Abstract
Bacillus subtilis, which grows under aerobic conditions, employs fatty acid desaturase (Des) to fluidize its membrane when subjected to temperature downshift. Des requires molecular oxygen for its activity, and its expression is regulated by DesK-DesR, a two-component system. Transcription of des is induced by the temperature downshift and is decreased when membrane fluidity is restored. B. subtilis is also capable of anaerobic growth by nitrate or nitrite respiration. We studied the mechanism of cold adaptation in B. subtilis under anaerobic conditions that were predicted to inhibit Des activity. We found that in anaerobiosis, in contrast to aerobic growth, the induction of des expression after temperature downshift (from 37°C to 25°C) was not downregulated. However, the transfer from anaerobic to aerobic conditions rapidly restored the downregulation. Under both aerobic and anaerobic conditions, the induction of des expression was substantially reduced by the addition of external fluidizing oleic acid and was fully dependent on the DesK-DesR two-component regulatory system. Fatty acid analysis proved that there was no desaturation after des induction under anaerobic conditions despite the presence of high levels of the des protein product, which was shown by immunoblot analysis. The cold adaptation of B. subtilis in anaerobiosis is therefore mediated exclusively by the increased anteiso/iso ratio of branched-chain fatty acids and not by the temporarily increased level of unsaturated fatty acids that is typical under aerobic conditions. The degrees of membrane fluidization, as measured by diphenylhexatriene fluorescence anisotropy, were found to be similar under both aerobic and anaerobic conditions.
Bacterial growth requires an appreciable fraction of the acyl chains of the membrane lipids to be in a disordered state. Such disordered states are brought about by fatty acids that act to offset the closely packed ordered arrangement of the lipid bilayer acyl chains that are imparted by the straight-chain saturated acyl chains. In most bacteria, the role of introducing acyl chain disorder is fulfilled by unsaturated fatty acids. Some bacteria synthesize UFAs by the process of desaturation, an oxygen-requiring reaction that introduces the double bond into a single concerted reaction. However, this is not an option for bacteria that grow under anaerobic conditions. For example, in the upper layers of soil, which are the natural habitat of Bacillus subtilis, the fluctuations in oxygen availability that are mostly caused by the changing water content are common.
Even though B. subtilis is still generally considered to be an obligate aerobe, several recent studies have proved that this bacterium is in fact a facultative anaerobe, which is capable of both fermentation and anaerobic respiration with either nitrate or nitrite used as the terminal electron acceptor (19, 20). The key role in the complex regulation of anaerobic metabolism of B. subtilis can be attributed to the genes resD and resE (21, 26) and fnr (23).
A decrease in the ambient temperature brings several additional problems for soil bacteria to cope with. One of them is the decrease of the fluidity of their membranes that prevents the membrane from functioning properly. B. subtilis cells use two distinct mechanisms of membrane adaptation to low temperatures. The long-term membrane adaptation employs an increase in low-melting-point anteiso-branched FAs (mostly a-15:0 and a-17:0) that fluidize the membrane effectively (27, 28). For branched-chain FA synthesis, branched-chain acyl-CoA molecules, which are derived from the amino acids valine (Val), leucine (Leu), and isoleucine (Ile), are used as primers (6, 15). Seven genes that code for the enzymes involved in the deamination of Ile and Val, and in the oxidative decarboxylation of branched-chain alpha-keto acids, are organized in an operon called bkd (10) that was shown to be induced by the presence of Ile and Val in the cultivation medium and also by a downshift in temperature (14). The induction is dependent on the alternative sigma factor SigL (29). The initial condensation step of FA synthesis is catalyzed by the FabH enzyme, which prefers branched-chain substrates to straight-chain ones. The substrate specificity of this enzyme is crucial for branched FA production. The B. subtilis genome contains two homologs of this enzyme: fabH1 and fabH2 (7). It has been suggested that fabH1 is constitutively expressed whereas fabH2 could be induced by an environmental or developmental signal. However, no direct evidence for this hypothesis has been provided so far. Therefore, the regulation of the anteiso/iso-branched FA ratio is still far from being fully understood, but it is evident that the availability of compounds, which serve as primers for FA synthesis, determines the membrane composition to a great extent. At the same time, it is not known whether anaerobic metabolism and its related changes of the global regulation have some impact on levels of these precursors.
After the temperature downshift, a different mechanism is employed for membrane fluidization, which induces the synthesis of the Des. Des is a membrane-bound protein with six transmembrane helices that span the lipid bilayer (11). It catalyzes the oxygen-dependent FA desaturation of membrane phospholipids (1, 2). The induction of des expression is regulated by a two-component system consisting of DesK, a sensor kinase, and DesR, a response regulator (2). The transmembrane domain of the DesK protein presumably serves as a sensor of the membrane fluidity (3). Des synthesis was shown to be inhibited by the presence of different types of UFAs in the medium; the oleic acid (18:1) had the most prominent impact (2). The authors presumed that the exogenous UFAs lowered the requirements for desaturation due to the fluidization of the cytoplasmic membrane. Inhibition of the Des synthesis was also reported for anteiso-branched FA precursors (9).
