google Scholar]
- 72.Saint-Dic, D., J. Kehrl, B. Frushour, and L. S. Kahng. 2008. Excess SeqA leads to replication arrest and a cell division defect in Vibrio cholerae. J. Bacteriol. 190:5870-5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skarstad, K., T. A. Baker, and A. Kornberg. 1990. Strand separation required for initiation of replication at the chromosomal origin of E. coli is facilitated by a distant RNA-DNA hybrid. EMBO J. 9:2341-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skarstad, K., E. Boye, and H. B. Steen. 1986. Timing of initiation of chromosome replication in individual Escherichia coli cells. EMBO J. 5:1711-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skarstad, K., and A. Løbner-Olesen. 2003. Stable co-existence of separate replicons in Escherichia coli is dependent on once-per-cell-cycle initiation. EMBO J. 22:140-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skovgaard, O., K. Olesen, and A. Wright. 1998. The central lysine in the P-loop motif of the Escherichia coli DnaA protein is essential for initiating DNA replication from the chromosomal origin, oriC, and the F factor origin, oriS, but is dispensable for initiation from the P1 plasmid origin, oriR. Plasmid 40:91-99. [DOI] [PubMed] [Google Scholar]
- 77.Slater, S., and R. Maurer. 1993. Simple phagemid-based system for generating allele replacements in Escherichia coli. J. Bacteriol. 175:4260-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Slater, S., S. Wold, M. Lu, E. Boye, K. Skarstad, and N. Kleckner. 1995. E. coli SeqA protein binds oriC in two different methyl-modulated reactions appropriate to its roles in DNA replication initiation and origin sequestration. Cell 82:927-936. [DOI] [PubMed] [Google Scholar]
- 79.Snoep, J. L., C. C. van der Weijden, H. W. Andersen, H. V. Westerhoff, and P. R. Jensen. 2002. DNA supercoiling in Escherichia coli is under tight and subtle homeostatic control, involving gene expression and metabolic regulation of both topoisomerase I and DNA gyrase. Eur. J. Biochem. 269:1662-1669. [DOI] [PubMed] [Google Scholar]
- 80.Speck, C., and W. Messer. 2001. Mechanism of origin unwinding: sequential binding of DnaA to double- and single-stranded DNA. EMBO J. 20:1469-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stuitje, A. R., N. de Wind, J. C. van der Spek, T. H. Pors, and M. Meijer. 1986. Dissection of promoter sequences involved in transcriptional activation of the Escherichia coli replication origin. Nucleic Acids Res. 14:2333-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.von Freiesleben, U., and K. V. Rasmussen. 1992. The level of supercoiling affects the regulation of DNA replication in Escherichia coli. Res. Microbiol. 143:655-663. [DOI] [PubMed] [Google Scholar]
- 83.Waldminghaus, T., and K. Skarstad. 2009. The Escherichia coli SeqA protein. Plasmid 61:141-150. [DOI] [PubMed] [Google Scholar]
- 84.Weigel, C., W. Messer, S. Preiss, M. Welzeck, Morigen, and E. Boye. 2001. The sequence requirements for a functional Escherichia coli replication origin are different for the chromosome and a minichromosome. Mol. Microbiol. 40:498-507. [DOI] [PubMed] [Google Scholar]
- 85.Weitao, T., K. Nordstrom, and S. Dasgupta. 2000. Escherichia coli cell cycle control genes affect chromosome superhelicity. EMBO Rep. 1:494-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamaki, H., E. Ohtsubo, K. Nagai, and Y. Maeda. 1988. The oriC unwinding by dam methylation in Escherichia coli. Nucleic Acids Res. 16:5067-5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zyskind, J. W., J. M. Cleary, W. S. Brusilow, N. E. Harding, and D. W. Smith. 1983. Chromosomal replication origin from the marine bacterium Vibrio harveyi functions in Escherichia coli: oriC consensus sequence. Proc. Natl. Acad. Sci. U. S. A. 80:1164-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]