Abstract
The heptad repeat of the RNA polymerase II (RNAPII) C-terminal domain is phosphorylated at serine 5 near gene 5′ ends and serine 2 near 3′ ends in order to recruit pre-mRNA processing factors. Ser-5(P) is associated with gene 5′ ends to recruit capping enzymes, whereas Ser-2(P) is associated with gene 3′ ends to recruit cleavage and polyadenylation factors. In the gene clusters called operons in Caenorhabditis elegans, there is generally only a single promoter, but each gene in the operon forms a 3′ end by the usual mechanism. Although downstream operon genes have 5′ ends, they receive their caps by trans splicing rather than by capping enzymes. Thus, they are predicted to not need Ser-5 phosphorylation. Here we show by RNAPII chromatin immunoprecipitation (ChIP) that internal operon gene 5′ ends do indeed lack Ser-5(P) peaks. In contrast, Ser-2(P) peaks occur at each mRNA 3′ end, where the 3′-end formation machinery binds. These results provide additional support for the idea that the serine phosphorylation of the C-terminal domain (CTD) serves to bring RNA-processing enzymes to the transcription complex. Furthermore, these results provide a novel demonstration that genes in operons are cotranscribed from a single upstream promoter.
Pre-mRNAs of protein-coding genes must be processed into mature mRNAs for translation. This transcription is carried out by RNA polymerase II (RNAPII) in association with a wide range of nuclear proteins that serve at different stages in the transcription cycle. Shortly after the nascent RNA emerges from RNAPII, its 5′ end is cotranscriptionally capped (6, 8, 26, 29). The addition of the cap is performed in three steps: first, the 5′ phosphate is removed by RNA triphosphatase; second, GMP is added by RNA guanyltransferase; and third, the cap is methylated by RNA methyltransferase (33). At the other end of the gene, the pre-mRNA is cotranscriptionally cleaved by the 3′-end formation machinery composed of the multisubunit proteins cleavage and polyadenylation specificity factor (CPSF) and cleavage-stimulatory factor (CstF) as well as several additional proteins. However, transcription does not terminate (i.e., release from the template) until the polymerase has continued synthesizing RNA for an additional kilobase or more (10, 12, 15). The 3′-end formation machinery, and perhaps pre-mRNA cleavage itself, plays a key role in the termination event. One popular idea is that cleavage exposes a free 5′ phosphate end on the downstream RNA, thereby allowing access to the 5′-to-3′ exonuclease XRN2 (11, 18, 38, 39).
To accommodate the large number of proteins required for these and other cotranscriptional events, RNAPII includes a unique and flexible tail-like domain at the carboxy terminus of its largest subunit, referred to as its carboxy-terminal domain (CTD). The CTD is composed of numerous heptad repeats with the consensus sequence Y1S2P3T4S5P6S7, a sequence conserved among all eukaryotes (35). The number of these heptad repeats correlates with genomic complexity, varying from 26 repeats in the budding yeast Saccharomyces cerevisiae to 42 repeats in Caenorhabditis elegans and 52 repeats in mammals (1, 30).
The deletion of the CTD in mammalian cells inhibits cotranscriptional capping, splicing, 3′-end cleavage, and polyadenylation, suggesting that the CTD functions in coupling transcription with pre-mRNA processing (22, 23). The CTD is dynamically and reversibly modified during transcription (20, 27), predominantly by phosphorylations at heptad repeats at serine 5 [Ser-5(P)] and serine 2 [Ser-2(P)] (9, 41). In vivo and in vitro evidence suggests that these phosphorylations of the RNAPII CTD heptad repeats facilitate the recruitment of processing factors to the transcription complex. Ser-5(P), phosphorylated primarily by cyclin-dependent kinase 7 (cdk7), is required for binding capping enzymes to RNAPII at the 5′ ends of genes (27, 28). On the other hand, the phosphorylation of Ser-2 by positive transcription elongation factor b (pTEFb) is required for binding 3′-end formation/termination factors to RNAPII at the 3′ end of genes (27, 28). In addition, chromatin immunoprecipitation (ChIP) experiments using antibodies specific for these phospho-epitopes in mammals and yeast have shown that Ser-5(P) is present at higher levels at the 5′ ends of genes, while Ser-2(P) levels peak closer to the 3′ ends (13, 19, 20, 31). These observations have led to the proposal that these phosphorylated serine residues guide the cotranscriptional processing of the pre-mRNA at different stages of the transcription cycle (27, 28).
