Abstract
The respiratory tract pathogen Streptococcus pneumoniae needs to adapt to the different levels of carbon dioxide (CO2) it encounters during transmission, colonization, and infection. Since CO2 is important for various cellular processes, factors that allow optimal CO2 sequestering are likely to be important for pneumococcal growth and survival. In this study, we showed that the putative pneumococcal carbonic anhydrase (PCA) is essential for in vitro growth of S. pneumoniae under the CO2-poor conditions found in environmental ambient air. Enzymatic analysis showed that PCA catalyzes the reversible hydration of CO2 to bicarbonate (HCO3−), an essential step to prevent the cellular release of CO2. The addition of unsaturated fatty acids (UFAs) reversed the CO2-dependent in vitro growth inhibition of S. pneumoniae strains lacking the pca gene (Δpca), indicating that PCA-mediated CO2 fixation is at least associated with HCO3−-dependent de novo biosynthesis of UFAs. Besides being necessary for growth in environmental ambient conditions, PCA-mediated CO2 fixation pathways appear to be required for intracellular survival in host cells. This effect was especially pronounced during invasion of human brain microvascular endothelial cells (HBMEC) and uptake by murine J774 macrophage cells but not during interaction of S. pneumoniae with Detroit 562 pharyngeal epithelial cells. Finally, the highly conserved pca gene was found to be invariably present in both CO2-independent and naturally circulating CO2-dependent strains, suggesting a conserved essential role for PCA and PCA-mediated CO2 fixation pathways for pneumococcal growth and survival.
The Gram-positive bacterium Streptococcus pneumoniae, or pneumococcus, is a human respiratory tract pathogen that contributes significantly to global mortality and morbidity. In addition, it is an important asymptomatic colonizer of the human nasopharynx, with carriage rates around 10% in adults and over 40% in children (6). Pneumococcal colonization and infection are closely linked, but knowledge of the factors that contribute to transmission, carriage, disease, and transition from carriage to disease is still limited. Research on components that physically contribute to host-pathogen interactions, such as capsular polysaccharides, adhesins, and toxins, has provided valuable insights into the process of pneumococcal pathogenesis (20). In contrast, the influence of environmental factors on pneumococcal growth and survival remains fairly unexplored.
S. pneumoniae needs to adapt to various aerobic and anaerobic conditions, reflecting the different niches it occupies during transmission, colonization, and invasive disease. During niche transition, oxygen (O2) levels change considerably. Levels of O2 are 21% in ambient air, decrease to 10 to 15% in the alveoli of the lungs, and are about 5% in resting cells. In O2-rich conditions, S. pneumoniae expresses pyruvate oxidase (SpxB), which generates acetyl-phosphate as a source of ATP and hydrogen peroxide (H2O2) for interspecies competition at the mucosal surfaces of the nasopharynx (41). The presence of O2 is also a prerequisite for the pneumococcal X state (4, 14), which is a physiological condition that allows for genetic transformation and an adequate response to environmental stress (32). Recently, it was shown that the fatty acid (FA) content of the pneumococcal cell membrane (31) and the expression of 69 genes (8) change in response to the availability of O2. Finally, changes in O2 levels can also affect production of the polysaccharide capsule (48), which is the major pneumococcal virulence determinant.
Similar to those of O2, the levels of carbon dioxide (CO2) vary considerably among the different pneumococcal niches inside and outside the host. Ambient levels of CO2 in the environment are 0.038%, while CO2 levels inside the human body, in particular in the lower respiratory tract, can reach 5% or more. The importance of this gaseous compound for S. pneumoniae is illustrated by the observation that the depletion of CO2 from ambient air completely inhibits pneumococcal growth (21). Moreover, about 8% of all clinical isolates require a CO2-enriched environment for growth in laboratory conditions (3). This intrinsic CO2 dependence of S. pneumoniae and many other (micro)organisms is most likely related to an anabolic need for CO2 or bicarbonate (HCO3−) during biosynthesis of nucleic acids, amino acids, and FAs (1). Pathogens can often sequester CO2 directly from host tissues, but in the absence of sufficient levels of extracellular CO2, endogenous CO2 needs to be enzymatically fixated. Carbonic anhydrases (CAs; EC 4.2.1.1) are enzymes that catalyze the reversible reaction CO2 + H2O ↔ HCO3− + H+. Because HCO3− cannot passively diffuse across biological membranes, its formation significantly delays the release of intracellular CO2. At least five different classes of CAs have been described, and most eukaryotic, prokaryotic, and archaeal species express at least one CA class (39, 40).
Genome analysis (39) has revealed that S. pneumoniae has one putative CA, a β-class CA that is highly conserved in all available pneumococcal genome sequences. Pneumococcal CA (PCA) is highly homologous to CAs in other streptococcal species, such as Streptococcus pyogenes. The closest nonstreptococcal PCA homologs are found in Mycobacterium species, while PCA homologs in other respiratory tract pathogens such as Neisseria meningitidis and Haemophilus influenzae are more divergent (40). The aim of this study was to investigate the functional characteristics of the pca gene and the encoded PCA enzyme in S. pneumoniae and to establish the relevance of PCA for pneumococcal growth and survival under CO2-poor conditions in vitro. Further, we examined the importance of PCA during host-pathogen interaction.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains that were used in this study are listed in Table 1. S. pneumoniae strains were routinely grown under static conditions in GM17 broth (23) or on blood agar (BA) plates composed of Colombia agar (Oxoid) supplemented with 5% sheep blood (Biotrading). Cultures were incubated in a 5%-CO2 incubator at 37°C. To compare growth under CO2-poor and -rich conditions, mid-log-phase cultures of pneumococcal strains in CO2-enriched GM17 were diluted 50-fold in medium that was exposed overnight to ambient air (0.038% CO2) or to ambient air enriched with 5% CO2, respectively. Pneumococcal genetic transformation was performed as described previously (10), and importantly, for preparation of competent S. pneumoniae strains lacking the pca gene (Δpca), all media were first exposed to ambient air enriched with 5% CO2. For transformation of the CO2-dependent carriage strains, a 1:1 mixture of competence-stimulating peptide 1 (CSP-1) (100 ng/ml) and CSP-2 (100 ng/ml) was used. Viable-bacteria counts were derived from CFUs after plating 10-fold serial dilutions in PBS. Escherichia coli strains were routinely grown at 37°C on Luria Bertani (LB) agar plates or in LB broth in a shaking incubator at 200 rpm. E. coli transformation was performed by the CaCl2 competence method (35). Lactococcus lactis strains were routinely grown on GM17 agar plates or in GM17 broth as static cultures at 30°C. L. lactis transformation was performed by electroporation (23). The antibiotics and stock solutions used for complementation studies were ampicillin, 100 μg/ml; spectinomycin, 150 μg/ml; kanamycin, 500 μg/ml for S. pneumoniae and 50 μg/ml for E. coli; trimethoprim, 0.25 μg/ml; chloroamphenicol, 2.5 μg/ml for S. pneumoniae and 5 μg/ml for L. lactis; adenine, 5 mg/ml in 0.05 M HCl; uracil, 2 mg/ml in 1% sodium carbonate (Na2CO3); arginine, 20 mg/ml; aspartic acid, 20 mg/ml (pH 7); palmitic acid or oleic acid, 200 mM in ethanol; sodium salicylate, 1 M; and bovine liver catalase, 200,000 U/ml (Sigma).