In our previous work (5), we showed that the cold adaptation of the B. subtilis membrane is influenced markedly by the available source of carbon. Aerobic growth on mineral medium with glycerol at 40°C (the near-optimum temperature for B. subtilis growth) was found to result in almost the same pattern of membrane FAs as did the growth on complex medium at 20°C. We assumed that the different energy metabolism during anaerobiosis must also have a profound impact on the carbon flow of the cells and, therefore, on the availability of the branched FA precursors. In addition, the cold adaptation in anaerobiosis should also be affected by the inability of FA desaturase to fluidize the membrane.
Therefore, we decided to study the effect of anaerobic cultivation on the long-term adaptation of B. subtilis to cold temperature and on the immediate response of B. subtilis cells to the temperature downshift. We focused on the changes in membrane composition, regulation of expression of FA desaturase, and the extent of adaptation of membrane fluidity.
MATERIALS AND METHODS
Abbreviations.
FA, fatty acid; UFA, unsaturated fatty acid; OD450, optical density at 450 nm; GFP, green fluorescent protein; i-, iso-branched fatty acid; a-, anteiso-branched fatty acid; n, nonbranched fatty acid; aero-, aerobically grown; anaero-, anaerobically grown; DPH, 1,6-diphenyl-1,3,5-hexatriene; rss, steady-state anisotropy of fluorescence; MU, Miller units; ONP, o-nitrophenol; ONPG, orthonitrophenyl-β-galactoside; HE, homeoviscous efficacy; CoA, coenzyme A; Des, fatty acid desaturase; LB, Luria broth; Amp, ampicillin; Cm, chloramphenicol; Km, kanamycin; Sp, spectinomycin.
Bacterial strain construction.
The bacterial strains and plasmids used in the present study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Bacillus subtilis strains | ||
| JH642 | trpC2 pheA1 | Laboratory stock |
| AKP3 | JH642 amyE::Pdes-lacZ | 2 |
| AKP4 | AKP3 des::Kmr | 2 |
| AKP21 | AKP3 desKR::Kmr | 2 |
| CM30 | JH642 desΩpAG19 (des-gfp) | This work |
| CM31 | CM30 amyE::Pdes-lacZ | This work |
| Plasmids | ||
| pAR11 | Pdes-lacZ; integrates at the amyE locus of B. subtilis; Cmr | 2 |
| pKL147 | gfp-carrying vector; Spr | 17 |
| pAG19 | Contains the 3′ end of des cloned in the EcoRI-XhoI sites of pKL147; Ampr | P. Aguilar, personal communication |
Ampr, Cmr, Kmr, and Spr denote resistance to ampicillin, chloramphenicol, kanamycin, and spectinomycin, respectively.
For monitoring the induction of the des promoter, we employed B. subtilis strains bearing a Pdes-lacZ fusion at the amyE locus. The strains used were AKP3, AKP4, and AKP21 (2) bearing a wild-type desaturase, an insertional mutation in the des gene, and an insertional mutation in the desKR genes, respectively.
The C-terminal fusion of desaturase to GFP was built by inserting a portion of the 3′ end of des into the gfp-carrying vector pKL147 (17) using the product of the primers desEco (5′-ATTTCAGAATTCAGGTTCAATCGGTGTTTG-3′) and desXho (5′-TATCACTCGAGATTCTTCCGCAGCTTCTGT-3′). The restriction sites are underlined. This pKL147 derivative, named pAG19, was transformed into B. subtilis strain JH642 via a Campbell-type integration, which yielded strain CM30. To add a transcriptional fusion between des and lacZ, plasmid pAR11 (2) was linearized with ScaI endonuclease and was introduced by a double crossover event at the amyE locus of strain CM30, which yielded strain CM31. Transformation of B. subtilis was carried out by the method described by Dubnau and Davidoff (12). Strain CM31 was used for monitoring of the Des expression.
All strains were stored at −20°C as the suspensions of spores in glycerol (15% [vol/vol]) frozen in small aliquots.
Growth conditions.
B. subtilis strains were routinely grown in Luria broth (Sigma) with the addition of 0.5% glucose. In the case of anaerobic cultivations, 0.2% KNO3 was also added. Aerobic cultivation was performed in 500-ml Erlenmeyer flasks with 60 ml of medium that was kept in a shaken incubator at 170 rpm. For the membrane and lipid isolation procedures, which required a higher volume of culture, 5,000-ml flasks with 600 ml of medium were used. Anaerobic cultivation was performed in 250-ml screw-cap bottles, which were completely filled with medium and placed in a temperate bath. Mild mixing with a magnetic stirrer was employed to prevent the aggregation of bacteria. Cultivation was started by inoculation of the overnight aerobic or anaerobic inoculum from the spore stock. In the morning this culture was diluted with the fresh medium and cultivated until exponential phase (OD450 of 0.5 to 0.6); finally, it was used for inoculation of the particular experimental system. Starting OD450s after dilution were always 0.005 and 0.05 for aerobic and anaerobic cultures, respectively. When the temperature downshift experiments were performed, cultures were grown at 37°C until they reached an OD450 of 0.15 to 0.2; afterwards, they were transferred to the lower temperature (25°C). The growth experiments were replicated at least five times for both aerobic and anaerobic systems.
