Abstract
The MarA protein of Escherichia coli can both activate and repress the initiation of transcription, depending on the position and orientation of its degenerate 20-bp binding site (“marbox”) at the promoter. For all three known repressed genes, the marbox overlaps the promoter. It has been reported that MarA represses the rob promoter via an RNA polymerase (RNAP)-DNA-MarA ternary complex. Under similar conditions, we found a ternary complex for the repressed purA promoter also. These findings, together with the backwards orientation of repressed marboxes, suggested a unique interaction of MarA with RNAP in repression. However, no repression-specific residues of MarA could be found among 38 single-alanine replacement mutations previously shown to retain activation function or among mutants from random mutagenesis. Mutations Thr12Ala, Arg36Ala, Thr95Ile, and Pro106Ala were more damaging for activation than for repression, some up to 10-fold, so these residues may play a specific role in activation. We found that nonspecific binding of RNAP to promoterless regions of DNA was presumably responsible for the ternary complexes seen previously. When RNAP binding was promoter specific, MarA reduced RNAP access to the rob promoter; there was little or no ternary complex. These findings strongly implicate steric hindrance as the mechanism of repression of rob by MarA.
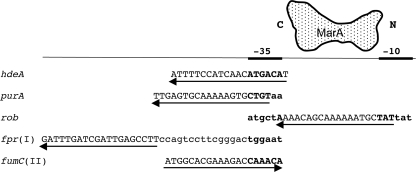
MarA is a regulator of transcriptional initiation in Escherichia coli that affects many genes (2). It directly activates 25 promoters, with about 30 more predicted (13, 14). MarA also directly represses 3 genes, hdeA and purA (23) and rob (24), in all cases by binding as a monomer to a degenerate asymmetrical 20-bp “marbox” in the promoter region. The orientation and location of the marbox influences the type of regulation by MarA. Most marboxes for class I-activated promoters are in the “backward” orientation and are located upstream of their promoters, while all class II activation marboxes are in the “forward” orientation and overlap the −35 region of their promoters (10) (see Fig. 1 for examples). The marboxes for all three repressed promoters have a backward orientation. The marboxes of hdeA and purA overlap only the −35 promoter motif (23), while that for rob lies between the −35 and −10 motifs and partly overlaps each (24) (Fig. 1). MarA protein binds to the 5′-to-3′ marbox in approximately an N terminus-to-C terminus direction (Fig. 1). All marboxes are aligned with the promoter motifs such that MarA is predicted to be on the same face of the DNA as RNA polymerase (RNAP).
FIG. 1.
Selected promoters repressed or activated by MarA. Marboxes are aligned with the −35 promoter motif. The first three sequences show all known directly repressed promoters, and the last two show typical directly activated promoters of classes I and II. Uppercase indicates the 20-bp marboxes; the putative −35 (and −10 for rob) promoter motifs are in boldface. Arrows show direction and location of the marboxes. A schematic MarA protein is shown with N and C termini oriented on the rob marbox. These data are according to references 10, 23, and 24.
Because of the degeneracy of the marbox, there are about 15,000 possible marboxes in the genome (13). There is evidence that activation by MarA (4) and its homolog SoxS (7, 25) involves the participation of a “prerecruitment” complex between the mobile carboxy-terminal domain of the α subunit (“αCTD”) of RNAP and MarA or SoxS that may help guide the two regulators to “true” mar-/soxboxes associated with promoters (14).
Repression by MarA at the rob promoter occurs before the formation of the RNAP-DNA open complex (24). Many transcriptional repressors act simply by interfering with the binding of RNAP to a promoter (20). However, even though the marbox for MarA at rob is located between the −35 and −10 motifs, such “steric hindrance” appeared not to occur there, since a MarA-RNAP-rob promoter ternary complex was seen in vitro (24).
In this work, we revisit the mechanism of repression by MarA.
MATERIALS AND METHODS
E. coli strains and promoter-lacZ fusions.
The host strain, with one exception, was N8452 (ΔlacU169, del1738 [Δ39 kb, including marRAB, but selected for loss of the originally linked zdd-239::Tn9] rob::kan) (12). Different activated or repressed promoter-lacZ fusions on chromosomal λ lysogens of N8452 were used. The host strain bearing λ-inaA-lacZ retained zdd-239::Tn9 and was rob+. MarA-activated lacZ promoter fusions (Kanr) were described previously (6). The repressed promoter regions cloned upstream of lacZ are (with respect to their transcriptional start sites in base pairs): hdeA, −349 to +23 (22), purA, −97 to +19 (T. M. Barbosa and L. M. McMurry, unpublished data), and rob, −88 to +136 (21, 24); the hdeA-lacZ and purA-lacZ λ lysogens are Ampr, while the rob-lacZ lysogen is Kanr and was obtained from R. G. Martin. The purA-lacZ and hdeA-lacZ fusions were transferred into host N8452 by phage P1 transduction and confirmed as single λ lysogens by PCR as described previously (18).
