Abstract
The TodS/TodT two-component system of Pseudomonas putida regulates the expression of the toluene dioxygenase (tod) operon for the metabolism of toluene, benzene, and ethylbenzene. The sensor kinase TodS has a complex domain arrangement containing two functional modules, each harboring a sensor and an autokinase domain separated by a receiver domain. The TodT protein is the cognate response regulator that activates transcription of the toluene dioxygenase (TOD) pathway genes at the PtodX promoter. We report in this study that the todST operon is transcribed from a main promoter and that the +1 initiation point is located 31 nucleotides upstream from the A of the first ATG codon and is preceded by a −10/−35 canonical promoter. Expression from PtodS is under catabolite control, and in cells growing with glucose, the level of expression from this promoter is reduced, which in turn translates to low levels of the TodS/TodT regulators and results in a decrease of transcription from the PtodX promoter. Thus, the main underlying regulatory mechanisms of the tod structural genes are at the levels of catabolite repression control from PtodS and transcription activation, mediated by the TodT response regulator through a regulatory cascade in which the effector enhances autophosphorylation of TodS by ATP, with subsequent transphosphorylation of TodT.
The adaptation of bacteria to changes in environmental signals at the transcriptional level is often mediated by two-component regulatory systems (TCS), which typically contain a sensor kinase and a response regulator (8, 9, 19, 20). The genome of Pseudomonas putida DOT-T1E was recently sequenced (our unpublished results), and more than 30 TCS were identified. Among these is the TodS/TodT TCS, which controls the expression of the tod catabolic genes, enabling this strain to use toxic compounds such as benzene, toluene, and ethylbenzene as sole carbon sources (10, 13, 15, 17, 21). The sensor kinase TodS has a complex domain arrangement containing two functional modules, each harboring a sensor domain (PAS-1 and PAS-2) and an autokinase domain (HK1 and HK2) separated by a receiver domain (RRR) (2, 3, 11). HK1, RRR, and HK2 each contain a phosphorylatable residue, namely, H190, D500, and H760, respectively (2). The TodT protein, on the other hand, shows the classic arrangement of an N-terminal input or phosphorylation site (D57) and a C-terminal helix-turn-helix (HTH) motif containing a DNA-binding domain (9, 11, 12).
In contrast to the narrow substrate range of the toluene dioxygenase (TOD) catabolic pathway, the TodS effector profile was shown to be broad both in vivo and in vitro (3). Parallel assays testing aromatic compounds in vivo for the capacity to activate transcription from PtodX and in vitro for the capacity to bind to the sensor kinase TodS allowed the effector profile to be established. Agonist effectors were mono- and biaromatic chemicals that bound to TodS and induced transcription of the tod pathway genes. The most potent effectors were toluene and m-chlorotoluene. Antagonistic compounds bound to TodS but did not induce transcription. Examples of antagonistic compounds are o-xylene and other ortho-substituted toluenes (3).
In vitro and in vivo analyses revealed that all agonistic and antagonistic compounds interact with the N-terminal input domain (PAS-1) (3). Agonist chemicals increase the autophosphorylation activity of HK1, which initiates the phosphorylation cascade through the TodS domains. All three phosphorylation sites (H190, D500, and H760) are essential to the signal transmission/transphosphorylation of TodT, as confirmed by the fact that H190A, D500A, and H760A single-amino-acid mutants were not able to induce transcription at PtodX in the presence of agonist compounds (2). This study is the first report of a phosphorelay involving two complete and active HK domains within a sensor kinase (2).
When transphosphorylated by TodS, the cognate response regulator TodT acts as the transcriptional activator of PtodX expression. TodT binds to two highly similar pseudopalindromic DNA binding sites, at positions −107 and −85, and to a half-site located at position −56 of the PtodX promoter. In addition to TodT, maximal expression requires that IHF binds between the −56 upstream TodT site and the −10 extended region. A functional model indicated that IHF favors contact between the TodT activator, bound farther upstream, and the α subunit of the RNA polymerase bound to the downstream promoter element. Once the scaffold is created and TodT is phosphorylated, the tod operon is transcribed efficiently (10, 11, 12, 13).
The todS and todT genes are located immediately downstream of the tod catabolic genes encoding the pathway enzymes. Regarding the transcriptional activation of PtodS, it remains to be established whether the todS and todT genes form an operon transcribed independently of the tod catabolic genes (Fig. 1 a), whether the expression of todS and todT is influenced by the presence of pathway substrates or TodS effector molecules, and whether the stability of TodS and/or TodT is affected by the presence of effectors. In this study, we show that the todS and todT genes are expressed from a σ70-dependent promoter and that the expression of the regulatory operon is under catabolite repression. In the presence of toluene, transphosphorylation of TodT by TodS is the sole relevant element in transcription from PtodX.