Antibiotics were added to the media for both aerobic and anaerobic cultivations according to the resistance properties of a particular strain (Table 1). The indicated concentrations were used: Amp, 100 μg·ml−1; Cm, 5 μg·ml−1; Km, 5 μg·ml−1; and Sp, 50 μg·ml−1.
β-Galactosidase assay.
B. subtilis strains harboring the Pdes-lacZ fusion were grown either aerobically or anaerobically at 37°C and transferred to 25°C. Culture samples were taken at 1-h intervals after the temperature downshift and assayed for β-galactosidase activity. To this end, cells were pelleted, diluted in Z buffer, and disrupted using lysozyme and Triton X-100. The formation of yellow ONP from ONPG was measured colorimetrically at 420 nm; see our previous article for details (5). The specific activity was expressed in MU (18). Each culture sample was analyzed in triplicate; at least 3 independent experiments (independent cultivations) were performed. To study the effect of fluidization by exogenously added UFAs, parallel cultures were supplemented with oleic acid (18:1) diluted in ethanol in a final concentration of 5 μM at the moment of the temperature downshift.
Cytoplasmic membrane isolation and fluorescence anisotropy measurement.
To estimate the impact of changes in FA composition on the physical properties of membranes, the isolated bacterial membranes from aerobic and anaerobic cultures at both 37°C and 25°C were labeled with DPH, a membrane fluorescence probe. Cytoplasmic membranes were isolated from the cells by enzymatic cell lysis using lysozyme with DNase and RNase. For fluorescence anisotropy measurements, the membrane vesicles were labeled 30 min at 37°C with DPH (final concentration, 10−6 M). DPH fluorescence anisotropy (rss) was then measured over the temperature range of 5°C to 45°C. The protocols used have been detailed in our previous work (5).
Lipid isolation and fatty acid analysis.
Chloroform-methanol extraction was used for lipid isolation. The pelleted cells were resuspended in the phosphate buffer and extracted with an appropriate volume of chloroform-methanol mixture for 2 h. Cells were then separated by centrifugation. Additional chloroform and distilled water were added, and the phases were allowed to separate. The lower lipid-containing phase was concentrated under the flow of nitrogen.
The lipids were then subjected to a mild alkaline methanolysis using methanol, toluene, and KOH. The resulting fatty acid methyl esters were extracted twice with a hexane-chloroform mixture and then separated by gas chromatography (Agilent 6850; Agilent Technologies) with a flame ionization detector on a capillary column (Ultra 2; 25 m by 0.20 mm by 0.33 μm; Agilent Technologies). Individual FA peaks were identified using the automatic identification system (MIDI Inc.). Detailed procedures have been described in our previous paper (5).
Nitrite concentration.
The concentration of nitrites in the culture was determined using a colorimetric assay based on determination of reddish-purple coloration produced by 1-aminonaphthalene with diazotized 4-aminobenzenesulfonic acid as described previously by Rider and Mellon (24).
Western blot analysis.
The analysis was performed basically as described in reference 25. One-milliliter aliquots of each culture were harvested, centrifuged, and frozen in liquid nitrogen. The pellets were resuspended in the lysis buffer (50 mM Tris-HCl, pH 8.0, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 0.1 mM dithiothreitol) (25), with addition of 160 μl of buffer per OD450 unit of the original culture. A 20-μl volume of the cell resuspension was disrupted by incubating it with lysozyme (500 μg/ml) for 30 min at 37°C followed by 5 min of boiling in the presence of the loading buffer. The final volume of the sample was 40 μl (20 μl of resuspended cells, 1 μl of lysozyme solution, 19 μl of sample buffer). A 20-μl aliquot from each sample was fractionated by sodium dodecyl sulfate-gel electrophoresis in a 12% acrylamide gel. Proteins were blotted onto a polyvinylidene difluoride membrane (Immobilon-P; Millipore) and detected using anti-GFP monoclonal mouse antibody (Roche), secondary goat anti-mouse IgG conjugated to horseradish peroxidase, and ECL Western blotting substrate (Pierce). The blots were exposed to film and developed using an ECL development device.
Statistics.
The differences in FA levels, anisotropy values, and doubling times were compared by Student's t test with an α value of 0.05. The numbers of independent experiments are indicated in the figure legends.
RESULTS
Growth and nitrite production of B. subtilis after the temperature downshift in aerobic and anaerobic conditions.