Plasmids bearing wild-type and mutant marA.
To avoid the clumping and lysis seen in our hands even at 30°C with the high-copy-number pUC plasmid constitutively expressing marA (6), we transferred the mutations to a low-copy-number, arabinose-inducible plasmid, pMPM-TmarA. This plasmid (5.54 kb, ori p15A, tetracycline resistant, marA regulated by the araBAD promoter) was constructed by PCR amplification of wild-type marA and cloning between the EcoRI and PstI sites of pMPM-T6Ω (15), obtained from the Cloning Vector Collection of the Japanese National Institute of Genetics. Control plasmid pMPM9, obtained from pMPM-TmarA by error-prone PCR mutagenesis (data not shown), had an inactivating 1-bp deletion in the 17th codon of MarA. A Thr95Ile mutation was also obtained by error-prone PCR. Alanine replacements in pMPM-TmarA in marA codons 16, 37, 58, 63, 89, 106, 107, 112, and 113 were obtained by a megaprimer method (3), followed by cloning into EcoRI-/PstI-restricted pMPM-TmarA. The remaining alanine replacements were made using mutant marA in pUC plasmids (6) (provided by R. G. Martin) as PCR templates, followed by cloning. The marA insert of each new plasmid was sequenced (Tufts University Core Facility) to verify that only the desired mutation was present.
LacZ (β-galactosidase) assay.
Cells with chromosomal promoter-lacZ fusions containing plasmid pMPM-TmarA derivatives were grown overnight in LB broth (22) with 20 μg/ml tetracycline at 37°C. They were then diluted 1/50 or to an optical density at 600 nm (OD600) of 0.04 in 4 ml of the same medium supplemented with 0.05% arabinose, enough to saturate the araBAD promoter (26). The induction of MarA specified by pMPM-TmarA did not begin until the OD600 reached about 0.3, irrespective of the number of generations of growth in arabinose. After growth for about 3 h (or 6 to 7 h for hdeA expression, which is maximally repressed in stationary phase [22]), the OD600 was determined and one or, usually, two sequential LacZ assays were done 45 min apart on 50 μl of cells in 0.5 ml of LacZ assay buffer (17) at room temperature with lysis by 0.005% SDS, 25 μl chloroform. Each experiment was repeated one or more times. Since LacZ activity varied somewhat with the growth phase of the culture, an OD600 between 0.8 and 1.2 was chosen for a given experiment and LacZ activity at that OD600 was determined for multipoint experiments from a plot of LacZ activity versus OD600; the results of different experiments were averaged. Two controls were used in each experiment, wild-type MarA (pMPM-TmarA) and defective MarA (pMPM9).
Since the LacZ activity induced via arabinose was stable after the removal of arabinose by washing (data not shown), measurement of repression using LacZ reporters was possible only because LacZ was diluted out during cell growth after MarA had blocked lacZ transcription. Wild-type MarA specified by pMPM-TmarA reduced rob and purA LacZ activities to 25% of the levels in the nonrepressing control pMPM9 by the time the OD600 of the culture had risen to 0.8, suggesting that the reduction in the rate of transcription initiation was actually closer to 100%.
PCR products for gel shift (electrophoretic mobility shift assay [EMSA]) experiments.
The DNA used in gel shift experiments was synthesized by PCR and included both promoter and marbox. All PCRs were done using a proofreading DNA polymerase. The template for PCR was chromosomal or plasmid DNA or a PCR product synthesized from the chromosome. The primers for purA were PurApF (5′-CGTTGTGGTCTACTACATGTTGAG) and PurApR (5′-CCCGTTCAGTCAGAAGATCGACGA), yielding a 225-bp product (−143 to +83 with respect to the transcriptional start site) that was employed in experiments for which data were not shown. For the wild-type and mutant rob promoter pair, a 186-bp wild-type rob PCR product (from −77 to +110) was first made using RobF4 (5′-CGTCAAGCCCTAAAACATACTCTA) and RobR (5′-ACAGGGGCTGATCCAGATGACCTTCC). With this product as template, a mutant megaprimer (3) was synthesized using forward mutant primer Rob10F (5′-CAAAAAATGCTATtcgccaattacc, in which 2 bp [in italics] of the −10 promoter motif [underlined] have been altered from the wild type TATtat; the marbox region, in bold uppercase, remained unchanged) with reverse primer RobR. A second PCR product was then synthesized using the mutant megaprimer with RobF4. The resulting 186-bp product was cloned into the SmaI site of pUC19, creating pUCrob10-1. A nonmutant clone, pUCrobWT, was also obtained. These were then used as PCR templates with primers RobF4 (above) and RobR2 (5′-CATAAAATATCCTCATCCTTTCAAC) (from −77 to +46) to synthesize the 122-bp “robWT” (wild-type rob promoter) and “rob10” (mutant rob promoter having a faulty −10 motif) DNA used for gel shift experiments. The primers for fumC were FumCF1 (5′-CGCTGTGTGAAATAAACAGAG) and FumCR1 (5′-GGTGGGCATTTTCTCCGTC), giving a 283-bp product (from −102 to +181).
