Abstract
Cyclic di-GMP (c-di-GMP) is a bacterial second messenger produced by GGDEF domain-containing proteins. The genome of Ehrlichia chaffeensis, an obligatory intracellular bacterium that causes human monocytic ehrlichiosis, encodes a single protein that contains a GGDEF domain, called PleD. In this study, we investigated the effects of c-di-GMP signaling on E. chaffeensis infection of the human monocytic cell line THP-1. Recombinant E. chaffeensis PleD showed diguanylate cyclase activity as it generated c-di-GMP in vitro. Because c-di-GMP is not cell permeable, the c-di-GMP hydrophobic analog 2′-O-di(tert-butyldimethylsilyl)-c-di-GMP (CDGA) was used to examine intracellular c-di-GMP signaling. CDGA activity was first tested with Salmonella enterica serovar Typhimurium. CDGA inhibited well-defined c-di-GMP-regulated phenomena, including cellulose synthesis, clumping, and upregulation of csgD and adrA mRNA, indicating that CDGA acts as an antagonist in c-di-GMP signaling. [32P]c-di-GMP bound several E. chaffeensis native proteins and two E. chaffeensis recombinant I-site proteins, and this binding was blocked by CDGA. Although pretreatment of E. chaffeensis with CDGA did not reduce bacterial binding to THP-1 cells, bacterial internalization was reduced. CDGA facilitated protease-dependent degradation of particular, but not all, bacterial surface-exposed proteins, including TRP120, which is associated with bacterial internalization. Indeed, the serine protease HtrA was detected on the surface of E. chaffeensis, and TRP120 was degraded by treatment of E. chaffeensis with recombinant E. chaffeensis HtrA. Furthermore, anti-HtrA inhibited CDGA-induced TRP120 degradation. Our results suggest that E. chaffeensis invasion is regulated by c-di-GMP signaling, which stabilizes some bacterial surface-exposed proteins against proteases.
Ehrlichia chaffeensis causes the potentially fatal infectious disease human monocytic ehrlichiosis. This disease is one of the most prevalent life-threatening tick-borne zoonoses in the United States, but it is less frequently reported to occur in other parts of the world (13, 42). E. chaffeensis is a Gram-negative bacterium that belongs to the order Rickettsiales in the Alphaproteobacteria. To survive, E. chaffeensis must infect eukaryotic host cells, as it lacks most of the genes for the biosynthesis of amino acids and the associated intermediary metabolism (47). E. chaffeensis invades human monocytes and macrophages and replicates in membrane-bound inclusions by inhibiting oxidative and nonoxidative microbicidal mechanisms in these cells (48).
Cyclic di-GMP (c-di-GMP) is a bacterial second messenger produced by the GGDEF domain-containing diguanylate cyclase (DGC). c-di-GMP is degraded by EAL or HD-GYP domain-containing c-di-GMP-specific phosphodiesterases (22, 29, 52, 53, 55, 63). Because GGDEF domain-containing proteins are found in most sequenced bacterial genomes, c-di-GMP signaling is considered to be ubiquitous (15, 28). c-di-GMP inversely regulates the planktonic traits (motility) and communal or sessile traits (synthesis of bacterial surface components such as biofilm, exopolysaccharide, pili, and stalks) of several bacterial groups, including Salmonella enterica serovar Typhimurium, Escherichia coli, Pseudomonas aeruginosa, Vibrio cholerae, Yersinia pestis, and Caulobacter crescentus (22, 23, 28, 29, 32, 37, 39, 40, 52, 53, 59, 63). Reported roles for c-di-GMP signaling in bacterial virulence are expanding. Overproduction of c-di-GMP inhibits invasion of S. Typhimurium into the gastrointestinal epithelial cell line HT-29 by overproducing the extracellular matrix component cellulose and inhibits interleukin-8 (IL-8) production by inhibiting the secretion of monomeric flagellin (36). A deletion mutant of a single EAL protein in S. Typhimurium is susceptible to host oxidative defense and is killed by mouse macrophages (24). c-di-GMP signaling is involved in the virulence of P. aeruginosa, S. Typhimurium, and V. cholerae in mice (32, 46, 62, 64), but the molecular targets of c-di-GMP and the signaling pathway remain to be investigated.
The above-mentioned bacterial groups contain multiple GGDEF domain-containing proteins, and thus, it is difficult to study the direct regulation of specific downstream events of each GGDEF domain-containing protein. In contrast, E. chaffeensis has a single gene encoding a GGDEF domain-containing protein, and thus, this species may serve as a simple model system for studying c-di-GMP signaling. We named this protein from E. chaffeensis PleD, because it shares a domain structure with C. crescentus PleD, including D1 (receiver), D2 (receiver-like), and a GGDEF domain, as well as amino acid sequences (41% identity) (7, 33). Despite the reductive evolution of genomes, PleD is conserved among members of the order Rickettsiales, suggesting its importance in obligatory intracellular bacteria (7, 51). PleD is expressed by E. chaffeensis and the related bacterium Anaplasma phagocytophilum during intracellular infection of human leukocytes, and recombinant PleD from each bacterium is phosphorylated by a recombinant cognate sensor kinase, named PleC, in each bacterium in vitro (33, 35). C. crescentus PleD and A. phagocytophilum PleD have DGC activity (35, 44). In this study, we showed that PleD from E. chaffeensis also has DGC activity. Because of the lack of a useful genetic system for E. chaffeensis and A. phagocytophilum and because c-di-GMP is not cell permeable, we employed the c-di-GMP hydrophobic analog 2′-O-di(tert-butyldimethylsilyl)-c-di-GMP (CDGA; C32H58N12O14P2Si2; molecular weight [MW] = 952.99; the structure is shown in Fig. 2A) (27) to explore c-di-GMP signaling. Because CDGA inhibits infection of the human promyelocytic leukemia cell line HL-60 by A. phagocytophilum (35), we determined whether CDGA inhibits well-known c-di-GMP-dependent phenomena, including cellulose synthesis and clumping in S. Typhimurium. Furthermore, using CDGA, we describe a novel role for c-di-GMP signaling in host cell invasion by stabilization of bacterial surface-exposed proteins, protecting them from degradation by endogenous proteases. Our results further expand the roles for c-di-GMP signaling in bacterial virulence.
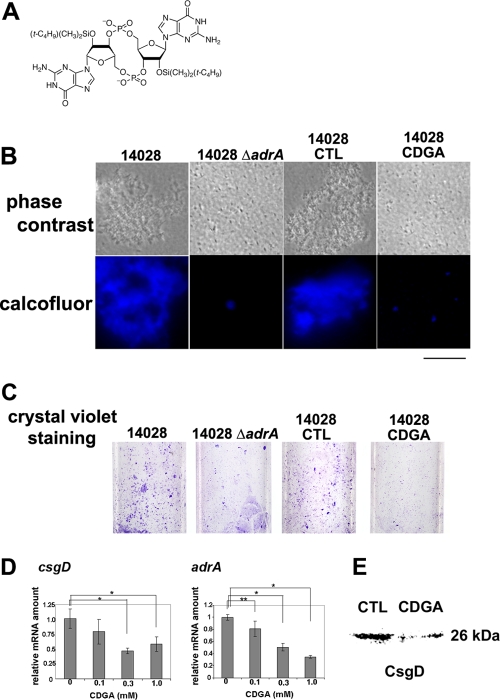
FIG. 2.
CDGA serves as a c-di-GMP antagonist. (A) Structure of CDGA. (B) Cultured S. Typhimurium 14028 and 14028 adrA::Km (14028 ΔadrA) were visualized with phase-contrast microscopy and calcofluor staining after growth for 48 h with no shaking. S. Typhimurium 14028 was cultured in the presence of CDGA (14028 CDGA) or solvent (1% [vol/vol] DMSO; 14028 CTL), and bacteria were visualized. Scale bar = 5 μm. CTL, control. (C) Bacteria that were attached to glass tubes were stained with crystal violet. (D) The mRNA levels of csgD and adrA were quantified by quantitative real-time PCR after S. Typhimurium 14028 was cultured as described for panel B in the absence or presence of CDGA. Columns represent relative values, with the control set at 1.0. The values are means ± standard deviations (n = 3). Asterisks show significant differences (**, P < 0.05; *, P < 0.01) between the control and CDGA. (E) Amount of CsgD as detected by Western blotting after S. Typhimurium 14028 was cultured in the presence of 0.3 mM CDGA or solvent (1% [vol/vol] DMSO; CTL).
MATERIALS AND METHODS
Bacterial strains and cell culture.
E. chaffeensis Arkansas was propagated in THP-1 cells (ATCC, Manassas, VA) in RPMI 1640 medium supplemented with 10% fetal bovine serum and 2 mM l-glutamine at 37°C in 5% CO2 and 95% air. The Escherichia coli strains NovaBlue (for DNA cloning; Novagen, Madison, WI) and BL21(DE3) (for recombinant protein induction; Novagen) were cultured in Luria-Bertani broth (58) supplemented with 50 μg/ml kanamycin, as needed.
Plasmid construction for expression of recombinant proteins.
To construct expression vectors for recombinant E. chaffeensis PleD with a C-terminal His tag (rEcPleD-CHis), recombinant Ech0345 with an N-terminal His tag (rEch0345), recombinant TRP120 (tandem repeat protein 120) with an N-terminal His tag (rTRP120), recombinant HtrA with a C-terminal His tag (rHtrA), and the C-terminal fragment of recombinant HtrA with a C-terminal His tag, the DNA fragments encoding these proteins were amplified by PCR using the primers listed in Table 1, using chromosomal DNA isolated from E. chaffeensis-infected THP-1 cells as a template. The PCR fragments were digested with the restriction enzymes described in Table 1 and ligated into pET33b (Novagen), which was digested with the same restriction enzymes. The mutant pleD alleles pleD(D53N) (phosphoryl acceptor site D was changed to N), pleD(I-VMGG) (I site RITD was changed to VMGG), and pleD(GG374DE) (GGEEF was changed to DEEEF) were constructed by site-directed mutagenesis using the primers listed in Table 1, with a plasmid containing wild-type pleD as a template, using a QuikChange site-directed mutagenesis kit (Agilent, Santa Clara, CA). Plasmid inserts and mutations were confirmed by DNA sequencing.
TABLE 1.