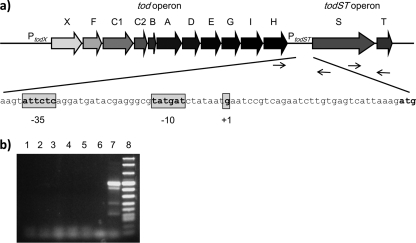
FIG. 1.
(a) Graphical overview of the tod catabolic and todST operons. The primers used to determine whether the todH and todST gene clusters form a transcriptional unit and whether todS and todT form an operon are indicated with arrows at the todH (forward) and todS (reverse) genes and at the todS (forward) and todT (reverse) genes, respectively. The promoter sequence with its −35 and −10 regions and the transcription initiation point (+1) are represented, as determined by primer extension analysis. (b) Reverse transcriptase PCR (RT-PCR) to determine transcriptional units. Experiments were carried out with primers including the todH-todS intergenic region and with RNAs isolated from wild-type P. putida DOT-T1E, without and with 1 mM toluene (lanes 1 and 2, respectively), and from P. putida DOT-T1E ΔC1C2, without and with 1 mM toluene (lanes 3 and 4, respectively). Lanes 5 and 6 are negative controls with RT enzyme but no RNA and with Taq polymerase enzyme with RNA, respectively. Lane 7 is the positive control, showing that todS and todT form an operon. Lane 8 is NEB molecular weight marker VIII.
TodS and TodT form an independent transcriptional unit.
To determine whether the todS and todT genes form an independent transcriptional unit or are transcribed in a read-through manner from the upstream tod catabolic operon, primers were designed to cover the intergenic region between the last genes of the tod catabolic operon, todH and todS. No common transcript between the todH and todST genes was detected, either with or without the TOD pathway inducer toluene (Fig. 1b). It should be noted that we have previously shown that todH and todS genes are expressed as independent cistrons (15).
Transcription from the PtodST promoter is constitutive.
To quantify the expression from the tod pathway and the todST promoters, we used PtodX::′lacZ (pMIR77 [17]) and PtodST::′lacZ (pTodST) transcriptional fusions, respectively (Table 1). Expression from these promoters was measured in wild-type P. putida DOT-T1E and in knockout mutant backgrounds in which todC (ΔC1C2), todS (ΔS), and todT (ΔT) were inactivated. Fresh medium was inoculated with a single colony from LB agar plates containing the appropriate antibiotics and cultured at 30°C overnight. These cultures were diluted 100-fold in the same medium, supplemented or not with 1 mM toluene, and cell growth was monitored over time. β-Galactosidase activity was determined when the cultures reached a turbidity at 600 nm of 0.8 unit. Expression from PtodX::lacZ was induced in the presence of toluene in the wild-type and ΔC1C2 strains, whereas expression was not observed in backgrounds deficient in todS or todT, in agreement with previous results (9, 10, 13). The results indicated that PtodST is expressed constitutively and does not vary significantly in the presence of toluene, the wild-type background, or any of the mutants tested (β-galactosidase levels were in the range of 110 to 150 Miller units). Because expression of PtodS in the ΔtodS and ΔtodT mutants was similar to that in the parental strain, self-regulation of the todST operon by either TodS or TodT was ruled out.
TABLE 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Relevant characteristics or sequence (5′-3′)a | Reference |
|---|---|---|
| Strains | ||
| P. putida DOT-T1E | Prototroph; Tol+ (tod pathway) | 7 |
| P. putida DOT-T1E todST | DOT-T1E todST::Km Tol− containing pMIR66; Gmr | 17 |
| P. putida DOT-T1E todT | DOT-T1E todT::Km Tol− containing pMIR66; Gmr | 17 |
| P. putida DOT-T1E ΔtodC1C2 | DOT-T1E todC2::Km Tol− containing pMIR66; Gmr | 17 |
| P. putida KT2440 | Prototroph | 7 |
| Plasmids | ||
| pMIR77 | Tcr; PtodX::′lacZ inserted in pMP220 | 17 |
| pTodST | Tcr; PtodST::′lacZ inserted in pMP220 | This work |
| pTodST S1 −10 | Tcr; PtodST::′lacZ inserted in pMP220, where at the putative S1 −10 site all T's are replaced by G's | This work |
| pTodST S2 −10 | Tcr; PtodST::′lacZ inserted in pMP220, where at the putative S2 −10 site all T's are replaced by G's | This work |
| pTodTD57A | pET28b(+) containing the todT gene | 12 |
| Primers | ||
| todHfor | GCT CAA GAC GGT GGA TAT TCT TG | |
| todSrev | GTG CAT GTT CTT CAC ACG TC | |
| todSfor | CTG TCA TTC TCA GGC AAT GAT ACA GG | |
| todTrev | GGA ATA TCA CCG TGG CCG CTG |
Gmr, Tcr, and Kmr indicate resistance to gentamicin, tetracycline, and kanamycin, respectively. Tol+ and Tol− indicate the ability to grow (+) or not (−) with toluene as the sole carbon source.
