Abstract
The NmlRsp transcription factor of Streptococcus pneumoniae is shown to induce adhC (alcohol dehydrogenase) expression in the presence of both formaldehyde and methylglyoxal. nmlRsp and adhC mutant strains display altered and opposite aerobic growth phenotypes. The nmlRsp strain exhibits increased resistance to high oxygen tension, attributable to decreased H2O2 production, which correlated with downregulation of carbamoyl phosphate synthase (carB). This indicates a possible role for AdhC in aldehyde metabolism and a broader role for NmlRsp in the regulation of carbon metabolism.
The Gram-positive bacterium Streptococcus pneumoniae is a human pathogen of major significance, causing approximately 1 million deaths in children under 5 years annually (11). The pneumococcus is carried asymptomatically in the nasopharynx of a large proportion of the human population but is capable of invading internal sites within the body, resulting in pathology such as otitis media, pneumonia, sepsis, and meningitis (3). Invasive pneumococcal disease is typically associated with an intense inflammatory response in host tissues and recruitment of cells involved in the innate immune response (2). It is clear that the pneumococcus must possess mechanisms to cope with the various stresses imposed by the host immune response in order to cause invasive disease. In a previous publication (17), we characterized two pneumococcal genes (nmlRsp and adhC) that are required for invasive disease but not colonization, using a mouse model of infection. NmlRsp was characterized as a transcription factor of the MerR/NmlR family that regulates the expression of a class III alcohol dehydrogenase (adhC) in the pneumococcus (17). Class III alcohol dehydrogenases are known to catalyze the metabolism of two electrophilic adducts of glutathione: the nitric oxide adduct (S-nitrosoglutathione [GSNO]) and the formaldehyde adduct (S-hydroxymethylglutathione) (16). Our earlier study led to the hypothesis that the S-nitrosoglutathione reductase activity of AdhC protects the pneumococcus against host-produced nitric oxide (NO) during systemic infection. In the current study we have explored an alternate/additional role for NmlRsp in the response to aldehyde stress and regulation of central carbon metabolism in the pneumococcus.
adhC expression is induced by NmlRsp in the presence of formaldehyde and methylglyoxal.
Given the known role of class III alcohol dehydrogenases in the detoxification of formaldehyde, a possible role for pneumococcal AdhC in formaldehyde metabolism was investigated. Real-time reverse transcription-PCR (RT-PCR) was used to measure the expression of adhC upon treatment of pneumococci with formaldehyde. S. pneumoniae D39 (virulent serotype 2 strain [1]) was grown to early exponential phase in Todd-Hewitt broth supplemented with 0.5% (wt/vol) yeast extract (THY) at 37°C before the culture was divided in half, and 0.5 mM formaldehyde (Sigma) was added to one sample. The cultures were incubated for a further 15 min before cells were collected by centrifugation, and total RNA was extracted using acid phenol-chloroform-isoamyl alcohol (125:24:1) as described elsewhere (12). The extract was precipitated at −80°C overnight in ethanol-sodium acetate and subsequently treated with RNase-free DNase (Promega), to remove DNA contamination. Relative levels of adhC mRNA transcripts were determined in relation to 16S rRNA levels using the