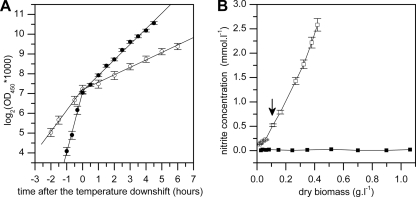
B. subtilis AKP3 (Pdes-lacZ) was cultivated aerobically or anaerobically in LB medium with glucose (5 g/liter) and nitrate (2 g/liter), at 37°C. The generation times were 23 ± 3 min and 64 ± 9 min (P < 0.001) for aerobic and anaerobic conditions, respectively. During the mid-exponential phase of culture growth, the growing cells were shifted to 25°C (see Materials and Methods). After the temperature downshift to 25°C, the cultures attained doubling times of 72 ± 4 min and 160 ± 17 min (P < 0.001) for aerobic and anaerobic conditions, respectively. No lag phase was observed after the downshift in either the aerobic or the anaerobic culture (Fig. 1A).
FIG. 1.
Growth and nitrite production of B. subtilis AKP3 after the temperature downshift under aerobic and anaerobic conditions. B. subtilis cultures were grown aerobically or anaerobically in LB medium with glucose and nitrate. After inoculation, the cells were grown at 37°C and transferred to 25°C at an OD450 of 0.13 to 0.16. (A) Growth under aerobic (closed circles) or anaerobic (open circles) conditions. The time of the transfer is identified as the zero point on the time scale. (B) Nitrite concentration in downshifted cultures grown anaerobically (open squares) or aerobically (closed squares). The time of transfer is indicated by the arrow. Data represent the means ± standard errors of the means from five (A) or two (B) independent experiments.
The production of nitrite from nitrate was examined to prove that our cultivation conditions enabled effective anaerobic nitrate respiration. In the anaerobic culture, the nitrite production was stable both before and after the transfer from 37°C to 25°C, while in aerobic culture supplemented with nitrate, no nitrite production was observed (Fig. 1B). The nitrite levels shown in Fig. 1B do not represent the net nitrite formation from nitrate because the nitrite produced from B. subtilis grown under anaerobic conditions was shown to be utilized for subsequent conversion into ammonia (13).
Effect of anaerobic growth on fatty acid profiles of B. subtilis cells after the temperature downshift and during long-term adaptation.
We compared the FA profiles of B. subtilis AKP3 cultures grown under aerobic and under anaerobic conditions at stable cultivation temperatures of either 25°C or 37°C (designated here as Aero-37°C, Anaero-37°C, Aero-25°C, and Anaero-25°C, respectively) and after the downshift from 37°C to 25°C. A longer time interval was used for B. subtilis anaerobic culture due to its longer doubling time at 25°C (Fig. 1A). The levels of nine main FA species are shown in Fig. 2B.
FIG. 2.
The effect of anaerobic respiration on cold adaptation at the level of membrane fatty acids in B. subtilis. AKP3 cells were grown under aerobic and anaerobic conditions at a constant temperature of 37°C or 25°C and harvested in the mid-log phase (OD450 of 0.5 to 0.6) for lipid isolation and fatty acid analysis. Parallel cultures were subjected to the temperature downshift (from 37°C to 25°C) in the mid-log phase and harvested 2 or 3 h (as indicated) after the temperature shift. A complete FA profile was recorded from each sample, and the frequencies of the main structural types (A) and the most prominent species (B) are shown. The prefixes “i,” “a,” and “n” indicate the respective branching patterns for iso-, anteiso-, and nonbranched FAs, respectively. The data shown here are the averages of two independent experiments (cultivation, membrane isolation, and fatty acid analysis) and represent the means ± standard errors of the means for each cultivation system. Abbreviations: unsat., unsaturated fatty acids; oth., others.
The trend in FA composition patterns that indicates the stepwise adaptation for lower cultivation temperatures was broadly similar in both the aerobic and anaerobic systems. However, some differences were found in the levels of i-15:0 and a-17:0. The level of i-15:0 FA was increased only in the aerobic system at the lower temperature (P < 0.04), and the level of a-17:0 was increased only in the anaerobic culture (P < 0.04). The increase of the major fluidizing FA a-15:0 at a lower temperature was less pronounced in Anaero-25°C than in Aero-25°C. There was no significant decrease of n-16:0 in Anaero-25°C, which was probably because this substrate could not be used for desaturation reactions under anaerobic conditions. In both aerobic and anaerobic cultures, the adaptation of the FA content to lower temperatures was noticeable within two generation times after the temperature downshift (data not shown).
Anaerobic cultures exhibited slightly higher overall levels of anteiso-, lower levels of iso-, and higher levels of nonbranched FAs than did aerobic ones (Fig. 2A). As for the UFA levels after the temperature downshift, we observed the increase (P < 0.045) in these under aerobic conditions where they reached approximately 5% of the total FAs 2 h after the shift. Under anaerobic conditions, no significant increase was observed; the proportion of UFAs remained stable at around 2% (Fig. 2).
Extent of membrane cold adaptation in aerobic and anaerobic conditions measured as fluorescence anisotropy of DPH.