Oligonucleotide primers
| Primer nameb | Sequence (5′-3′)a | Enzyme | Purpose |
|---|---|---|---|
| EcpleD33-CHis F | GGCATATGACTGCAAAAGTATTAATAG | NdeI | Construction of rEcPleD-CHis expression vector |
| EcpleD33-CHis R | GGCTCGAGTGATAGATATGTGACAACTTTG | XhoI | |
| TRP120 F | CGAGGATCCGATGGATATTGATAATAGTAACATAAG | BamHI | Construction of rTRP120 expression vector |
| TRP120 R | GGTGCGGCCGCTTATACAATATCATTTACTACATTG | NotI | |
| HtrA F | GGACCATGGATCAGAAAGCAGAACAATATG | NcoI | Construction of rHtrA expression vectors |
| HtrAC F | GAACCATGGGCAACGGAATGCCTAAGGATG C | NcoI | Construction of the expression vector for the HtrA C-terminal fragment |
| HtrA R | GGAGTCGACCT CTTCAGCTTGACAGTCA | SalI | Construction of the expression vectors for rHtrA and the HtrA C-terminal fragment |
| Ech0345 F | CCGAAGCTTAATCACTTCGTGGTTGCAAGA | HindIII | Construction of rEch0345 expression vector |
| Ech0345 R | CCGCTCGAGTTATGAAATGTTAGTACTTTTAATTG | XhoI | |
| pleD(I-VMGG) sense | GCAACAGTTTAAAAAACGTGTATCAGACAATATAGTCATGGGCGGCCTATTAGCAAGATTTGGTGGAGAAGAATTTG | Introduction of mutation in PleD I site | |
| pleD(I-VMGG) antisense | CAAATTCTTCTCCACCAAATCTTGCTAATAGGCCGCCCATGACTATATTGTCTGATACACGTTTTTTAAACTGTTGC | ||
| pleD(GG374DE) sense | CGCATTACAGACCTATTAGCAAGATTTGATGAAGAAGAATTTGTTGTAATATTACC | Introduction of mutation in PleD GGDEF motif | |
| pleD(GG374DE) antisense | GGTAATATTACAACAAATTCTTCTTCATCAAATCTTGCTAATAGGTCTGTAATGCG | ||
| adrA real time F | GTCATGTCTGGTGACGTTGC | Detection of adrA mRNA level | |
| adrA real time R | AAGCGGATACAACATAATGACG | ||
| csgD real time F | ACGCTACTGAAGACCAGGAAC | Detection of csgD mRNA level | |
| csgD real time R | GCATTCGCCACGCAGAATAC | ||
| TRP120 gene real time F | GATGACACAGTCTCTCAACC | Detection of TRP120 gene mRNA level | |
| TRP120 gene real time R | CTGATCTGATACACCAGATTC | ||
| ompA real time F | GCAAAAGGCAGAAGAGTATCC | Detection of ompA mRNA level | |
| ompA real time R | GCATTTGCACGCTGTTTG | ||
| p28 real time F | CAGCAGGTAGTGGTATTAACG | Detection of p28 mRNA level | |
| p28 real time R | TCAGTCCAAACACTCCAACTG |
Restriction enzyme sites and mutation sites are underlined.
F, forward; R, reverse.
Recombinant protein purification.
For the DGC assay, rEcPleD-CHis and rEcPleD(D53N)CHis were purified from the E. coli soluble fraction using His-Select nickel affinity gel (Sigma-Aldrich, St. Louis, MO) (33) with one modification: the column binding buffer was 50 mM Tris-HCl (pH 8.0), 500 mM NaCl, 5 mM β-mercaptoethanol, and 10% glycerol. rEcPleD(GG374DE)CHis was purified from the E. coli insoluble fraction using His-Select nickel affinity gel and refolded (33). rEcPleD-CHis was also purified from the insoluble fraction and refolded as a positive control for rEcPleD(GG374DE)CHis. For the [32P]c-di-GMP binding assay, rEcPleD-CHis was purified from both E. coli soluble and insoluble fractions, and rEcPleD(I-VMGG)CHis was purified from the E. coli insoluble fraction (33). rEch0345 was purified from an E. coli lysate by the batch method using His-Select nickel affinity gel. Recombinant P. aeruginosa WspR (rWspR) (32), rTRP120, rHtrA, and the C-terminal fragment of recombinant HtrA were purified from the E. coli soluble fraction (33).
DGC activity.
For high-pressure liquid chromatography (HPLC), rEcPleD-CHis and rEcPleD(D53N)CHis purified from E. coli soluble fractions were dialyzed against 50 mM sodium phosphate (pH 7.0)-500 mM NaCl and loaded onto a TSK-G3000 SWXL gel filtration column (Tosho, Tokyo, Japan) equipped with an ÄKTA purifier (GE Healthcare, Piscataway, NJ) to separate the monomer form of the protein from the multimers. Proteins were eluted at a flow rate of 1 ml/min. Gel filtration standards (Bio-Rad, Hercules, CA) were used to determine the molecular masses of the proteins in the peaks. The eluted monomers of rEcPleD-CHis and rEcPleD(D53N)CHis as well as rEcPleD-CHis and rEcPleD(GG374DE)CHis purified from E. coli insoluble fractions were extensively dialyzed against 50 mM Tris-HCl (pH 8.0), 250 mM NaCl, and 5 mM β-mercaptoethanol, and the protein samples (100 μg) were incubated with 1 mM BeCl2, 10 mM NaF, and 10 mM MgCl2 for 30 min at room temperature in DGC buffer (50 mM Tris-HCl [pH 8.0], 250 mM NaCl, 10 mM MgCl2, 5 mM β-mercaptoethanol). The proteins were then incubated with 50 nmol GTP for 1 to 4 h at room temperature. Samples were resolved by reversed-phase HPLC (35).
CDGA.
The purities of the synthesized c-di-GMP and CDGA were >95% as measured by the Waters 2695 HPLC system (Waters, Milford, MA), which was equipped with a reversed-phase HPLC column (COSMOSIL 5C18-AR-II; Nacalai Tesque, Kyoto, Japan). The sample was eluted with a linear gradient of 0 to 48% acetonitrile in 0.9% NaCl. c-di-GMP was dissolved in 0.9% NaCl, and CDGA was dissolved in dimethyl sulfoxide (DMSO).
Antibodies.
Rabbit anti-HtrA was raised by immunizing rabbits with the C-terminal fragment of recombinant HtrA (amino acids [aa] 367 to 471; New England Peptide, Gardner, MA). The other antibodies used were rabbit anti-CsgD (61), rabbit anti-P28 (41) (to detect both P28 and Omp-1F [34]), rabbit anti-TRP120 (45), rabbit anti-OmpA (25), rabbit anti-NtrX (7), rabbit anti-VirB9 (4), rabbit anti-VirB6-2 (4), rabbit anti-Hsp60 (69), horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (KPL, Gaithersburg, MD), dog anti-E. chaffeensis (25), fluorescein isothiocyanate (FITC)-conjugated goat anti-dog IgG (Rockland, Gaithersburg, MD), Texas Red-conjugated goat anti-dog IgG (Rockland), and Alexa Fluor 555-conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA).
Cellulose synthesis and clumping assay with S. Typhimurium in the presence of CDGA.
S. Typhimurium strains 14028 and 14028 adrA::Km (16) were cultured overnight, inoculated (1:100 dilution) in a glass tube containing Luria-Bertani medium without salt, and cultured for 48 h without shaking at room temperature. Bacterial clumping was observed by phase-contrast microscopy using a Nikon Eclipse E400 microscope (Nikon Instruments, Inc., Melville, NY). Cellulose synthesis was assessed by staining bacteria with 200 μg/ml calcofluor and analyzing them with a microscope equipped with a xenon-mercury light source. To examine bacterial attachment to the glass wall, the culture was rotated for 1 day at room temperature, and the glass tube was stained with 0.1% (wt/vol) crystal violet. Strain 14028 was cultured in the presence of 0.3 mM CDGA or its solvent DMSO (1%, vol/vol), and the phenotypes were examined under the same conditions. After S. Typhimurium strain 14028 was cultured in the presence of CDGA (0.1 mM, 0.3 mM, or 1 mM) or the solvent control, RNA was purified with an RNeasy minikit (Qiagen, Valencia, CA), treated with DNase I, and reverse transcribed (7). The expression levels of csgD and adrA were quantified by real-time PCR using the primers listed in Table 1 (7). CsgD protein level was determined by Western blotting using anti-CsgD.
[32P]c-di-GMP binding assay.
[32P]c-di-GMP was enzymatically synthesized by incubating purified rWspR (20 μg), the substrate [α-32P]GTP (20 pmol), and carrier GTP (80 pmol) (35). Nonreacted GTP was cleaved by incubation with 10 U of calf intestinal alkaline phosphatase (New England Biolabs, Ipswich, MA). The mixture was heated for 5 min at 95°C and centrifuged to eliminate proteins. c-di-GMP production and removal of nonreacted GTP were verified by thin-layer chromatography on a polyethyleneimine-cellulose plate (Macherey-Nagel, Düren, Germany) in 1.5 M KH2PO4 (pH 3.65). Alternatively, after incubation of rWspR (20 μg) and unlabeled GTP (100 pmol), reversed-phase HPLC was performed to detect c-di-GMP as described above.
Host cell-free E. chaffeensis at the mid-exponential growth phase from a synchronous culture was isolated (34). E. chaffeensis lysate (60 μg), rEcPleD-CHis purified from either the E. coli soluble fraction or the E. coli insoluble fraction (1 μg each), rEcPleD(I-VMGG)CHis (1 μg), and rEch0345 (1 μg) were incubated with 1 μCi (0.1 pmol) of [32P]c-di-GMP and chemically synthesized c-di-GMP (50 pmol) as a carrier in cross-linking buffer (50 mM Tris-HCl [pH 7.5], 250 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol [DTT]) in the absence or presence of 150 pmol, 450 pmol, or 1.5 nmol c-di-GMP or CDGA as a competitor. Samples were UV cross-linked, trichloroacetic acid (TCA) precipitated, dissolved in SDS-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and subjected to SDS-PAGE (35). The gel was exposed to a storage phosphor screen (Molecular Dynamics, Sunnyvale, CA), which was scanned with a PhosphorImager 445 Si (Molecular Dynamics).
Determination of E. chaffeensis binding and internalization.