In the nematode C. elegans, many genes are organized into operons, which are polycistronic transcription units in which several genes are cotranscribed from a single promoter at the 5′ end of the gene cluster (3). These operons can contain from 2 to 8 genes, and each gene's mRNA is independently cleaved and polyadenylated at its 3′ end. Transcription termination has been shown to be prevented from occurring at these internal 3′ ends in order for the downstream gene to be expressed (15). The site for the cleavage and polyadenylation of the upstream gene typically occurs only ∼100 bp from the 5′ end of the next gene in the operon. The 3′ end of this short intercistronic region (ICR) is defined by a trans-splice site. The SL2 snRNP is specialized for processing at these sites by a process similar to that of cis splicing, in which a 22-nucleotide (nt) SL2 exon containing a trimethylguanosine cap at its 5′ end is donated to the 5′ end of downstream gene mRNAs. This 22-nt spliced leader RNA provides a cap for these downstream operon RNAs. We know that trans splicing at these internal sites is cotranscriptional, since it is normally difficult or impossible to detect the polycistronic precursors. Thus, downstream operon transcripts are provided with a cap cotranscriptionally by trans splicing rather than by direct processing by the capping enzymes. Thus, they are predicted to be processed without any need for a cotranscriptional binding of capping enzymes to the CTD, and so Ser-5(P) at these 5′ ends should be unnecessary. However, all operon 3′ ends are formed cotranscriptionally by the normal mechanism, which would presumably require Ser-2(P) at the 3′ end of each gene in the cluster. This provides a unique opportunity to test whether Ser-5(P) is indeed associated only with sites requiring a cotranscriptional association of capping enzymes or whether it occurs also at 5′ ends of genes expressed downstream in the cluster at sites distant from the promoter. Furthermore, it provides an opportunity to test whether Ser-2(P) is associated with all RNA 3′ ends in the cluster or only those accompanied by transcription termination at the 3′ end of the entire cluster.
Here we present high-resolution mapping of Ser-5(P) and Ser-2(P) by ChIP quantitative PCR (qPCR) experiments with three different C. elegans operons. In all cases, Ser-5(P) is associated with promoter locations but not with locations specifying 5′ ends of mRNAs distant from promoters, whereas Ser-2(P) is associated with all 3′ ends, even those at large distances from transcription termination sites. These data provide strong support for the idea that these phosphorylation events mark RNA-processing sites rather than gene ends and also provide a novel demonstration that genes in C. elegans operons are cotranscribed as predicted.
MATERIALS AND METHODS
Antibodies.
Ser-5(P) and Ser-2(P) antibodies were obtained from Bethyl Laboratories (catalog numbers A300-655A and A300-654A, respectively). The “total RNAPII” antibody was a rabbit polyclonal antibody raised against glutathione S-transferase (GST)-mouse CTD (positions 1 to 52) and was a gift from David Bentley (32).
ChIP.
Mixed-stage Bristol (N2) worms were maintained and grown as described previously by Sulston and Brenner (36) in liquid culture and harvested in water. The worms were washed three times in water, and the bacteria were cleared by sucrose flotation. The worms were then frozen in liquid nitrogen and ground to a powder with a mortar and pestle. The resulting worm powder was transferred into cross-linking buffer (1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM EDTA, 1 mM EGTA, 1% formaldehyde, phosphate-buffered saline [PBS]) for 10 min at room temperature. The reaction was quenched for 5 min at room temperature by the addition of glycine to the mixture to a final concentration of 125 mM and sedimented at 4,000 × g. Pellets were washed three times with FA buffer plus 0.1% SDS (50 mM HEPES [pH 7.5], 1 mM EDTA, 1% Triton, 0.1% deoxycholic acid, 150 mM NaCl, 0.1% SDS) containing protease inhibitors. Each wash mixture was incubated for 5 min at 4°C and then sedimented at 4,000 × g. The pellets were then divided into 500-μl aliquots and stored at −80°C. Each aliquot was resuspended in 1.5 ml of FA buffer plus 0.3% SDS containing protease (Complete cocktail tablets, catalog number 11697498001; Roche) and phosphatase inhibitors (catalog number 786-450; GBiosciences). The samples were sonicated with a Virsonic digital 600 sonicator using a microtip (20 pulses of 11 s each at 30% amplitude with bursts of 0.9 s on and 0.5 s off) to generate DNA fragments of approximately 500 bp, as determined experimentally. Samples were sedimented at 13,000 × g for 15 min at 4°C, and the supernatant was transferred into a new tube, which was then diluted to 4.5 ml with FA buffer containing protein and phosphatase inhibitors. The extract was divided into four 1-ml samples.