TABLE 1.
Bacterial strains used in this study
| Species and strain | Relevant characteristics | Reference |
|---|---|---|
| S. pneumoniae | ||
| R6 | Wild-type strain, unencapsulated | 19 |
| D39 | Wild-type strain, serotype 2 | 25 |
| TIGR4 | Wild-type strain, serotype 4 | 45 |
| R6Δpca | Δspr0026 Spr | This study |
| D39Δpca | ΔSPD_0030 Spr | This study |
| TIGR4Δpca | ΔSP_0024 Spr | This study |
| R6bga::nisRK | Nisin-responsive R6 strain; spr0565::nisRK Tmpr | 23 |
| R6bga::nisRKΔpca | Nisin-responsive R6 strain; spr0565::nisRK Δspr0026 Tmpr Spr | This study |
| R6ΔpcaΔspxB | Δspr0026 Δspr0642 Spr Kmr | This study |
| TIGR4ΔpcaΔspxB | ΔSP_0024 ΔSP_0730 Spr Kmr | This study |
| TIGR4Δcps | Unencapsulated TIGR4 strain; ΔSP_0343-0365 Kmr | 11 |
| TIGR4ΔcpsΔpca | Unencapsulated TIGR4 strain; ΔSP_0343-0365 ΔSP_0024 Kmr Spr | This study |
| D39Δcps | Unencapsulated D39 strain; ΔSPD_0312-0333 Kmr | 11 |
| D39ΔcpsΔpca | Unencapsulated D39 strain; ΔSPD_0312-0333 ΔSPD_0030 Kmr Spr | This study |
| H23 | CO2-dependent carriage isolate | Laboratory collection |
| H26 | CO2-dependent carriage isolate | Laboratory collection |
| E. coli | ||
| DH5α | Cloning strain | 17 |
| BL21 | Expression strain | Novagen |
| L. lactis | ||
| NZ9000 | Cloning strain | 24 |
Spr, spectinomycin resistant; Tmpr, trimethoprim resistant; Kmr, kanamycin resistant.
DNA extraction and PCR conditions.
Chromosomal DNA was isolated from S. pneumoniae and E. coli broth cultures by cetyltrimethylammonium bromide (CTAB) extraction as described previously (47). Plasmids were isolated from E. coli and L. lactis broth cultures with a Qiaprep mini- or midikit (Qiagen). For construction of directed-deletion mutants and glutathione S-transferase (GST) fusion protein cloning, the proofreading Pwo DNA polymerase (Roche) was used. For other PCR-based approaches, AmpliTaq DNA polymerase (Applied Biosystems) was applied. The primers (Biolegio, Nijmegen, Netherlands) that were used in this study are listed in Table S1 in the supplemental material.
Construction of pneumococcal mutants.
Directed-deletion mutants of S. pneumoniae were generated by allelic exchange of the target gene with an antibiotic resistance marker as described previously (10). Briefly, overlap extension PCR was applied to insert the kanamycin or spectinomycin resistance cassette of the pR410 or pR412 plasmid (Table 2), respectively, between the two 500-bp flanking sequences surrounding the target gene. The overlap extension PCR products were transformed into S. pneumoniae, and directed mutants were obtained by selective plating. Correct integration of the antibiotic resistance cassette into the target gene was validated by PCR. Gene deletion mutants were crossed back to the wild-type strain, using chromosomal DNA of the mutant strains as the donor during transformation. Since the flanking sequences of all the target genes used in this study were homologous in the R6, D39, and TIGR4 strains, gene deletions were introduced into D39 and TIGR4 by transformation with the PCR-amplified gene deletions and 500-bp flanking sequences of the R6 mutant derivatives.
TABLE 2.
Plasmids used in this study
| Plasmid | Relevant characteristicsa | Reference |
|---|---|---|
| pR410 | Donor for kanamycin resistance cassette | 43 |
| pR412 | Donor for spectinomycin resistance cassette | 28 |
| pCR2.1 | Cloning vector; Apr Kmr | Invitrogen |
| pGEX-1N | Expression vector with N-terminal GST tag; Apr | Novagen |
| pNG8048E | Expression vector with nisin-inducible promoter; Car | 23 |
| pWA1 | pCR2.1 with pca gene, BamHI site; Apr Kmr | This study |
| pWA4 | pGEX-1N with gst-pca construct; Apr | This study |
| pCR2.1-PCA_L | pCR2.1 with pca gene, BsaI site; Apr Kmr | This study |
| pCR2.1-ECCA | pCR2.1 with ecca gene, BsaI site; Apr Kmr | This study |
| pUO1 | pNG8048 with ecca gene behind nisin-inducible promoter; Car | This study |
| pUO3 | pNG8048 with pca gene behind nisin-inducible promoter; Car | This study |
Apr, ampicillin resistant; Kmr, kanamycin resistant; Car, chloroamphenicol resistant.
Plasmid construction.
All plasmids used in this study are listed in Table 2. To obtain the plasmids for complementation of the CO2-dependent growth defect of the Δpca strains, the pca gene of S. pneumoniae TIGR4 and the ecca gene (ECDH10B_106) of E. coli DH5α were PCR amplified with the PBNISPCA_L/PBNISPCA_R and PB_NISECCA_L/PB_NISECCA_R primer pairs, respectively. The PCR products were cloned into the pCR2.1 cloning vector of a TA cloning kit (Invitrogen) to obtain pCR2.1-PCA_L and pCR2.1-ECCA, respectively. In the next step, the genes were excised by BsaI/EcoRI digestion and ligated to the NcoI/EcoRI-digested pNG8048 plasmid to obtain pUO3 and pUO1, respectively. To obtain the plasmid for overproduction of GST-PCA, the pca gene of S. pneumoniae TIGR4 was PCR amplified with the PBPCA_S/PBPCA_E primer pair and cloned into pCR2.1 to obtain pWA1. In the next step, the pca gene was excised by BamHI/EcoRI digestion and subcloned behind the GST gene in a BamHI/EcoRI-digested pGEX-1N vector to obtain pWA4. Cloning of the pCR2.1 and pGEX-1N plasmids was performed in E. coli DH5α, and cloning of the pNG8048E plasmids was performed in L. lactis NZ9000. The nucleotide sequences of the PCR products in the pCR2.1 plasmid were confirmed by sequencing.
Production and purification of recombinant GST-PCA.
For GST-PCA production, an overnight culture of E. coli BL21 (pWA4) was diluted 50-fold in prewarmed (37°C) 2× LB supplemented with 0.5% glucose. At an optical density at 600 nm (OD600) of 0.6 to 0.8, 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added, and cultures were shifted to room temperature. After 4 h, cells were placed on ice, pelleted by centrifugation, resuspended in ice-cold lysis buffer (50 mM Tris-HCl [pH 7.5], 300 mM NaCl, 0.5 mM dithiothreitol [DTT], 1.5 mM MgCl2, 0.2 mM EDTA, 1% Triton X-100) with 1× protease inhibitor mixture (Complete Mini; Roche Applied Science) to a cell density equivalent to an OD600 of 100, and lysed by sonication. Insoluble debris in the lysate was removed by centrifugation at 16,000 × g for 10 min at 4°C, and the supernatant was incubated overnight with prewashed (1× PBS) glutathione Sepharose 4 Fast Flow beads (GE Healthcare) at 4°C. Nonspecifically bound proteins were removed by washing the beads three times with lysis buffer for 15 min at 4°C. GST-PCA was eluted with elution buffer (50 mM Tris-HCl [pH 8.0], 300 mM NaCl, 10 mM glutathione, and 0.5 mM DTT). The eluate was dialyzed against 50 mM Tris, pH 7.5. The protein concentration in the GST-PCA solution was determined with a bicinchoninic acid (BCA) protein assay kit (Pierce).