Host cell-free bacteria isolated from heavily infected cells (close to cell rupture) were suspended in SPK medium (200 mM sucrose-50 mM potassium phosphate buffer, pH 7.4) supplemented with 2 mM l-glutamine and incubated with 0.3 mM CDGA or 1% (vol/vol) DMSO for 2 h at 37°C. Bacteria were washed twice with SPK medium and added to THP-1 cells at a multiplicity of infection (MOI; the ratio of the number of bacteria to the number of host cells) of 100. For the binding assay, the mixture was incubated for 1 h at room temperature, and cells were washed twice with phosphate-buffered saline (PBS; 37 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1 mM KH2PO4, pH 7.4) or RPMI medium to remove unbound bacteria. Cells were cytocentrifuged, fixed with 2 or 4% paraformaldehyde, and stained with dog anti-E. chaffeensis (1:100), followed by FITC-conjugated goat anti-dog IgG (1:100). Then, the number of bacteria per 100 cells was determined. For the internalization assay, the bacteria and THP-1 cell mixture were incubated for 2 h at 37°C and washed twice with PBS to remove unbound bacteria. Cells were cytocentrifuged and stained as described above. Then, cells were permeabilized with 0.3% saponin and stained with dog anti-E. chaffeensis (1:100), followed by Texas Red-conjugated goat anti-dog IgG (1:100). The fluorescent images were analyzed with a Nikon Eclipse E400 fluorescence microscope with a xenon-mercury light source (Nikon Instruments, Inc.). The numbers of bacteria stained yellow (noninternalized) and red (internalized) in the merged photos were counted. To assess the combined effect of CDGA and rHtrA on E. chaffeensis binding and internalization, bacteria were pretreated with 1% (vol/vol) DMSO or 0.3 mM CDGA with rHtrA (0, 0.25, 0.5, 0.75, and 1 μg) and then mixed with THP-1 cells (MOI of 200), followed by determination of binding and internalization as described above. For the infection assay, the pretreated E. chaffeensis and THP-1 cell mixtures were incubated for 2 h at 37°C and washed twice with PBS to remove unbound bacteria. After culturing for 2 to 4 days, infected cells were counted. The assays were conducted in triplicate.
E. chaffeensis surface protein labeling.
Host cell-free E. chaffeensis was cytocentrifuged and fixed with 2% or 4% paraformaldehyde in PBS for 15 min. Bacteria were then quenched with 50 mM NH4Cl in PBS for 15 min, incubated with primary antibodies for 1 h, and then incubated with secondary antibodies for 1 h. All procedures were done at room temperature, and antibodies were diluted in 2% fetal bovine serum and 1% bovine serum albumin in PBS to 1:100 unless otherwise noted. As a negative control, E. chaffeensis was incubated with preimmune rabbit serum, followed by incubation with the appropriate fluorochrome-conjugated secondary antibody.
Detection of E. chaffeensis surface-exposed proteins.
Host cell-free bacteria were incubated with 0.3 mM CDGA or 1% (vol/vol) DMSO in the presence or absence of protease inhibitor cocktail (EMB Biosciences, La Jolla, CA) (35) supplemented with 5 mM EDTA or were incubated with diisopropyl fluorophosphate (DFP; 0.1 or 1 mM) only for 2 h at 37°C. Proteins were detected by Western blotting using antibodies recognizing TRP120, P28, OmpA, VirB6-2, VirB9, and Hsp60.
Protease assays.
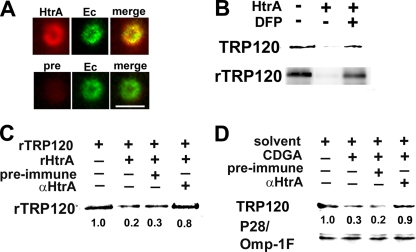
Purified rHtrA was dialyzed against 10 mM HEPES (pH 7.8), 5 mM MgCl2, and 100 mM NaCl, and protease activity was confirmed using a general protease assay kit (QuantiCleave protease assay kit; Pierce, Rockford, IL) in the presence or absence of 1 or 10 mM DFP. rHtrA was incubated with rTRP120, host cell-free E. chaffeensis, or an E. chaffeensis membrane fraction isolated as described in reference 33 and supplemented with 10 mM tosyl-l-lysine chloromethyl ketone and 5 mM EDTA in the presence or absence of 10 mM DFP. TRP120 and P28/Omp-1F were detected by Western blotting using anti-TRP120 and anti-P28, respectively.
Effects of anti-HtrA on CDGA-induced downregulation of TRP120.
Gamma globulin fractions were partially purified from rabbit HtrA antiserum and preimmune serum with sodium sulfate (4). To examine whether anti-HtrA inhibits the protease activity of rHtrA, TRP120 (1 μg) was incubated with rHtrA (1 μg) in the presence of 5 μg of anti-HtrA IgG or preimmune IgG for 2 h at 37°C. Host cell-free E. chaffeensis isolated from 106 cells was incubated with 5 μg of anti-HtrA IgG or preimmune IgG in the presence of 0.3 mM CDGA for 2 h at 37°C. The amount of TRP120 was determined by Western blotting using anti-rTRP120.
Statistical analysis.
An unpaired Student t test and analysis of variance were used for statistical analysis. A P value of <0.05 was considered significant.
RESULTS
E. chaffeensis PleD has DGC activity.
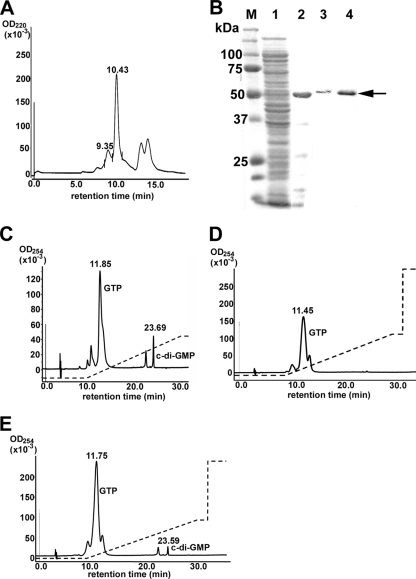
Although DGC activity has not been reported to occur in purified native proteins, recombinant GGDEF domain-containing proteins from several bacterial groups do have DGC activity (44, 57). When rEcPleD-CHis was overexpressed in E. coli (33), most of the purified protein formed multimers as determined by HPLC gel filtration chromatography, and no DGC activity was detected (data not shown). Thus, this protein was purified in the presence of a high salt concentration and glycerol under reducing conditions using Ni affinity chromatography, followed by separation of the rEcPleD-CHis monomer with HPLC gel filtration. The major peak eluted from the HPLC column at 10.43 min (Fig. 1 A) and contained the monomer form of rEcPleD-CHis. No other protein was detected by SDS-PAGE (Fig. 1B). The peak at 9.35 min (Fig. 1A) contained the dimer form of rEcPleD-CHis, and no other protein was detected in this fraction (Fig. 1B). The approximate ratio of monomer to dimer was 4 to 1. The DGC activity was very weak in the monomer fraction (data not shown). For C. crescentus rPleD, the phosphor mimic BeF3− induces dimerization, leading to a higher level of DGC activity (43, 66). Therefore, we incubated the HPLC-separated monomer rEcPleD-CHis with BeF3− and assayed for DGC activity. When BeF3-treated rEcPleD-CHis (100 μg) was incubated with 50 nmol GTP, about 0.5 nmol c-di-GMP was produced as detected by reversed-phase HPLC (Fig. 1C). To further investigate the activation of PleD by phosphorylation, we constructed a phosphoryl group acceptor mutant, rEcPleD(D53N)CHis. In C. crescentus PleD, the D53N mutation is not activated by BeF3− (43). Similarly, when rEcPleD(D53N)CHis monomer purified by Ni column and HPLC gel filtration chromatography was incubated with BeF3−, the DGC activity was not detected (Fig. 1D). DGC activity was dependent on the GGDEF motif, because the mutant rEcPleD(GG374DE)CHis had diminished DGC activity (Fig. 1E).
FIG. 1.
rEcPleD-CHis has DGC activity. (A) Separation of monomer and dimer forms of rEcPleD-CHis using HPLC gel filtration chromatography. The solid line is a representative elution profile of the dimer (9.35 min) and monomer (10.43 min) of rEcPleD-CHis. (B) SDS-PAGE gel showing the purification of rEcPleD-CHis. M, molecular size marker; lane 1, the soluble fraction from BL21(DE3)/pET33b-pleDCHis; lane 2, Ni column-purified rEcPleD-CHis; lane 3, HPLC-purified rEcPleD-CHis dimer; lane 4, HPLC-purified rEcPleD-CHis monomer. The arrow indicates rEcPleD-CHis. The gel was stained with Coomassie brilliant blue. (C, D, and E) One-hundred-microgram amounts of rEcPleD-CHis monomer (C) and rEcPleD(D53N)CHis monomer (D) as well as rEcPleD(GG374DE)CHis purified from the E. coli insoluble fraction (E) were incubated with BeCl2, NaF, and MgCl2 and then with 50 nmol GTP for 4 h at room temperature, were denatured, and were precipitated by centrifugation. The supernatant was resolved by reversed-phase HPLC. The solid line is an elution profile. The dashed line depicts the 3-to-20% methanol gradient. Numbers above the peaks in panels A, C, D, and E are retention times (min).
CDGA inhibits c-di-GMP-dependent biological events in Salmonella.
