Abstract
Type 1 secretion systems (T1SS) are present in a wide range of Gram-negative bacteria and are involved in the secretion of diverse substrates such as proteases, lipases, and hemophores. T1SS consist of three proteins: an inner membrane ABC (ATP binding cassette) protein, a periplasmic adaptor, and an outer membrane channel of the TolC family. Assembly of the tripartite complex is transient and induced upon binding of the substrate to the ABC protein. It is generally accepted that T1SS-secreted proteins have a C-terminal secretion signal required for secretion and that this signal interacts with the ABC protein. However, we have previously shown that for the Serratia marcescens hemophore HasA, interactions with the ABC protein and subsequent T1SS assembly require additional regions. In this work, we characterize these regions and demonstrate that they are numerous, distributed throughout the HasA polypeptide, and most likely linear. Together with the C-terminal signal, these elements maximize the secretion of HasA. The data also show that the C-terminal signal of HasA triggers HasD-driven ATP hydrolysis, leading to disassembly of the complex. These data support a model of type 1 secretion involving a multistep interaction between the substrate and the ABC protein that stabilizes the assembled secretion system until the C terminus is presented. This model also supports tight coupling between synthesis and secretion.
The targeting of proteins to their proper ultimate compartments is an essential task of all cell types, which have evolved a variety of trafficking pathways. In particular, Gram-negative bacteria possess several multicomponent secretion pathways to transport proteins to the extracellular medium across the inner membrane, the periplasm, and the outer membrane (15). To date, six different secretion system types have been identified (T1SS [type 1 secretion system] to T6SS). T2SS and T5SS secrete proteins in a two-step process. Substrates are synthesized with a consensus N-terminal signal peptide that first allows targeting to the Sec or Tat translocon to cross the inner membrane (9, 34, 41). The periplasmic intermediates are then transported across the outer membrane. On the other hand, T1SS and T3SS bypass the periplasm and are able to export proteins that lack a cleavable N-terminal signal peptide in a single-step manner (10, 14).
Type 1 secretion is widespread among Gram-negative bacteria and is notable for its apparent simplicity. The T1SS directs the secretion of a wide range of proteins of different sizes and activities (14). These include pore-forming hemolysins (HlyA), adenylate cyclases, lipases, proteases, surface layers, and hemophores (HasA). HasA hemophores are small extracellular proteins produced by several species of Gram-negative bacteria. They scavenge extracellular heme and deliver it to specific outer membrane receptors (16, 42). HasA of Serratia marcescens is secreted by an archetypal T1SS comprising an inner membrane ABC (ATP binding cassette) protein (HasD), a periplasmic adaptor (HasE), and an outer membrane channel-forming protein of the TolC family (HasF) (4, 26). When expressed in Escherichia coli K-12, the hybrid secretion apparatus formed by HasD, HasE, and endogenous TolC allows the secretion of HasA (26).
T1SS components are not permanently associated. Their assembly is initiated by binding of the substrate to the ABC protein (27, 39). The majority of T1SS-secreted substrates carry a secretion signal located at the extreme C terminus (16, 21, 31). In vitro studies have demonstrated that C-terminal fragments of HlyA and Erwinia chrysanthemi metalloproteases were able to interact with their cognate ABC protein independently of the presence of other system components and modulate their ATPase activity (3, 12). No consensus sequence has been identified within the C-terminal signals, except in protein substrates of the same subfamily that can be secreted by the same T1SS (28, 32). Although it is absolutely required for secretion, the role of the C-terminal signal is still poorly understood.
Due to the C-terminal location of the secretion signal, the common model implies that secretion of T1SS-secreted substrates occurs posttranslationally rather than cotranslationally. How the majority of the polypeptides remain in the cytoplasm in a secretion-suitable conformation after complete synthesis is unclear. Secretion of hemophores depends on the presence of the general chaperone SecB (13). SecB holds premade HasA molecules in an unfolded (or loosely folded) conformation compatible with secretion but does not function to deliver HasA to HasD (35, 45). However, we have previously shown that delayed expression of HasDE causes HasA to fold in the cytoplasm, accumulate, and inhibit the secretion of newly synthesized molecules, indicating that synthesis and secretion must be coupled (11).
Our previous work on the hemophore HasA of S. marcescens showed that a nonsecreted mutant form of HasA lacking its last 14 C-terminal amino acids was still able to interact with the ABC protein HasD and induced stable assembly of the HasDE-TolC secretion apparatus (7). E. coli cells producing the secretion components HasD and HasE and the truncated substrate—henceforth referred to as HasA(1-174)—are hypersensitive to antimicrobials. This occurs because HasA(1-174) locks TolC in the T1SS and prevents it from associating with drug efflux proteins (AcrA and AcrB). Such frozen HasDE-TolC complexes are dissociated only when the C-terminal signal of HasA is provided in trans as a distinct peptide. This work demonstrated that the C-terminal signal of HasA was not the unique sequence that bound to the ABC protein. In addition, rather than promoting the association of T1SS components, the C-terminal signal of HasA appeared to be involved in their dissociation. In the present work, we expanded our data and found that a mutant form of HasA(1-174) with folding defects was sufficient to block the T1SS, demonstrating that the sites responsible for binding to HasD are linear rather than conformational. By analyzing the functions of HasA(1-174) insertion mutant forms, we identified multiple regions distributed along HasA(1-174) as additional HasD binding sites. Together with the C-terminal signal, these elements act in concert to promote the maximum secretion of full-length HasA. The HasA C terminus is needed for disassembly of the secretion system, and this step requires an intact Walker B box in HasD for ATP hydrolysis. These findings provide new insights into the molecular picture of the mechanism and assembly of T1SS.
MATERIALS AND METHODS
Bacterial strains and media.
