Covalent modification of eukaryotic proteins by ubiquitin and the distantly related ubiquitin-like protein called SUMO contributes to an extraordinary assortment of biological regulatory mechanisms (2). These attachments have different consequences, depending not only on which of the proteins is attached but also on whether they are in the form of a polymer. Polyubiquitin chains connected by amide (isopeptide) bonds involving certain ubiquitin lysine side chains, for instance, are often signals for directing a substrate to the proteasome for degradation. The ubiquitylation and sumoylation pathways intersect in a variety of ways. An intriguing example is the recently unearthed group of ubiquitin-ligating enzymes called SUMO-targeted ubiquitin ligases (STUbLs), which recognize (poly)SUMO-modified proteins and attach ubiquitin to the conjugates, leading to their degradation by the proteasome (3, 9). Discovery of the STUbLs has raised many interesting questions, such as whether the ubiquitin is attached to the SUMO moiety or the substrate itself in the SUMO-substrate conjugate and how SUMO and ubiquitin are recycled from these conjugates once they have reached the proteasome. In this issue, the study by Mullen et al. (8) throws fresh light on these questions through the characterization of an unusual SUMO-directed protease that may associate with the proteasome and seems able to cleave both ubiquitin and SUMO from proteins.
The protease in question is S. cerevisiae Wss1 (weak suppressor of smt3), which had initially been identified as a high-copy-number suppressor of a mutation in SMT3, the lone yeast gene encoding SUMO (1). Subsequent sequence analysis led to the prediction that Wss1 is the prototype of a novel family of SUMO- or ubiquitin-cleaving enzymes called the WLM family (Wss1-like metalloproteases), which are broadly distributed in fungi and plants and a few additional species (5). Until now, this prediction had never been tested. Mullen et al. (8) report that Wss1 is indeed a SUMO-dependent protease, with interesting genetic interactions with other components of the ubiquitin/SUMO system and some unusual biochemical properties.
Yeast cells lacking Wss1 do not suffer major growth defects, but the protein becomes important in strains that are also without Sgs1, a DNA helicase related to the human Bloom and Werner syndrome helicases (8). Notably, the heterodimeric STUbL Slx5-Slx8 had also been found to be essential for viability in cells lacking Sgs1, leading the authors to investigate a possible link between Wss1 and Slx5-Slx8. Overexpressed Wss1 could suppress the synthetic lethality associated with sgs1Δ slx5Δ or sgs1Δ slx8Δ double mutants. Although no enhancement of slx5Δ growth defects was seen for wss1Δ slx5Δ double mutants, a slight increase in the accumulation of large poly-SUMO-linked proteins typical of slx5Δ single mutants (but not wss1Δ mutants) was observed by anti-SUMO immunoblotting. Poly-SUMO-modified proteins are believed to be preferred substrates of the Slx5-Slx8 ubiquitin ligase, which normally would lead to their elimination by the proteasome.
These genetic links between Wss1, Slx5-Slx8, and the SUMO system prompted a biochemical analysis of the Wss1 protein. In its central region, Wss1 has a putative Zn-binding motif commonly found in Zn-dependent metalloproteases (5). Mullen et al. created a mutant derivative, Wss1-pd (protease deficient), in which two of the three histidines in this motif were changed to alanines. Expression of the wss1-pd allele did not complement the slow growth of wss1Δ sgs1Δ yeast cells. Both Wss1 and Wss1-pd were then overproduced in yeast and partially purified. Based on glutathione S-transferase (GST)-ubiquitin and GST-SUMO pulldown assays, Wss1 binds to SUMO but not ubiquitin. This is consistent with the presence of two predicted SUMO-interacting motifs (SIMs) in the C-terminal region of Wss1, which was previously shown to associate with SUMO (4). Incubation of Wss1 with high-molecular-weight 32P-labeled poly-SUMO chains led to release of free SUMO and short SUMO oligomers. Minimal cleavage was seen with Wss1-pd. In contrast, wild-type Wss1 had little if any activity against 32P-labeled polyubiquitin chains. These data would seem to point to a straightforward model in which Wss1 functions as a SUMO isopeptidase that does not act on ubiquitin-modified proteins.
But matters turn out not to be so simple. A substrate that consisted of a poly-SUMO chain capped on its distal monomer by one or a small number of 32P-labeled ubiquitin molecules was also prepared (Fig. 1) (7). Unlike what was observed with pure polyubiquitin chains, labeled ubiquitin monomers were released by treatment with Wss1, suggesting a ubiquitin isopeptidase activity when the ubiquitin is attached to SUMO or a SUMO chain. Furthermore, when a translational fusion between ubiquitin and SUMO followed by a peptide tag was tested as a substrate in vitro, the authors observed a strong preference for cleavage at the C terminus of ubiquitin rather than after SUMO. This appears to indicate that, while Wss1 protease activity depends on SUMO, presumably through binding to its C-terminal SIMs, Wss1 actually might prefer to cleave after ubiquitin. In other words, Wss1 behaves in these assays as a SUMO-targeted deubiquitylating enzyme (DUB), the inverse of a STUbL.