Our previous study suggested that the hydrophobic c-di-GMP analog, CDGA, is useful in studying the c-di-GMP signaling in live bacteria (35). To define the functions of CDGA, we examined its effects in well-established c-di-GMP-dependent phenomena, including the biosynthesis of cellulose and curli fimbriae in S. Typhimurium (30, 52, 60, 61, 70). c-di-GMP induces the master transcriptional regulator CsgD, which in turn induces the expression of curli biosynthesis genes. CsgD induces the GGDEF domain-containing protein AdrA as well, which regulates cellulose synthesis through putative creation of a different c-di-GMP pool (30, 52). An S. Typhimurium strain deficient in adrA displays reduced cellulose synthesis as detected by reduced staining of colonies on a calcofluor-containing plate (16). Similarly, the adrA mutant showed less-intense staining with calcofluor than did the parent strain 14028 after culturing in liquid medium without shaking (Fig. 2 B). The adrA mutant adheres to glass walls when cultured at 37°C with rapid shaking in a nutrient-deficient medium (16). Similarly, with crystal violet staining, we observed that the mutant was less adhesive to glass walls than the parent strain after bacteria were cultured with rotation (Fig. 2C). When the 14028 strain was cultured in the presence of CDGA, cellulose biosynthesis was reduced compared with a culture grown in the presence of the solvent DMSO (Fig. 2B and C). Bacterial clump formation mediated by curli fimbriae (54) was also reduced when the 14028 strain was cultured in the presence of CDGA, as in the case of the 14028 adrA mutant, as detected by phase-contrast microscopy (Fig. 2B). In contrast, addition of c-di-GMP did not influence cellulose and curli fimbriae synthesis under these experimental conditions (data not shown). Neither c-di-GMP nor CDGA changed the S. Typhimurium growth rate in the liquid medium (data not shown). We also examined the effects of CDGA on the protein level of CsgD and the mRNA levels of csgD and adrA, which are regulated by c-di-GMP (30). CDGA induced downregulation of csgD and adrA mRNA in a dose-dependent manner in the 14028 strain (Fig. 2D and E). These results showed that CDGA acts as antagonist of c-di-GMP signaling in Salmonella.
c-di-GMP binds to E. chaffeensis native proteins and I-site proteins and is inhibited by CDGA.
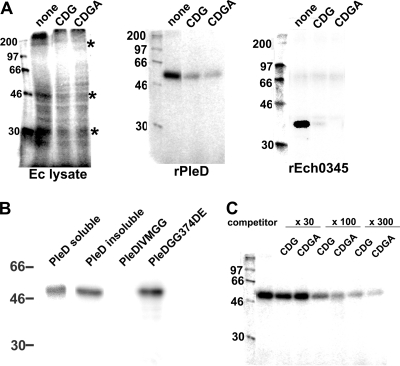
c-di-GMP functions via c-di-GMP-binding proteins, including several PilZ domain-containing proteins (3), such as C. crescentus DgrA (9), E. coli YcgR (56), P. aeruginosa Alg44 (40), and V. cholerae PlzABCDE (46), and several non-PilZ proteins, such as P. aeruginosa PelD (37), FleQ (23), and C. crescentus PopA (14). In silico analysis of the E. chaffeensis genome detected no protein that contains the characteristic PilZ domain motif R-Xa (produces hydrogen bond)-X-X-R-Xb (hydrophobic amino acid)-(1 to 50 of any amino acids)-D/N-Xb-S-X-X-G (5). c-di-GMP binds to the I site of C. crescentus PleD, which is associated with allosteric inhibition of its DGC activity (6, 8). C. crescentus PopA also binds c-di-GMP at the I site (14). The majority (>60%) of annotated GGDEF domain-containing proteins contain an I site (8). E. chaffeensis PleD has a typical I-site sequence (RIID) six amino acids upstream of the GGDEF motif. Our in silico analysis showed another potential I-site protein (Ech0345) with a deduced molecular mass of 33 kDa. This protein is weakly homologous to the S. Typhimurium GGDEF domain-containing protein AdrA (E value is 6e-05). Ech0345 contains a degenerate GGDEF motif, and the candidate I site (RIID) is also six amino acid residues upstream of the motif. Therefore, we examined [32P]c-di-GMP binding to the native E. chaffeensis lysate proteins, rPleD, and rECH0345. At least three E. chaffeensis-specific [32P]c-di-GMP-binding proteins (molecular masses of ∼32, 48, and 150 kDa) were detected in the E. chaffeensis lysate, all of which were specific to [32P]c-di-GMP because excess unlabeled c-di-GMP inhibited the [32P]c-di-GMP binding (Fig. 3 A), but excess GTP did not (data not shown). Two other bands of >200 kDa and 28 kDa may also be E. chaffeensis proteins, although bands of similar sizes were also detected in uninfected THP-1 cell lysates (data not shown). The two candidate I-site proteins, PleD and Ech0345, displayed specific binding to [32P]c-di-GMP as well, because the binding was inhibited by unlabeled c-di-GMP (Fig. 3A). The binding to PleD was I site dependent because the refolded I-site mutant rEcPleD(I-VMGG)CHis purified from the E. coli insoluble fraction did not bind [32P]c-di-GMP, whereas wild-type PleD purified and refolded in the same manner as the I-site mutant bound to [32P]c-di-GMP (Fig. 3B).
FIG. 3.
c-di-GMP specifically binds to E. chaffeensis native proteins and recombinant I-site proteins and is inhibited by CDGA. (A) The lysates (60 μg) prepared from host cell-free E. chaffeensis (Ec lysate), rEcPleD-CHis purified from the E. coli soluble fraction (1 μg), and rEch0345 (1 μg) were incubated with 1 μCi (0.1 pmol) of [32P]c-di-GMP and 50 pmol carrier unlabeled c-di-GMP in the absence (none) or presence of 1.5 nmol of synthesized c-di-GMP (CDG) or CDGA. Asterisks show E. chaffeensis native proteins that bind c-di-GMP. (B) The PleD I site is involved in c-di-GMP binding. rEcPleD-CHis purified from the E. coli soluble fraction (PleD soluble) or from the E. coli insoluble fraction (PleD insoluble), as well as rEcPleD(I-VMGG)CHis (PleDIVMGG) and rEcPleD(GG374DE)CHis (PleDGG374DE) purified from the E. coli insoluble fraction (1 μg each), were incubated with 1 μCi (0.1 pmol) of [32P]c-di-GMP and 50 pmol carrier unlabeled c-di-GMP. (C) CDGA binds to rPleD with an affinity similar to that of c-di-GMP. rEcPleD-CHis purified from the E. coli soluble fraction (1 μg) was incubated with 1 μCi (0.1 pmol) of [32P]c-di-GMP and 50 pmol carrier unlabeled c-di-GMP in the presence of 150 pmol, (×30), 450 pmol, (×100), or 1.5 nmol (×300) of unlabeled c-di-GMP (CDG) or CDGA. Numbers on the left are the molecular mass markers (in kDa). Asterisks show E. chaffeensis native proteins that bind c-di-GMP.
Because CDGA inhibited c-di-GMP signaling in Salmonella (Fig. 2), we examined whether CDGA inhibits c-di-GMP binding to E. chaffeensis target proteins. c-di-GMP binding to the three native proteins, rPleD, and rEch0345 was inhibited by CDGA (Fig. 3A). The relative affinities for binding of c-di-GMP and CDGA to the target proteins were likely similar, because the inhibitory effects on [32P]c-di-GMP binding induced by unlabeled c-di-GMP and CDGA were similar (Fig. 3C).
Pretreatment of E. chaffeensis with CDGA inhibits internalization.
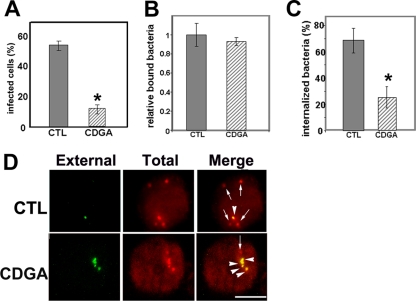
S. Typhimurium grows in the absence of eukaryotic cells and survives in eukaryotic cells. c-di-GMP is not essential for growth in vitro, because the growth rate of a strain lacking all 12 GGDEF domain-containing proteins is similar to that of the wild-type strain (62). To the best of our knowledge, there is no report showing a requirement of c-di-GMP for intracellular S. Typhimurium. Unlike Salmonella, E. chaffeensis exclusively survives and replicates in eukaryotic host cells. Because CDGA inhibits c-di-GMP-dependent biological activity of Salmonella as well as binding of c-di-GMP to E. chaffeensis proteins, we used CDGA to study whether c-di-GMP signaling is required for E. chaffeensis infection. First, host cell-free E. chaffeensis was pretreated with CDGA or DMSO. After being washed, bacteria were mixed with THP-1 cells, and infected cells were counted. The infectivity of E. chaffeensis pretreated with CDGA was significantly lower than that of E. chaffeensis pretreated with DMSO alone (Fig. 4 A). We then examined the inhibitory mechanisms of E. chaffeensis infection by CDGA in detail. E. chaffeensis binding to THP-1 cells was not inhibited by CDGA, because the number of CDGA-pretreated bacteria that were bound to host cells was comparable to that of solvent-pretreated bacteria (Fig. 4B). In contrast, E. chaffeensis internalization was significantly inhibited by CDGA, because the number of internalized bacteria was lower than that of solvent-pretreated bacteria (Fig. 4C and D). As a control, when isolated E. chaffeensis was preincubated with c-di-GMP or the solvent, no difference in infectivity was observed (data not shown).
FIG. 4.
CDGA pretreatment inhibits E. chaffeensis internalization. Host cell-free E. chaffeensis was pretreated with 0.3 mM CDGA (CDGA) or solvent (1% [vol/vol] DMSO; CTL) and incubated with THP-1 cells at an MOI of 100. (A) Cells were cultured for 96 h, and 100 THP-1 cells were scored for infection. (B) Bacteria bound to 100 THP-1 cells were counted. Bars represent relative numbers of bound bacteria, with the level for DMSO-treated bacteria set to 1.0. (C) After E. chaffeensis and THP-1 cells were incubated for 2 h, surface bacteria and internalized bacteria were differentially stained, bacteria in 100 THP-1 cells were counted, and the percentage of internalized bacteria was determined. The values are the means ± standard deviations (n = 3). Asterisks indicate significant differences between control and CDGA-treated E. chaffeensis (P < 0.01). (D) Differentially stained E. chaffeensis bacteria: external (green), total (internalized and external; red), and merge (red [arrows], internalized; yellow [arrowheads], noninternalized). Scale bar = 10 μm.