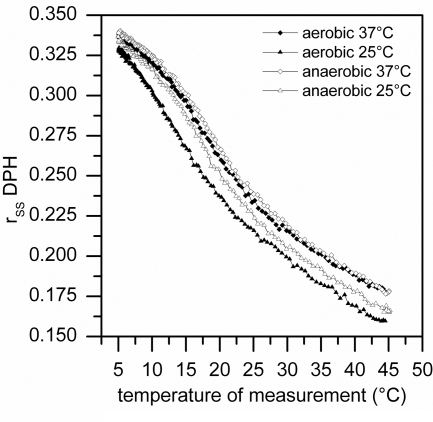
As can be seen from Fig. 3, the DPH anisotropy values for the membranes from cells cultivated at 25°C were always lower than those of the cells cultivated at 37°C. These differences are obvious for both aerobic and anaerobic cultivation, and they reflect a fluidization of the membranes of cold-adapted cells. The extent of adaptation reached by anaerobically grown cells was then compared to that of cells grown aerobically. The difference in the values of DPH anisotropy between Anaero-37°C and Anaero-25°C obtained for the reference temperature of 25°C was slightly lower than in the case of aerobically cultivated cells (P < 0.001). Therefore, B. subtilis grown under anaerobic conditions was able to fluidize its membrane but the extent of fluidization was lower than that under aerobic conditions.
FIG. 3.
The dependence of DPH fluorescence anisotropy on temperature in cytoplasmic membranes isolated from B. subtilis cultivated aerobically and anaerobically at a constant temperature. Cytoplasmic membranes were isolated from B. subtilis AKP3 cells grown aerobically or anaerobically at a constant temperature of 37°C or 25°C (mid-exponential-phase cells, OD450 of 0.5 to 0.6) and were labeled with DPH, as described in Materials and Methods. The steady-state fluorescence anisotropy (rss) of DPH embedded in cytoplasmic membrane vesicles was measured along the temperature scale as indicated on the x axis.
Induction of fatty acid desaturase synthesis after the temperature downshift under anaerobic conditions.
In our experiments, we tested whether desaturase is still synthesized under anaerobic conditions despite being unable to function properly and whether its transcription is regulated by the DesK-DesR two-component system as is found under aerobic conditions.
Under aerobic conditions (Fig. 4A ), β-galactosidase expression in strain AKP3 started to increase after an approximately 30-min delay after the temperature downshift and reached the maximum level of induction in 2 h. The rate of synthesis then decreased, and β-galactosidase activity was stabilized. The final OD450 reached by all four cultures was not higher than 1.4 (not shown). When AKP3 culture was supplemented with oleic acid, β-galactosidase activity was induced by the temperature decrease in a similar way as in the culture without the addition of UFA, but its synthesis was suppressed earlier and more effectively.
FIG. 4.
Effect of anaerobic respiration on the cold-induced des expression and on membrane fluidization by external oleic acid. Induction of Pdes-controlled β-galactosidase synthesis was followed in the AKP3 (des+ Pdes-lacZ), AKP4 (carrying a mutation in des; Pdes-lacZ), and AKP21 (carrying a mutation in desKR; Pdes-lacZ) strains after the temperature downshift from 37°C to 25°C (performed at an OD450 of 0.15 to 0.2). Panels A and B show data from experiments under aerobic and anaerobic growth conditions, respectively. The addition of 5 μM oleic acid at the moment of the temperature shift is indicated as “+ UFA.” Data represent the means ± standard errors of the means from three independent experiments.
In the des mutant strain, AKP4, the stable Pdes induction was inhibited only if the external UFA was added. However, the decrease in β-galactosidase activity induced by oleic acid in AKP4 did not reach the level obtained for AKP3 with UFA (Fig. 4A). The more effective inhibition of Pdes induction in the case of the AKP3 strain can be explained by a higher fluidization of the membrane due to the combined effect of desaturase activity and exogenous UFA.
Under anaerobic conditions (Fig. 4B), we observed similar patterns of Pdes induction for the two strains. In AKP3 and AKP4 cultured without exogenously added UFA, a stable increase of β-galactosidase activity was observed, which was almost identical to that found in the aerobic AKP4 system. In anaerobic AKP3 and AKP4 cultures supplemented with UFA, we observed the strongest decrease of β-galactosidase activity.
To verify that this induction under anaerobic conditions is also exclusively controlled by the DesK-DesR two-component system, we used strain AKP21, which has a nonfunctional DesK-DesR system. In this mutant strain, no β-galactosidase expression was observed. These results showed that the induction of des expression after the temperature downshift occurred under both aerobic and anaerobic conditions despite the fact that Des synthesis without available oxygen could not support any fluidization. Under both conditions, des expression was regulated by the DesK-DesR two-component system and could be affected by the isothermal membrane fluidity changes.