SuperScript III Platinum SYBR green One-Step quantitative RT-PCR (qRT-PCR) kit (Invitrogen) and the LightCycler 480 II (Roche). The nucleotide sequences of primers used for real-time RT-PCR can be found in Table 1. adhC expression was found to be approximately 31-fold higher in the sample treated with formaldehyde than in the nontreated sample (Fig. 1A). To determine whether this formaldehyde-dependent induction is mediated by NmlRsp, the experiment was repeated using an S. pneumoniae nmlRsp mutant strain (described in reference 17). adhC was found to be expressed in the sample treated with formaldehyde to a level similar to that in the nontreated sample (Fig. 1A). Thus, induction of adhC expression in the presence of formaldehyde is dependent on the cells expressing an active NmlRsp protein. The results presented in Fig. 1A also indicate that NmlRsp acts as a repressor of adhC expression under standard culture conditions (no formaldehyde), as indicated by the fact that adhC expression is almost 5-fold higher in the nmlRsp strain than in wild-type pneumococci. This is consistent with the unique mode of action of MerR family transcription factors acting as both activators and repressors of transcription (6). Despite the significant transcriptional response of adhC to formaldehyde, neither the S. pneumoniae nmlRsp strain nor the adhC strain (described in reference 17) exhibited an altered growth phenotype upon challenge with formaldehyde, compared with wild-type pneumococci (data not shown). It has recently been shown that the NmlRsp homologue in Bacillus subtilis upregulates the expression of its regulon (including an alcohol dehydrogenase) in response to both formaldehyde and methylglyoxal (10). To test if NmlRsp also induces adhC expression in the presence of methylglyoxal, the above experiment was repeated using 0.01% (vol/vol) methylglyoxal (Sigma). adhC expression was found to be induced approximately 100-fold in the wild-type strain upon treatment with methylglyoxal, while treatment of the nmlRsp strain did not result in differential expression (Fig. 1B). Thus, it seems likely that NmlRsp is a general aldehyde-responsive regulator, as opposed to selectively responding to a particular compound. NmlRsp shares a conserved cysteine residue with its B. subtilis homologue (17), which was shown to be essential for activation of transcription in response to formaldehyde and methylglyoxal (10). This suggests that thiol-(S)-alkylation of this residue by aldehyde compounds may be responsible for NmlRsp-mediated activation of adhC in pneumococci.
TABLE 1.
Primers used for real-time RT-PCR
| Primer name | Nucleotide sequence (5′-3′) |
|---|---|
| 16S-F | GGTGAGTAACGCGTAGGTAA |
| 16S-R | ACGATCCGAAAACCTTCTTC |
| adhC-RT-F | CGGATGATGTGATTATTCGTGT |
| adhC-RT-R | CCTGGTTTCACCGTCGTAATGGC |
| spxB-RT-F | CAACATGTGCTACCCAGACG |
| spxB-RT-R | CGAGCATCGATGACAACAGT |
| lox-RT-F | GCGCCTTTAACCACAAACTC |
| lox-RT-R | AACCAAACTCATGCACACCA |
| carB-RT-F | GTGGTATGTGTGCCAACGAG |
| carB-RT-R | AATGGAATCCCCTGTGTGAA |
FIG. 1.
Real-time quantification of adhC in S. pneumoniae D39 wild-type (wt) and nmlRsp strains grown in the presence or absence (NA) of formaldehyde (+form) (A) and methylglyoxal (+MG) (B). Error bars indicate standard deviations from the means. Experiments were conducted in triplicate.
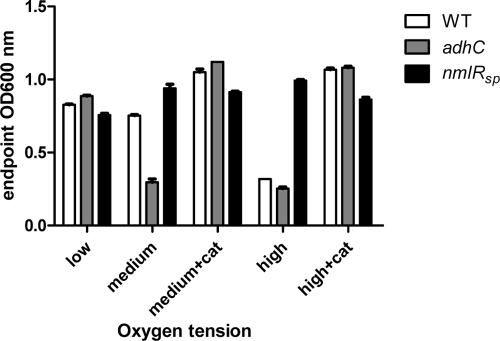
NmlRsp and AdhC have a role the in growth of the pneumococcus under different oxygen tensions.