Gel shift assays.
DNA templates for gel shift assays synthesized by PCR as described above were purified using a Qiagen PCR purification kit. Sigma-saturated holo-RNAP (1 μM stock) was obtained from Epicentre. 6H-MarA (57 μM stock in a 1 M NaCl buffer [24]) was a gift from Paratek Pharmaceuticals, Boston, MA. The default reaction buffer EMSAII/E contained 40 mM Tris·HCl, pH 7.5, at 4°C, 5 mM MgCl2, 100 mM KCl, 1 mM dithiothreitol, 0.1 mM EDTA, 10% glycerol, 100 μg/ml bovine serum albumin. For the “37°C assay,” DNA (5 to 10 nM) and ribonucleotides (100 μM; for rob, UTP, CTP, and ATP, and for fumC, UTP, CTP, and GTP) were mixed in EMSAII/E buffer on ice. Then, MarA followed by RNAP was added (the order was reversed for the experiment whose results are shown in Fig. 3) and the tubes were incubated for 10 min on ice and for 20 min at 37°C and then chilled on ice, and sheared salmon sperm DNA (Ambion) was added at 5 μg/ml, followed by one-quarter volume of gel-loading buffer containing bromophenol blue in 45% glycerol. A 5% acrylamide gel (14 cm by 14 cm, 1.5 mm thick, 19:1 ratio of acrylamide/bis [National Diagnostics]) was preelectrophoresed in one-quarter TBE (22.5 mM Tris-borate, 0.5 mM EDTA) backwards (toward the cathode) for 1 h at 300V (20V/cm) at 4°C, and fresh cathode (top) buffer was added. Assay samples were loaded immediately and electrophoresed toward the anode for 0.7 to 3 h at 4°C. The gel was stained for 30 min in 50 ml of prefiltered 20 mM Tris·HCl, pH 7.5, to which SYBR green I stain (Invitrogen) at a 1/10,000 dilution had been freshly added. UV-excited fluorescence of the gel was recorded by a charge-coupled device (CCD) camera in a Kodak Gel Doc 1000 system in which the default (ethidium bromide) filter was replaced with a GG495 Schott longpass filter (Chroma) that was transparent at ≥495 nm. Images were taken at a variety of exposures.
FIG. 3.
Results of gel shift experiment showing the effects of increasing concentrations of MarA on initiation complex (RD) formation at the rob promoter when RNAP was added before MarA. The 37°C assay was used, except that RNAP was added 10 min before MarA at 4°C; after the addition of MarA, the usual 10-min incubation at 4°C was performed before placing the samples at 37°C. The rob DNA concentration was 10 nM. Band labeling is as described for Fig. 2.
The “4°C assay” for rob DNA was similar except that it was all done on ice, preelectrophoresis was toward the anode, no nucleotides (or salmon sperm DNA, unless stated otherwise) were added, and the mixture was incubated for 10 min with MarA before the addition of RNAP, after which the mixture was incubated for another 10 min at 4°C, as described previously (24).
The 4°C assay for purA DNA (data not shown) was done in buffer EMSAI, in which there was no MgCl2 or bovine serum albumin, NaCl replaced KCl, and 0.5 μg/ml sheared salmon sperm DNA was present during the assay (but did not form bands in the gel). The purA DNA concentration was 7.5 nM.
Western blot analysis for MarA.
Some SYBR green-stained gels were subjected to Western blot analysis to locate MarA protein. The stained gel was shaken in 4 volumes of gel blot buffer (48 mM Tris, 39 mM glycine, 20% methanol, 0.0375% SDS, pH 9.2) for 20 min and electroblotted onto a polyvinylidene difluoride membrane for 1 h at 150 mA with a semidry blotter (Bio-Rad). The membrane was blocked for 1 h in 3% powdered milk in TBS (25 mM Tris base, 22 mM HCl, 17 mM NaCl, 5 mM KCl) and probed in TBS containing 0.1% milk, 0.05% Tween 20, and 1/3,000 rabbit anti-MarA (16) followed by 1/10,000 anti-rabbit horseradish peroxidase conjugate (Cell Signaling). The luminescence signal (Western Light; Perkin Elmer) was captured on Kodak MR film, which was scanned using Adobe Photoshop.
RESULTS AND DISCUSSION
Ternary complex formed with purA promoter at 4°C.