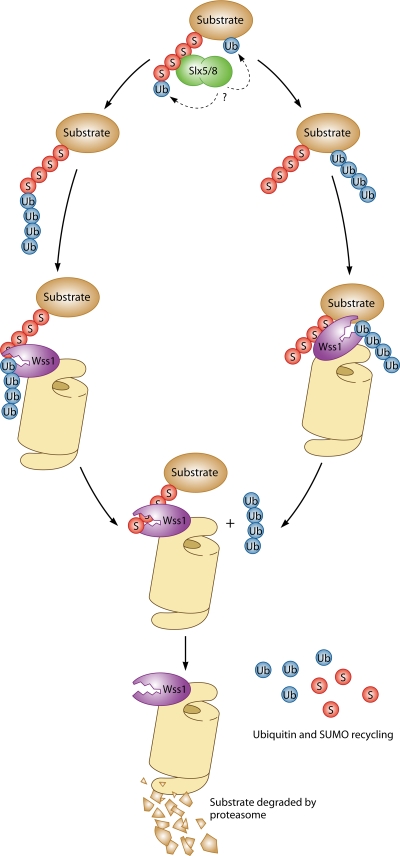
FIG. 1.
Model for the role of Wss1 in proteasomal degradation of polysumoylated proteins. The Slx5-Slx8 ubiquitin (Ub) ligase conjugates mono-Ub either to the terminal SUMO moiety of polysumoylated protein (left) (7) or directly to the target protein (right). Additional Ub molecules might be added to form a poly-Ub chain in vivo, although in vitro assays showed that only a single Ub was added to the poly-SUMO chain (7). In the model, Wss1 associates with the proteasome and binds to the SUMO moieties of Slx5-Slx8 substrates. The latter interaction stimulates Wss1-dependent cleavage of Ub from the substrate. Wss1 may also help recycle SUMO from the conjugates.
While these in vitro analyses of Wss1 represent the heart of the study by Mullen et al. (8), the authors also described some striking genetic interactions with the two known yeast SUMO proteases, Ulp1 and Ulp2. A surprising early finding with the latter enzymes was that their simultaneous mutation partially rescued cells from the deleterious effects of mutating only one or the other (6). Deletion of WSS1 caused a similar suppression of ulp2Δ defects, such as hypersensitivity to the DNA-damaging agent hydroxyurea. Deletion of WSS1 did not suppress a temperature-sensitive ulp1ts mutant, but it did abrogate the ability of ulp1ts to suppress ulp2Δ. Finally, overexpression of Wss1 allowed growth of ulp1ts cells at high temperature (if mature SUMO was also provided). This is precisely what had been observed previously upon overexpression of Slx5 (10). Hence, Wss1 might either supply a critical SUMO isopeptidase activity in the absence of Ulp1 or bypass the requirement for Ulp1 desumoylation activity altogether by stimulating proteasome-dependent degradation of SUMO conjugates, as had been proposed for Slx5-Slx8. The possibility of a direct link between Wss1 and proteasomal activity was suggested by the coprecipitation of Wss1 with proteasomes, although this was done under conditions of strong overexpression of Wss1. These data are summarized in the model shown in Fig. 1.
Many questions are raised by the new study. The most obvious are whether Wss1 is a SUMO protease or a ubiquitin protease (or both) and whether Wss1 is truly a metalloprotease. These questions cannot yet be answered definitively. First, it should be noted that the apparent SUMO isopeptidase activity of Wss1 is substantially weaker under the conditions used than those of the two known yeast SUMO proteases. Second, both ubiquitin and SUMO have flexible C-terminal tails that terminate with a pair of glycines. True isopeptidase activity would involve cleavage between the last of these glycines and the lysine side chain to which it was attached. This was not directly examined by Mullen et al. One testable hypothesis consistent with all the data in the paper is that Wss1 is a generally acting but weak protease that preferentially cleaves conformationally flexible regions of target proteins. When Wss1 binds via its C-terminal SIMs to SUMO, this might position its active site and possibly activate it allosterically to cleave nearby flexible regions. These nearby flexible sites might be either SUMO-protein or ubiquitin-protein linkages. The notion of a less restricted cleavage site specificity compared to those of classical SUMO and ubiquitin proteases is suggested by the additional in vitro fragments generated by Wss1 from the ubiquitin-SUMO-peptide fusion substrate employed by the authors.