All strains used in this study are derivatives of E. coli MC4100 (6) and are listed in Table 1. Unless otherwise noted, strains were routinely grown at 37°C in Luria broth (LB) or on agar (LBA). M9 minimal medium supplemented with vitamin B1 and glycerol as a carbon source was used to grow the cells for spectroscopic analysis. Antibiotics were added to the growth medium at the following final concentrations: ampicillin (Ap), 100 μg/ml; chloramphenicol (Cm), 25 μg/ml; spectinomycin, 50 μg/ml. Isopropyl-β-d-thiogalactopyranoside (IPTG; 0.1 to 0.5 mM) and arabinose (0.002 to 0.2%) were used to induce protein expression.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| MC4100 | F−araD139 Δ(argF-lac)U139 rpsL150 flbB5301 ptsF25 deoC1 thi-1 rbsR relA | 6 |
| JP313 | MC4100 Δara-174 | Laboratory collection |
| LMD439 | JP313 att λ::hasDE | 11 |
| MC4100 ΔsecB | 35 | |
| DH5α | ||
| Plasmids | ||
| pAM238 | Low-copy-number expression vector; Spcr | Laboratory collection |
| pAM-HasA | Wild-type HasA in pAM238 | 30 |
| pAM-HasAE148A, D167A | 45 | |
| pAM-HasAX | pAM-HasA mutant derivatives with X representing the positions of pentapeptide insertions | 30 |
| pAM-HasA(1-174) | HasA shorn of its 14 C-terminal amino acids in pAM238 | 7 |
| pAM-HasA(133-188) | 56 C-terminal amino acids of HasA in pAM238 | 7 |
| pTrc99A | Expression vector; Apr | Pharmacia |
| pTrc99A-HasA | 11 | |
| pTrc99A-HasA(1-174) | 7 | |
| pTrc99A-HasA(133-188) | 7 | |
| pTrc99A-HasA(1-174)X | This work | |
| pTrc99A-HasA(1-174)E148A, D167A | This work | |
| pTrc99C | Expression vector; Cmr | 11 |
| pTrc99C-HasA | 11 | |
| pTrc99C-HasA(1-174) | 7 | |
| pTrc99C-HasA(133-188) | This work | |
| pTrc99C-HasA(1-174)X | This work | |
| pTrc99C-HasA(1-174)E148A, D167A | This work | |
| pSyc150 | hasDE in pACYC184; Cmr | 26 |
| pAM-HasISRADE | hasISRADE in pAM238 | 17 |
| pBAD24-HisHasDE | 6His-HasD and HasE in pBAD24 | P. Delepelaire, personal communication |
Plasmid constructions.
All plasmids used in this study are listed in Table 1. pAM238-HasA and derivative plasmids carrying HasAE148A, D167A or HasA mutant forms with pentapeptide insertions were described elsewhere (29, 30, 45). hasA(1-174) alleles were amplified by PCR from the appropriate plasmid templates with the forward primer 5′-TTCACCATGGCATTTTCAGTCAATTATGAC-3′ and the reverse primer 5′-CGACTCTAGATCACGCCGTCGCCGCCGCCA-3′. NcoI (introducing a start codon, underlined) and XbaI (introducing a stop codon, underlined) restriction sites were used for cloning of the PCR products under the control of an IPTG-inducible promoter into the plasmid vector pTrc99A (Pharmacia). For construction of pTrc99A plasmids carrying HasA(1-174) with two pentapeptide insertions, plasmids carrying HasA(1-174) with single-pentapeptide insertions were first digested with BstEII. BstEII cuts pTrc99A 855 bp upstream of the hasA start codon and downstream of codon 63 inside hasA. Digestion products containing the 5′ and 3′ portions from two distinct hasA(1-174) alleles were gel purified and religated. pTrc99C is an Aps Cmr derivative of pTrc99A. pTrc99C plasmids coding for HasA(1-174) derivative mutant proteins were constructed as described previously (7).
The Walker B box substitution D474Q in HasD was created by site-directed mutagenesis using plasmid pBAD24-HisHasDE as the template and a QuikChange mutagenesis kit (Stratagene) according to the manufacturer's procedure.
Secretion assays.
Overnight cultures of E. coli strains harboring the appropriate recombinant plasmids were diluted 1:100 into fresh LB supplemented with antibiotics. Cells carrying the defined plasmid combinations were routinely grown to an optical density at 600 nm (OD600) of ∼0.2 before IPTG or arabinose was added to induce the expression of plasmid-borne proteins. When indicated, cells with plasmid pAM-HasISRADE, which carries the complete has operon under the control of its native iron-repressible promoter, were subcultured in fresh medium containing 0.2 mM 2,2′-dipyridyl. After 3 h of induction, aliquots of cultures were centrifuged. Proteins in the supernatants were precipitated with 20% trichloroacetic acid for 30 min at 4°C. The precipitated proteins were collected by centrifugation and washed in 80% acetone. Cell pellets were washed once in 20 mM Tris (pH 8.0)-1 mM EDTA. Cell pellets and precipitated supernatants were resuspended in 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and amounts equivalent to 0.2 OD unit were analyzed by SDS-PAGE and immunoblotting.
Purification of HasA(1-174) and derivatives.
HasA(1-174) and derivative mutant proteins were expressed from recombinant pTrc99A plasmids in E. coli MC4100. The solubility of HasA(1-174) and derivative mutant proteins in the cytoplasm was tested by using gentle solubilization conditions. Five-milliliter cultures were grown in LB to an OD600 of ∼0.2 and induced with 0.4 mM IPTG for 3 h at 37°C. Cells were collected by centrifugation and resuspended in 1 ml BugBuster Protein Extraction Reagent (Novagen)-0.5 μl Benzonase nuclease (Novagen) for 20 min at room temperature. The mixtures were centrifuged for 25 min at 20,000 × g, and supernatants containing the solubilized proteins were saved. Protein purification was performed as described previously (7). Protein concentrations were calculated from the values of absorbance at 280 nm.
Purification of (His)HasD.
One liter of LB supplemented with 0.02% arabinose was inoculated with an overnight culture of E. coli JP313 containing pBAD24-(His)HasD-HasE and incubated at 30°C to an OD600 of ∼1. Cells were resuspended in lysis buffer containing 60 mM Tris-HCl (pH 7.5), 5 mM MgCl2, and traces of DNase I. Bacterial cells were broken by one passage through a French pressure cell at 750 lb/in2. Unlysed cells were removed by low-speed centrifugation (8,000 × g for 20 min), and supernatant containing the whole-cell extract was centrifuged at 100,000 × g for 1 h. Membranes were homogenized in buffer A (60 mM Tris [pH 7.5], 150 mM NaCl, 20% glycerol, 0.7% lauryl maltoside, 20 mM imidazole) and stirred for 1 h at 4°C. Insoluble material was then removed by centrifugation at 10,000 × g for 30 min. Solubilized membrane extracts (10 ml) were incubated with 1 ml of Ni-nitrilotriacetic acid beads (Qiagen) equilibrated with buffer B (60 mM Tris [pH 7.5], 150 mM NaCl, 20% glycerol) containing 0.03% lauryl maltoside and 20 mM imidazole. The mixture was incubated for 1 h at 4°C on a wheel and centrifuged for 5 min at 5,000 × g. The beads were washed three times with buffer B containing 0.03% lauryl maltoside and 20 mM imidazole. 6His-HasD was eluted with buffer B containing 0.03% lauryl maltoside and 250 mM imidazole. Fractions were collected and analyzed by SDS-PAGE.
Dot blot overlay.
Dot blot overlay assays were carried out as previously described (7). Briefly, aliquots of purified (His)HasD were adsorbed on nitrocellulose membranes. Membranes were overlaid without or with purified HasA(1-174) or a derivative mutant protein diluted in 10 ml Tris-buffered saline (TBS) to a final concentration of 5 μM, washed in TBS-Tween, and probed with rabbit polyclonal antibodies directed against HasA.
Proteinase K sensitivity assay.