Immunoprecipitation and elution were performed according to methods described previously by Lee et al. (21), with some modifications. For each 1-ml extract, 100 μl of protein A Dynabeads (Invitrogen), conjugated with antibody, was added. After incubation at 4°C overnight, the beads were washed five times with radioimmunoprecipitation assay (RIPA) buffer (50 mM HEPES [pH 7.5], 1 mM EDTA, 1% NP-40, 0.7% deoxycholic acid, 0.5 M LiCl) and once with Tris-EDTA (TE) plus 50 mM NaCl. After the removal of residual wash buffer, 210 μl of elution buffer (50 mM Tris-HCl [pH 8], 10 mM EDTA, 1% SDS) was added and incubated at 65°C for 30 min, with intense mixing briefly every 5 min. The beads were removed by a spin column (catalog number CLS8160; Sigma). Ten microliters of proteinase K (20 mg/ml) was added to the eluted fraction and incubated at 55°C for 2 to 3 h. The tubes were transferred to 65°C overnight to reverse the cross-links. DNA was purified by using a Qiagen PCR purification kit eluted twice with 50 μl of water.
Real-time PCR.
A fraction of the DNA was used as a template for real-time PCRs. The primers used were designed with Roche LightCycler Probe design software 2.0, and their sequences are shown in Table S1 in the supplemental material. PCR products were between 75 and 150 bp. PCRs were performed with a total volume of 10 μl containing 1× SYBR green mix (Applied Biosynthesis), a 1/200 fraction of ChIP-enriched DNA, and 100 nM primers in a 384-well plate using an Eppendorf epMotion 5070 robot. Standard curves were generated by using sonicated genomic DNA samples run concurrently with ChIP samples. All standards and samples were run in triplicate. Plates were read with an Applied Biosystems 7900HT real-time PCR machine (absolute quantification method). Enrichment of ChIP DNA was calculated by using the standard-curve method, and the numbers were corrected by removing outlier points from samples that had a greater-than-17% coefficient of variation. Input DNA values were used to normalize results following subtraction of the control without antibody.
RESULTS
Ser-5(P) and Ser-2(P) across a single C. elegans gene.
To determine whether the CTD of RNAPII is phosphorylated in a pattern similar to those of yeast and mammalian RNAPII (24), we performed ChIP qPCR experiments using antibodies specific to Ser-5(P) and Ser-2(P) in a nonoperon C. elegans gene, R10E4.2, chosen because of its isolated genomic location and its relatively high transcript level (16). By quantifying the immunoprecipitated DNA using multiple primer sets spanning the entire transcription unit, we were able to map Ser-5 and Ser-2 phosphorylations across the gene. We are confident that these antibodies recognize these epitopes because (i) the C. elegans consensus CTD is identical in sequence to the CTD of other organisms and (ii) we saw patterns seen with Ser-5(P) and Ser-2(P) that have been reported previously for other organisms. Figure 1 a shows that the pattern of Ser-5(P) is high near the promoter and stays high throughout the body of the gene, although its level decreases in the latter half of the gene. In contrast, the level of Ser-2(P) is relatively low near the promoter and higher at the 3′ end (Fig. 1b). If the data from Fig. 1a and b are plotted as a ratio of a ChIP with an antibody against the total RNAPII CTD, we can clearly distinguish two distinct peaks of phosphorylated RNAPII (Fig. 1c), where Ser-5(P) peaks at a more 5′ position than Ser-2(P). This indicates that nonoperon C. elegans genes behave much like yeast and mammalian genes with respect to CTD phosphorylation (5, 13, 20, 24).
FIG. 1.
Ser-2 and Ser-5 phosphorylation across a C. elegans gene. The gene is depicted by filled boxes and angled lines representing exons and introns, respectively. Flanking intergenic regions are shown as lines. The arrow indicates the location of the promoter (2). (a and b) ChIP qPCR experiments using antibodies against Ser-5(P) (a) and Ser-2(P) (b) in the R10E4.2 gene, each normalized to the highest value. The results from each primer set are positioned immediately above the corresponding genomic location. Error bars represent standard errors of the means of data from two independent immunoprecipitation experiments. (c) ChIP qPCR signals of Ser-5(P) and Ser-2(P) as a ratio of total RNAPII in the R10E4.2 gene. Normalized ChIP signals of Ser-5(P) and Ser-2(P) (as in a and b) were divided by the normalized total RNAPII at each primer set. The graph shows best-fit lines.
Ser-5(P) and Ser-2(P) across C. elegans operons.
Next, we wanted to determine if Ser-5 CTD phosphorylation is specific to promoter regions of operons or whether it also marks sites of mRNA 5′-end formation by SL2 trans splicing. Similarly, we asked whether Ser-2(P) marks sites of 3′-end processing or transcription termination. Using antibodies against Ser-5(P), Ser-2(P), and total RNAPII, ChIP qPCR experiments were performed with three operons containing two, four, or eight genes.