Carbonic anhydrase activity assay.
The activity of CAs was determined by the changing-pH/dye indicator method (22) on an RX.2000 rapid-mixing stopped-flow unit (Applied Photophysics, United Kingdom). Briefly, enzyme samples were diluted in reaction buffer at pH 7.5 (50 mM HEPES [pH 7.5], 200 mM phenol red, and 200 mM Na2SO4) or at pH 8.4 (50 mM TAPS [pH 8.4], 200 mM m-cresol purple, and 200 mM Na2SO4), and the reaction was initiated by the addition of an equivalent amount of CO2-saturated water. The subsequent restoration of CO2/HCO3− balance was monitored by the color conversion of the pH-sensitive dye indicators at 558 nm (pH 7.5) or 578 nm (pH 8.4). All reactions were performed at 25°C. The CA activities of GST-PCA and human CA II ([hCAII] Sigma) were measured at final concentrations of 100 μg/ml and 0.5 μg/ml, respectively. When appropriate, 50 mM Tris-HCl (pH 7.5) and dimethyl sulfoxide (DMSO) were included as nonenzymatic controls. The stock solutions for CA inhibition studies were 100 mM acetazolamide ([AZA] Sigma) and 100 mM ethoxyzolamide ([EZA] Sigma) in DMSO.
Cell lines, culture conditions, and host-pathogen studies.
The human pharyngeal epithelial cell line Detroit 562 (CCL-138; ATCC) was routinely grown in RPMI 1640 medium without phenol red (Invitrogen, Netherlands) supplemented with 1 mM sodium pyruvate and 10% fetal calf serum (FCS). The human brain microvascular endothelial cell (HBMEC) line was cultivated in RPMI 1640 medium supplemented with 10% FCS, 10% Nu-Serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 1% minimal essential medium (MEM)-vitamins, and 1% nonessential amino acids (42). Prior to infection, HBMEC monolayers were incubated for 1 h in culture medium with 10 ng/ml of tumor necrosis factor alpha (TNF-α). The murine macrophage-like cell line J774 (TIB 67; ATCC) was cultured in Dulbecco's modified Eagle's medium (DMEM) GlutaMAX-I (Invitrogen, Netherlands) with 10% FCS. All cells were cultured in a 5%-CO2 incubator at 37°C.
Pneumococcal adherence, invasion, and intracellular survival studies were performed essentially as described previously (7, 11, 16). Briefly, monolayers of J774, Detroit 562, or HBMECs were infected with bacteria in 5%-CO2-enriched culture medium with only 1% FCS (infection medium). Subsequently, the pneumococci were allowed to adhere to the cells for 0.5 h, 1 h, or 2 h, respectively, and nonadherent bacteria were removed by washing. To quantify adherence, host cells were detached from the wells and lysed with 0.025% Triton X-100 or 1% saponin and trypsin-EDTA (0.05%-0.02%). To determine the level of invasion into the host cells, extracellular S. pneumoniae cells were killed by a 1-h incubation with 1 ml 5%-CO2-enriched infection medium supplemented with gentamicin (200 μg/ml) and penicillin G (10 μg/ml) before cells were lysed. To examine intracellular survival, cells were infected and treated with gentamicin and penicillin G as described above, after which cells were washed once and fresh 5%-CO2-enriched medium containing gentamicin (13.34 μg/ml) and penicillin G (0.67 μg/ml) (1/15 of beginning antibiotic concentration) was added to each well for prolonged incubation. For all in vitro cell culture studies, the pneumococcal wild-type and mutant strains grew comparably in infection medium alone. Results were corrected mathematically to account for small differences in count in the initial inoculum.
In vivo colonization and bacteremia experiments.
Bacteremia and nasopharyngeal colonization experiments with mice were conducted with 9-week-old female outbred CD-1 mice (Harlan, Horst, Netherlands) as described recently (18). Briefly, for the colonization experiments, 1 × 106 CFU in 10 μl of PBS were administered to the nostrils of groups of five mice for each strain, and bacteria were recovered from the nasopharynx by flushing the nose with 2 ml of sterile PBS at 96 h. Bacteremia experiments were performed twice with groups of at least five mice for each strain. Mice were infected intravenously in the tail vein with 1 × 106 CFU in 100 μl of PBS, and bacteria were recovered from the blood by retro-orbital puncture. For mice in which no bacteria were found, a lower limit of detection (22 CFU/ml) was used. Results were corrected mathematically to account for small differences in bacterial count in the initial inoculum. All experiments were performed with the approval of the Animal Experimentation Committee (DEC) of the Radboud University Nijmegen Medical Centre.
In silico analysis.
The subcellular location of PCA enzyme was predicted by various online prediction servers, such as PSORTb (http://www.psort.org) and TMHMM (http://www.cbs.dtu.dk/services/TMHMM). Conservation of the pca gene and PCA protein was performed by the genomic BLAST service on the website of the National Center for Biotechnology Information (NCBI) of the U.S. National Library of Medicine (http://www.ncbi.nlm.nih.gov/).
Statistical analysis.
For in vitro host-pathogen studies, data were analyzed using an unpaired Student's t test, with P values of <0.05 considered significant. All statistical analyses were performed using GraphPad Prism version 4.0.
RESULTS
The pca gene is required for pneumococcal growth under CO2-poor conditions.
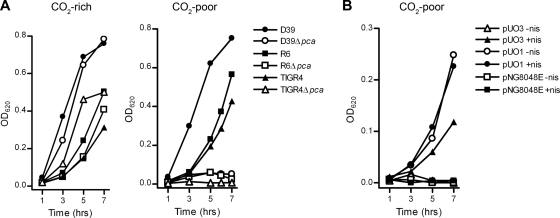
To determine the importance of the pca gene for pneumococcal growth, pca deletion mutants (Δpca) were constructed from three S. pneumoniae strains, i.e., R6 (Δspr0026), D39 (ΔSPD_0030), and TIGR4 (ΔSP_0024). All Δpca strains were able to grow normally on BA plates and Trypticase soy broth (TSB) agar plates supplemented with catalase (Trypticase soy agar [TSA]) under ambient air enriched with 5% CO2 (data not shown). In vitro growth rates in 5%-CO2-enriched GM17 broth medium were similar for the Δpca and wild-type strains, with cultures reaching an OD620 of 0.3 or more (Fig. 1 A, left panel). In GM17 broth medium that was exposed to ambient air, the wild-type strains were also able to reach a high OD620. In contrast, growth of all Δpca strains under these CO2-poor (0.038%) growth conditions was attenuated, and cultures did not reach an OD620 above 0.1 (Fig. 1A, right panel). Growth of the Δpca strains under CO2-poor conditions was also impaired on TSA plates and reduced on BA plates (data not shown).