As was the case with formaldehyde, neither the S. pneumoniae nmlRsp strain nor the adhC strain exhibited an altered growth phenotype upon challenge with methylglyoxal (data not shown). However, an altered growth phenotype for these strains was observed in standard culture medium (THY) during growth at increased oxygen tension. Figure 2 shows endpoint growth measurements of wild-type, adhC, and nmlRsp pneumococcal cultures grown overnight in THY with 0.5% (wt/vol) choline chloride under different oxygen tensions at 37°C. Differential oxygenation was achieved through varying the culture volume-to-tube volume ratio as well as the shaking speed. Low-oxygen cultures were 5-ml cultures in 10-ml tubes incubated without shaking. Medium-oxygen cultures were 5-ml cultures in 50-ml tubes with shaking at 125 rpm. High-oxygen cultures were 5-ml cultures in 50-ml tubes with shaking at 150 rpm. While all strains exhibited similar growth characteristics under low oxygen tension, the adhC strain showed impaired growth under medium oxygen tension compared with the wild-type strain (P < 0.0001). However, growth could be restored completely by supplementing the medium with 6 μg/ml beef liver catalase (Roche), indicating that hydrogen peroxide (H2O2) toxicity is responsible for growth inhibition. Conversely, the nmlRsp strain exhibited increased resistance to aerobic growth as evidenced by the fact that wild-type pneumococci were unable to grow at high oxygen tensions, while growth of the nmlRsp strain was not inhibited (P < 0.0001). This growth difference also appears to be due to H2O2 toxicity, as wild-type growth was restored by supplementing the medium with catalase. Pneumococci are known to produce high levels of H2O2 under aerobic conditions, primarily through the action of pyruvate oxidase (SpxB), which, together with acetate kinase, provides a pathway to increased ATP generation in the presence of oxygen (15). Despite the fact that pneumococci produce high concentrations of H2O2, little is known about the mechanisms employed by the cell to defend against this toxic compound. Pneumococci do not possess catalase or the well-described peroxide-responsive regulators OxyR and PerR (19). To investigate if the different aerobic growth phenotypes of the strains may be due to variation in the levels of H2O2 that accumulates during growth of each strain, H2O2 production assays were performed on each of the strains under low oxygen tension using the horseradish peroxidase/phenol red assay described elsewhere (14). S. pneumoniae wild-type and adhC strains were found to produce identical amounts of H2O2 under these conditions (146 ± 4 μM and 147 ± 10 μM, respectively [P = 0.89]). However, the nmlRsp strain was found to produce approximately 6-fold-less H2O2 (26 ± 1 μM [P < 0.0001]). Thus, the increased resistance of the nmlRsp mutant to growth at high oxygen tension appears to be due to reduced H2O2 production/accumulation in this strain.
FIG. 2.
Growth of S. pneumoniae wild-type (WT), adhC, and nmlRsp strains in THY broth under different oxygen tensions. +cat, exogenous catalase added to cultures. Error bars indicate standard deviations from the mean. Experiments were conducted in triplicate.
Variation in peroxide production is due to downregulation of carB.
To further investigate the low-H2O2-producing phenotype of the nmlRsp strain, the expression of 3 genes known to be associated with H2O2 production in S. pneumoniae was determined using real-time RT-PCR. Pyruvate oxidase (SpxB) is known to be responsible for the majority of the H2O2 produced by pneumococci (15). Lactate oxidase (Lox) has recently been described in the pneumococcus and produces H2O2 as a by-product of converting lactate to pyruvate (18). Carbamoyl phosphate synthase (CarB) also influences H2O2 production, as evidenced by the fact that a carB mutant produces amounts of H2O2 similar to those produced by an spxB mutant (8), although the mechanism by which this occurs is unknown. The expression of spxB, lox, and carB was measured in S. pneumoniae wild-type and nmlRsp strains grown with and without formaldehyde supplementation as described previously. The levels of spxB and lox transcripts were found to be essentially identical in the two strains and under the two growth conditions (Fig. 3A and B). However, the level of carB transcript was found to be approximately 5-fold lower in the nmlRsp strain than in the wild-type strain (Fig. 3C). Treatment of the cultures with formaldehyde resulted in a small (approximately 2-fold) increase in carB expression in both strains. Thus, the low-H2O2-producing phenotype of the nmlRsp strain appears to be due to reduced levels of CarB in this strain. Furthermore, the fact that carB expression was not decreased in wild-type pneumococci in the presence of formaldehyde (conditions that lead to activation of adhC) suggests that differential regulation of carB in the nmlRsp strain is not due to derepression of adhC. Carbamoyl phosphate synthase (composed of 2 subunits; CarAB) catalyzes the reaction of bicarbonate with ATP and glutamine to produce carbamoyl phosphate, glutamic acid, ADP, and inorganic phosphate (Pi) (9). The link between CarAB activity and hydrogen peroxide production in pneumococci is unclear; however, it should be noted that one of the products of the CarAB-catalyzed reaction (Pi) is a required substrate for SpxB. The major product of CarAB activity, carbamoyl phosphate, is a central intermediate in both the pyrimidine and arginine biosynthetic pathways (4). Regulation of carB in S. pneumoniae has not been studied, but in other organisms carB expression has been shown to be influenced by both pyrimidine and arginine availability, as well as the intracellular inorganic carbon concentration (CO2/bicarbonate) (4, 5). Analysis of the carB promoter sequence of S. pneumoniae D39 did not reveal the presence of a typical MerR family regulator binding site; thus, it is expected that NmlRsp mediates differential expression of carB via an indirect mechanism.