CDGA induces downregulation of E. chaffeensis surface-exposed proteins.
c-di-GMP signaling regulates the synthesis of bacterial surface components (exopolysaccharide, pili, flagella, and stalks) (22, 23, 28, 29, 32, 37, 39, 40, 52, 53, 59, 63). The E. chaffeensis genome does not encode complete sets of proteins required for biosynthesis of exopolysaccharide capsules, common pili, or flagella. Because CDGA pretreatment led to impaired internalization into host cells, we investigated the amounts of known E. chaffeensis surface-exposed proteins recovered when E. chaffeensis was cultured in the presence of CDGA or DMSO. Immunoelectron microscopic studies have demonstrated that TRP120 and P28 are bacterial surface-exposed proteins (41, 45, 68). The inclusion matrix comprising E. chaffeensis and Ehrlichia canis is filled with filamentous capsule-like materials (45, 49), and E. chaffeensis TRP120 is associated with filamentous material in the matrix (45). Proteomic analysis has also shown that P28 is exposed on the E. chaffeensis surface (17). P28 is predicted to form a β barrel in the outer membrane consisting of 12 transmembrane segments and functions as a porin (34). OmpA (peptidoglycan-associated lipoprotein) induces delayed-type hypersensitivity reactions in dogs (25). OmpA is predicted to be an outer membrane protein, because homologous proteins are found on the outer membranes of most Gram-negative bacterial species, such as Helicobacter pylori (31) and Haemophilus influenzae (11). Because of the lack of data regarding OmpA localization on E. chaffeensis, we first examined the surface expression of OmpA by immunofluorescence labeling. Anti-OmpA bound to the surfaces of paraformaldehyde-prefixed nonpermeabilized bacteria (65) (Fig. 5 A), indicating OmpA surface exposure. Normal rabbit serum (data not shown) and antiserum against the E. chaffeensis cytoplasmic protein NtrX (7, 33) did not bind to the bacterial surface (Fig. 5A), confirming the specificity of the antibody and surface exposure of OmpA. As detected by Western blotting with Hsp60 as a loading control, when host cell-free E. chaffeensis was incubated with CDGA for 2 h, the protein amounts of TRP120 and OmpA were reduced, whereas the P28 protein amounts did not change (Fig. 5B).
FIG. 5.
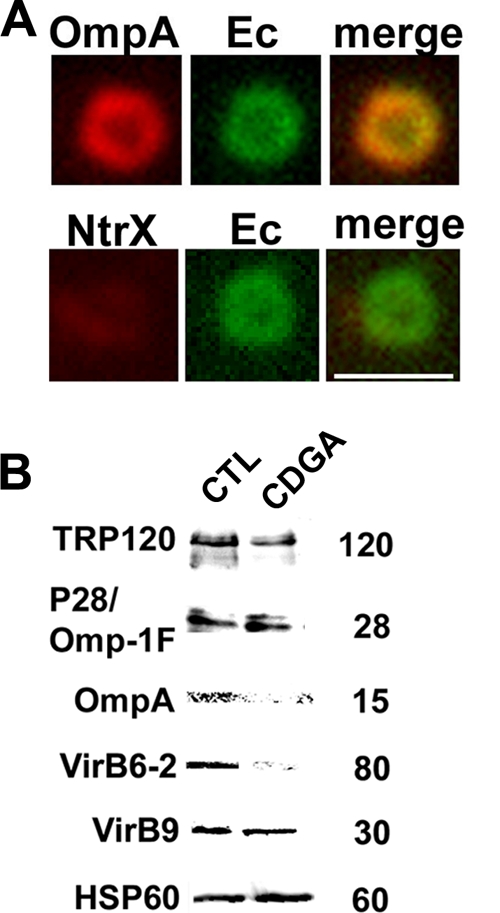
CDGA differentially downregulates E. chaffeensis surface-exposed proteins. (A) Surface expression of OmpA on a representative bacterium. Host cell-free bacteria (Ec) were stained with rabbit anti-OmpA or anti-NtrX (1:25), followed by Alexa Fluor 555-conjugated anti-rabbit IgG, and with dog anti-E. chaffeensis (Ec), followed by FITC-conjugated anti-dog IgG. The right panels are merged images. Scale bar = 2 μm. (B) Host cell-free E. chaffeensis was incubated with 0.3 mM CDGA (CDGA) or solvent (1% [vol/vol] DMSO; CTL) for 2 h. Proteins were detected in the E. chaffeensis lysates with rabbit antibodies to TRP120, P28, OmpA, VirB6-2, VirB9, and Hsp60.
The type IV secretion system (T4SS) is a virulence factor in several host cell-associated pathogens (2). The T4SS apparatus traverses the two bacterial membranes and transports macromolecules such as DNA and proteins into host cells or into or out of bacteria (2). In E. chaffeensis, proteomic analysis identified VirB9 as a surface-exposed protein (17). The 120-kDa VirB6-2 protein from E. chaffeensis is cleaved to produce an 80-kDa fragment (4). Most VirB6-2 80-kDa fragments are localized outside bacteria within the inclusion, and these fragments interact with VirB9 and VirB6-3 (4). Therefore, we determined the amounts of VirB9 and VirB6-2 in host cell-free bacteria treated with CDGA. CDGA reduced the amount of the 80-kDa VirB6-2 fragment but not that of VirB9 (Fig. 5B).
The reduction in E. chaffeensis surface-exposed proteins by CDGA is blocked by protease inhibitors.
E. chaffeensis is an obligatory intracellular bacterium lacking genes for most amino acid biosynthesis and for intermediary metabolism (47) and is not expected to synthesize significant new proteins within 2 h in SPK medium which contains only a single amino acid, l-glutamine. Thus, we first examined whether the reductions in TRP120, OmpA, and VirB6-2 protein levels was due to the degradation of these proteins by proteases. When host cell-free bacteria were incubated with CDGA in the presence of protease inhibitor cocktail, the reductions of the three proteins by CDGA were blocked (Fig. 6 A), implying that proteases are involved in the CDGA-dependent degradation of these proteins. The degradation of surface-exposed proteins is likely dependent on bacterial proteases on the bacterial surface, in the periplasm, or in the outer membrane. Besides several proteases with unknown localizations and specificity, our P-sort analysis (http://psort.ims.u-tokyo.ac.jp/) has predicted that two serine proteases of E. chaffeensis are localized at these sites. Therefore, we examined degradation of E. chaffeensis surface proteins in the presence of a serine protease inhibitor, DFP. TRP120 and VirB6-2 degradation was blocked by DFP, and OmpA degradation was slightly inhibited by DFP (Fig. 6B). These results suggest that an endogenous serine protease(s) is involved in CDGA-induced degradation of surface-exposed proteins.
FIG. 6.
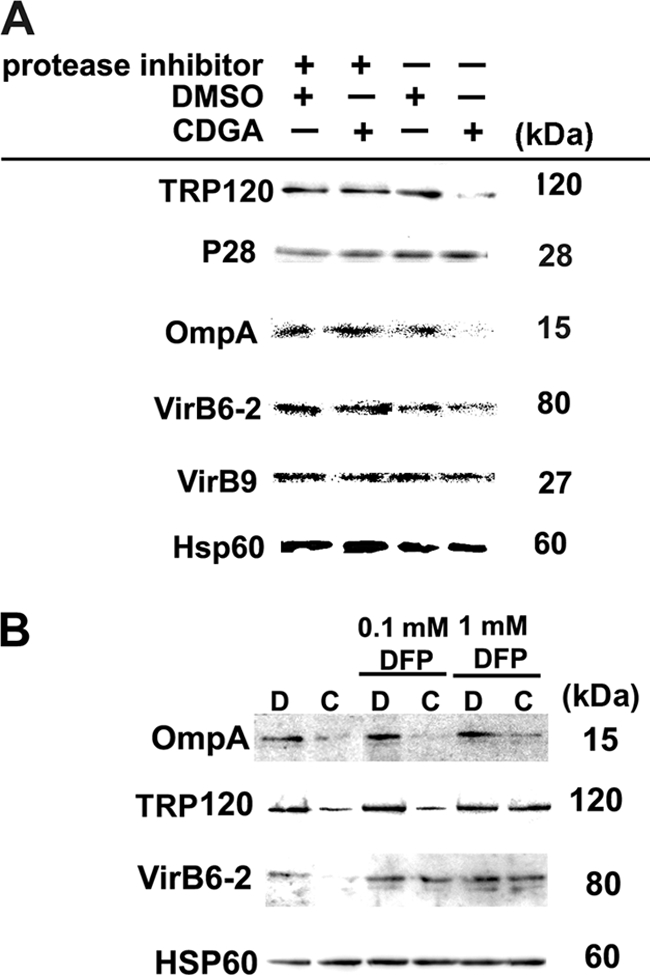
Protease inhibitors prevent CDGA-induced downregulation of E. chaffeensis surface-exposed proteins. (A) Host cell-free E. chaffeensis was incubated with 0.3 mM CDGA (CDGA) or solvent (1% [vol/vol] DMSO) for 2 h in the presence or absence of a protease inhibitor cocktail supplemented with 5 mM EDTA. (B) Host cell-free E. chaffeensis was incubated with 0.3 mM CDGA (C) or solvent (1% [vol/vol] DMSO) (D) for 2 h in the presence or absence of DFP. Proteins were visualized by Western blotting as described for Fig. 5B.
The surface serine protease HtrA degrades TRP120.
Of the two predicted surface-exposed serine proteases, an HtrA ortholog (Ech1052; YP_507837) was detected on the E. chaffeensis surface by biotin surface labeling of bacteria followed by proteomic analysis (17). The HtrA family of proteases consists of widely conserved endopeptidases from bacteria to humans, and members contain a trypsin-like protease domain and one or more PDZ domains that are involved in interaction with substrates (10). E. chaffeensis HtrA has two PDZ domains, similar to E. coli DegP/DegQ, that belong to the HtrA family (10). The C-terminal PDZ domain does not have significant homology with human HtrA. Therefore, anti-E. chaffeensis HtrA was raised against the C-terminal PDZ domain (anti-HtrA) and displayed no cross-reactivity against THP-1 cell proteins (data not shown). We confirmed surface exposure of HtrA using immunofluorescence labeling of nonpermeabilized E. chaffeensis. The surfaces of host cell-free bacteria were stained with anti-HtrA but not with preimmune serum (Fig. 7 A). E. chaffeensis rHtrA degraded succinylated casein, which is used in general protease assays (20), and the activity was blocked by DFP (data not shown), indicating that E. chaffeensis HtrA is, indeed, a serine protease.