Under conditions similar to those used with the rob promoter (24), we performed gel shift experiments using purA promoter DNA, followed by Western blotting with anti-MarA. We indeed found an apparent ternary complex for purA at 4°C comprising RNAP, DNA, and MarA (data not shown).
No repression-specific MarA mutations were found.
The appearance of a ternary complex not only for the rob promoter (24) but now also for the purA promoter encouraged us to think, as suggested earlier (24), that MarA might repress both promoters not by steric hindrance but via an interaction between marbox-bound MarA and promoter-bound RNAP to prevent proper initiation. This is a mechanism known for other repressors (20). Given the unique placement and backwards orientation of the repressed marboxes, mutants of MarA specific for repression might indicate a region of MarA interacting with RNAP. We therefore undertook a search for repression-specific MarA mutations.
We tested many of the alanine replacement mutations from the collection of Gillette et al. (6), avoiding those which prevent binding of MarA to marbox DNA, for their effect on repression using chromosomal lacZ promoter fusions. We examined the following three categories of mutations: (i) some of those known to affect activation, namely, at residues 19, 52, 54, 77, 80, and 91; (ii) those in contiguous codons at the amino terminus (in or near the first helix of MarA at residues 5, 6, 7, 8, 9, 11, 12, 13, 14, 15, 16, 17, and 18), since this region of MarA would be potentially close to RNAP for at least hdeA and purA; and (iii) all those known not to affect activation. This third group comprised the remaining mutants of MarA reported by Gillette et al. (6) to have more than 60 to 80% of the wild-type activity for three or more of five activated promoters and not less than 60% for any single promoter. Since mutants in the third group had activity, they would not fail to repress due to an unstable MarA protein. They might, however, fail to bind uniquely to our repressed promoters. These were substitutions of alanine (or arginine at codon 25) at codons 22, 25(R), 26, 27, 31, 34, 35, 37, 55, 57, 58, 62, 67, 71, 74, 84, 85, 89, 92, 93, 97, 100, 104, 107, 108, 111, 112, and 113. We also tested substitutions at positions 63 and 106, which were not examined by Gillette et al.
In the first and second categories, we found that most MarA mutations that reduced activation also reduced repression to a similar degree and so were not of interest. MarA mutants Leu80Ala and Gln91Ala were able to function at those class I but not class II activated promoters tested (as expected; see reference 6). They repressed all three repressed promoters very well (data not shown). Ile13Ala had poor activity with all promoters except micF, where activation was excellent, while the newly made mutant Ile16Ala had poor activity with all promoters (data not shown). In the third category, we found Ser26Ala to have ≤45% activity at all promoters (disagreeing with Gillette et al. [6]) and to produce a smaller amount of MarA protein (one-third or less of the wild-type level), while Arg63Ala had no activity on any promoter (data not shown). The remaining substitutions in the third category all had good (≥65%) repression activity at purA (data not shown).
Therefore, none of the mutations tested affected the repressing activity of MarA more than its activating activity.
MarA likely blocks RNAP access to the rob promoter by steric hindrance.
Since we found no repression-specific mutants of MarA, either by PCR-mediated error-prone random mutagenesis (40 independent but mostly unsequenced mutants, tested using hdeA and fumC promoters [unpublished results]) or by alanine substitution (above), we considered the following possible explanations: (i) we had not tested the pertinent codon substitution in MarA; (ii) more than a single amino acid change was required to see the phenotype; (iii) repression-specific interactions with RNAP involved only backbone atoms of MarA, not side chains; and (iv) MarA acted by steric hindrance, not by interacting with RNAP, so repression-specific mutants would never be found.
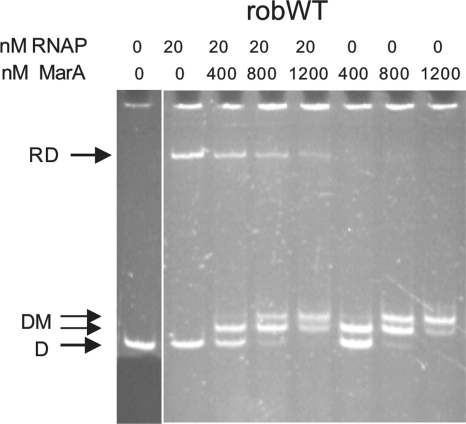
We suspected that the third explanation would be unlikely for all interactions. Since our mutagenesis was not saturating, the first and second explanations remained theoretical possibilities. Nevertheless, we decided to examine the last possibility, that of steric hindrance. Using gel shift assays, we confirmed the previous report (24) that at 4°C, MarA did not block binding of RNAP to wild-type rob DNA (robWT), at least at the major RNAP-DNA band, but rather formed a ternary complex of RNAP-DNA-MarA (Fig. 2A, 4°C, RDM, lanes 3, 4, and 9). If salmon sperm DNA was added at 5 μg/ml at the end of this reaction, the ternary complex was destroyed (Fig. 2A, 4°C, lanes 5 and 10), showing that it was not stable. A similar heparin-induced dissociation of the ternary complex formed at 4°C was reported previously (24).