FIG. 3.
Real-time quantification of spxB (A), lox (B), and carB (C) in S. pneumoniae D39 wild-type (wt) and nmlRsp strains grown with (+form) or without (−form) formaldehyde. Error bars indicate standard deviations from the mean. Experiments were conducted in triplicate.
Conclusions.
The results presented here indicate that the NmlRsp regulator has a broader function in S. pneumoniae than previously recognized. NmlRsp was shown to induce the expression of the class III alcohol dehydrogenase gene adhC in the presence of both formaldehyde and methylglyoxal, suggesting that it may function as a general aldehyde-responsive regulator. The physiological aldehydic compound for NmlRsp and AdhC in the pneumococcus remains to be elucidated. It seems unlikely that it will be formaldehyde, since pneumococcus does not oxidize methanol, the principal source of this aldehyde. Similarly, S. pneumoniae lacks the methylglyoxal synthase that is found in Escherichia coli and is associated with a response to phosphate limitation (7). However, the requirement for AdhC at medium and high oxygen tension suggests that this enzyme may be required to protect cells against reactive aldehydes and dicarbonyl compounds that can arise from C3 sugars during carbon metabolism (13). In S. pneumoniae, carbon metabolism under aerobic conditions coincides with the production of H2O2 via the action of SpxB. Although pneumococcus lacks catalase, it can protect itself against this oxidant by using glutathione peroxidase (PsaD) (20). However, PsaD is dependent upon a supply of glutathione that is likely to be limiting in pneumococcus, since it cannot synthesize this tripeptide. It follows that the central role of AdhC may actually be to regenerate reduced glutathione following the formation of a glutathione-aldehyde adduct. Loss of the ability to perform this catalytic step results in glutathione limitation and a failure to tolerate hydrogen peroxide. The fact that NmlRsp influences carbamoyl phosphate synthase expression via a mechanism independent of adhC regulation suggests that there may be additional members of the NmlRsp regulon that are yet to be described. Our data are consistent with the view that NmlRsp activates AdhC and modulates gene expression either directly or indirectly to provide protection against hydrogen peroxide stress. We suggest that this functionality is essential for pneumococci to cause invasive disease.
Acknowledgments
This research was funded by program grant 565526 from the National Health and Medical Research Council (NHMRC) of Australia. J.C.P. is an NHMRC Australia Fellow.
Footnotes
Published ahead of print on 4 June 2010.
REFERENCES
- 1.Avery, O. T., C. M. MacLeod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types, induction of transformation by a deoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergeron, Y., N. Ouellet, A. M. Deslauriers, M. Simard, M. Olivier, and M. G. Bergeron. 1998. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect. Immun. 66:912-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogaert, D., R. de Groot, and P. W. M. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144-154. [DOI] [PubMed] [Google Scholar]
- 4.Bringel, F., P. Hammann, V. Kugler, and F. Arsene-Ploetze. 2008. Lactobacillus plantarum response to inorganic carbon concentrations: PyrR(2)-dependent and -independent transcription regulation of genes involved in arginine and nucleotide metabolism. Microbiology 154:2629-2640. [DOI] [PubMed] [Google Scholar]
- 5.Bringel, F., S. Vuilleumier, and F. Arsene-Ploetze. 2008. Low carbamoyl phosphate pools may drive Lactobacillus plantarum CO2-dependent growth phenotype. J. Mol. Microbiol. Biotechnol. 14:22-30. [DOI] [PubMed] [Google Scholar]