FIG. 1.
Disruption of the pca gene in S. pneumoniae leads to CO2-dependent growth inhibition. (A) Growth characteristics of the S. pneumoniae R6, D39, and TIGR4 wild-type and Δpca strains in CO2-rich and CO2-poor GM17 broth medium. (B) Growth of the S. pneumoniae R6bga::nisRK Δpca strain harboring either pNG8048E (empty vector), pUO1 (ecca), or pUO3 (pca) in CO2-poor GM17 broth medium without (−nis) and with (+nis) 20 ng/ml nisin. Growth of the pneumococcal cultures was monitored by recording the OD620. All curves in the graph present the averages of the results of three independent growth experiments.
To exclude polar effects due to disruption of the pca gene, we provided the pca gene in trans on the pUO3 plasmid behind a nisin-inducible promoter. Induction of pca gene expression by the addition of nisin restored growth of the nisin-responsive R6bga::nisRKΔpca (pUO3) strain in CO2-poor GM17 broth (Fig. 1B). Introduction of the pUO1 plasmid with the gene for E. coli carbonic anhydrase (ECCA) (12) into R6bga::nisRKΔpca also reversed the CO2 dependence of this strain (Fig. 1B). Interestingly, complementation by ECCA did not appear to require induction with nisin. Because pUO1 could not restore the CO2 dependence of the R6Δpca strain lacking the NisRK sensor for nisin (data not shown), it is likely that autoinduction of the NisRK two-component signal transduction system resulted in expression of small but sufficient amounts of ECCA.
PCA has carbonic anhydrase activity.
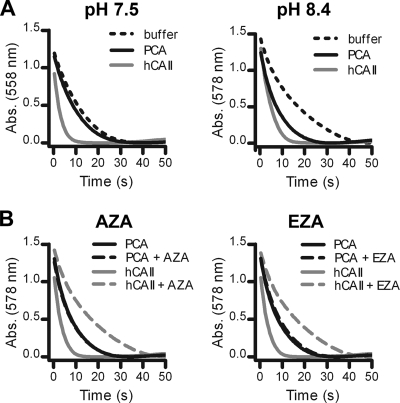
The PCA enzyme was further characterized with enzymatic activity and inhibition assays. To facilitate the measurement of PCA enzymatic activity, PCA was overproduced as a GST fusion protein in E. coli. Since no endogenous E. coli CA activity was detected in the lysates of control cells expressing only the GST protein, the CA activity in E. coli cells expressing GST-PCA can be fully ascribed to the presence of the recombinant protein (data not shown). The affinity-purified recombinant GST-PCA protein catalyzed the conversion of CO2 to HCO3− at pH 8.4, whereas the enzymatic activity was almost completely abrogated at pH 7.5 (Fig. 2 A). Sulfonamides such as AZA and EZA are broad-range CA inhibitors that are active against most CAs (39), including the homologous Rv1284 CA in Mycobacterium tuberculosis (29). Interestingly, the presence of 100 μM AZA or 100 μM EZA did not reduce the CA activity of recombinant GST-PCA, whereas that of hCAII was completely inhibited (Fig. 2B). Since both compounds also did not induce CO2 dependence in S. pneumoniae wild-type strains (data not shown), these sulfonamides are unlikely to have high affinity for PCA.
FIG. 2.
Enzymatic activity and inhibition studies of recombinant GST-PCA. (A) The CA activity of GST-PCA (100 μg/ml) was measured by the changing-pH/dye indicator method at pH 7.5 and pH 8.4. (B) Inhibitory effect of the sulfonamides AZA (100 μM) and EZA (100 μM) on the CA activity of GST-PCA at pH 8.4. Under all conditions tested, hCAII (0.5 μg/ml) and nonenzymatic reactions were included as positive and negative controls, respectively. The curves for the nonenzymatic control reactions of the inhibition study overlapped with the curves for hCAII with an inhibitor and for the clarity of the graph were not displayed. All curves in the graphs present the averages of the results of three independent CA activity assays. Abs., absorbance.
PCA is linked to UFA biosynthesis.
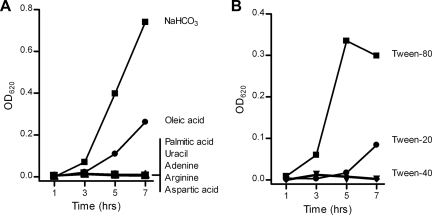
The biosynthesis pathways for nucleic acids, fatty acids, and several amino acids all contain an essential HCO3−-dependent carboxylation step that could potentially account for the observed growth defect of microbial CA mutants in CO2-poor conditions (1). To investigate if one or more of these carboxylation steps are responsible for the growth inhibition of the S. pneumoniae Δpca strains in CO2-poor GM17 broth medium, we complemented pneumococcal cultures with sodium hydrogen carbonate (NaHCO3) or various metabolic intermediates (i.e., adenine, uracil, arginine, aspartic acid, palmitic acid [in 0.1% Tween 40], or oleic acid [in 0.1% Tween 40]) (Fig. 3 A). As predicted, NaHCO3 fully reversed growth of the S. pneumoniae TIGR4Δpca strain under CO2-poor conditions. The unsaturated fatty acid (UFA) oleic acid was the only metabolic intermediate that could partially restore growth as well, although not to the same level as NaHCO3. Other sources of UFAs, such as Tween 20 and Tween 80 (30), could also (partially) reverse the CO2 dependence of the S. pneumoniae TIGR4Δpca strain (Fig. 3B). In contrast, the saturated fatty acid (SFA) palmitic acid (Fig. 3A) or Tween 40 (Fig. 3B), which is a Tween derivative that is solely composed of SFA, was ineffective.
FIG. 3.
Bicarbonate and oleic acid revert the CO2 dependence of Δpca strains. (A) Growth of the S. pneumoniae TIGR4Δpca strain in CO2-poor GM17 broth medium supplemented with NaHCO3 (10 mM), adenine (200 μg/ml), uracil (200 μg/ml), arginine (200 μg/ml), aspartic acid (200 μg/ml), palmitic acid (0.01 mM in 0.1% Tween 40), or oleic acid (0.01 mM in 0.1% Tween 40). (B) Growth of the S. pneumoniae TIGR4Δpca strain in CO2-poor GM17 broth medium with 0.1% Tween 20, Tween 40, or Tween 80. The growth of all pneumococcal broth cultures was monitored by recording the OD620. All curves in the graph present the averages of the results of three independent growth experiments.