E. coli JP313 cells carrying pBAD24 with (His)HasD-HasE or (His)HasDE474Q-HasE and pTrc99A with fragments of HasA were grown to an OD600 of ∼0.2 and induced with 0.2% arabinose and 0.4 mM IPTG for 2 h. Proteinase K treatment of whole cells in vivo has been described previously (44). Briefly, cells (5 ml) were gently permeabilized with a buffer containing 20% sucrose, 20 mM Tris-HCl (pH 8.0), 10 mM EDTA, and 0.5% Triton X-100. Whole-cell samples were left untreated or mixed with proteinase K (10 to 50 μg/ml, final concentration) and incubated at 30°C for 10 min. The reaction was stopped with phenylmethylsulfonyl fluoride (PMSF; 1 mM). Samples were immediately mixed with 2× SDS-PAGE loading buffer, boiled, and analyzed by SDS-PAGE and immunoblotting.
SDS sensitivity.
The growth of strains expressing plasmid-borne hasA(1-174) alleles in the LMD439 background was tested by incubating the strains at 37°C for 18 h on LBA plates containing 1% SDS and 0.02% arabinose without or with 0.4 mM IPTG.
Spectroscopy.
Spectroscopic analysis was performed using MC4100 with HasA(1-174) or derivatives expressed from pTrc99A as described previously (11).
Electrophoresis and Western blotting.
Protein samples were analyzed on 11% or 14% polyacrylamide minigels and transferred onto nitrocellulose membranes. Membranes were probed with primary antibodies raised against HasA (1:5,000), TolC (1:1,000), or HasD (1:5,000). Alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G secondary antibodies and an nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (BCIP) mixture (Pierce) were used for detection. For histidine tag detection, membranes were probed with a 1:5,000 dilution of HisProbe-HRP (Pierce) according to the manufacturer's instructions. Protein levels were quantified with ImageQuant software (Amersham).
RESULTS
HasA (1-174) folding is not required for T1SS blocking.
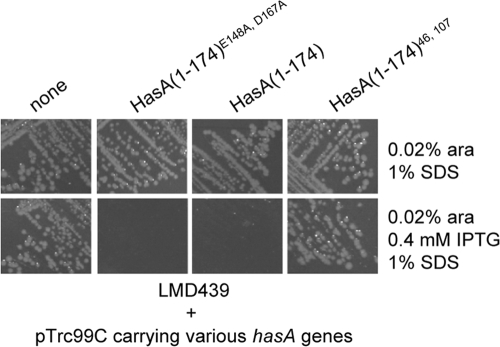
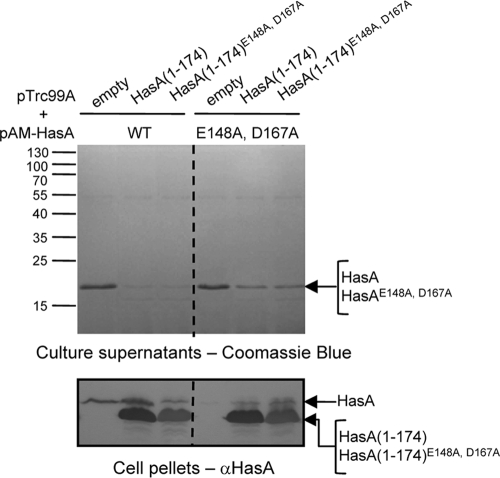
To localize the HasA(1-174) sequences involved in direct interactions with HasD and to determine whether they involve linear or folded regions, we used a mutant form of HasA that carries the C-terminal substitutions E148A and D167A. These mutations were shown to abolish hydrogen bonds with the N-terminal residues Y7 and H17, which normally stabilize the folded structure of HasA (45). Consequently, the double-mutant protein HasAE148A, D167A showed refolding kinetics that were much slower than those of wild-type HasA (45). This double-mutant protein was subsequently shorn of its last 14 amino acids and cloned into pTrc99A under the control of the IPTG-inducible promoter Ptrc. E. coli MC4100 was then transformed with pTrc99A-HasA(1-174) or pTrc99A-HasA(1-174)E148A, D167A, and intracellular folding of the truncated proteins was monitored on the basis of their capacities to bind heme in vivo. The absorption spectrum of cells expressing HasA(1-174) showed a peak—the Soret band—centered at 407 nm (7). In contrast, the Soret band was not observed for cells expressing HasA(1-174)E148A, D167A, indicating that cytoplasmic HasA(1-174), but not HasA(1-174)E148A, D167A, was folded into a conformation compatible with heme loading (see Fig. S1 in the supplemental material). Next, we tested blocking substrate-related phenotypes in different strain backgrounds. Expression of HasA(1-174) or HasA(1-174)E148A, D167A in LMD439 (att λ::hasDE) rendered the cells sensitive to growth in the presence of SDS, suggesting that both truncated substrates were able to enter the secretion pathway and induce the assembly and blocking of HasDE-TolC (Fig. 1). The effect of the blocking substrates on HasA secretion was tested in MC4100 carrying plasmids pSyc150 (HasDE), pAM-HasA, and either pTrc99A-HasA(1-174) or pTrc99A-HasA(1-174)E148A, D167A. HasA(1-174) and HasA(1-174)E148A, D167A strongly inhibited the secretion of wild-type HasA to similar extents. This suggested that folding of HasA(1-174) was compatible with although not essential for the inhibition of type 1 secretion (Fig. 2).
FIG. 1.
Effects of HasA 1-174 mutations on SDS sensitivity. E. coli strain LMD439 (MC4100 Δara att λ::hasDE) was transformed with the control plasmid vector pTrc99C or a derivative recombinant plasmid containing hasA alleles under the control of an IPTG-inducible promoter. Strains were streaked onto LB plates supplemented with SDS or arabinose (ara) with or without IPTG and incubated overnight at 37°C.
FIG. 2.
Effect of HasA(1-174)E148A, D167A on HasA secretion. Cultures of E. coli MC4100 with plasmids pSyc150 (HasDE), pAM-HasA, or pAM-HasAE148A, D167A and pTrc99A-HasA(1-174) or pTrc99A-HasA(1-174)E148A, D167A were grown and induced with 0.5 mM IPTG. After 3 h of induction, culture aliquots were centrifuged. Proteins in the supernatants were precipitated with trichloroacetic acid, separated by 14% SDS-PAGE, and visualized by Coomassie blue staining (top panel). Proteins present in the cell pellets were electrotransferred to a nitrocellulose membrane and immunoblotted with anti-HasA polyclonal antiserum (bottom panel). Loaded samples were normalized to an OD600 of 0.2. The molecular masses of the markers (kilodaltons) are indicated at the left.
Secretion of wild-type HasA is dependent on SecB, which acts to maintain HasA in a mostly unfolded conformation suitable for secretion (13, 45). Owing to its slow folding kinetics, secretion of the double-mutant protein HasAE148A, D167A is not affected in the absence of SecB (45). However, as both wild-type HasA and HasAE148A, D167A interact with SecB in vitro (45), the intracellular accumulation of HasA(1-174)E148A, D167A could titrate SecB, resulting in a decrease in HasA secretion. The inhibitory effects of both HasA(1-174) and HasA(1-174)E148A, D167A were tested on a SecB-independent substrate, HasAE148A, D167A. Although inhibition of HasAE148A, D167A secretion was lower than that of wild-type HasA, the truncated proteins HasA(1-174) and HasA(1-174)E148A, D167A were able to inhibit HasAE148A, D167A secretion to similar extents (Fig. 2). Thus, regions responsible for T1SS blocking are present in unfolded HasA(1-174), suggesting that they are linear rather than conformational.