FIG. 7.
rHtrA degrades rTRP120 and native TRP120. (A) Surface expression of HtrA on a representative bacterium. Host cell-free bacteria (Ec) were stained with rabbit anti-HtrA (HtrA) or preimmune serum (pre), followed by Alexa Fluor 555-conjugated anti-rabbit IgG and dog anti-E. chaffeensis, followed by FITC-conjugated anti-dog IgG. The antibody dilution was 1:100. The right panels are merged images. Scale bar = 2 μm. (B) rHtrA (1 μg) was incubated with 5 μg E. chaffeensis membrane fraction (TRP120) or 1 μg rTRP120 for 12 h at room temperature in the presence or absence of 10 mM DFP. (C) Anti-HtrA (αHtrA) IgG inhibits rTRP120 degradation by rHtrA. rTRP120 (1 μg) was incubated with rHtrA (1 μg) in the presence of anti-HtrA IgG (5 μg) or preimmune IgG (5 μg) for 5 h at room temperature. (D) Anti-HtrA inhibits CDGA-induced degradation of TRP120. Host cell-free bacteria isolated from 106 infected cells were incubated with 0.3 mM CDGA in the presence of anti-HtrA IgG (5 μg) or preimmune IgG (5 μg) or with solvent (1% [vol/vol] DMSO) alone. TRP120 was detected by Western blotting. The relative densities of rTRP120 or native TRP120 bands observed following different treatments are shown below each lane.
Although the detailed mechanism of E. chaffeensis internalization into host cells is unknown, TRP120-expressing E. coli bacteria are internalized into eukaryotic cells (45). This observation suggests that the reduction in E. chaffeensis internalization by CDGA treatment is related to the downregulation of TRP120 on the bacterial surface. We therefore examined whether E. chaffeensis TRP120 can be degraded by E. chaffeensis rHtrA. rHtrA degraded rTRP120 as well as native TRP120 in the E. chaffeensis membrane fraction and was inhibited by DFP (Fig. 7B). When rHtrA was incubated with the substrate rTRP120 in the presence of anti-HtrA, degradation of rTRP120 was partially inhibited, whereas no inhibition by the preimmune serum control was observed (Fig. 7C), indicating that the antibody blocked rHtrA protease activity for rTRP120. To examine whether endogenous HtrA is involved in CDGA-induced degradation of TRP120, we examined whether anti-HtrA inhibited CDGA-induced downregulation of endogenous TRP120. The amount of TRP120 was reduced in the presence of CDGA, and this reduction was blocked by anti-HtrA. No inhibition was detected when preimmune serum was used as a control (Fig. 7D). No change in the amount of P28/Omp-1F was observed under the same conditions (Fig. 7D). The CDGA-induced downregulation of TRP120 is not likely to be due to a direct interaction of CDGA with rHtrA or with the substrate TRP120, because the degradation of rTRP120 by rHtrA was not affected by CDGA (data not shown). Taken together, our data suggest that endogenous HtrA is involved in the CDGA-induced degradation of native TRP120.
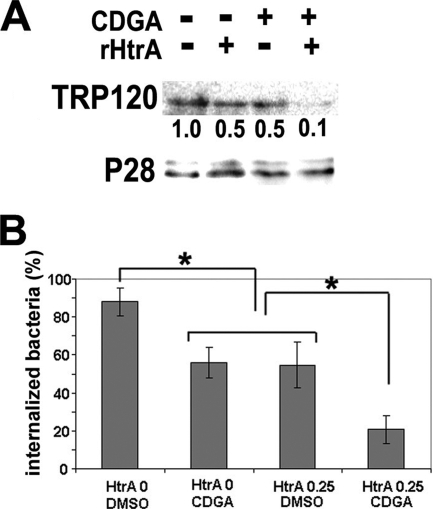
rHtrA enhances CDGA-induced downregulation of TRP120 and E. chaffeensis internalization.
Because rTRP120 degradation by rHtrA was not affected by CDGA, we examined whether rHtrA enhanced CDGA-induced native TRP120 degradation as well as bacterial internalization. When E. chaffeensis was incubated with 0.25 μg rHtrA or CDGA alone, internalization was reduced, and when CDGA and HtrA were combined, inhibitory effects were increased (Fig. 8 A and B).
FIG. 8.
CDGA and rHtrA have additive effects on the downregulation of TRP120 and reduction in E. chaffeensis internalization. (A) Host cell-free E. chaffeensis isolated from 2 × 106 heavily infected cells was incubated with exogenous rHtrA (0.25 μg) in the presence or absence of 0.3 mM CDGA for 2 h at 37°C. The amounts of TRP120 and P28/Omp-1F were detected by Western blotting. Relative densities of TRP120 bands are shown below each lane. (B) After incubation with rHtrA, bacteria were incubated with THP-1 cells for 2 h at 37°C. External bacteria and internalized bacteria were differentially stained with FITC-conjugated and Alexa Fluor 555-conjugated anti-dog IgG, respectively, as described for Fig. 4D. The percentage of internalized bacteria in 100 THP-1 cells was determined. The values are the means ± standard deviations (n = 3). Asterisks indicate significant differences (P < 0.01).
DISCUSSION
This study demonstrated that CDGA blocks well-established c-di-GMP-regulated phenomena, including the synthesis of cellulose and curli fimbriae by S. Typhimurium. Our data support the utility of CDGA for probing c-di-GMP signaling in live bacteria. CDGA contains two hydrophobic residues, and it exerted biological activity in Salmonella, A. phagocytophilum, and E. chaffeensis when exogenously added, implying that CDGA indeed penetrates across bacterial membranes. As demonstrated in the S. Typhimurium system, CDGA likely acts as a c-di-GMP functional antagonist by competitively inhibiting c-di-GMP binding to E. chaffeensis target proteins, as CDGA inhibited [32P]c-di-GMP binding of the three endogenous E. chaffeensis proteins in vitro. We cannot, however, exclude an alternative or concurrent possibility that the effects of CDGA are mediated by direct inhibition of E. chaffeensis PleD through binding to the I site and a consequent reduction of c-di-GMP in bacterial cells.
CDGA inhibited E. chaffeensis internalization and infection, implying that c-di-GMP signaling is indispensable for E. chaffeensis survival. Although c-di-GMP signaling pathways remain to be studied, E. chaffeensis has at least three c-di-GMP-binding proteins (32, 48, and 150 kDa) that may mediate c-di-GMP signaling. The 48-kDa band likely contains PleD, as E. chaffeensis rPleD bound [32P]c-di-GMP. Interestingly, a new I-site protein (a hypothetical protein, Ech0345, whose predicted molecular mass is 33 kDa) with a degenerate GGDEF domain was found to bind c-di-GMP, and thus, the 32-kDa band likely includes Ech0345.
Our results suggest that c-di-GMP signaling regulates the stability of several surface-exposed proteins (TRP120, OmpA, and VirB6-2) in E. chaffeensis. Although the details of the signaling pathway are unknown, during the C. crescentus cell cycle, marked changes in bacterial surface components that mediate functions such as flagellum ejection, holdfast formation, and stalk formation take place at the same pole, and these surface events are spatially and temporally controlled by polar-localized activated PleD (1, 38, 44). Proper positioning of a c-di-GMP PDE is required for flagellum biogenesis at the newly emerging cell pole (26). E. chaffeensis lacks these bacterial surface organelles or an obvious pole; however, E. chaffeensis PleD may share a fundamental principle with Caulobacter PleD in regulating bacterial surface components. We also examined the influences of CDGA on bacterial mRNA levels. E. chaffeensis mRNA levels of several genes normalized by the rpoA mRNA level were reduced by 0 to 40% in CDGA-treated bacteria (see Fig. S1 in the supplemental material). Thus, CDGA destabilizes certain mRNA; however, reduced mRNA is unlikely the cause of the reductions of TRP120, OmpA, and VirB6-2 protein amounts by CDGA under the present assay condition. Because (i) this reduction is blocked by protease inhibitors, (ii) any change in mRNA is unlikely translated to the protein levels, as new protein synthesis by host cell-free bacteria is minimum, and (iii) there is no correlation between changes in mRNA and protein levels, reduction was not specific to ompA or the TRP120 gene, and p28 was also reduced.
Anti-HtrA inhibited CDGA-induced TRP120 degradation, suggesting TRP120 degradation by endogenous HtrA. The serine protease HtrA and the metalloprotease PepA aminopeptidase are present on the surface of E. chaffeensis, as shown by biotin surface labeling followed by proteomic analysis (17); however, neither of the proteases had been characterized. Our study showed that HtrA is indeed surface exposed on E. chaffeensis and has the ability to degrade endogenous surface-exposed proteins. Members of the HtrA family of proteases in bacteria are generally localized in the periplasm (10), and to the best of our knowledge, no report has shown HtrA localization on the bacterial surface. E. chaffeensis HtrA has a signal peptide, but the mature protein does not contain a potential transmembrane domain. Thus, the protein is associated with the bacterial surface but is not likely to be anchored to the outer membrane. Although the localization of E. chaffeensis HtrA in the periplasm has not been examined, it may also be localized in the periplasm. Several bacterial surface proteases function as virulence factors by facilitating infection of the host. For example, Pla protease produced by Y. pestis mediates bacterial adherence, invasion, and attack of the host innate immune system (18). Pic protease, which is produced by enteroaggregative E. coli, is involved in bacterial colonization in the mouse intestine (19). To the best of our knowledge, no study has reported the involvement of c-di-GMP in any protease functions that inhibit bacterial infection. CDGA per se did not activate rHtrA, as degradation of casein by rHtrA was not accelerated by CDGA (data not shown). CDGA may, however, indirectly activate endogenous HtrA. Alternatively, CDGA renders certain surface-exposed proteins, such as TRP120, accessible or susceptible to bacterial surface protease actions, such as HtrA. The additive effects of rHtrA and CDGA on degradation of TRP120 suggest that rHtrA and CDGA act on the nonoverlapping target independently.
It remains to be elucidated how cytoplasmic c-di-GMP regulates the susceptibility of surface-exposed E. chaffeensis proteins. c-di-GMP is associated with protease function in C. crescentus. The c-di-GMP-binding I-site protein PopA mediates the degradation of CtrA, an inhibitor of chromosomal DNA replication, at the G1/S cell cycle transition in C. crescentus by directing CtrA to the cell pole where the ClpXP protease functions (14). Ech0345 is a c-di-GMP-binding I-site protein with a degenerate GGDEF motif similar to that of PopA, and it is possible that this protein is involved in linking c-di-GMP to proteases in E. chaffeensis.