In the two-gene operon (CEOPX012), the level of Ser-5(P) is highest at the promoter and drops unevenly throughout the body of the operon (Fig. 2 a). In contrast, the level of Ser-2(P) is low at the 5′ end of the operon, peaks at the 3′ end of the first gene, and then drops within the second gene and peaks a second time at the 3′ end of the operon (Fig. 2b). Note that here and in subsequent figures, the peaks at 3′ ends of operon genes often extend to the 5′ end of the next gene. We presume that this is due to the fact that the genes are only 100 bp apart, and the ChIP fragment size averages ∼500 bp. Thus, the experiments lack the resolution to enable us to determine whether the level of Ser-2(P) decreases immediately following the 3′-end formation site.
FIG. 2.
In a two-gene operon (CEOPX012), the level of Ser-5(P) is high at the promoter and the level of Ser-2(P) is high at both 3′ ends. Shown are ChIP qPCR signals of Ser-5(P) (a) and Ser-2(P) (b) in a two-gene operon, each normalized to the highest value. The dashed lines separate genes. A gene in the opposite orientation, just 3′ of C44C1.2, is expressed at a very low level, and primers that query this region were not tested. The most 3′ primer pair is a negative control located in the center of a region lacking annotated genes; it is not adjacent to this operon. Error bars represent standard errors of the means of data from two independent immunoprecipitation experiments.
In the four-gene operon (CEOP3156), the level of Ser-5(P) is high near the promoter and decreases throughout the body of the operon (Fig. 3 a). Importantly, Ser-5(P) RNAPII does not peak at the internal 5′ ends (trans-splice sites), which are not close to promoters. We have confirmed this result by using a different Ser-5(P) antibody (data not shown). In contrast, the level of Ser-2(P) peaks at all four 3′ ends in the operon, dropping to a lower level following each 3′ end (Fig. 3b). Do these peaks represent pause sites where the level of total RNAPII would be expected to increase as well, or do they represent increases in the fraction of RNAPII that is Ser-2 phosphorylated? To distinguish between these two possibilities, we determined the amount of total RNAPII with each primer set and then divided the ChIP signal of Ser-2(P) by the ChIP signal for total RNAPII. This ratio clearly delineates the four peaks of Ser-2(P) representing the 3′ end of each gene in the operon (Fig. 3c), consistent with these peaks representing a specific enrichment of Ser-2 phosphorylation. Another interesting question is whether RNAPII pauses at internal 3′-end formation sites. Unfortunately, our data do not resolve this question, since we saw peaks in levels of total RNAPII at some, but not all, 3′-end formation sites (data not shown).
FIG. 3.
In a four-gene operon (CEOP3156), the level of Ser-5(P) is high at the promoter and the level of Ser-2(P) is high at each 3′ end. (a and b) ChIP qPCR signals of Ser-5(P) (a) and Ser-2(P) (b) in a four-gene operon, each normalized to the highest value. Error bars represent standard errors of the means of data from three independent immunoprecipitation experiments. (c) ChIP qPCR signals of Ser-2(P) as a ratio of total RNAPII in a four-gene operon. Normalization was done as described in the legend to Fig. 1c. The line connects adjacent points.
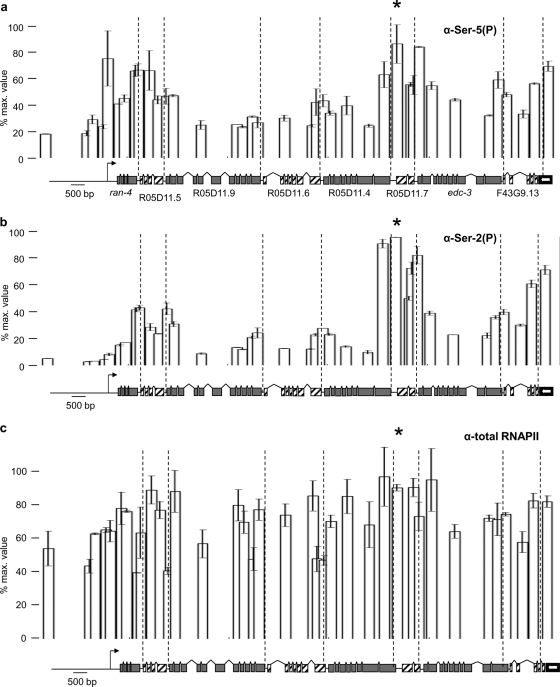
In the eight-gene operon (CEOP1484), the level of Ser-5(P) is high near the promoter of the operon and then drops throughout the first five genes. However, there is a second Ser-5(P) peak at an internal site near the 5′ end of the sixth gene (Fig. 4 a). Interestingly, this internal Ser-5(P) peak occurs at a previously uncharacterized internal promoter. The existence of this promoter is supported by the presence of a peak of paused RNAPII in starved worms that is released upon feeding and that is characteristic of promoter sites (2). It is also supported by the fact that there is a high level of trans splicing by SL1 to the R05D11.7 trans-splice site, which generally occurs at the first gene downstream of a transcription start site but not at downstream genes (16). Thus, the level of Ser-5(P) peaks at promoter locations, even a promoter situated within an operon, but it does not peak at most positions where mRNAs are capped by trans splicing. We note that Ser-5(P) does appear to peak at a few 3′ ends, especially those near the 3′ end of this operon, a result for which we lack an explanation. Finally, in this operon, as in the two operons described above, the level of Ser-2(P) peaks near each gene's 3′ end, falling to a lower level within each gene and rising again at each gene's 3′ end (Fig. 4b).