Identification and in vivo analysis of secondary mutations in HasA(1-174) that relieve T1SS blocking.
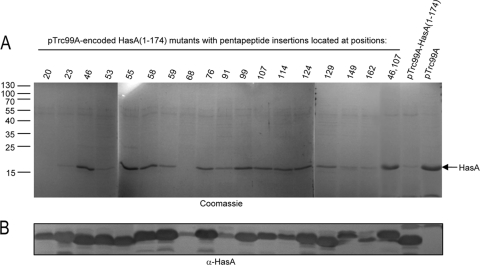
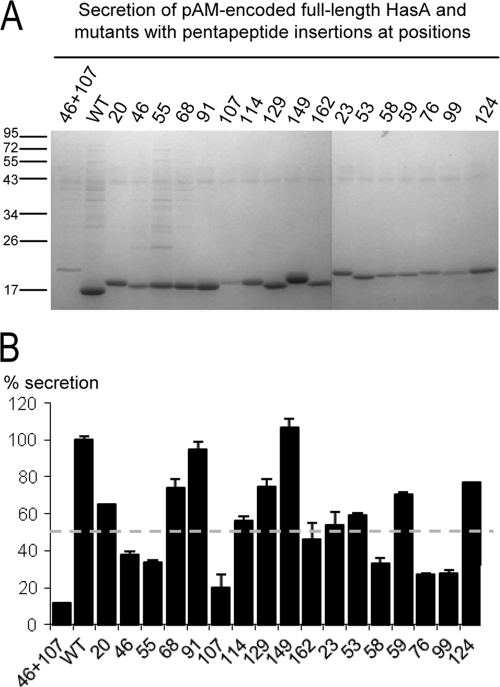
To identify HasA(1-174) sequences required for T1SS blocking, we used a laboratory collection of hasA mutant forms carrying pentapeptide insertions covering the entire hasA sequence (30). To test whether these mutations interrupt sequences that are required for T1SS blocking, 17 hasA alleles were shorn of their 3′ end encoding the 14 C-terminal amino acids and cloned into pTrc99A. The resulting plasmids were expressed in MC4100 to quantify the expression of mutant proteins. Western blot analysis of the soluble fractions showed that 13 mutant proteins (with insertions at positions 20, 23, 46, 53, 55, 58, 59, 76, 99, 107, 124, 129, and 162) have intracellular levels similar to that of the nonmutated HasA(1-174) protein (Fig. 3B). This indicated that these mutant proteins were not subjected to proteolytic degradation or aggregation in the cytoplasm. The mutant plasmids were used to transform MC4100(pAM-HasISRADE) and tested for inhibition of wild-type HasA secretion as shown in Fig. 2. Among these, seven pentapeptide mutant forms with insertions at positions 46, 55, 58, 76, 99, 107, and 124 blocked HasA secretion less efficiently than HasA(1-174), although they were expressed to a similar extent. In such mutant proteins, the HasA secretion level reached at least 50% of that observed in the absence of HasA(1-174) (Fig. 3A).
FIG. 3.
Effects of HasA(1-174) mutant forms carrying pentapeptide insertions on wild-type HasA secretion. (A) Wild-type HasA was expressed from pAM-HasISRADE; HasA(1-174) and derivative mutant proteins carrying pentapeptide insertions at various positions were under the control the IPTG-inducible promoter Ptrc in pTrc99A. Cultures of E. coli strain MC4100 were first induced for type 1 secretion with 0.2 mM 2,2′-dipyridyl for 1 h. Cultures were then grown in the presence of 0.5 mM IPTG for 3 h to induce the expression of mutant HasA(1-174) proteins. Following centrifugation of culture aliquots, proteins in the supernatants were precipitated with trichloroacetic acid, separated by SDS-PAGE, and visualized by Coomassie blue staining. (B) Cultures of MC4100 with pTrc99A-HasA(1-174) were induced with IPTG. Cells were harvested by centrifugation, followed by suspension in BugBuster reagent at room temperature and Benzonase treatment according to the manufacturer's instructions. Insoluble proteins and cell debris were removed by centrifugation. Proteins released in the soluble fractions were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and detected with anti-HasA polyclonal antiserum. Loaded samples were normalized to an OD600 of 0.2. HasA(1-174) and HasA mutant proteins showed different migrations on SDS-PAGE due to the nature of the inserted amino acids, as previously reported (31). The molecular masses of the markers (kilodaltons) are indicated at the left.
The seven mutant forms of HasA(1-174) that enabled some secretion of wild-type HasA were expressed in LMD439 and tested for their effects on SDS sensitivity. None of them allowed cell growth on SDS selective medium (data not shown). Thus, single-pentapeptide insertions were still able to impose some T1SS blockade, suggesting that HasA(1-174) interacts with components of the T1SS via multiple discrete motifs. To further test this hypothesis, we constructed a set of HasA(1-174) mutant forms carrying two pentapeptide insertions, of which two—carrying pentapeptide insertions at positions 46 and 99 and positions 46 and 107—were stable (Fig. 3B and data not shown). Expression of the double-mutant protein HasA(1-174)46, 107 in MC4100(pAM-HasISRADE) did not reduce HasA secretion (Fig. 3A). In addition, when expressed in LMD439, HasA(1-174)46, 107 did not lead to SDS sensitivity (Fig. 1). Similar results were obtained with HasA(1-174)46, 99 (data not shown). Thus, the combination of two mutations had an additive effect and abolished the T1SS-blocking properties of HasA(1-174).
HasA(1-174)-HasD interactions.
The observation that HasA(1-174)46, 107 was unable to block T1SS could result from the loss of its capacity to interact with HasD. To test this hypothesis, (His)HasD containing a six-histidine tag at its N terminus was purified by affinity chromatography under native conditions (see Materials and Methods and Fig. S2 in the supplemental material) and aliquots of purified protein were dotted onto nitrocellulose membranes. Membranes were then incubated with equal amounts of purified HasA(1-174) or a derivative mutant protein and probed with polyclonal anti-HasA antibodies (Fig. 4). Both HasD-HasA(1-174) and HasD-HasA(1-174)E148A, D167A complexes were detected, indicating successful interaction of HasD with both the folded and unfolded forms of HasA(1-174) (Fig. 4). Derivative mutant proteins with single-pentapeptide insertions HasA(1-174)46 and HasA(1-174)107 showed a binding capacity similar to that of HasA(1-174) (Fig. 4). In contrast, binding was undetectable when HasA(1-174)46, 107 was used in the overlay (Fig. 4). Thus, the HasA-HasD interaction involves multiple sites on HasA(1-174). These sites are subsequently referred to as primary recognition sites.