Besides the quantification of des promoter activity using LacZ reporter, the synthesis of desaturase under anaerobic conditions was also tested by Western blot analysis using a monoclonal anti-GFP antibody. The CM31 strain of B. subtilis, which bears the isotopic Des-GFP fusion, was initially cultivated under aerobic or anaerobic conditions at 37°C and then transferred to 25°C. From the results shown in Fig. 5, one can clearly conclude that Des is synthesized in both aerobic and anaerobic systems.
FIG. 5.
Expression of Des-GFP under aerobic and anaerobic conditions at 37°C and after the temperature downshift. Strain CM31, bearing the Des-GFP fusion, was cultivated aerobically and anaerobically at 37°C and then shifted to 25°C. The samples for immunoblot analysis were taken at 1-h intervals after temperature downshift, as indicated, and analyzed as described in Materials and Methods.
In our further experiments, we studied the effect of the rapid transfer from anaerobic to aerobic conditions on Pdes induction after the temperature downshift. This approach allowed us to confirm whether it is the desaturase fluidizing activity that negatively regulates des expression under aerobic conditions. The AKP3 strain was cultivated anaerobically at 37°C and was then transferred to 25°C. β-Galactosidase activity was then assayed. After a substantial increase of β-galactosidase activity, e.g., 2 h after the temperature downshift, the culture was transferred into aerobic conditions. Figure 6 shows that Pdes induction was inhibited shortly after the shift to aerobic conditions. Probably, the high level of desaturase that was accumulated during anaerobic growth resulted in the effective fluidization after the transfer to aerobic conditions and consequently blocked further des transcription.
FIG. 6.
Effect of transfer from anaerobic to aerobic conditions on the induction of Pdes. LB medium was inoculated with B. subtilis AKP3 (starting OD450 of 0.05) and grown anaerobically, and the induction of Pdes-controlled β-galactosidase synthesis was followed continually after the temperature downshift (performed at an OD450 of 0.15). Two hours after the temperature downshift (arrow), the culture was transferred into aerobic conditions (open squares). The AKP3 strain, which was cultivated under anaerobic conditions, was used as the control (closed squares). Data represent the means ± standard errors of the means from three independent experiments.
DISCUSSION
The aim of our current work was to elucidate the effect of anaerobic metabolism on the adaptation of B. subtilis cytoplasmic membrane to the low cultivation temperature. We studied this adaptation at the level of membrane FA composition, as well as the membrane fluidization by cold-induced FA desaturase controlled by the DesK-DesR two-component system.
The composition of fatty acids in membranes at low temperatures has been reported to be dependent on the presence of isoleucine in the cultivation medium (16). We showed in our previous paper (5) that the membrane FA composition of B. subtilis was also very sensitive to the carbon source in the cultivation medium. When it was cultured in mineral medium with glycerol at 40°C, we found increased levels of a-15:0 and a-17:0 fatty acids that are usually considered typical for a cultivation at low temperature. At the same time, the FA adaptation for low temperature was markedly decreased. We concluded that the high levels of a-15:0 and a-17:0 appeared as a consequence of increased levels of the precursors for anteiso-branched FAs. In our present work, we expected that the changes in basic metabolic pathways due to anaerobiosis would also affect the level of available FA precursors and, therefore, the pattern of cold adaptation. Besides, anaerobic conditions should also abolish the ability of FA desaturase to perform a short-term adaptation by the fluidization of membranes after the temperature downshift.
The analysis of the FA profiles shown in Fig. 2 verified our assumption that B. subtilis JH642 cells were not able to synthesize new UFAs under the conditions that favored anaerobic respiration (in contrast to aerobically grown cells) and, therefore, that the short-term mechanism of rapid membrane fluidization is blocked. However, even in aerobic culture the level of UFAs was rather low, comprising approximately 5% of the total cellular FAs. Comparable low levels of UFAs in B. subtilis JH642 grown aerobically on mineral medium after the temperature downshift were reported by Altabe and coworkers (4). The authors found that an increase in the production of UFAs requires overproduction of response regulator DesR, in the absence of the sensor kinase DesK. Therefore, anaerobically grown B. subtilis employs for membrane fluidization the mechanism based exclusively on an increased anteiso/iso ratio of branched-chain fatty acids. The same aerobically grown organism additionally exploits an increased level of unsaturated fatty acids after the temperature downshift; at stable low temperature, however, its membrane is fluidized by an increased anteiso/iso ratio similarly as under anaerobic conditions.
In our work, we correlated the changes in the composition of membrane FAs with the level of membrane fluidization as measured by fluorescence anisotropy of the membrane fluorescence probe DPH. The extent of adaptation for 25°C compared with that for 37°C was quantified according to the method of Cossins and Sinensky (8) as the HE. HE values of 40 and 30% were found for aerobic and anaerobic conditions, respectively.