FIG. 4.
In an eight-gene operon (CEOP1484), the level of Ser-5(P) is high at the operon promoter and at an internal promoter and the level of Ser-2(P) is high at each 3′ end. Shown are data from ChIP qPCR experiments using antibodies against Ser-5(P) (a), Ser-2(P) (b), and total RNAPII (c) in an eight-gene operon, each normalized to the highest value. The location of a previously unknown internal promoter is marked by an asterisk. Error bars represent standard errors of the means of data from two independent immunoprecipitation experiments.
These data provide strong support for the idea that each gene is treated as a separate entity with respect to Ser-2 phosphorylation, supporting the idea that Ser-2 phosphorylation is associated with RNA 3′-end formation. However, one could argue that a small population of RNAPII molecules does terminate at internal sites, allowing the possibility that Ser-2(P) could be associated with sites of transcription termination. To investigate this possibility, we performed ChIP qPCR experiments using an antibody to total RNAPII in the eight-gene operon (Fig. 4c). Clearly, total RNAPII levels do not drop from one gene to the next in the operon, providing evidence that premature transcription termination is not occurring at significant levels at most 3′-end formation sites. However, the increase in total RNAPII levels following the internal promoter is small, suggesting that this promoter may compensate for some transcription termination following R05D11.4.
DISCUSSION
The patterns of phosphorylation of Ser-2 and Ser-5 on the RNAPII CTD correlate with RNA-processing events that occur near gene ends: Ser-5 is phosphorylated near the 5′ ends of genes, whereas Ser-2 is phosphorylated near 3′ ends. Because some pre-mRNA processing enzymes can bind to Ser-5(P) or Ser-2(P), it has been postulated that these processing events are facilitated by the binding of processing proteins to the CTD phosphorylated at these sites (27, 28). However, this idea requires additional support and testing with more experimental systems; it remains possible that these phosphorylation events are associated with sites of pre-mRNA processing but do not direct them. Capping enzymes do bind preferentially to RNAPII containing Ser-5(P), the level of which peaks close to the 5′ ends of genes, the site of pre-mRNA capping. However, this site also occurs close to the promoter. Since, in general, all 5′ ends of genes occur at the promoter, Ser-5(P) could be correlated with either promoters or sites of mRNA 5′-end formation. Similarly, Ser-2(P) peaks near 3′ ends of genes, but mRNA 3′-end formation and transcription termination are, in general, inextricably linked (31). Thus, it is possible that Ser-2(P) plays a role in transcription termination as well as mRNA 3′-end formation. The events occur simply too close together to allow them to be distinguished based solely on chromatin immunoprecipitation in yeast or mammalian experimental systems.
C. elegans operons have allowed us to bring a new experimental system to bear on these questions. Our results make it clear that Ser-5(P) marks the location where pre-mRNAs are cotranscriptionally capped near the transcription start site. However, Ser-5(P) does not mark downstream positions, where capped 5′ ends are formed by trans splicing (Fig. 2a, 3a, and 4a). The only site within any of the three operons that we studied with a large Ser-5(P) peak was at a site in the eight-gene operon where there is a promoter (Fig. 4a). The existence of an internal promoter was not entirely surprising since internal promoters in C. elegans operons were noted previously (17, 40). Thus, our results support the idea that Ser-5 phosphorylation serves to facilitate cotranscriptional capping, presumably by binding capping enzymes.