FIG. 2.
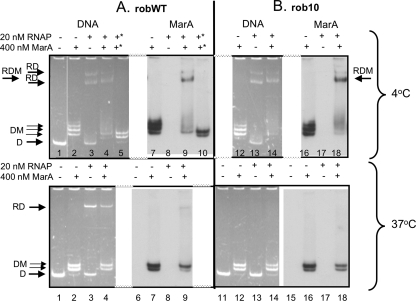
Results of gel shift experiment showing MarA and RNAP binding to wild-type robWT (A) and mutant rob10 (B) promoters at 4°C and 37°C. 4°C, incubation was all at 4°C. 37°C, MarA and RNAP were preincubated together at 4°C with DNA followed by the formation of stable RNAP-DNA initiation complexes in the presence of three nucleotides at 37°C. All samples are from the same gel and experiment. For details, see Materials and Methods. The rob DNA concentration was 10 nM. For panel A at 4°C, the lanes indicated by an asterisk received salmon sperm DNA at 5 μg/ml at the end of the reaction. The fluorescent band in the top of the wells was seen even in the absence of sample. DNA was detected by fluorescence; MarA was detected by anti-MarA. D, free DNA; RD, RNAP-DNA; RDM, RNAP-DNA-MarA; DM, DNA-MarA.
FIG. 4.
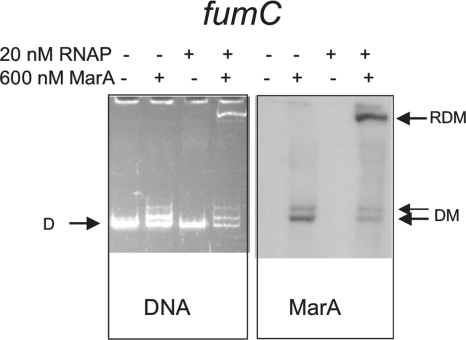
Results of gel shift experiment showing the effect of preincubation with MarA on initiation complex (RDM) formation by RNAP at activated promoter fumC. The 37°C assay was used. DNA and MarA were detected as described for Fig. 2. The fumC DNA concentration was 10 nM. Band labeling is as described for Fig. 2.
However, upon further investigation we found that, at 4°C, RNAP bound even to promoterless E. coli PCR products and promiscuously and strongly to DNA such as salmon sperm DNA and dI/dC (data not shown). We further noted that RNAP bound at 4°C even to a mutant rob promoter having a faulty −10 motif (rob10), forming two bands (Fig. 2B, 4°C, RD, lane 13), and formed a ternary complex (migrating slightly slower than the faster RD band) if MarA was present (Fig. 2B, 4°C, RDM, lanes 14 and 18). Therefore, at 4°C, a ternary complex would result simply by binding of RNAP nonspecifically to nonpromoter sequences, while MarA bound correctly to its marbox on the same DNA molecule.
To increase the specificity of RNAP for promoter regions, we altered various conditions, including the reaction buffer, the temperature of both the assay and the gel, the cross-linking of the gel, and the addition of nonspecific DNAs. Only when we performed the experiments at 37°C for 20 min in the presence of three ribonucleotides (U, A, and C) to allow the formation of stable initiation RNAP-rob promoter complexes and added salmon sperm DNA at the end of the reaction to eliminate nonstable complexes did we achieve the desired specificity (see “Gel shift assays” in Materials and Methods).
When we now compared the same wild-type and mutant rob promoter DNA using this 37°C assay, an RNAP-DNA band appeared only for the wild-type rob promoter, robWT (Fig. 2A, 37°C, RD, lane 3), and not for the mutated one, rob10 (Fig. 2B, 37°C, lane 13). As expected, when MarA alone was added, both DNA templates bound MarA equally (Fig. 2, 37°C, DM, lanes 7 and 9 versus lanes 16 and 18). Even though MarA has been shown to bind DNA as a monomer (19), we often saw more than one MarA-DNA band in gel shifts (see Fig. 2, 3, and 4), as has been reported by others for MarA at other promoters (1, 11).