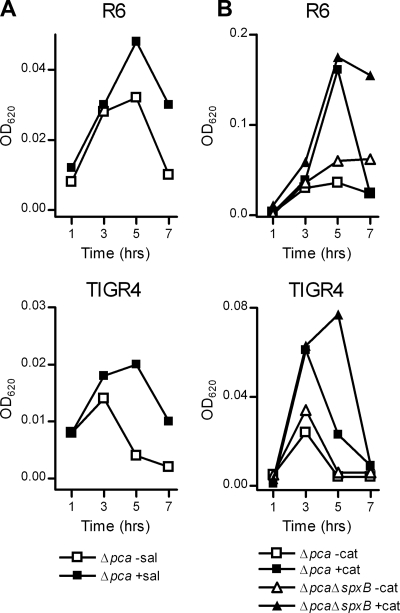
Because supplementation with SFAs could not reverse the CO2-dependent growth inhibition of the Δpca strains, CO2 fixation by PCA appears to be essential when insufficient UFAs are available in the growth medium. The synthesis of UFAs and SFAs in S. pneumoniae occurs essentially by the same pathway (27). The dependency of the Δpca strains on UFA supplementation for growth under CO2-poor conditions therefore suggests that under this condition UFAs are more readily depleted. Recently, it was reported that the reactive oxygen species (ROS) scavenger salicylate increased the unsaturation index of bacterial-membrane fatty-acyl chains under aerobic (thus CO2-poor) growth conditions by protecting UFAs against endogenous oxidative stress (31). In line with this observation, cultures of the S. pneumoniae R6Δpca and TIGR4Δpca strains grown under CO2-poor conditions reached an almost-2-fold-higher optical density when supplemented with salicylate (Fig. 4 A). Neutralization of endogenous H2O2, which also plays an important role in lipid peroxidation (38), through the addition of high concentrations of catalase restored growth of the S. pneumoniae R6Δpca and TIGR4Δpca strains to an almost-3-fold-higher optical density (Fig. 4B). Despite the involvement of pyruvate oxidase (SpxB) in endogenous H2O2 production (41), disruption of the spxB gene in the TIGR4Δpca and R6Δpca strains did not restore growth to the same level as that in the catalase-complemented cultures (Fig. 4B). Moreover, the addition of catalase still promoted growth of the S. pneumoniae TIGR4ΔspxBΔpca and R6ΔspxBΔpca strains (Fig. 4B).
FIG. 4.
Scavengers for endogenous ROS delay the CO2-dependent growth defect of Δpca strains. (A) Growth of the the R6 and TIGR4 S. pneumoniae Δpca strains in CO2-poor GM17 broth medium without (−sal) or with 5 mM (+sal) sodium salicylate. (B) Growth of the S. pneumoniae R6 and TIGR4Δpca and ΔpcaΔspxB strains in CO2-poor GM17 broth medium without (−cat) or with (+cat) 10,000 U/ml of catalase. The growth of all pneumococcal broth cultures was monitored by recording the OD620. All curves in the graphs present the results of a single experiment that are characteristic of those for three independent growth experiments.
PCA is required for intracellular survival inside host cells.
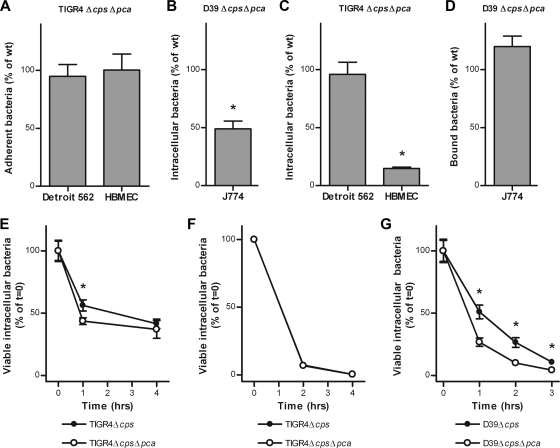
Membrane fatty acids are essential for pneumococcal growth and survival (26) and are important targets for host defense mechanisms (38). Because our experiments suggest that PCA activity and UFA biosynthesis are linked, we investigated the specific contribution of PCA to pneumococcal host-pathogen interactions. To identify PCA-mediated effects on the interaction of S. pneumoniae with host cells, we assessed the ability of the Δpca strains to adhere to, invade, and survive in different cell lines. First, we studied the interaction of S. pneumoniae with human pharyngeal epithelial Detroit 562 cells, which are representative of the host cells encountered by S. pneumoniae during colonization of human upper airways. Disruption of the pca gene in the unencapsulated (Δcps) derivative of the S. pneumoniae TIGR4 strain did not lead to decreased adherence to (Fig. 5 A) or invasion of (Fig. 5C) these epithelial cells. However, at 1 h after pneumococcal invasion of the host cells, we observed a statistically significant 1.3-fold reduction in intracellular survival of the TIGR4ΔcpsΔpca strain in Detroit 562 cells (Fig. 5E). Next, we examined the role of PCA during interaction of S. pneumoniae with the HBMEC line. Endothelial cells are the main component of the blood-brain barrier, and penetration of this barrier by pathogens can lead to meningitis. Adherence to HBMEC was not significantly different between the TIGR4Δcps and TIGR4ΔcpsΔpca strains (Fig. 5A). In contrast, the number of viable intracellular bacteria that could be recovered from HBMECs directly after pneumococcal invasion was reduced 7-fold for the TIGR4ΔcpsΔpca strain compared to that of the TIGR4Δcps strain (Fig. 5C). Interestingly, at 2 and 4 h after invasion, the relative decreases in the numbers of viable intracellular bacteria were equal for the two strains (Fig. 5F). Finally, we investigated the role of PCA during interaction of S. pneumoniae with mouse J774 macrophage cells, which are primary immune cells important for clearance of bacterial infections. Exposure of J774 cells to TIGR4Δcps induced morphological and phenotypical changes in J774 cells, such as surface detachment and cell lysis, making readout unreliable and leading to nonreproducible results. J774 interaction studies were therefore continued with the S. pneumoniae D39Δcps and D39ΔcpsΔpca strains. Although disruption of the pca gene had no significant effect on the binding of S. pneumoniae by host immune cells (Fig. 5B), the number of viable intracellular bacteria directly after uptake by the macrophages was 2-fold lower for the D39ΔcpsΔpca strain than for the D39Δcps strain (Fig. 5D). Moreover, temporal monitoring revealed that phagocytic killing of intracellular bacteria continued to be significantly faster for the D39ΔcpsΔpca strain than for the D39Δcps strain (Fig. 5G). Despite the in vitro contribution of PCA to pneumococcal intracellular survival, no significant difference between the S. pneumoniae TIGR4 wild-type and TIGR4Δpca strains was observed in mouse models of pneumococcal nasopharyngeal carriage and bacteremia (see Fig. S1 in the supplemental material).
FIG. 5.
PCA is required for invasion and intracellular survival in host cells. (A and B) In vitro adherence of the TIGR4ΔcpsΔpca strain to Detroit 562 cells and HBMECs (A) and binding of the D39ΔcpsΔpca strain by J774 cells (B). The relative adherence and binding efficiencies were correlated to those of the TIGR4Δcps and D39Δcps strains, respectively. (C and D) Invasive properties of the TIGR4ΔcpsΔpca strain toward Detroit 562 cells and HBMECs (C) and uptake of the D39ΔcpsΔpca strain by J774 cells (D). The relative invasion and uptake efficiencies were correlated to the number of viable intracellular cells of the TIGR4Δcps and D39Δcps strains, respectively. (E and F) Intracellular survival kinetics of the TIGR4Δcps and TIGR4ΔcpsΔpca strains in Detroit 562 cells (E) and HBMECs (F). (G) Phagocytic killing of the D39Δcps and D39ΔcpsΔpca strains in J774 cells. Intracellular survival and phagocytic killing were correlated to viable-bacteria counts at time zero. *, statistically significant differences (P < 0.05).