If Wss1 is indeed a Zn-dependent metalloprotease, it would seem to have some unusual properties. First, it remains active in the presence of 1 mM EDTA, a divalent cation chelator that was present in the assay buffers and that inhibits most metalloproteases. Second, a mutant protein affecting two of the three histidines in the putative Zn-binding motif retained detectable protease activity. Third, the DUB activity of Wss1 was strongly inhibited by ubiquitin aldehyde, an inhibitor that normally forms a thiohemiacetal linkage to DUBs bearing active-site cysteine nucleophiles. It does not inhibit other known DUBs that utilize a metalloenzyme mechanism (11). Finally, because Wss1 was purified from yeast cells, it is conceivable that the isopeptidase activities derived at least in part from a copurifying protein(s); the lower activity of the Wss1-pd preparation could be explained by structural changes in Wss1-pd that reduced binding to the copurifying enzyme(s).
Regardless of these caveats, Mullen and coworkers have clearly located Wss1 at a key intersection of the SUMO and ubiquitin modification systems. Both the genetic and biochemical data indicate that Wss1 regulation of these pathways is closely related to the activity of the Slx5-Slx8 STUbL. Potentially, Wss1 can facilitate the degradation of Slx5-Slx8 substrates that are both sumoylated and ubiquitylated by recycling one or both of these modifiers once the substrates have docked onto the proteasome (Fig. 1). Experiments to test these and other ideas suggested by the provocative study of Mullen et al. should be illuminating.
Acknowledgments
We are grateful to Rachael Felberbaum and Keith Wilkinson for comments on the manuscript.
Related work from our laboratory is supported by grants from the U.S. National Institutes of Health.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Footnotes
Published ahead of print on 21 June 2010.
REFERENCES
- 1.Biggins, S., N. Bhalla, A. Chang, D. L. Smith, and A. W. Murray. 2001. Genes involved in sister chromatid separation and segregation in the budding yeast Saccharomyces cerevisiae. Genetics 159:453-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denuc, A., and G. Marfany. 2010. SUMO and ubiquitin paths converge. Biochem. Soc. Trans. 38:34-39. [DOI] [PubMed] [Google Scholar]
- 3.Geoffroy, M. C., and R. T. Hay. 2009. An additional role for SUMO in ubiquitin-mediated proteolysis. Nat. Rev. Mol. Cell Biol. 10:564-568. [DOI] [PubMed] [Google Scholar]
- 4.Hannich, J. T., A. Lewis, M. B. Kroetz, S. J. Li, H. Heide, A. Emili, and M. Hochstrasser. 2005. Defining the SUMO-modified proteome by multiple approaches in Saccharomyces cerevisiae. J. Biol. Chem. 280:4102-4110. [DOI] [PubMed] [Google Scholar]
- 5.Iyer, L. M., E. V. Koonin, and L. Aravind. 2004. Novel predicted peptidases with a potential role in the ubiquitin signaling pathway. Cell Cycle 3:1440-1450. [DOI] [PubMed] [Google Scholar]
- 6.Li, S. J., and M. Hochstrasser. 2000. The yeast ULP2 (SMT4) gene encodes a novel protease specific for the ubiquitin-like Smt3 protein. Mol. Cell. Biol. 20:2367-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullen, J. R., and S. J. Brill. 2008. Activation of the Slx5-Slx8 ubiquitin ligase by poly-small ubiquitin-like modifier conjugates. J. Biol. Chem. 283:19912-19921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullen, J. R., C.-F. Chen, and S. J. Brill. 2010. Wss1 is a SUMO-dependent isopeptidase that interacts genetically with the Slx5-Slx8 SUMO-targeted ubiquitin ligase. Mol. Cell. Biol. 30:3737-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry, J. J., J. A. Tainer, and M. N. Boddy. 2008. A SIM-ultaneous role for SUMO and ubiquitin. Trends Biochem. Sci. 33:201-208. [DOI] [PubMed] [Google Scholar]
- 10.Xie, Y., O. Kerscher, M. B. Kroetz, H. F. McConchie, P. Sung, and M. Hochstrasser. 2007. The yeast Hex3.Slx8 heterodimer is a ubiquitin ligase stimulated by substrate sumoylation. J. Biol. Chem. 282:34176-34184. [DOI] [PubMed] [Google Scholar]
- 11.Yao, T., and R. E. Cohen. 2002. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature 419:403-407. [DOI] [PubMed] [Google Scholar]