If MarA was incubated on ice with RNAP prior to formation of the wild-type rob initiation complex at 37°C, the amount of RNAP-DNA complex was reduced (Fig. 2A, 37°C, RD, lane 4 versus lane 3). Very little ternary complex was seen (Fig. 2A, 37°C, at the RD position in lane 9): nearly all MarA was bound alone (to the marbox), not with promoter-bound RNAP (Fig. 2A, 37°C, DM, lane 9). In a repeat experiment at 37°C, no ternary complex at all was seen (data not shown). By binding to the promoter, RNAP also competed with MarA at the overlapping MarA binding site (marbox), in turn reducing MarA binding to DNA (Fig. 2A, 37°C, DM, lane 2 versus 4 and lane 7 versus 9). The 1 M NaCl buffer in which MarA was dissolved had no effect by itself (data not shown). The amount of RNAP-DNA complex decreased with increasing concentrations of MarA (Fig. 3, RD). In these experiments, in contrast to those whose results are shown in Fig. 2, RNAP was added prior to MarA, but it was still displaced from DNA by MarA, suggesting that MarA and RNAP reach equilibrium binding with DNA at 4°C. We concluded that MarA and RNAP competed for binding to DNA in the rob promoter region, with MarA blocking RNAP from binding by steric hindrance.
As a final control, we looked at the effect of MarA on the formation of an initiation complex at the class I-activated promoter fumC. In this case, no RNAP was bound to the fumC promoter in the absence of MarA, but when MarA was added, a ternary complex containing both MarA and RNAP bound to promoter-containing DNA clearly appeared (Fig. 4, RDM), as expected (9).
Previous experiments had shown that MarA represses rob at a step prior to the formation of the RNAP-DNA open complex and that MarA greatly reduces the RNAP-dependent cleavage of promoter DNA by potassium permanganate without altering the pattern of that cleavage (24). These findings are all consistent with a steric hindrance model for repression.
From the crystal structures of RNAP-DNA and MarA-DNA, the MarA and RNAP binding sites are predicted to be on the same face of the DNA for all MarA-regulated promoters (see reference 24). This alone is highly suggestive of a steric hindrance mechanism for repression at the rob promoter. The rob marbox lies between the −10 and −35 motifs to which RNAP binds (Fig. 1), so that RNAP would have to sit on top of MarA if both were bound together in a ternary complex. That MarA must bind the rob promoter before formation of the open complex to inhibit transcription, as reported earlier (24), is consistent with a steric hindrance mechanism.
Four mutations in MarA were specifically defective for activation.
Although we did not find any MarA mutations that were defective more for repression than for activation, we did observe the opposite, MarA mutants that were much (Thr12Ala and Pro106Ala) or somewhat (Arg36Ala and Thr95Ile) more defective for activation than for repression (Table 1).
TABLE 1.
Activity of selected MarA mutants at repressed and activated promotersa
| Role of residue | MarA mutation | % Repression |
% Activation |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type 1 |
Type 2 | Class I |
Class II |
|||||||
| purA | hdeA | rob | fpr | mar | zwf | fumC | inaAb | micF | ||
| Binds αCTD | Trp19Ala | 10 | 40 | 30 | 0 | — | 5c | 5c | — | 10 |
| Relatively activation | Arg36Ala | 95 | >100 | 110 | — | — | 35 | — | 50 | — |
| specific | Thr12Ala | 90 | 95 | 85 | 15 | 45 | 15 | 15 | 30 | 30 |
| Thr95Ile | 70 | 50 | 70 | 0 | 50 | 10 | −10 | 5 | 0 | |
| Pro106Ala | 40 | 55 | 25 | 0 | 5 | 0 | 0 | — | 0 | |
Assays were performed in duplicate or triplicate (see Materials and Methods) except where noted. Values (±10%) are given to the nearest 5% and are normalized to the results for wild-type MarA as 100% repression or activation. Specifically, the percent repression by a MarA protein is determined by the equation [(P − M)/P]100 and the percent activation by a MarA protein is determined by the equation [(M − P)/P]100, where P and M are the LacZ activities of cells containing pMPM9 (completely inactive MarA control) and the MarA protein in question, respectively. Finally, the values in the table were obtained by normalizing the percent for a MarA mutant to that of wild-type MarA, set as 100%. —, not determined.
One experiment, two points.
Two experiments, one point each; negative control has plasmid pMPM-T6Ω instead of pMPM9.
It is possible that some of these effects are at the level of DNA binding. For example, nuclear magnetic resonance (NMR) studies comparing MarA bound to different activated marboxes suggested that Thr12 may modulate binding of MarA to DNA (5). Thr95 makes van der Waals contacts with the thymidine residues complementing conserved adenines 16 and 17 of the mar promoter marbox (19); it might be predicted, therefore, that only promoters having one or both adenines would be affected by the Thr95Ile mutation. This was not quite the case, however. The micF marbox lacks both adenines, yet micF was still affected by Thr95Ile, as were all other activated promoters (Table 1), each of which has both marbox adenines. The marboxes of the repressed promoters purA and rob have neither of these adenines and were indeed not affected by the Thr95Ile mutation, but hdeA, with both adenines, was also not affected very much (Table 1). Although Pro106 itself does not bind to DNA, a Pro106Ala mutation might affect binding by the adjacent residues His107 and Lys108, both of which bind to the same backbone phosphate in the mar marbox (19). Arg36 is reported to interact with the αCTD of RNAP and is discussed below.