FIG. 4.
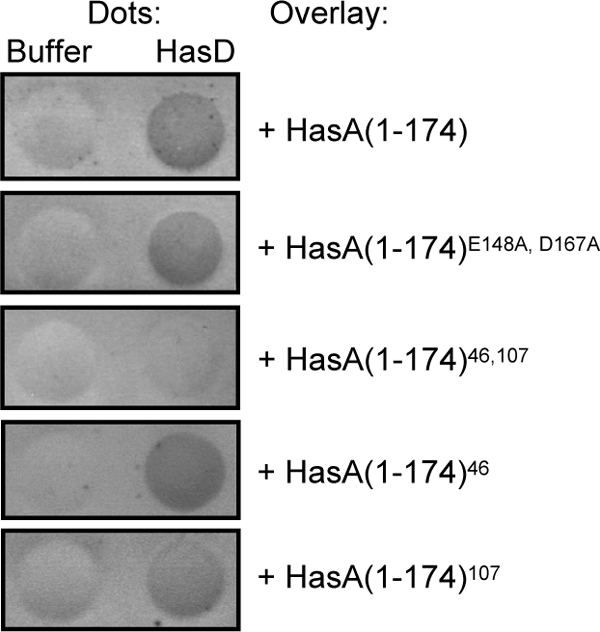
Dot blot overlay analysis of the interactions between HasD and HasA(1-174) mutant proteins. His-tagged HasD was overexpressed and purified by affinity chromatography as described in Materials and Methods. HasA(1-174) and derivative mutant proteins were purified by anion-exchange chromatography, followed by gel filtration. Dots of (His)HasD were incubated with HasA(1-174) or a derivative mutant protein for 1 h. After extensive washing, the HasD-HasA(1-174) complexes were detected with a rabbit anti-HasA polyclonal antiserum used at a dilution of 1:5,000. Note that the data generated are qualitative and not quantitative. Each assay was repeated three times.
Respective roles of the primary recognition sites and the C-terminal signal of HasA in T1SS assembly dynamics.
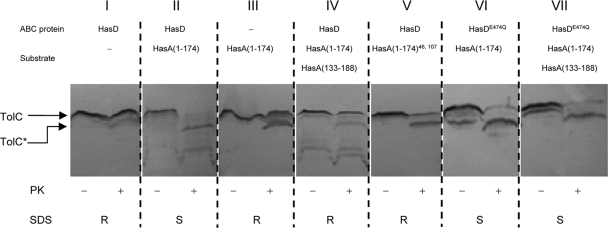
HasA(1-174) was shown to induce the assembly of HasDE-TolC in vivo. This was observed by copurification of HasE and TolC via (His)HasD using nickel affinity chromatography (7). In order to investigate the recruitment of TolC in the presence of different HasA mutant forms, we monitored concomitant conformational changes in TolC by assaying its susceptibility to proteinase K. Previous studies have shown that the C-terminal end of the 471-residue mature TolC protein is accessible from the periplasm and that treatment of permeabilized cells with proteinase K typically generates a stable membrane-bound N-terminal fragment (residues 1 to 451 of the mature TolC protein) of 46 kDa (TolC* in Fig. 5) (24, 44). Figure 5 shows that TolC displays a typical pattern of proteinase K susceptibility upon the treatment of cells expressing all three T1SS components in the absence of substrate (Fig. 5I). In contrast, TolC was completely degraded in cells expressing HasD, HasE, and HasA(1-174) (Fig. 5II). TolC is clearly more accessible to proteolytic cleavage when it is recruited into the tripartite complex. Thus, the susceptibility of TolC to proteinase K reflects the status of T1SS assembly. A control experiment confirmed that the substrate-induced hypersusceptibility of TolC to protease was dependent on the presence of HasD and HasE (Fig. 5III). TolC also displayed a typical pattern of proteolytic degradation in cells expressing HasA(1-174)46, 107, showing that a mutant substrate unable to interact with HasD cannot promote system assembly (Fig. 5V).
FIG. 5.
Accessibility of TolC to proteinase K reflects its recruitment to T1SS. The proteinase K (PK) accessibility of TolC was assessed on permeabilized cells expressing combinations of T1SS components and substrates as indicated for each panel. Cultures (5 ml) were permeabilized and treated with protease at 10 μg/ml for 10 min at 30°C. Protease treatment was terminated with 0.1 mM PMSF. Proteins were resolved by 11% SDS-PAGE, electrotransferred onto a nitrocellulose membrane, and detected with an anti-TolC polyclonal antiserum. TolC and TolC*, which corresponds to the 46-kDa degradation product generated by treatment with proteinase K, are indicated. The same strains were streak onto SDS-supplemented plates and incubated overnight at 37°C. Growth was recorded as follows: R, SDS resistant; S, SDS sensitive.
Lastly, a 6-kDa C-terminal fragment of HasA containing the C-terminal signal—HasA(133-188)—also suppressed TolC hypersusceptibility to protease in cells expressing HasDE and HasA(1-174) (Fig. 5IV). Expression of this peptide does not entirely restore complete proteinase K resistance, most likely because TolC alternates between two states of sensitivity to proteinase K that correspond to its dynamic association with and dissociation from HasDE.
Previous results showed that HasA(133-188) suppressed the SDS sensitivity of strain LMD439 expressing HasA(1-174) (7). Together, these data confirm that the C-terminal fragment of HasA can promote the release of TolC from HasDE.
Role of ATP hydrolysis in HasDE-TolC association-dissociation.
Previous studies have demonstrated that ATP hydrolysis by the ABC protein is essential for type 1 secretion but not for assembly of the secretion system (23, 39). To examine whether ATP hydrolysis is required for disassembly of the secretion system, we used the mutant protein HasDE474Q, in which the catalytic glutamate that follows the Walker B motif was substituted. As monitored by protease susceptibility, TolC was still recruited in strains expressing HasDE474Q and HasA(1-174) (Fig. 5VI). Moreover, this occurred even in the presence of HasA(133-188) (Fig. 5VII). Accordingly, the same strains exhibited sensitivity to SDS, indicating stable interactions among the three secretion proteins HasD, HasE, and TolC (Fig. 5). Our data support the notion that the Walker B substitution in HasD does not affect system assembly. Instead, it interferes with the function of the HasA C terminus as an inducer of TolC dissociation.
The results presented above indicate two types of interactions between HasA and HasD. (i) Interaction with the primary recognition sites initiates T1SS assembly, and (ii) interaction with the C terminus signals complex dissociation. The C-terminal signal is essential for secretion, yet previous studies have shown that several N-terminally deleted mutant forms of HasA were secreted with variable efficiency, raising the question of the role of the primary recognition sites in secretion (16).
Role of the primary recognition sites in HasA secretion.