The pca gene is present in CO2-dependent circulating strains.
The pca gene appears to be a highly conserved gene, which is present in all 11 complete and 18 draft S. pneumoniae genomes that are currently available in the public databases. Still, about 8% of all S. pneumoniae isolates from various sources have been reported as CO2 dependent (3). To exclude the possibility that the pca gene is absent in these circulating strains, we investigated whether the CO2 dependence of these isolates is related to the absence of a functional pca gene. Two out of 126 carriage strains (H23 and H26) isolated from healthy Venezuelan children (our unpublished data) did not grow on BA and TSA plates unless the environment was enriched with 5% CO2. PCR analysis indicated that the pca gene was present in both CO2-dependent strains (Table 3), and genetic transformation of these strains with chromosomal DNA from both the S. pneumoniae R6 wild-type and R6Δpca strains resulted in CO2-independent colonies (Table 3). These results suggest that the observed CO2 dependence of the H23 and H26 strains is associated with a genetic defect or a missing gene other than pca. In addition, further phenotypical characterization of these strains showed that their CO2 dependence was different from that of the S. pneumoniae Δpca strains used in this study. Although both strains were completely CO2 dependent for growth on BA plates, the H23 strains reached high optical densities in CO2-poor GM17 broth medium. In contrast, the H26 strain did not grow at all in CO2-poor GM17 broth medium, not even when it was supplemented with UFA (Tween 80) (Table 3).
TABLE 3.
Characteristics of CO2-dependent carriage isolates
| Straina | Transformation (CFU/ml) with DNA from: |
Growth on or in indicated plate or brothc |
||||
|---|---|---|---|---|---|---|
| R6 | R6Δpca | BA plates | TSA plates | GM17 broth | GM17 broth (0.1% Tween 80) | |
| H23 | 19,800 | 14,900 | − | − | + | + |
| H26 | 67 | 67 | − | − | − | − |
Both strains carry the pca gene.
No. of colonies growing on BA plates under CO2-poor conditions.
+, growth; −, no growth.
DISCUSSION
The respiratory tract pathogen S. pneumoniae needs to adapt to the various conditions it encounters during transmission, colonization, and disease. Currently, relatively little is known about the genetic and metabolic factors that contribute to an adequate response of this bacterium to changes in CO2 availability. In this study, we showed that the putative carbonic anhydrase in S. pneumoniae has an important role for growth in CO2-poor conditions.
Our experiments clearly showed that the pca gene encodes a functionally active carbonic anhydrase. All the Δpca strains were growth deficient in CO2-poor conditions but could be complemented by the addition of HCO3−, the expected end product of PCA enzymatic activity. In addition, growth of the Δpca strains could be restored by in trans expression of the well-characterized homologous β-CA (ECCA) from E. coli. Finally, recombinant GST-PCA was able to catalyze the conversion of CO2 to HCO3−. Interestingly, PCA did not appear to be active at the physiological pH of 7.5. This is not unusual for β-CAs and has been observed for ECCA and the H. influenzae CA (HICA). Most likely, this pH-dependent behavior is linked to the pH-dependent coordination of Zn2+ in the active site (13). Furthermore, both ECCA and HICA appear to have an alternative bicarbonate binding site that renders the enzyme inactive at physiological pH when sufficient substrate is present (13). Although PCA appears to miss essential amino acids that form the alternative bicarbonate binding site, it is also not unlikely that differences exist between its CA activity in enzymatic assays and in physiological conditions. Another striking characteristic of PCA is its lack of affinity for broad-range carbonic anhydrase inhibitors. This indicates that this enzyme is deviant from other well-characterized CAs, which is not surprising, as there is huge variation among the different CAs, and CA inhibitors were often developed against unrelated hCAs. In fact, differences between PCA and hCAs could benefit the therapeutic potential of PCA inhibitors.
Our metabolic complementation experiments revealed, in analogy to the role of CAs in other microorganisms (1, 5), that the cellular function of PCA in CO2-poor conditions is at least linked to FA biosynthesis. This implies that PCA provides HCO3− required for the carboxylation of acetyl coenzyme A (acetyl-CoA) by acetyl-CoA carboxylase to form malonyl-CoA, which is the first committed step of FA biosynthesis (15). We did not observe a stimulating effect of any of the other tested metabolic intermediates on the growth of the S. pneumoniae Δpca strains in CO2-poor GM17 broth medium. This implies that GM17 medium contains limiting amounts of UFAs but sufficient levels of the other metabolites to support growth. Based on the UFA supplementation experiments and previous observations of other microorganisms (1, 5), we can predict that other carboxylation reactions, e.g., those involved in biosynthesis of some amino acids, pyrimidines, and purines, also depend on PCA activity when CO2 levels are low. Still, we feel that support of UFA biosynthesis is one of the most relevant aspects of PCA function. Although S. pneumoniae is able to tolerate low levels of membrane SFAs, insufficient UFAs lead to decreased cell viability (2). In ambient-air conditions, both environmental and cellular UFAs are prone to oxidation and can be replaced only by the PCA-supported de novo biosynthesis of UFAs. In addition, endogenous production of ROS by S. pneumoniae itself leads to increased cellular UFA peroxidation (31). Due to the transient phenotype of the pca mutation, it was not possible to perform a straightforward experiment to directly link the disruption of the pca gene to an alteration in the membrane FA composition or increased ROS sensitivity. In the absence of CO2, the Δpca strains do not grow, whereas in the presence of CO2, the Δpca and wild-type strains are phenotypically identical. In analogy with studies of S. pneumoniae UFA auxotrophs (27), we did attempt to complement cultures of the Δpca strains in CO2-poor conditions with UFAs to restore growth and allow characterization of membrane FAs. However, supplementation of cultures of the pneumococcal wild-type and Δpca strains with UFAs completely repressed expression of the FA biosynthesis gene cluster (our unpublished data), which inevitably results in a membrane that is predominantly composed of exogenous FAs (9).
It is tempting to speculate about the role of PCA in neutralizing the detrimental effect of pneumococcal SpxB activity. In ambient air, SpxB produces H2O2, acetyl-phosphate, and CO2. Production of H2O2 leads to UFA peroxidation (31), whereas acetyl-phosphate can readily be converted to acetyl-CoA by phosphate acetyl-transferase to support de novo FA biosynthesis. PCA then acts to convert CO2 to HCO3−, allowing carboxylation of acetyl-CoA to form malonyl-CoA. Currently, this hypothesis is not supported by our own observations, as catalase improved growth of both the S. pneumoniae Δpca and ΔpcaΔspxB strain cultures. However, the interconnection between SpxB activity and FA biosynthesis is still poorly understood and might involve different metabolic and regulatory pathways (31, 44). Alternatively, this suggests that other sources of endogenous oxidative stress, such as the Fenton reaction (31) or lactate oxidase activity (44), have a profound impact on the growth arrest of the Δpca strains in CO2-poor conditions as well.