Similarly, our data support the conclusion that Ser-2(P) serves to facilitate RNA 3′-end cleavage, presumably by binding 3′-end formation proteins to RNAPII, since we found a peak of Ser-2(P) levels at all 3′-end formation sites that we examined, many of which occur at large distances from sites of transcription termination (Fig. 2b, 3b, and 4b). Indeed, it was sometimes the case that the lowest peak of Ser-2(P) levels occurred at the 3′ end of the entire gene cluster. Of course, it is possible that some RNAPII is terminating at each internal poly(A) site, but this possibility is made unlikely by the fact that total RNAPII levels remained flat throughout the eight-gene operon (Fig. 4c). If transcription were terminating at internal sites, we would have expected to see a gradual reduction of RNAPII levels from the 5′ end to the 3′ end of the operon. These results argue strongly that Ser-2(P) facilitates RNA 3′-end formation as previously proposed, although they do not eliminate the possibility that Ser-2(P) could facilitate transcription termination directly as well. If that is the case, our results indicate that Ser-2 phosphorylation is not sufficient to direct transcription termination, since the level of internal Ser-2(P) peaks occur at sites quite distant from transcription termination.
Interestingly, it is clear that with respect to Ser-5(P), the entire operon is treated as a single gene, while with respect to Ser-2(P), it is treated as a cluster of individual genes. The mechanism by which the phosphorylation and dephosphorylation enzymes can make this distinction will be an interesting subject for further study. For example, one of the phosphatases for Ser-5(P), Rtr1, is required for the transition from Ser-5(P) to Ser-2(P) enrichment in yeast genes (25). Therefore, it will be of interest to determine whether the worm Rtr1 ortholog dephosphorylates Ser-5(P) in operon genes without causing an immediate increase in levels of Ser-2(P). In addition, our observation that the level of Ser-2(P) peaks at each 3′ end and then drops to lower levels in the body of the next gene before peaking again at the next 3′ end argues that the heptad Ser-2 residues are actively dephosphorylated following 3′-end formation and then rephosphorylated near the 3′ end of the next gene. Clearly, it would be interesting to examine the patterns of Ser-5 and Ser-2 phosphatases as well as their kinases across these operons once antibodies capable of recognizing the C. elegans versions of these proteins become available. Furthermore, we presume that the capping enzymes and 3′-end formation proteins are interacting with the Ser-5(P) and Ser-2(P) CTD, respectively, as RNAPII traverses the operons, but that remains to be demonstrated.
The evidence for the existence of C. elegans operons is overwhelming but circumstantial. It has rested on the very strong association of SL2-accepting trans-splice sites with downstream positions in unusually tightly linked genes. Virtually all SL2 trans splicing occurs at such positions, and typically, only ∼100 bp separates the site of 3′ cleavage of the upstream gene and the SL2 trans-splice site of the downstream gene (4; M. A. Allen, L. W. Hillier, R. H. Waterston, and T. Blumenthal, manuscript in preparation). This observation, however, does not demonstrate cotranscription, although this has been demonstrated in a few cases (e.g., see references 34 and 37). The data presented here provide a different kind of evidence for cotranscription. The fact that Ser-5(P) does not peak at these SL2 trans-splice sites suggests strongly that transcription is not initiating there, implying that these are not sites of cotranscriptional capping. We conclude that, at least for the three operons investigated here, the gene clusters are, in fact, cotranscribed. Only one of these operons also has an internal promoter (Fig. 4A).
Why do internal 3′-end formation sites in operons not result in transcription termination? We show here that the RNAPII CTD is phosphorylated on Ser-2 residues near internal 3′-end formation sites just like terminal sites are, so something else must differentiate internal from terminal sites. Internal sites could have a sequence that prevents termination (14), or they might lack a sequence needed for termination. It was recently shown that cis splicing of the first intron of downstream genes in operons may play a role in preventing transcription termination from occurring at these internal sites (15). Alternatively, the trans-splicing event itself, which generally occurs quite close to the 3′-end cleavage event, could prevent transcription termination. If the torpedo model for transcription termination (7, 18, 39) is at least partly correct, then the cap provided by trans splicing would be expected to prevent the 5′-to-3′ degradation of downstream transcripts in operons, thereby preventing termination until after the final cleavage event. In sum, ChIP analysis of RNAPII CTD phosphorylation on several C. elegans operons has provided strong support for the idea that these modifications facilitate cotranscriptional processing in C. elegans just as they do in mammals. Our Ser-5(P) data also provide a novel demonstration that the genes in C. elegans operons are in fact parts of a single transcription unit. Some C. elegans operons also have internal promoters, and these can be revealed by Ser-5(P) peaks.
Supplementary Material
Acknowledgments
We thank David Bentley (University of Colorado Health Sciences Center) for the total RNAPII antibody, for advice throughout this project, and for comments on the manuscript. We thank Haibo Liu (Han laboratory) and Christina Whittle (Lieb laboratory) for critical advice with the ChIP protocol. We are also grateful to Joaquin Espinosa for considerable technical advice. Finally, we thank members of our laboratory for their many helpful comments throughout this work and on the manuscript.
This work was supported by National Institute of General Medical Science grant GM42432.