Although the regulation mechanisms involved in the adaptation of B. subtilis to a low cultivation temperature have been elucidated to a considerable extent, almost no data are available that would link the adaptation to cold with the adaptation to a limited oxygen supply. During anaerobic growth, the metabolism of B. subtilis undergoes a necessary remodeling that involves several hundreds of genes, including those involved in basic metabolic pathways (30). The tricarboxylic acid branch of the Krebs cycle was shown to be reduced during anaerobiosis (20). The related changes in key metabolites available in the cell are therefore quite likely to have a substantial influence on the biosynthesis of FAs. For our experiments, a complex cultivation medium (LB with glucose) was used that provided sufficient amino acids for branched FA synthesis. We concluded from the differences in FA composition that were found between aerobically and anaerobically grown cells at 37°C (Fig. 2) that the regulation of biosynthetic pathways leading to branched FA formation also depends on the type of energy metabolism. Despite the fact that the detailed mechanism of such regulation has yet to be elucidated, our present work clearly shows that in anaerobiosis, the different strategy of cold adaptation observed at the level of FA composition resulted in a lesser adaptation of membrane fluidity.
DesK, the histidine kinase from B. subtilis, is the founding example of a membrane-bound thermosensor, which is suited to the purpose of remodeling membrane fluidity when the ambient temperature drops below approximately 30°C. We proved that under anaerobic conditions, the regulatory loop composed of the DesK-DesR two-component system and UFAs is functional. Without oxygen, Des is synthesized seemingly without any reason because it cannot fluidize the membrane. As seen both here and in a previous paper (2), strain AKP4, which contains an insertional mutation in the desaturase gene, allows for the monitoring of the low-temperature inducibility of a des-lacZ fusion in the absence of FA desaturation (Fig. 4). The transcription of the desaturase gene is increased in this strain and is not downregulated by a prolonged incubation of cells at low temperatures. It was concluded that UFAs act as negative signaling molecules of the autokinase activity of DesK (2). Nevertheless, with this experimental approach it is not possible to rule out the possibility that the membrane-bound desaturase per se affects DesK kinase activity. Recent estimations performed with proteomic and lipidomic approaches indicate that biological membranes are crowded (22). In fact, the medium-center spacing between membrane proteins is about 10 nm, raising the possibility that Des might be able to influence DesK activity through the intervening membrane. However, we show here that the transcription of the des gene of strain AKP3 under anaerobic low-temperature conditions is not downregulated even though the desaturase is synthesized. Moreover, when anaerobic cultures of this strain were supplemented with UFA, a decrease in des transcription was observed. Therefore, these experiments directly demonstrate that membrane fluidity rather than a Des-DesK protein-protein interaction shuts off the kinase activity of DesK, which terminates des transcription.
In summary, our results show that despite the different compositions of membrane FAs caused by anaerobic metabolism, B. subtilis is able to adapt effectively to cold conditions both at stable temperatures and after a sudden temperature downshift. We can also conclude that the control mechanism of FA desaturase synthesis is the same as that under aerobic conditions, in spite of the fact that the enzyme is not functional under anoxic conditions.
Acknowledgments
This work was supported by grants Research Center LC06066 and Research Plan MSM0021620858 from the Ministry of Education, Youth and Sports of the Czech Republic and in Argentina by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and the Agencia Nacional de Promoción Científica y Tecnológica (FONCYT). M. C. Mansilla and D. de Mendoza are Career Investigators from CONICET. D. de Mendoza is an International Research Scholar from Howard Hughes Medical Institute.
We thank Radovan Fišer for assistance with fluorescence measurements and Lucie Jánská for excellent technical help.
Footnotes
Published ahead of print on 25 June 2010.
REFERENCES
- 1.Aguilar, P. S., J. E. Cronan, Jr., and D. de Mendoza. 1998. A Bacillus subtilis gene induced by cold shock encodes a membrane phospholipid desaturase. J. Bacteriol. 180:2194-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar, P. S., A. M. Hernandez-Arriaga, L. E. Cybulski, A. C. Erazo, and D. de Mendoza. 2001. Molecular basis of thermosensing: a two-component signal transduction thermometer in Bacillus subtilis. EMBO J. 20:1681-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albanesi, D., M. C. Mansilla, and D. de Mendoza. 2004. The membrane fluidity sensor DesK of Bacillus subtilis controls the signal decay of its cognate response regulator. J. Bacteriol. 186:2655-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altabe, S. G., P. Aguilar, G. M. Caballero, and D. de Mendoza. 2003. The Bacillus subtilis acyl lipid desaturase is a delta5 desaturase. J. Bacteriol. 185:3228-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beranova, J., M. Jemiola-Rzeminska, D. Elhottova, K. Strzalka, and I. Konopasek. 2008. Metabolic control of the membrane fluidity in Bacillus subtilis during cold adaptation. Biochim. Biophys. Acta Biomembr. 1778:445-453. [DOI] [PubMed] [Google Scholar]
- 6.Butterworth, P. H., and K. Bloch. 1970. Comparative aspects of fatty acid synthesis in Bacillus subtilis and Escherichia coli. Eur. J. Biochem. 12:496-501. [DOI] [PubMed] [Google Scholar]
- 7.Choi, K. H., R. J. Heath, and C. O. Rock. 2000. Beta-ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis. J. Bacteriol. 182:365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cossins, A. R., and M. Sinensky. 1984. Adaptation of membranes to temperature, pressure and exogenous lipids. In M. Shinitzky (ed.), Physiology of membrane fluidity, vol. II. CRC Press Inc., Boca Raton, FL.