We assumed that pentapeptide insertions in HasA(1-174) which affected the blocking phenotype may also influence the recognition of full-length HasA by the secretion apparatus and its subsequent secretion. We compared the level of secretion of HasA with that of derivative mutant proteins carrying single- and double-pentapeptide insertions in strain LM439 (Fig. 6A). An arbitrary threshold was set at ≥50% reduction of secretion (Fig. 6B). Consistent with the above findings, six of the seven previously identified single-pentapeptide insertions significantly reduced the secretion level of HasA—namely, those located at positions 46, 55, 58, 76, 99, and 107. The presence of the two pentapeptide insertions at positions 46 and 107 resulted in a strong reduction in secretion of only ∼15% relative to the wild-type protein. Therefore, the behavior of HasA mutant forms in this experiment indicated that primary recognition sites responsible for the interaction with HasD and subsequent T1SS assembly also improved secretion efficiency.
FIG. 6.
Secretion of full-length mutant HasA proteins with pentapeptide insertions. (A) Cells of LMD439 (att λ::hasDE) expressing a pAM-encoded wild-type (WT) or mutant form of HasA were induced for type 1 secretion with 0.02% arabinose until the late exponential phase. Following centrifugation of culture aliquots, proteins in the supernatants were precipitated with trichloroacetic acid, separated by SDS-PAGE, and visualized by Coomassie blue staining. The molecular masses of the markers (kilodaltons) are indicated at the left. (B) The levels of secretion were quantified by densitometry analysis. The results generated are based on three independent experiments and are expressed in percentages of the wild-type HasA level of secretion (100%). The dashed line indicates the arbitrary threshold of 50% reduction of secretion. Note that in the absence of arabinose, the expression levels of HasA and the mutant proteins retained in the cytoplasm are comparable (data not shown).
DISCUSSION
T1SS are widespread in Gram-negative bacteria and secrete a range of proteins with various lengths and functions (14). To date, one of the central questions is how these proteins are recognized as substrates by their cognate machineries. A secretion signal resides within the extreme C terminus of the substrates. However, previous studies pointed out that additional domains must be involved in the interaction with the secretion complex. First, efficient secretion of heterologous proteins fused to E. coli HlyA or E. chrysanthemi PrtB requires C-terminal fragments much larger than the minimal secretion signals (20, 25, 33). Second, hemophore HasA(1-174) without its C-terminal signal retains its capacity to interact with HasD and induce the assembly of the tripartite complex (7). In an effort to understand how proteins are recognized as substrates for T1SS and how they regulate T1SS assembly dynamics, we focused on HasADE-TolC as a working model. These questions were addressed by analyzing the interactions of the blocking substrate HasA(1-174) and its variants with the secretion machinery. Several combined approaches were used, and the blocking activity of HasA(1-174) was defined along with four criteria: (i) inhibition of wild-type HasA secretion, (ii) SDS sensitivity associated with TolC trapping in the protein secretion complex, (iii) TolC accessibility to proteinase K that correlates with its recruitment into the secretion complex, and (iv) in vitro interactions between HasD and HasA variants and derived peptides.
Heme binding capacity was used to monitor the in vivo folding of HasA(1-174) mutant forms. Accordingly, it was concluded that cytoplasmic HasA(1-174) acquired its tertiary structure whereas the amino acid substitutions E148A and D167A affected the cytoplasmic folding of HasA(1-174), as was also the case for the full-length HasAE148A, D167A mutant protein (45). HasA(1-174)E148A, D167A showed secretion machinery-blocking activity similar to that of HasA(1-174). This strongly suggested that the motifs responsible for the interaction between HasA(1-174) and HasD are linear rather than conformational. However, we cannot exclude the possibility that cytoplasmic HasA(1-174)E148A, D167A acquires folded secondary structures that are sufficient to block the secretion machinery.
Along with the four criteria established above, we identified recognition sites responsible for the blocking activity of HasA(1-174). The data obtained led us to the conclusion that insertion of pentapeptides interrupted some sequences that are required for direct interaction with HasD. Another explanation could be that these insertions produced slight structural changes in HasA(1-174) so that one or several key regions are less well presented to HasD. These regions are scattered throughout its primary sequence and do not correspond to either specific conserved or repeated motifs (data not shown). In addition, when reported in the three-dimensional structure of HasA (2), none of the seven insertions were clustered to form a conformational motif.
The finding that HasA contains multiple sites that recognize HasD was quite unexpected and raises several questions. Do these sites contribute to HasD binding concurrently or successively during secretion? What are their relative contributions? Both in vivo and in vitro results showed that only mutant forms of HasA(1-174) carrying two insertions had lost their interaction with HasD. In vitro assays do not reflect the dynamics of HasA-HasD interactions, as multiple motifs within HasA might act synergistically to promote efficient interaction with HasD. In vivo, these sites might rather interact sequentially with HasD. Preliminary results from our laboratory indicate that a mutant form of E. coli HlyA without its 60 C-terminal amino acids that constitute the minimal C-terminal signal cannot be secreted and induces SDS sensitivity. This indicates that HlyA also contains regions located outside its C-terminal signal that are probably able to recognize HlyB and induce blocking of the HlyBD-TolC system (our unpublished data). Thus, the presence of multiple binding sites might be a general characteristic of T1SS. Together, our data are consistent with the common view that HasA and other T1SS-secreted substrates are transported in an unfolded or loosely folded state.
The notion that secreted proteins require multiple noncontiguous secretion signals (or sequences required for secretion) is not new and first emerged for type 3 secretion. Strikingly, one feature of both T1SS and T3SS is the lack of a single clearly conserved signal sequence to direct substrate proteins for secretion. Although the model of type 3 secretion is still making progress, the N terminus of the secreted proteins is thought to contain a secretion signal (10, 36, 37). In addition, while many of the T3SS-secreted substrates are also known to possess specific chaperones that are required for their secretion (8, 36, 43), some are not but possess two secretion signals (1, 5). Similarly, T1SS-secreted substrates usually do not have specific chaperones. In the particular case of HasA, SecB only has an antifolding function (13, 45), excluding the possibility of a chaperone-dependent secretion signal. Here, the primary recognition sites ensure efficient interaction between HasA and HasD, which then triggers the recruitment of TolC. Thus, they play an important role in the efficiency of HasA secretion. On the other hand, several lines of evidence indicate that the C-terminal signal induces the dissociation of TolC. First, a fragment of HasA corresponding to its 56 C-terminal amino acids suppresses the SDS sensitivity of strains expressing HasDE-TolC and HasA(1-174), suggesting that it induces the dissociation of TolC (7). Second, as monitored in limited proteolysis assays, TolC reverts to its unengaged resting state in the presence of the HasA C terminus.