Footnotes
Published ahead of print on 24 May 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Allison, L. A., J. K. Wong, V. D. Fitzpatrick, M. Moyle, and C. J. Ingles. 1988. The C-terminal domain of the largest subunit of RNA polymerase II of Saccharomyces cerevisiae, Drosophila melanogaster, and mammals: a conserved structure with an essential function. Mol. Cell. Biol. 8:321-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baugh, L. R., J. Demodena, and P. W. Sternberg. 2009. RNA Pol II accumulates at promoters of growth genes during developmental arrest. Science 324:92-94. [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal, T. 2005. Trans-splicing and operons. WormBook 2005:1-9. [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal, T., D. Evans, C. D. Link, A. Guffanti, D. Lawson, J. Thierry-Mieg, D. Thierry-Mieg, W. L. Chiu, K. Duke, M. Kiraly, and S. K. Kim. 2002. A global analysis of Caenorhabditis elegans operons. Nature 417:851-854. [DOI] [PubMed] [Google Scholar]
- 5.Chapman, R. D., M. Heidemann, T. K. Albert, R. Mailhammer, A. Flatley, M. Meisterernst, E. Kremmer, and D. Eick. 2007. Transcribing RNA polymerase II is phosphorylated at CTD residue serine-7. Science 318:1780-1782. [DOI] [PubMed] [Google Scholar]
- 6.Chiu, Y. L., C. K. Ho, N. Saha, B. Schwer, S. Shuman, and T. M. Rana. 2002. Tat stimulates cotranscriptional capping of HIV mRNA. Mol. Cell 10:585-597. [DOI] [PubMed] [Google Scholar]
- 7.Connelly, S., and J. L. Manley. 1988. A functional mRNA polyadenylation signal is required for transcription termination by RNA polymerase II. Genes Dev. 2:440-452. [DOI] [PubMed] [Google Scholar]
- 8.Coppola, J. A., A. S. Field, and D. S. Luse. 1983. Promoter-proximal pausing by RNA polymerase II in vitro: transcripts shorter than 20 nucleotides are not capped. Proc. Natl. Acad. Sci. U. S. A. 80:1251-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahmus, M. E. 1996. Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J. Biol. Chem. 271:19009-19012. [DOI] [PubMed] [Google Scholar]
- 10.Dye, M. J., and N. J. Proudfoot. 1999. Terminal exon definition occurs cotranscriptionally and promotes termination of RNA polymerase II. Mol. Cell 3:371-378. [DOI] [PubMed] [Google Scholar]
- 11.Dye, M. J., and N. J. Proudfoot. 2001. Multiple transcript cleavage precedes polymerase release in termination by RNA polymerase II. Cell 105:669-681. [DOI] [PubMed] [Google Scholar]
- 12.Glover-Cutter, K., S. Kim, J. Espinosa, and D. L. Bentley. 2008. RNA polymerase II pauses and associates with pre-mRNA processing factors at both ends of genes. Nat. Struct. Mol. Biol. 15:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes, N. P., G. Bjerke, B. Llorente, S. A. Szostek, B. M. Emerson, and J. M. Espinosa. 2006. Gene-specific requirement for P-TEFb activity and RNA polymerase II phosphorylation within the p53 transcriptional program. Genes Dev. 20:601-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graber, J. H., J. Salisbury, L. N. Hutchins, and T. Blumenthal. 2007. C. elegans sequences that control trans-splicing and operon pre-mRNA processing. RNA 13:1409-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haenni, S., H. E. Sharpe, M. Gravato Nobre, K. Zechner, C. Browne, J. Hodgkin, and A. Furger. 2009. Regulation of transcription termination in the nematode Caenorhabditis elegans. Nucleic Acids Res. 37:6723-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillier, L. W., V. Reinke, P. Green, M. Hirst, M. A. Marra, and R. H. Waterston. 2009. Massively parallel sequencing of the polyadenylated transcriptome of C. elegans. Genome Res. 19:657-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, P., E. D. Pleasance, J. S. Maydan, R. Hunt-Newbury, N. J. O'Neil, A. Mah, D. L. Baillie, M. A. Marra, D. G. Moerman, and S. J. Jones. 2007. Identification and analysis of internal promoters in Caenorhabditis elegans operons. Genome Res. 17:1478-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, M., N. J. Krogan, L. Vasiljeva, O. J. Rando, E. Nedea, J. F. Greenblatt, and S. Buratowski. 2004. The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature 432:517-522. [DOI] [PubMed] [Google Scholar]