- 9.Cybulski, L. E., D. Albanesi, M. C. Mansilla, S. Altabe, P. S. Aguilar, and D. de Mendoza. 2002. Mechanism of membrane fluidity optimization: isothermal control of the Bacillus subtilis acyl-lipid desaturase. Mol. Microbiol. 45:1379-1388. [DOI] [PubMed] [Google Scholar]
- 10.Debarbouille, M., R. Gardan, M. Arnaud, and G. Rapoport. 1999. Role of BkdR, a transcriptional activator of the SigL-dependent isoleucine and valine degradation pathway in Bacillus subtilis. J. Bacteriol. 181:2059-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz, A. R., M. C. Mansilla, A. J. Vila, and D. de Mendoza. 2002. Membrane topology of the acyl-lipid desaturase from Bacillus subtilis. J. Biol. Chem. 277:48099-48106. [DOI] [PubMed] [Google Scholar]
- 12.Dubnau, D., and R. Davidoff. 1971. Fate of transforming DNA following uptake by competent Bacillus subtilis. J. Mol. Biol. 56:209-221. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann, T., N. Frankenberg, M. Marino, and D. Jahn. 1998. Ammonification in Bacillus subtilis utilizing dissimilatory nitrite reductase is dependent on resDE. J. Bacteriol. 180:186-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaan, T., G. Homuth, U. Mader, J. Bandow, and T. Schweder. 2002. Genome-wide transcriptional profiling of the Bacillus subtilis cold shock response. Microbiology 148:3441-3455. [DOI] [PubMed] [Google Scholar]
- 15.Kaneda, T. 1977. Fatty acids of the genus Bacillus: an example of branched-chain preference. Bacteriol. Rev. 41:391-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein, W., M. H. W. Weber, and M. A. Marahiel. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 181:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemon, K. P., and A. D. Grossman. 1998. Localization of bacterial DNA polymerase: evidence for a factory model of replication. Science 282:1516-1519. [DOI] [PubMed] [Google Scholar]
- 18.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 19.Nakano, M. M., and F. M. Hulett. 1997. Adaptation of Bacillus subtilis to oxygen limitation. FEMS Microbiol. Lett. 157:1-7. [DOI] [PubMed] [Google Scholar]
- 20.Nakano, M. M., and P. Zuber. 1998. Anaerobic growth of a “strict aerobe” Bacillus subtilis. Annu. Rev. Microbiol. 52:165-190. [DOI] [PubMed] [Google Scholar]
- 21.Nakano, M. M., P. Zuber, P. Glaser, A. Danchin, and F. M. Hulett. 1996. Two-component regulatory proteins ResD-ResE are required for transcriptional activation of fnr upon oxygen limitation in Bacillus subtilis. J. Bacteriol. 178:3796-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips, R., T. Ursell, P. Wiggins, and P. Sens. 2009. Emerging roles for lipids in shaping membrane-protein function. Nature 459:379-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reents, H., R. Munch, T. Dammeyer, D. Jahn, and E. Hartig. 2006. The Fnr regulon of Bacillus subtilis. J. Bacteriol. 188:1103-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rider, B. F., and M. G. Mellon. 1946. Colorimetric determination of nitrites. Ind. Eng. Chem. Anal. Ed. 18:96-99. [Google Scholar]
- 25.Schujman, G. E., K. H. Choi, S. Altabe, C. O. Rock, and D. de Mendoza. 2001. Response of Bacillus subtilis to cerulenin and acquisition of resistance. J. Bacteriol. 183:3032-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun, G. F., E. Sharkova, R. Chesnut, S. Birkey, M. F. Duggan, A. Sorokin, P. Pujic, S. D. Ehrlich, and F. M. Hulett. 1996. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J. Bacteriol. 178:1374-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suutari, M., and S. Laakso. 1992. Unsaturated and branched-chain fatty acids in temperature adaptation of Bacillus subtilis and Bacillus megaterium. Biochim. Biophys. Acta 1126:119-124. [DOI] [PubMed] [Google Scholar]
- 28.Svobodova, J., and P. Svoboda. 1988. Membrane fluidity in Bacillus subtilis—physical change and biological adaptation. Folia Microbiol. 33:161-169. [DOI] [PubMed] [Google Scholar]
- 29.Wiegeshoff, F., C. L. Beckering, M. Debarbouille, and M. A. Marahiel. 2006. Sigma L is important for cold shock adaptation of Bacillus subtilis. J. Bacteriol. 188:3130-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye, R. W., W. Tao, L. Bedzyk, T. Young, M. Chen, and L. Li. 2000. Global gene expression profiles of Bacillus subtilis grown under anaerobic conditions. J. Bacteriol. 182:4458-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]