Involvement of c-di-GMP signaling in stabilization of the TRP120 surface protein is consistent with a critical role for this molecule in obligatory intracellular bacterial infection. TRP120 is expressed in mature dense-core cells that have a high level of internalization ability (68), and recombinant E. coli bacteria that express E. chaffeensis TRP120 invade HeLa cells (45). E. coli bacteria that express Ehrlichia ruminantium “mucin-like protein” (a TRP120 homolog) bind to tick IDE8 cells, and the recombinant mucin-like protein binds to tick IDE8 cell lysate proteins (12). Although the host cell receptors for these binding events and the matter of how they are involved in the internalization process are currently unknown, it is possible that besides TRP120, other bacterial surface-exposed proteins are associated with bacterial internalization. OmpA may be one such protein, because levels were reduced by CDGA treatment and because the E. coli OmpA ortholog is involved in bacterial invasion of astrocytes and E. coli-induced meningitis (67). One interesting finding in our study is that CDGA treatment and consequent degradation of TRP120 or OmpA did not inhibit E. chaffeensis binding to THP-1 cells, indicating that the remaining proteins are sufficient for bacterial binding but that TRP120 and OmpA are required for efficient internalization. Moreover, one component of the T4SS, VirB6-2 (4), was reduced by CDGA treatment. Because the T4SS is a virulence factor in several pathogens for establishing infection in host cells (2), it is possible that in E. chaffeensis, c-di-GMP contributes to bacterial infection by regulating T4SS function by modulating bacterial surface-exposed T4SS components.
Despite the sequence similarity between E. chaffeensis PleD and C. crescentus PleD (41% identical), several intriguing differences are apparent. First, the specific DGC activity of E. chaffeensis rPleD was much lower than that of C. crescentus rPleD, even after activation by BeF3− (data not shown). The E. chaffeensis genome does not encode EAL (29, 50) or HD-GYP (55) proteins associated with c-di-GMP PDE activity, suggesting that intrabacterial levels of c-di-GMP depend only on the weak DGC activity of PleD in E. chaffeensis. Second, PleD in free-living C. crescentus is involved in bacterial differentiation by regulating the expression of bacterial surface organelles, whereas PleD in the obligatory intracellular bacterium E. chaffeensis is involved in the host-bacteria interaction (internalization) by regulating the expression of interfacing surface proteins. Third, and most importantly, on the basis of the proposed function of c-di-GMP, PleD is likely to be essential for E. chaffeensis survival, whereas PleD is not essential for C. crescentus survival (21).
Like E. chaffeensis and A. phagocytophilum (35), bacteria belonging to the order Rickettsiales have only a single GGDEF domain-containing protein, PleD (51). Except for the two species E. chaffeensis and A. phagocytophilum, PleD orthologs in Rickettsiales have not been characterized. The results presented here are the first data that link c-di-GMP to bacterial surface-exposed proteins and bacterial invasion, providing an interesting model for studying c-di-GMP functions in obligatory intracellular bacteria and beyond.
Supplementary Material
Acknowledgments
We thank David Wemmer at the University of California, Berkeley (Berkeley, CA), for BeCl2, Iñigo Lasa at the Universidad Pública de Navarra (Navarra, Spain) for the S. Typhimurium strains 14028 and 14028 adrA::Km, Ute Römling at Karolinska Institutet (Stockholm, Sweden) for anti-S. Typhimurium CsgD, Stephen Lory at Harvard Medical School (Boston, MA) for the E. coli strain that overexpresses P. aeruginosa rWspR, Matthias Christen at the University of Basel (Basel, Switzerland) for detailed methods for the c-di-GMP binding assay and for the E. coli strain that overexpresses C. crescentus PleD, Koshiro Miura in our laboratory for in silico analysis of an I-site protein, and Xue-jie Yu at the University of Texas Medical Branch at Galveston (Galveston, TX) for anti-TRP120.
This work was supported by National Institutes of Health grant R01 AI054476.
Footnotes
Published ahead of print on 18 June 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aldridge, P., R. Paul, P. Goymer, P. Rainey, and U. Jenal. 2003. Role of the GGDEF regulator PleD in polar development of Caulobacter crescentus. Mol. Microbiol. 47:1695-1708. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Martinez, C. E., and P. J. Christie. 2009. Biological diversity of prokaryotic type IV secretion systems. Microbiol. Mol. Biol. Rev. 73:775-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amikam, D., and M. Y. Galperin. 2006. PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics 22:3-6. [DOI] [PubMed] [Google Scholar]
- 4.Bao, W., Y. Kumagai, H. Niu, M. Yamaguchi, K. Miura, and Y. Rikihisa. 2009. Four VirB6 paralogs and VirB9 are expressed and interact in Ehrlichia chaffeensis-containing vacuoles. J. Bacteriol. 191:278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benach, J., S. S. Swaminathan, R. Tamayo, S. K. Handelman, E. Folta-Stogniew, J. E. Ramos, F. Forouhar, H. Neely, J. Seetharaman, A. Camilli, and J. F. Hunt. 2007. The structural basis of cyclic diguanylate signal transduction by PilZ domains. EMBO J. 26:5153-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, C., R. Paul, D. Samoray, N. C. Amiot, B. Giese, U. Jenal, and T. Schirmer. 2004. Structural basis of activity and allosteric control of diguanylate cyclase. Proc. Natl. Acad. Sci. U. S. A. 101:17084-17089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, Z., Y. Kumagai, M. Lin, C. Zhang, and Y. Rikihisa. 2006. Intra-leukocyte expression of two-component systems in Ehrlichia chaffeensis and Anaplasma phagocytophilum and effects of the histidine kinase inhibitor closantel. Cell. Microbiol. 8:1241-1252. [DOI] [PubMed] [Google Scholar]
- 8.Christen, B., M. Christen, R. Paul, F. Schmid, M. Folcher, P. Jenoe, M. Meuwly, and U. Jenal. 2006. Allosteric control of cyclic di-GMP signaling. J. Biol. Chem. 281:32015-32024. [DOI] [PubMed] [Google Scholar]
- 9.Christen, M., B. Christen, M. G. Allan, M. Folcher, P. Jeno, S. Grzesiek, and U. Jenal. 2007. DgrA is a member of a new family of cyclic diguanosine monophosphate receptors and controls flagellar motor function in Caulobacter crescentus. Proc. Natl. Acad. Sci. U. S. A. 104:4112-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clausen, T., C. Southan, and M. Ehrmann. 2002. The HtrA family of proteases: implications for protein composition and cell fate. Mol. Cell 10:443-455. [DOI] [PubMed] [Google Scholar]
- 11.Deich, R. A., B. J. Metcalf, C. W. Finn, J. E. Farley, and B. A. Green. 1988. Cloning of genes encoding a 15,000-dalton peptidoglycan-associated outer membrane lipoprotein and an antigenically related 15,000-dalton protein from Haemophilus influenzae. J. Bacteriol. 170:489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Fuente, J., J. C. Garcia-Garcia, A. F. Barbet, E. F. Blouin, and K. M. Kocan. 2004. Adhesion of outer membrane proteins containing tandem repeats of Anaplasma and Ehrlichia species (Rickettsiales: Anaplasmataceae) to tick cells. Vet. Microbiol. 98:313-322. [DOI] [PubMed] [Google Scholar]
- 13.Demma, L. J., R. C. Holman, J. H. McQuiston, J. W. Krebs, and D. L. Swerdlow. 2006. Human monocytic ehrlichiosis and human granulocytic anaplasmosis in the United States, 2001-2002. Ann. N. Y. Acad. Sci. 1078:118-119. [DOI] [PubMed] [Google Scholar]
- 14.Duerig, A., S. Abel, M. Folcher, M. Nicollier, T. Schwede, N. Amiot, B. Giese, and U. Jenal. 2009. Second messenger-mediated spatiotemporal control of protein degradation regulates bacterial cell cycle progression. Genes Dev. 23:93-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galperin, M. Y., A. N. Nikolskaya, and E. V. Koonin. 2001. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol. Lett. 203:11-21. [DOI] [PubMed] [Google Scholar]
- 16.Garcia, B., C. Latasa, C. Solano, F. Garcia-del Portillo, C. Gamazo, and I. Lasa. 2004. Role of the GGDEF protein family in Salmonella cellulose biosynthesis and biofilm formation. Mol. Microbiol. 54:264-277. [DOI] [PubMed] [Google Scholar]
- 17.Ge, Y., and Y. Rikihisa. 2007. Surface-exposed proteins of Ehrlichia chaffeensis. Infect. Immun. 75:3833-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haiko, J., M. Suomalainen, T. Ojala, K. Lahteenmaki, and T. K. Korhonen. 2009. Invited review: breaking barriers—attack on innate immune defences by omptin surface proteases of enterobacterial pathogens. Innate Immun. 15:67-80. [DOI] [PubMed] [Google Scholar]
- 19.Harrington, S. M., J. Sheikh, I. R. Henderson, F. Ruiz-Perez, P. S. Cohen, and J. P. Nataro. 2009. The Pic protease of enteroaggregative Escherichia coli promotes intestinal colonization and growth in the presence of mucin. Infect. Immun. 77:2465-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatakeyama, T., H. Kohzaki, and N. Yamasaki. 1992. A microassay for proteases using succinylcasein as a substrate. Anal. Biochem. 204:181-184. [DOI] [PubMed] [Google Scholar]
- 21.Hecht, G. B., and A. Newton. 1995. Identification of a novel response regulator required for the swarmer-to-stalked-cell transition in Caulobacter crescentus. J. Bacteriol. 177:6223-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hengge, R. 2009. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 7:263-273. [DOI] [PubMed] [Google Scholar]
- 23.Hickman, J. W., and C. S. Harwood. 2008. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol. Microbiol. 69:376-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hisert, K. B., M. MacCoss, M. U. Shiloh, K. H. Darwin, S. Singh, R. A. Jones, S. Ehrt, Z. Zhang, B. L. Gaffney, S. Gandotra, D. W. Holden, D. Murray, and C. Nathan. 2005. A glutamate-alanine-leucine (EAL) domain protein of Salmonella controls bacterial survival in mice, antioxidant defence and killing of macrophages: role of cyclic diGMP. Mol. Microbiol. 56:1234-1245. [DOI] [PubMed] [Google Scholar]