- 6.Brown, N. L., J. V. Stoyanov, S. P. Kidd, and J. L. Hobman. 2003. The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 27:145-163. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson, G. P., S. Totemeyer, M. J. MacLean, and I. R. Booth. 1998. Methylglyoxal production in bacteria: suicide or survival? Arch. Microbiol. 170:209-219. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann, O., J. Zweigner, S. H. Smith, D. Freyer, C. Mahrhofer, E. Dagand, E. I. Tuomanen, and J. R. Weber. 2006. Interplay of pneumococcal hydrogen peroxide and host-derived nitric oxide. Infect. Immun. 74:5058-5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holden, H. M., J. B. Thoden, and F. M. Raushel. 1999. Carbamoyl phosphate synthetase: an amazing biochemical odyssey from substrate to product. Cell. Mol. Life Sci. 56:507-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen, T. T., W. Eiamphungporn, U. Mader, M. Liebeke, M. Lalk, M. Hecker, J. D. Helmann, and H. Antelmann. 2009. Genome-wide responses to carbonyl electrophiles in Bacillus subtilis: control of the thiol-dependent formaldehyde dehydrogenase AdhA and cysteine proteinase YraA by the MerR-family regulator YraB (AdhR). Mol. Microbiol. 71:876-894. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien, K. L., L. J. Wolfson, J. P. Watt, E. Henkle, M. Deloria-Knoll, N. McCall, E. Lee, K. Mulholland, O. S. Levine, and T. Cherian. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893-902. [DOI] [PubMed] [Google Scholar]
- 12.Ogunniyi, A. D., P. Giammarinaro, and J. C. Paton. 2002. The genes encoding virulence-associated proteins and the capsule of Streptococcus pneumoniae are upregulated and differentially expressed in vivo. Microbiology 148:2045-2053. [DOI] [PubMed] [Google Scholar]
- 13.Okado-Matsumoto, A., and I. Fridovich. 2000. The role of alpha, beta-dicarbonyl compounds in the toxicity of short chain sugars. J. Biol. Chem. 275:34853-34857. [DOI] [PubMed] [Google Scholar]
- 14.Pesakhov, S., R. Benisty, N. Sikron, Z. Cohen, P. Gomelsky, I. Khozin-Goldberg, R. Dagan, and N. Porat. 2007. Effect of hydrogen peroxide production and the Fenton reaction on membrane composition of Streptococcus pneumoniae. Biochim. Biophys. Acta 1768:590-597. [DOI] [PubMed] [Google Scholar]
- 15.Spellerberg, B., D. R. Cundell, J. Sandros, B. J. Pearce, I. IdanpaanHeikkila, C. Rosenow, and H. R. Masure. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19:803-813. [DOI] [PubMed] [Google Scholar]
- 16.Staab, C. A., M. Hellgren, and J. O. Hoog. 2008. Dual functions of alcohol dehydrogenase 3: implications with focus on formaldehyde dehydrogenase and S-nitrosoglutathione reductase activities. Cell. Mol. Life Sci. 65:3950-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroeher, U. H., S. P. Kidd, S. L. Stafford, M. P. Jennings, J. C. Paton, and A. G. McEwan. 2007. A pneumococcal MerR-like regulator and S-nitrosoglutathione reductase are required for systemic virulence. J. Infect. Dis. 196:1820-1826. [DOI] [PubMed] [Google Scholar]
- 18.Taniai, H., K. Iida, M. Seki, M. Saito, S. Shiota, H. Nakayama, and S. Yoshida. 2008. Concerted action of lactate oxidase and pyruvate oxidase in aerobic growth of Streptococcus pneumoniae: role of lactate as an energy source. J. Bacteriol. 190:3572-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tettelin, H., V. Masignani, M. J. Cieslewicz, J. A. Eisen, S. Peterson, M. R. Wessels, I. T. Paulsen, K. E. Nelson, I. Margarit, T. D. Read, L. C. Madoff, A. M. Wolf, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, R. T. DeBoy, A. S. Durkin, J. F. Kolonay, R. Madupu, M. R. Lewis, D. Radune, N. B. Fedorova, D. Scanlan, H. Khouri, S. Mulligan, H. A. Carty, R. T. Cline, S. E. Van Aken, J. Gill, M. Scarselli, M. Mora, E. T. Iacobini, C. Brettoni, G. Galli, M. Mariani, F. Vegni, D. Maione, D. Rinaudo, R. Rappuoli, J. L. Telford, D. L. Kasper, G. Grandi, and C. M. Fraser. 2002. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc. Natl. Acad. Sci. U. S. A. 99:12391-12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tseng, H. J., A. G. McEwan, J. C. Paton, and M. P. Jennings. 2002. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect. Immun. 70:1635-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]