Genetic and biochemical data showed that type 1 secretion requires ATP hydrolysis by the ABC protein (22, 23). As the C-terminal signal binds to HasD (unpublished data), perhaps directly to the nucleotide binding domain (NBD), it is tempting to speculate that it also regulates ATP hydrolysis. The system containing HasDE474Q cannot release TolC, suggesting that the energy of ATP hydrolysis could be used to facilitate disassembly of the system and release of the translocating substrate. Molecular events coupled to substrate transport have been examined in detail for various other ABC proteins. The LolCDE complex catalyzes the release of lipoproteins from the inner membrane of E. coli in an ATP-dependent manner, leading to the formation of a complex between a lipoprotein substrate and the periplasmic chaperone LolA (40). Recent results showed that ATP binding and hydrolysis weaken the interaction between LolCDE and lipoproteins, thereby causing dissociation of substrates from LolCDE in a detergent solution (19, 38). For other ABC proteins, the ATP switch model proposes that ATP binding and ATP hydrolysis induce the formation and dissociation of the NBD dimer, respectively (18).
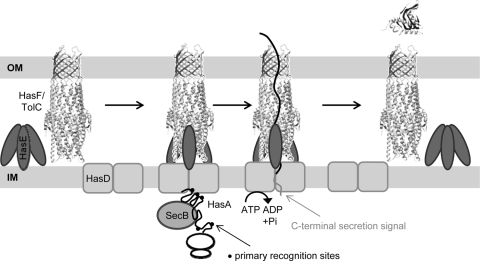
Our work indicates two modes of interaction between HasA and HasD: (i) primary interactions via discrete linear motifs that promote anchoring to HasD and recruitment of TolC and (ii) secondary interactions via the C-terminal signal that induce ATP hydrolysis and dissociation of TolC. Together with previous studies in that field, we propose a model for the secretion of HasA (Fig. 7). First, the nascent polypeptide interacts with SecB, which is only needed for its antifolding activity (13, 35, 45). Then, HasA binds to HasD via primary recognition sites. These interactions also complete the formation of the transmembrane machine with recruitment of TolC. As synthesis of HasA without binding to HasD would prevent its subsequent secretion, this step could be cotranslational (11). Such tight coupling between synthesis and secretion would enable sequential interaction between unfolded HasA and HasD. Finally, substrate translocation ends with the presentation of the C-terminal releasing signal. Its specific interaction with HasD NBD induces ATP hydrolysis and dissociation of TolC for the next cycle.
FIG. 7.
Steps of the type I secretion pathway. The model shown focuses on the functions of different regions of HasA in the assembly dynamics of the tripartite TolC-HasD-HasE system. (Step 1) Outer membrane (OM) TolC is recruited to HasDE only when HasA interacts with the ABC protein HasD (IM, inner membrane). These primary interactions between HasD and newly synthesized HasA are driven by linear sites which are sequentially exposed on the unfolded molecules during or soon after protein translation. (Step 2) The secondary interaction between HasD and HasA via C-terminal signaling induces ATP hydrolysis and separation of TolC to its preengagement state.
Supplementary Material
Acknowledgments
We thank all members of the C.W. lab, particularly Philippe Delepelaire, for several insightful discussions. We are grateful to Elie Dassa, Laurent Debarbieux, and Jeffrey Mellin for critically reading the manuscript and to Sandra Cescau for constructing some strains used in this study.
M.M. was supported by the Institut Pasteur Paris (Roux fellowship).
Footnotes
Published ahead of print on 23 April 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Anderson, D. M., and O. Schneewind. 1997. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science 278:1140-1143. [DOI] [PubMed] [Google Scholar]
- 2.Arnoux, P., R. Haser, N. Izadi, A. Lecroisey, M. Delepierre, C. Wandersman, and M. Czjzek. 1999. The crystal structure of HasA, a hemophore secreted by Serratia marcescens. Nat. Struct. Biol. 6:516-520. [DOI] [PubMed] [Google Scholar]
- 3.Benabdelhak, H., S. Kiontke, C. Horn, R. Ernst, M. A. Blight, I. B. Holland, and L. Schmitt. 2003. A specific interaction between the NBD of the ABC-transporter HlyB and a C-terminal fragment of its transport substrate haemolysin A. J. Mol. Biol. 327:1169-1179. [DOI] [PubMed] [Google Scholar]
- 4.Binet, R., and C. Wandersman. 1996. Cloning of the Serratia marcescens hasF gene encoding the Has ABC exporter outer membrane component: a TolC analogue. Mol. Microbiol. 22:265-273. [DOI] [PubMed] [Google Scholar]
- 5.Blaylock, B., J. A. Sorg, and O. Schneewind. 2008. Yersinia enterocolitica type III secretion of YopR requires a structure in its mRNA. Mol. Microbiol. 70:1210-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541-555. [DOI] [PubMed] [Google Scholar]
- 7.Cescau, S., L. Debarbieux, and C. Wandersman. 2007. Probing the in vivo dynamics of type I protein secretion complex association through sensitivity to detergents. J. Bacteriol. 189:1496-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, L. W., D. M. Anderson, and O. Schneewind. 1997. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol. Microbiol. 24:757-765. [DOI] [PubMed] [Google Scholar]
- 9.Chevalier, N., M. Moser, H. G. Koch, K. L. Schimz, E. Willery, C. Locht, F. Jacob-Dubuisson, and M. Muller. 2004. Membrane targeting of a bacterial virulence factor harbouring an extended signal peptide. J. Mol. Microbiol. Biotechnol. 8:7-18. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 11.Debarbieux, L., and C. Wandersman. 2001. Folded HasA inhibits its own secretion through its ABC exporter. EMBO J. 20:4657-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delepelaire, P. 1994. PrtD, the integral membrane ATP-binding cassette component of the Erwinia chrysanthemi metalloprotease secretion system, exhibits a secretion signal-regulated ATPase activity. J. Biol. Chem. 269:27952-27957. [PubMed] [Google Scholar]
- 13.Delepelaire, P., and C. Wandersman. 1998. The SecB chaperone is involved in the secretion of the Serratia marcescens HasA protein through an ABC transporter. EMBO J. 17:936-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delepelaire, P. 2004. Type I secretion in gram-negative bacteria. Biochim. Biophys. Acta 1694:149-161. [DOI] [PubMed] [Google Scholar]
- 15.Economou, A., P. J. Christie, R. C. Fernandez, T. Palmer, G. V. Plano, and A. P. Pugsley. 2006. Secretion by numbers: protein traffic in prokaryotes. Mol. Microbiol. 62:308-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghigo, J. M., and C. Wandersman. 1994. A carboxyl-terminal four-amino acid motif is required for secretion of the metalloprotease PrtG through the Erwinia chrysanthemi protease secretion pathway. J. Biol. Chem. 269:8979-8985. [PubMed] [Google Scholar]
- 17.Ghigo, J. M., S. Létoffé, and C. Wandersman. 1997. A new type of hemophore-dependent heme acquisition system of Serratia marcescens reconstituted in Escherichia coli. J. Bacteriol. 179:3572-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins, C. F., and K. J. Linton. 2004. The ATP switch model for ABC transporters. Nat. Struct. Mol. Biol. 11:918-926. [DOI] [PubMed] [Google Scholar]