- 19.Kim, M., H. Suh, E. J. Cho, and S. Buratowski. 2009. Phosphorylation of the yeast Rpb1 C-terminal domain at serines 2, 5, and 7. J. Biol. Chem. 284:26421-26426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komarnitsky, P., E. J. Cho, and S. Buratowski. 2000. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 14:2452-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, T. I., S. E. Johnstone, and R. A. Young. 2006. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat. Protoc. 1:729-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCracken, S., N. Fong, K. Yankulov, S. Ballantyne, G. Pan, J. Greenblatt, S. D. Patterson, M. Wickens, and D. L. Bentley. 1997. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature 385:357-361. [DOI] [PubMed] [Google Scholar]
- 23.McCracken, S., N. Fong, E. Rosonina, K. Yankulov, G. Brothers, D. Siderovski, A. Hessel, S. Foster, S. Shuman, and D. L. Bentley. 1997. 5′-capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II. Genes Dev. 11:3306-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris, D. P., G. A. Michelotti, and D. A. Schwinn. 2005. Evidence that phosphorylation of the RNA polymerase II carboxyl-terminal repeats is similar in yeast and humans. J. Biol. Chem. 280:31368-31377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosley, A. L., S. G. Pattenden, M. Carey, S. Venkatesh, J. M. Gilmore, L. Florens, J. L. Workman, and M. P. Washburn. 2009. Rtr1 is a CTD phosphatase that regulates RNA polymerase II during the transition from serine 5 to serine 2 phosphorylation. Mol. Cell 34:168-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moteki, S., and D. Price. 2002. Functional coupling of capping and transcription of mRNA. Mol. Cell 10:599-609. [DOI] [PubMed] [Google Scholar]
- 27.Perales, R., and D. Bentley. 2009. “Cotranscriptionality”: the transcription elongation complex as a nexus for nuclear transactions. Mol. Cell 36:178-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phatnani, H. P., and A. L. Greenleaf. 2006. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 20:2922-2936. [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen, E. B., and J. T. Lis. 1993. In vivo transcriptional pausing and cap formation on three Drosophila heat shock genes. Proc. Natl. Acad. Sci. U. S. A. 90:7923-7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosonina, E., and B. J. Blencowe. 2004. Analysis of the requirement for RNA polymerase II CTD heptapeptide repeats in pre-mRNA splicing and 3′-end cleavage. RNA 10:581-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosonina, E., S. Kaneko, and J. L. Manley. 2006. Terminating the transcript: breaking up is hard to do. Genes Dev. 20:1050-1056. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder, S. C., B. Schwer, S. Shuman, and D. Bentley. 2000. Dynamic association of capping enzymes with transcribing RNA polymerase II. Genes Dev. 14:2435-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shuman, S. 2001. Structure, mechanism, and evolution of the mRNA capping apparatus. Prog. Nucleic Acid Res. Mol. Biol. 66:1-40. [DOI] [PubMed] [Google Scholar]
- 34.Spieth, J., G. Brooke, S. Kuersten, K. Lea, and T. Blumenthal. 1993. Operons in C. elegans: polycistronic mRNA precursors are processed by trans-splicing of SL2 to downstream coding regions. Cell 73:521-532. [DOI] [PubMed] [Google Scholar]
- 35.Stiller, J. W., and B. D. Hall. 2002. Evolution of the RNA polymerase II C-terminal domain. Proc. Natl. Acad. Sci. U. S. A. 99:6091-6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sulston, J. E., and S. Brenner. 1974. The DNA of Caenorhabditis elegans. Genetics 77:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka, Y., A. Ohta, K. Terashima, and H. Sakamoto. 1997. Polycistronic expression and RNA-binding specificity of the C. elegans homologue of the spliceosome-associated protein SAP49. J. Biochem. 121:739-745. [DOI] [PubMed] [Google Scholar]
- 38.Teixeira, A., A. Tahiri-Alaoui, S. West, B. Thomas, A. Ramadass, I. Martianov, M. Dye, W. James, N. J. Proudfoot, and A. Akoulitchev. 2004. Autocatalytic RNA cleavage in the human beta-globin pre-mRNA promotes transcription termination. Nature 432:526-530. [DOI] [PubMed] [Google Scholar]
- 39.West, S., N. Gromak, and N. J. Proudfoot. 2004. Human 5′→3′ exonuclease Xrn2 promotes transcription termination at co-transcriptional cleavage sites. Nature 432:522-525. [DOI] [PubMed] [Google Scholar]
- 40.Whittle, C. M., K. N. McClinic, S. Ercan, X. Zhang, R. D. Green, W. G. Kelly, and J. D. Lieb. 2008. The genomic distribution and function of histone variant HTZ-1 during C. elegans embryogenesis. PLoS Genet. 4:e1000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, J., and J. L. Corden. 1991. Identification of phosphorylation sites in the repetitive carboxyl-terminal domain of the mouse RNA polymerase II largest subunit. J. Biol. Chem. 266:2290-2296. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.