The role of PCA in the de novo biosynthesis of UFAs and, possibly, other metabolites could also explain the decreased viability of the S. pneumoniae Δpca strains after invasion of endothelial cells and uptake by macrophages. During phagocytosis, and possibly endocytosis (33), a substantial portion of the intracellular bacteria is sorted to the host-cell lysosome. The low pH of this compartment reduces HCO3− availability, and the production of ROS leads to peroxidation of bacterial membrane UFAs (38) and nucleic acids (37). Interestingly, the effect of pca disruption on S. pneumoniae invasion and intracellular survival inside Detroit 562 pharyngeal epithelial cells was not as pronounced as in the two other cell types. Whether this difference reflects on the different routes for pneumococcal invasion of Detroit 562 cells by interaction with the polymeric immunoglobulin receptor (pIgR) (49) and HBMECs by interaction with the platelet-activating factor receptor (PAFr) (34) remains to be studied. A role for microbial carbonic anhydrases inside host cells was earlier suggested for a Salmonella enterica serovar Typhimurium CA (mig-5), which was expressed after uptake in macrophages and a mutant of which had a marked decrease in spleen colonization of mice (46). In contrast to findings for Salmonella CA mutants, we were not able to link PCA with virulence in animal models of bacteremia. However, this observation is in line with the outcome of a previous study showing that mice deficient in the NADPH oxidase subunit gp91, which is essential for lysosomal ROS production, were as sensitive to pneumococcal infection as wild-type mice (36). Furthermore, it is known that pneumococcal capsular polysaccharides prevent recognition and uptake of the bacterium by host immune cells, and once S. pneumoniae remains extracellular during infection of the blood, it might utilize serum HCO3− and FAs (9). Possibly, the role of PCA in pneumococcal disease is more pronounced in animal models of disease in which the bacterium needs to traverse the boundaries of epithelial and endothelial cells for dissemination from the respiratory tract to the blood and cerebrospinal fluid.
Finally, the role of PCA in S. pneumoniae can be projected onto CAs in other (respiratory tract) pathogens. Although CAs are ubiquitous enzymes in many microorganisms, most studies have investigated the role of CAs that are exposed to the surface or periplasm, have species-specific functions, or do not belong to the class of β-CAs (39). Here, we show that cytosolic β-CAs related to PCA are involved in FA biosynthesis and may offer novel opportunities for the design of broad-range therapies. Furthermore, PCA is probably only one of the factors that contribute to the adaptation of S. pneumoniae to CO2-poor conditions, which might be relevant for pneumococcal transmission in environmental ambient air. Detailed examination of the metabolic pathways that depend on PCA-mediated CO2 fixation and the identification of the genetic basis for the CO2 dependence observed in approximately 8% of all circulating pneumococcal isolates is expected to lead to novel insights into the way respiratory pathogens adapt to the CO2- and HCO3−-poor environments they encounter during transmission, colonization, and disease.
Supplementary Material
Acknowledgments
We thank Wresti Anggayasti, Emiel Hoeboer, Ugur Özturk, and Marijke Kamsteeg (UMC St. Radboud, Nijmegen, Netherlands) for assistance with constructing pWA4, purification of recombinant GST-PCA, and genetic complementation of Δpca strains and for providing hCAII, respectively. Furthermore, we thank Marjan Smeulders (Radboud University, Nijmegen, Netherlands) for assistance with the CA activity assays and K. S. Kim (Johns Hopkins University, Baltimore, MD) for HBMECs.
This work was financially supported by Horizon Breakthrough grant 93518023 from the Netherlands Genomics Initiative and Pneumopath grant 222983 from the European Union Seventh Framework Programme (FP7).
Footnotes
Published ahead of print on 4 June 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aguilera, J., J. P. Van Dijken, J. H. De Winde, and J. T. Pronk. 2005. Carbonic anhydrase (Nce103p): an essential biosynthetic enzyme for growth of Saccharomyces cerevisiae at atmospheric carbon dioxide pressure. Biochem. J. 391:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altabe, S., P. Lopez, and D. de Mendoza. 2007. Isolation and characterization of unsaturated fatty acid auxotrophs of Streptococcus pneumoniae and Streptococcus mutans. J. Bacteriol. 189:8139-8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austrian, R., and P. Collins. 1966. Importance of carbon dioxide in the isolation of pneumococci. J. Bacteriol. 92:1281-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auzat, I., S. Chapuy-Regaud, G. Le Bras, D. Dos Santos, A. D. Ogunniyi, I. Le Thomas, J. R. Garel, J. C. Paton, and M. C. Trombe. 1999. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol. Microbiol. 34:1018-1028. [DOI] [PubMed] [Google Scholar]
- 5.Bahn, Y. S., G. M. Cox, J. R. Perfect, and J. Heitman. 2005. Carbonic anhydrase and CO2 sensing during Cryptococcus neoformans growth, differentiation, and virulence. Curr. Biol. 15:2013-2020. [DOI] [PubMed] [Google Scholar]
- 6.Bogaert, D., R. de Groot, and P. W. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144-154. [DOI] [PubMed] [Google Scholar]
- 7.Bootsma, H. J., M. Egmont-Petersen, and P. W. Hermans. 2007. Analysis of the in vitro transcriptional response of human pharyngeal epithelial cells to adherent Streptococcus pneumoniae: evidence for a distinct response to encapsulated strains. Infect. Immun. 75:5489-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bortoni, M. E., V. S. Terra, J. Hinds, P. W. Andrew, and H. Yesilkaya. 2009. The pneumococcal response to oxidative stress includes a role for Rgg. Microbiology 155:4123-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinster, S., G. Lamberet, B. Staels, P. Trieu-Cuot, A. Gruss, and C. Poyart. 2009. Type II fatty acid synthesis is not a suitable antibiotic target for Gram-positive pathogens. Nature 458:83-86. [DOI] [PubMed] [Google Scholar]
- 10.Burghout, P., H. J. Bootsma, T. G. Kloosterman, J. J. Bijlsma, C. E. de Jongh, O. P. Kuipers, and P. W. Hermans. 2007. Search for genes essential for pneumococcal transformation: the RadA DNA repair protein plays a role in genomic recombination of donor DNA. J. Bacteriol. 189:6540-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cron, L. E., H. J. Bootsma, N. Noske, P. Burghout, S. Hammerschmidt, and P. W. Hermans. 2009. Surface-associated lipoprotein PpmA of Streptococcus pneumoniae is involved in colonization in a strain-specific manner. Microbiology 155:2401-2410. [DOI] [PubMed] [Google Scholar]
- 12.Cronk, J. D., J. A. Endrizzi, M. R. Cronk, J. W. O'Neill, and K. Y. Zhang. 2001. Crystal structure of E. coli beta-carbonic anhydrase, an enzyme with an unusual pH-dependent activity. Protein Sci. 10:911-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cronk, J. D., R. S. Rowlett, K. Y. Zhang, C. Tu, J. A. Endrizzi, J. Lee, P. C. Gareiss, and J. R. Preiss. 2006. Identification of a novel noncatalytic bicarbonate binding site in eubacterial beta-carbonic anhydrase. Biochemistry 45:4351-4361. [DOI] [PubMed] [Google Scholar]