- 19.Ito, Y., K. Kanamaru, N. Taniguchi, S. Miyamoto, and H. Tokuda. 2006. A novel ligand bound ABC transporter, LolCDE, provides insights into the molecular mechanisms underlying membrane detachment of bacterial lipoproteins. Mol. Microbiol. 62:1064-1075. [DOI] [PubMed] [Google Scholar]
- 20.Kenny, B., R. Haigh, and I. B. Holland. 1991. Analysis of the haemolysin transport process through the secretion from Escherichia coli of PCM, CAT or beta-galactosidase fused to the Hly C-terminal signal domain. Mol. Microbiol. 5:2557-2568. [DOI] [PubMed] [Google Scholar]
- 21.Koronakis, V., E. Koronakis, and C. Hughes. 1989. Isolation and analysis of the C-terminal signal directing export of Escherichia coli hemolysin protein across both bacterial membranes. EMBO J. 8:595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koronakis, V., C. Hughes, and E. Koronakis. 1991. Energetically distinct early and late stages of HlyB/HlyD-dependent secretion across both Escherichia coli membranes. EMBO J. 10:3263-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koronakis, E., C. Hughes, I. Milisav, and V. Koronakis. 1995. Protein exporter function and in vitro ATPase activity are correlated in ABC-domain mutants of HlyB. Mol. Microbiol. 16:87-96. [DOI] [PubMed] [Google Scholar]
- 24.Koronakis, V., J. Li, E. Koronakis, and K. Stauffer. 1997. Structure of TolC, the outer membrane component of the bacterial type I efflux system, derived from two-dimensional crystals. Mol. Microbiol. 23:617-626. [DOI] [PubMed] [Google Scholar]
- 25.Létoffé, S., and C. Wandersman. 1992. Secretion of CyaA-PrtB and HlyA-PrtB fusion proteins in Escherichia coli: involvement of the glycine-rich repeat domain of Erwinia chrysanthemi protease B. J. Bacteriol. 174:4920-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Létoffé, S., J. M. Ghigo, and C. Wandersman. 1994. Secretion of the Serratia marcescens HasA protein by an ABC transporter. J. Bacteriol. 176:5372-5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Létoffé, S., P. Delepelaire, and C. Wandersman. 1996. Protein secretion in gram-negative bacteria: assembly of the three components of ABC protein-mediated exporters is ordered and promoted by substrate binding. EMBO J. 15:5804-5811. [PMC free article] [PubMed] [Google Scholar]
- 28.Létoffé, S., K. Omori, and C. Wandersman. 2000. Functional characterization of the HasA(PF) hemophore and its truncated and chimeric variants: determination of a region involved in binding to the hemophore receptor. J. Bacteriol. 182:4401-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Létoffé, S., C. Deniau, N. Wolff, E. Dassa, P. Delepelaire, A. Lecroisey, and C. Wandersman. 2001. Haemophore-mediated bacterial haem transport: evidence for a common or overlapping site for haem-free and haem-loaded haemophore on its specific outer membrane receptor. Mol. Microbiol. 41:439-450. [DOI] [PubMed] [Google Scholar]
- 30.Létoffé, S., L. Debarbieux, N. Izadi, P. Delepelaire, and C. Wandersman. 2003. Ligand delivery by haem carrier proteins: the binding of Serratia marcescens haemophore to its outer membrane receptor is mediated by two distinct peptide regions. Mol. Microbiol. 50:77-88. [DOI] [PubMed] [Google Scholar]
- 31.Nicaud, J. M., N. Mackman, L. Gray, and I. B. Holland. 1986. The C-terminal, 23 kDa peptide of E. coli haemolysin 2001 contains all the information necessary for its secretion by the haemolysin (Hly) export machinery. FEBS Lett. 204:331-335. [DOI] [PubMed] [Google Scholar]
- 32.Omori, K., A. Idei, and H. Akatsuka. 2001. Serratia ATP-binding cassette protein exporter, Lip, recognizes a protein region upstream of the C terminus for specific secretion. J. Biol. Chem. 276:27111-27119. [DOI] [PubMed] [Google Scholar]
- 33.Palacios, J. L., I. Zaror, P. Martinez, F. Uribe, P. Opazo, T. Socias, M. Gidekel, and A. Venegas. 2001. Subset of hybrid eukaryotic proteins is exported by the type I secretion system of Erwinia chrysanthemi. J. Bacteriol. 183:1346-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pugsley, A. P., M. G. Kornacker, and I. Poquet. 1991. The general protein-export pathway is directly required for extracellular pullulanase secretion in Escherichia coli K12. Mol. Microbiol. 5:343-352. [DOI] [PubMed] [Google Scholar]
- 35.Sapriel, G., C. Wandersman, and P. Delepelaire. 2003. The SecB chaperone is bifunctional in Serratia marcescens: SecB is involved in the Sec pathway and required for HasA secretion by the ABC transporter. J. Bacteriol. 185:80-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schesser, K., E. Frithz-Lindsten, and H. Wolf-Watz. 1996. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J. Bacteriol. 178:7227-7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sory, M. P., A. Boland, I. Lambermont, and G. R. Cornelis. 1995. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl. Acad. Sci. U. S. A. 92:11998-12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taniguchi, N., and H. Tokuda. 2008. Molecular events involved in a single cycle of ligand transfer from an ATP binding cassette transporter, LolCDE, to a molecular chaperone, LolA. J. Biol. Chem. 283:8538-8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thanabalu, T., E. Koronakis, C. Hughes, and V. Koronakis. 1998. Substrate-induced assembly of a contiguous channel for protein export from E. coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J. 17:6487-6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tokuda, H., and S. Matsuyama. 2004. Sorting of lipoproteins to the outer membrane in E. coli. Biochim. Biophys. Acta 1693:5-13. [DOI] [PubMed] [Google Scholar]
- 41.Voulhoux, R., G. Ball, B. Ize, M. L. Vasil, A. Lazdunski, L. F. Wu, and A. Filloux. 2001. Involvement of the twin-arginine translocation system in protein secretion via the type II pathway. EMBO J. 20:6735-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wandersman, C., and P. Delepelaire. 2004. Bacterial iron sources: from siderophores to hemophores. Annu. Rev. Microbiol. 58:611-647. [DOI] [PubMed] [Google Scholar]
- 43.Wattiau, P., B. Bernier, P. Deslee, T. Michiels, and G. R. Cornelis. 1994. Individual chaperones required for Yop secretion by Yersinia. Proc. Natl. Acad. Sci. U. S. A. 91:10493-10497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werner, J., A. M. Augustus, and R. Misra. 2003. Assembly of TolC, a structurally unique and multifunctional outer membrane protein of Escherichia coli K-12. J. Bacteriol. 185:6540-6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolff, N., G. Sapriel, C. Bodenreider, A. Chaffotte, and P. Delepelaire. 2003. Antifolding activity of the SecB chaperone is essential for secretion of HasA, a quickly folding ABC pathway substrate. J. Biol. Chem. 278:38247-38253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.