- 25.Huang, H., M. Lin, X. Wang, T. Kikuchi, H. Mottaz, A. Norbeck, and Y. Rikihisa. 2008. Proteomic analysis of and immune responses to Ehrlichia chaffeensis lipoproteins. Infect. Immun. 76:3405-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huitema, E., S. Pritchard, D. Matteson, S. K. Radhakrishnan, and P. H. Viollier. 2006. Bacterial birth scar proteins mark future flagellum assembly site. Cell 124:1025-1037. [DOI] [PubMed] [Google Scholar]
- 27.Hyodo, M., and Y. Hayakawa. 2004. An improved method for synthesizing cyclic bis(3′-5′)diguanylic acid (c-di-GMP). Bull. Chem. Soc. Jpn. 77:2089-2093. [Google Scholar]
- 28.Jenal, U. 2004. Cyclic di-guanosine-monophosphate comes of age: a novel secondary messenger involved in modulating cell surface structures in bacteria? Curr. Opin. Microbiol. 7:185-191. [DOI] [PubMed] [Google Scholar]
- 29.Jenal, U., and J. Malone. 2006. Mechanisms of cyclic-di-GMP signaling in bacteria. Annu. Rev. Genet. 40:385-407. [DOI] [PubMed] [Google Scholar]
- 30.Kader, A., R. Simm, U. Gerstel, M. Morr, and U. Römling. 2006. Hierarchical involvement of various GGDEF domain proteins in rdar morphotype development of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 60:602-616. [DOI] [PubMed] [Google Scholar]
- 31.Kim, J. S., J. H. Chang, W. Y. Seo, G. J. Yu, S. I. Chung, and J. S. Yum. 2000. Cloning and characterization of a 22 kDa outer-membrane protein (Omp22) from Helicobacter pylori. Mol. Cells 10:633-641. [DOI] [PubMed] [Google Scholar]
- 32.Kulasakara, H., V. Lee, A. Brencic, N. Liberati, J. Urbach, S. Miyata, D. G. Lee, A. N. Neely, M. Hyodo, Y. Hayakawa, F. M. Ausubel, and S. Lory. 2006. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3′-5′)-cyclic-GMP in virulence. Proc. Natl. Acad. Sci. U. S. A. 103:2839-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumagai, Y., Z. Cheng, M. Lin, and Y. Rikihisa. 2006. Biochemical activities of three pairs of Ehrlichia chaffeensis two-component regulatory system proteins involved in inhibition of lysosomal fusion. Infect. Immun. 74:5014-5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumagai, Y., H. Huang, and Y. Rikihisa. 2008. Expression and porin activity of P28 and OMP-1F during intracellular Ehrlichia chaffeensis development. J. Bacteriol. 190:3597-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai, T. H., Y. Kumagai, M. Hyodo, Y. Hayakawa, and Y. Rikihisa. 2009. The Anaplasma phagocytophilum PleC histidine kinase and PleD diguanylate cyclase two-component system and role of cyclic di-GMP in host cell infection. J. Bacteriol. 191:693-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamprokostopoulou, A., C. Monteiro, M. Rhen, and U. Römling. 2010. Cyclic di-GMP signalling controls virulence properties of Salmonella enterica serovar Typhimurium at the mucosal lining. Environ. Microbiol. 12:40-53. [DOI] [PubMed] [Google Scholar]
- 37.Lee, V. T., J. M. Matewish, J. L. Kessler, M. Hyodo, Y. Hayakawa, and S. Lory. 2007. A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol. Microbiol. 65:1474-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levi, A., and U. Jenal. 2006. Holdfast formation in motile swarmer cells optimizes surface attachment during Caulobacter crescentus development. J. Bacteriol. 188:5315-5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim, B., S. Beyhan, J. Meir, and F. H. Yildiz. 2006. Cyclic-diGMP signal transduction systems in Vibrio cholerae: modulation of rugosity and biofilm formation. Mol. Microbiol. 60:331-348. [DOI] [PubMed] [Google Scholar]
- 40.Merighi, M., V. T. Lee, M. Hyodo, Y. Hayakawa, and S. Lory. 2007. The second messenger bis-(3′-5′)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol. Microbiol. 65:876-895. [DOI] [PubMed] [Google Scholar]
- 41.Ohashi, N., N. Zhi, Y. Zhang, and Y. Rikihisa. 1998. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paddock, C. D., and J. E. Childs. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 16:37-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paul, R., S. Abel, P. Wassmann, A. Beck, H. Heerklotz, and U. Jenal. 2007. Activation of the diguanylate cyclase PleD by phosphorylation-mediated dimerization. J. Biol. Chem. 282:29170-29177. [DOI] [PubMed] [Google Scholar]
- 44.Paul, R., S. Weiser, N. C. Amiot, C. Chan, T. Schirmer, B. Giese, and U. Jenal. 2004. Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Genes Dev. 18:715-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Popov, V. L., X. Yu, and D. H. Walker. 2000. The 120 kDa outer membrane protein of Ehrlichia chaffeensis: preferential expression on dense-core cells and gene expression in Escherichia coli associated with attachment and entry. Microb. Pathog. 28:71-80. [DOI] [PubMed] [Google Scholar]
- 46.Pratt, J. T., R. Tamayo, A. D. Tischler, and A. Camilli. 2007. PilZ domain proteins bind cyclic diguanylate and regulate diverse processes in Vibrio cholerae. J. Biol. Chem. 282:12860-12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rikihisa, Y. 2010. Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells. Nat. Rev. Microbiol. 8:328-339. [DOI] [PubMed] [Google Scholar]
- 48.Rikihisa, Y. 2010. Molecular events involved in cellular invasion by Ehrlichia chaffeensis and Anaplasma phagocytophilum. Vet. Parasitol. 167:155-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rikihisa, Y. 1991. The tribe Ehrlichieae and ehrlichial diseases. Clin. Microbiol. Rev. 4:286-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Römling, U. 2005. Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell. Mol. Life Sci. 62:1234-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Römling, U. 2009. Cyclic Di-GMP (c-di-GMP) goes into host cells—c-di-GMP signaling in the obligate intracellular pathogen Anaplasma phagocytophilum. J. Bacteriol. 191:683-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Römling, U., and D. Amikam. 2006. Cyclic di-GMP as a second messenger. Curr. Opin. Microbiol. 9:218-228. [DOI] [PubMed] [Google Scholar]
- 53.Römling, U., M. Gomelsky, and M. Y. Galperin. 2005. C-di-GMP: the dawning of a novel bacterial signalling system. Mol. Microbiol. 57:629-639. [DOI] [PubMed] [Google Scholar]
- 54.Römling, U., W. D. Sierralta, K. Eriksson, and S. Normark. 1998. Multicellular and aggregative behaviour of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Mol. Microbiol. 28:249-264. [DOI] [PubMed] [Google Scholar]
- 55.Ryan, R. P., Y. Fouhy, J. F. Lucey, L. C. Crossman, S. Spiro, Y. W. He, L. H. Zhang, S. Heeb, M. Camara, P. Williams, and J. M. Dow. 2006. Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proc. Natl. Acad. Sci. U. S. A. 103:6712-6717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Ryjenkov, D. A., R. Simm, U. Römling, and M. Gomelsky. 2006. The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J. Biol. Chem. 281:30310-30314. [DOI] [PubMed] [Google Scholar]
- 57.Ryjenkov, D. A., M. Tarutina, O. V. Moskvin, and M. Gomelsky. 2005. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J. Bacteriol. 187:1792-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 59.Simm, R., J. D. Fetherston, A. Kader, U. Römling, and R. D. Perry. 2005. Phenotypic convergence mediated by GGDEF-domain-containing proteins. J. Bacteriol. 187:6816-6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simm, R., A. Lusch, A. Kader, M. Andersson, and U. Römling. 2007. Role of EAL-containing proteins in multicellular behavior of Salmonella enterica serovar Typhimurium. J. Bacteriol. 189:3613-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simm, R., U. Remminghorst, I. Ahmad, K. Zakikhany, and U. Römling. 2009. A role for the EAL-like protein STM1344 in regulation of CsgD expression and motility in Salmonella enterica serovar Typhimurium. J. Bacteriol. 191:3928-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Solano, C., B. Garcia, C. Latasa, A. Toledo-Arana, V. Zorraquino, J. Valle, J. Casals, E. Pedroso, and I. Lasa. 2009. Genetic reductionist approach for dissecting individual roles of GGDEF proteins within the c-di-GMP signaling network in Salmonella. Proc. Natl. Acad. Sci. U. S. A. 106:7997-8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tamayo, R., J. T. Pratt, and A. Camilli. 2007. Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu. Rev. Microbiol. 61:131-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tischler, A. D., and A. Camilli. 2005. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect. Immun. 73:5873-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, X., T. Kikuchi, and Y. Rikihisa. 2006. Two monoclonal antibodies with defined epitopes of P44 major surface proteins neutralize Anaplasma phagocytophilum by distinct mechanisms. Infect. Immun. 74:1873-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wassmann, P., C. Chan, R. Paul, A. Beck, H. Heerklotz, U. Jenal, and T. Schirmer. 2007. Structure of BeF3−-modified response regulator PleD: implications for diguanylate cyclase activation, catalysis, and feedback inhibition. Structure 15:915-927. [DOI] [PubMed] [Google Scholar]
- 67.Wu, H. H., Y. Y. Yang, W. S. Hsieh, C. H. Lee, S. J. Leu, and M. R. Chen. 2009. OmpA is the critical component for Escherichia coli invasion-induced astrocyte activation. J. Neuropathol. Exp. Neurol. 68:677-690. [DOI] [PubMed] [Google Scholar]
- 68.Zhang, J. Z., V. L. Popov, S. Gao, D. H. Walker, and X. J. Yu. 2007. The developmental cycle of Ehrlichia chaffeensis in vertebrate cells. Cell. Microbiol. 9:610-618. [DOI] [PubMed] [Google Scholar]
- 69.Zhang, Y., N. Ohashi, E. H. Lee, A. Tamura, and Y. Rikihisa. 1997. Ehrlichia sennetsu groE operon and antigenic properties of the GroEL homolog. FEMS Immunol. Med. Microbiol. 18:39-46. [DOI] [PubMed] [Google Scholar]
- 70.Zogaj, X., M. Nimtz, M. Rohde, W. Bokranz, and U. Römling. 2001. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 39:1452-1463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.