- 14.Echenique, J. R., S. Chapuy-Regaud, and M. C. Trombe. 2000. Competence regulation by oxygen in Streptococcus pneumoniae: involvement of ciaRH and comCDE. Mol. Microbiol. 36:688-696. [DOI] [PubMed] [Google Scholar]
- 15.Fujita, Y., H. Matsuoka, and K. Hirooka. 2007. Regulation of fatty acid metabolism in bacteria. Mol. Microbiol. 66:829-839. [DOI] [PubMed] [Google Scholar]
- 16.Hammerschmidt, S., S. Wolff, A. Hocke, S. Rosseau, E. Muller, and M. Rohde. 2005. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect. Immun. 73:4653-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 18.Hendriksen, W. T., H. J. Bootsma, S. Estevao, T. Hoogenboezem, A. de Jong, R. de Groot, O. P. Kuipers, and P. W. Hermans. 2008. CodY of Streptococcus pneumoniae: link between nutritional gene regulation and colonization. J. Bacteriol. 190:590-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadioglu, A., J. N. Weiser, J. C. Paton, and P. W. Andrew. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6:288-301. [DOI] [PubMed] [Google Scholar]
- 21.Kempner, W., and C. Schlayer. 1942. Effect of CO2 on the growth rate of the pneumococcus. J. Bacteriol. 43:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalifah, R. G. 1971. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 246:2561-2573. [PubMed] [Google Scholar]
- 23.Kloosterman, T. G., J. J. Bijlsma, J. Kok, and O. P. Kuipers. 2006. To have neighbour's fare: extending the molecular toolbox for Streptococcus pneumoniae. Microbiology 152:351-359. [DOI] [PubMed] [Google Scholar]
- 24.Kuipers, O. P., P. G. Ruyter, M. Kleerebezem, and W. M. Vos. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15-21. [Google Scholar]
- 25.Lanie, J. A., W. L. Ng, K. M. Kazmierczak, T. M. Andrzejewski, T. M. Davidsen, K. J. Wayne, H. Tettelin, J. I. Glass, and M. E. Winkler. 2007. Genome sequence of Avery's virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J. Bacteriol. 189:38-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu, Y. J., and C. O. Rock. 2006. Transcriptional regulation of fatty acid biosynthesis in Streptococcus pneumoniae. Mol. Microbiol. 59:551-566. [DOI] [PubMed] [Google Scholar]
- 27.Marrakchi, H., K. H. Choi, and C. O. Rock. 2002. A new mechanism for anaerobic unsaturated fatty acid formation in Streptococcus pneumoniae. J. Biol. Chem. 277:44809-44816. [DOI] [PubMed] [Google Scholar]
- 28.Martin, B., M. Prudhomme, G. Alloing, C. Granadel, and J. P. Claverys. 2000. Cross-regulation of competence pheromone production and export in the early control of transformation in Streptococcus pneumoniae. Mol. Microbiol. 38:867-878. [DOI] [PubMed] [Google Scholar]
- 29.Minakuchi, T., I. Nishimori, D. Vullo, A. Scozzafava, and C. T. Supuran. 2009. Molecular cloning, characterization, and inhibition studies of the Rv1284 beta-carbonic anhydrase from Mycobacterium tuberculosis with sulfonamides and a sulfamate. J. Med. Chem. 52:2226-2232. [DOI] [PubMed] [Google Scholar]
- 30.Partanen, L., N. Marttinen, and T. Alatossava. 2001. Fats and fatty acids as growth factors for Lactobacillus delbrueckii. Syst. Appl. Microbiol. 24:500-506. [DOI] [PubMed] [Google Scholar]
- 31.Pesakhov, S., R. Benisty, N. Sikron, Z. Cohen, P. Gomelsky, I. Khozin-Goldberg, R. Dagan, and N. Porat. 2007. Effect of hydrogen peroxide production and the Fenton reaction on membrane composition of Streptococcus pneumoniae. Biochim. Biophys. Acta 1768:590-597. [DOI] [PubMed] [Google Scholar]
- 32.Prudhomme, M., L. Attaiech, G. Sanchez, B. Martin, and J. P. Claverys. 2006. Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science 313:89-92. [DOI] [PubMed] [Google Scholar]
- 33.Radin, J. N., C. J. Orihuela, G. Murti, C. Guglielmo, P. J. Murray, and E. I. Tuomanen. 2005. β-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect. Immun. 73:7827-7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ring, A., J. N. Weiser, and E. I. Tuomanen. 1998. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J. Clin. Invest. 102:347-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samsbrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 36.Schaper, M., S. L. Leib, D. N. Meli, R. P. Brandes, M. G. Tauber, and S. Christen. 2003. Differential effect of p47phox and gp91phox deficiency on the course of pneumococcal meningitis. Infect. Immun. 71:4087-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlosser-Silverman, E., M. Elgrably-Weiss, I. Rosenshine, R. Kohen, and S. Altuvia. 2000. Characterization of Escherichia coli DNA lesions generated within J774 macrophages. J. Bacteriol. 182:5225-5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shohet, S. B., J. Pitt, R. L. Baehner, and D. G. Poplack. 1974. Lipid peroxidation in the killing of phagocytized pneumococci. Infect. Immun. 10:1321-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith, K. S., and J. G. Ferry. 2000. Prokaryotic carbonic anhydrases. FEMS Microbiol. Rev. 24:335-366. [DOI] [PubMed] [Google Scholar]
- 40.Smith, K. S., C. Jakubzick, T. S. Whittam, and J. G. Ferry. 1999. Carbonic anhydrase is an ancient enzyme widespread in prokaryotes. Proc. Natl. Acad. Sci. U. S. A. 96:15184-15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spellerberg, B., D. R. Cundell, J. Sandros, B. J. Pearce, I. Idanpaan-Heikkila, C. Rosenow, and H. R. Masure. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19:803-813. [DOI] [PubMed] [Google Scholar]
- 42.Stins, M. F., J. Badger, and K. K. Sik. 2001. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb. Pathog. 30:19-28. [DOI] [PubMed] [Google Scholar]
- 43.Sung, C. K., H. Li, J. P. Claverys, and D. A. Morrison. 2001. An rpsL cassette, janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taniai, H., K. Iida, M. Seki, M. Saito, S. Shiota, H. Nakayama, and S. Yoshida. 2008. Concerted action of lactate oxidase and pyruvate oxidase in aerobic growth of Streptococcus pneumoniae: role of lactate as an energy source. J. Bacteriol. 190:3572-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 46.Valdivia, R. H., and S. Falkow. 1997. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 277:2007-2011. [DOI] [PubMed] [Google Scholar]
- 47.van Soolingen, D., P. E. de Haas, P. W. Hermans, and J. D. Van Embden. 1994. DNA fingerprinting of Mycobacterium tuberculosis. Methods Enzymol. 235:196-205. [DOI] [PubMed] [Google Scholar]
- 48.Weiser, J. N., D. Bae, H. Epino, S. B. Gordon, M. Kapoor, L. A. Zenewicz, and M. Shchepetov. 2001. Changes in availability of oxygen accentuate differences in capsular polysaccharide expression by phenotypic variants and clinical isolates of Streptococcus pneumoniae. Infect. Immun. 69:5430-5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, J. R., K. E. Mostov, M. E. Lamm, M. Nanno, S. Shimida, M. Ohwaki, and E. Tuomanen. 2000. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102:827-837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.