If the activation specificity of the mutant proteins were to occur solely via DNA binding, there should be a definable difference between the sequences of activated marboxes and repressed marboxes. We think it more likely that one or more of the four activation-specific mutations decreases the productive interaction of MarA with RNAP at activated promoters. A similar effect would not be expected for repression, at least at rob, since it occurs via steric hindrance.
Repression and prerecruitment.
An NMR study on mixtures of a MarA-marbox complex with the αCTD of RNAP indicates that Trp19 and Arg36 of MarA interact with αCTD at a “265-like DNA-binding determinant” of αCTD; the Trp19Ala and Arg36Ala mutations only minimally affect binding to the mar marbox in EMSA assays (4). Furthermore, the Trp19Ala mutation of MarA decreases binding in vitro of the MarA-marbox to the αCTD and is proposed therefore to reduce prerecruitment of MarA by RNAP (4). We found that Trp19Ala did not function at fpr, zwf, fumC, and micF (all activated promoters), as expected (Table 1). It also functioned poorly at purA but moderately for rob and hdeA (all repressed promoters) (Table 1). The Arg36Ala mutation was also reported to reduce activation to about 50% of the wild-type level (4). We found a similar effect (35% to 50% of the wild-type level) on the activation controls zwf and inaA (Table 1). However, Arg36Ala repressed purA, hdeA, and rob as well as or better than did wild-type MarA (Table 1). Therefore, while our results with Trp19Ala suggest that prerecruitment might play some role in repression, our Arg36Ala data suggest the contrary, so the possible role of prerecruitment in repression is unclear.
Conclusions.
We found no MarA mutations specific for repression. We did identify four residues more specific for activation that may have a unique role there. The importance of using promoterless controls for gel shift experiments with RNAP was shown. Steric hindrance appears to be the mechanism for repression of rob by MarA.
Acknowledgments
We are grateful to R. G. Martin for providing most of the alanine scan mutations used in this work, as well as host strain N8452 and all promoter-lacZ fusions therein except for hdeA and purA. We thank Paratek Pharmaceuticals, Boston, MA, for MarA protein, the National Institute of Genetics of Japan for pMPM-T6Ω, A. L. Sonenshein for the idea to use initiation complexes, T. Schneiders for performing the construction of pMPM-TmarA and for helpful discussions, and an anonymous reviewer for suggestions.
This work was supported by United States Public Health Service grant AI56012 from the National Institutes of Health.
Footnotes
Published ahead of print on 7 May 2010.
REFERENCES
- 1.Barbosa, T. M., and S. B. Levy. 2002. Activation of the Escherichia coli nfnB gene by MarA through a highly divergent marbox in a class II promoter. Mol. Microbiol. 45:191-202. [DOI] [PubMed] [Google Scholar]
- 2.Barbosa, T. M., and P. J. Pomposiello. 2005. The mar regulon, p. 209-223. In D. G. White, M. N. Alekshun, and P. F. McDermott (ed.), Frontiers in antimicrobial resistance: a tribute to Stuart B. Levy. ASM Press, Washington, DC.
- 3.Colosimo, A., Z. Xu, G. Novelli, B. Dallapiccola, and D. C. Gruenert. 1999. Simple version of “megaprimer” PCR for site-directed mutagenesis. Biotechniques 26:870-873. [DOI] [PubMed] [Google Scholar]
- 4.Dangi, B., A. M. Gronenborn, J. L. Rosner, and R. G. Martin. 2004. Versatility of the carboxy-terminal domain of the alpha subunit of RNA polymerase in transcriptional activation: use of the DNA contact site as a protein contact site for MarA. Mol. Microbiol. 54:45-59. [DOI] [PubMed] [Google Scholar]
- 5.Dangi, B., P. Pelupessey, R. G. Martin, J. L. Rosner, J. M. Louis, and A. M. Gronenborn. 2001. Structure and dynamics of MarA-DNA complexes: an NMR investigation. J. Mol. Biol. 314:113-127. [DOI] [PubMed] [Google Scholar]
- 6.Gillette, W. K., R. G. Martin, and J. L. Rosner. 2000. Probing the Escherichia coli transcriptional activator MarA using alanine-scanning mutagenesis: residues important for DNA binding and activation. J. Mol. Biol. 299:1245-1255. [DOI] [PubMed] [Google Scholar]
- 7.Griffith, K. L., and R. E. Wolf, Jr. 2004. Genetic evidence for pre-recruitment as the mechanism of transcription activation by SoxS of Escherichia coli: the dominance of DNA binding mutations of SoxS. J. Mol. Biol. 344:1-10. [DOI] [PubMed] [Google Scholar]
- 8.Reference deleted.
- 9.Martin, R. G., W. K. Gillette, N. I. Martin, and J. L. Rosner. 2002. Complex formation between activator and RNA polymerase as the basis for transcriptional activation by MarA and SoxS in Escherichia coli. Mol. Microbiol. 43:355-370. [DOI] [PubMed] [Google Scholar]
- 10.Martin, R. G., W. K. Gillette, S. Rhee, and J. L. Rosner. 1999. Structural requirements for marbox function in transcriptional activation of mar/sox/rob regulon promoters in Escherichia coli: sequence, orientation and spatial relationship to the core promoter. Mol. Microbiol. 34:431-441. [DOI] [PubMed] [Google Scholar]
- 11.Martin, R. G., K. W. Jair, R. E. Wolf, Jr., and J. L. Rosner. 1996. Autoactivation of the marRAB multiple antibiotic resistance operon by the MarA transcriptional activator in Escherichia coli. J. Bacteriol. 178:2216-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin, R. G., and J. L. Rosner. 1997. Fis, an accessorial factor for transcriptional activation of the mar (multiple antibiotic resistance) promoter of Escherichia coli in the presence of the activator MarA, SoxS, or Rob. J. Bacteriol. 179:7410-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin, R. G., and J. L. Rosner. 2002. Genomics of the marA/soxS/rob regulon of Escherichia coli: identification of directly activated promoters by application of molecular genetics and informatics to microarray data. Mol. Microbiol. 44:1611-1624. [DOI] [PubMed] [Google Scholar]
- 14.Martin, R. G., and J. L. Rosner. 2005. Structure and function of MarA and its homologs, p. 235-246. In D. G. White, M. N. Alekshun, and P. F. McDermott (ed.), Frontiers in antimicrobial resistance: a tribute to Stuart B. Levy. ASM Press, Washington, DC.
- 15.Mayer, M. P. 1995. A new set of useful cloning and expression vectors derived from pBlueScript. Gene 163:41-46. [DOI] [PubMed] [Google Scholar]
- 16.McDermott, P. F., D. G. White, I. Podglajen, M. N. Alekshun, and S. B. Levy. 1998. Multidrug resistance following expression of the Escherichia coli marA gene in Mycobacterium smegmatis. J. Bacteriol. 180:2995-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratories Press, Cold Spring Harbor, NY.
- 18.Powell, B. S., M. P. Rivas, D. L. Court, Y. Nakamura, and C. L. Turnbough, Jr. 1994. Rapid confirmation of single copy lambda prophage integration by PCR. Nucleic Acids Res. 22:5765-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhee, S., R. G. Martin, J. L. Rosner, and D. R. Davies. 1998. A novel DNA-binding motif in MarA: the first structure for an AraC family transcriptional activator. Proc. Natl. Acad. Sci. U. S. A. 95:10413-10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rojo, F. 2001. Mechanisms of transcriptional repression. Curr. Opin. Microbiol. 4:145-151. [DOI] [PubMed] [Google Scholar]
- 21.Rosner, J. L., B. Dangi, A. M. Gronenborn, and R. G. Martin. 2002. Posttranscriptional activation of the transcriptional activator Rob by dipyridyl in Escherichia coli. J. Bacteriol. 184:1407-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz, C., L. M. McMurry, and S. B. Levy. 2008. Role of the multidrug resistance regulator MarA in global regulation of the hdeAB acid resistance operon in Escherichia coli. J. Bacteriol. 190:1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneiders, T., T. M. Barbosa, L. M. McMurry, and S. B. Levy. 2004. The Escherichia coli transcriptional regulator MarA directly represses transcription of purA and hdeA. J. Biol. Chem. 279:9037-9042. [DOI] [PubMed] [Google Scholar]
- 24.Schneiders, T., and S. B. Levy. 2006. MarA mediated transcriptional repression of the rob promoter. J. Biol. Chem. 281:10049-10055. [DOI] [PubMed] [Google Scholar]
- 25.Shah, I. M., and R. E. Wolf, Jr. 2004. Novel protein-protein interaction between Escherichia coli SoxS and the DNA binding determinant of the RNA polymerase alpha subunit: SoxS functions as a co-sigma factor and redeploys RNA polymerase from UP-element-containing promoters to SoxS-dependent promoters during oxidative stress. J. Mol. Biol. 343:513-532. [DOI] [PubMed] [Google Scholar]
- 26.Siegele, D. A., and J. C. Hu. 1997. Gene expression from plasmids containing the araBAD promoter at subsaturating inducer concentrations represents mixed populations. Proc. Natl. Acad. Sci. U. S. A. 94:8168-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]