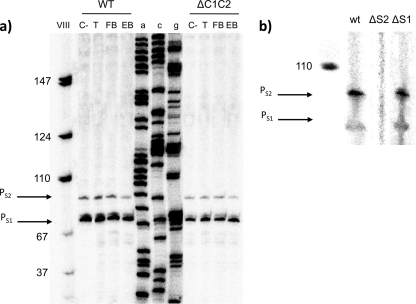
To determine the transcription initiation point at the PtodST promoter, primer extension analysis was carried out. To this end, wild-type cells and ΔC1C2 cells were grown overnight in LB medium in the absence and presence of 1 mM toluene, ethylbenzene, or fluorobenzene at 30°C. Cells (10 ml) were harvested by centrifugation (9,000 × g for 15 min) in disposable plastic tubes precooled in liquid nitrogen and were kept at −80°C until use. RNA was extracted using Tri reagent (Ambion, Austin, TX) according to a modified protocol, as previously described (14). The RNA concentration was determined spectrophotometrically at 260 nm, and its integrity was assessed by agarose gel electrophoresis. Primer extension assays were then performed under the conditions given in the legend for Fig. 2. Figure 2 shows two main extension bands under all conditions, either in the wild-type strain or in the ΔC1C2 mutant background. The putative +1 sites were S1, located 25 nucleotides from the A of the first ATG of todS, and S2, located 31 nucleotides from the A of the first ATG. The potential −10 regions of S1 and S2 corresponded to the sequences 5′-TATAAT-3′ and 5′-TATGAT-3′, respectively.
FIG. 2.
(a) Primer extension analysis of the PtodST promoter region in P. putida DOT-T1E. Wild-type (WT) and ΔC1C2 mutant cells were used to determine the transcription initiation point (+1), in both cases in the absence of an effector (C−) or in the presence of a 1 mM concentration of the agonist toluene (T), fluorobenzene (FB), or ethylbenzene (EB). For primer extension analysis, primers were labeled at their 5′ ends with [γ-32P]ATP and T4 polynucleotide kinase. About 105 cpm of the labeled primers was hybridized to 30 μg of total RNA, and extension was carried out using avian myeloblastosis virus RT as previously described (14). Electrophoresis of cDNA products was done using a urea-polyacrylamide sequencing gel to separate the reaction products, and gels were exposed to a phosphor screen (Fuji Photo Film Co., Ltd.) for 24 to 48 h. Phosphor screens were scanned using a phosphorimaging instrument (Molecular Imager FX; Bio-Rad). (b) Primer extension analysis to determine whether both putative transcription initiation sites (S1 and S2) are active or if the lower band (S1) is a degradation product of S2. See the text for experimental procedures.
To determine whether both putative promoters were active or whether the smaller band (S1) corresponded to degradation of the larger RNA transcript, both potential −10 regions were mutated and primer extension analysis was repeated. For these assays, the pTodST plasmid containing the todH-todS intergenic region was used as the template. Overlapping PCR was done according to the Qiaquick site-directed mutagenesis kit (Qiagen) protocol, using additional primers containing the mutant sequences of the putative S1 and S2 −10 regions. In the S1 −10 region promoter and the S2 −10 region promoter, every “T” was replaced by a “G” to disrupt the putative −10 regions. The plasmids were called pS1-10 and pS2-10, and the transcriptional start sites were determined by primer extension as described above. Primer extension revealed that mutation of the −10 region of the largest mRNA yielded no band, whereas the extension of the mRNA of the mutant corresponding to the shortest band yielded two bands, as it did with the wild-type promoter. We therefore suggest that the S1 band is a degradation product of S2, which implies that the +1 site corresponds to the larger RNA.
Stability of TodS and TodT is not influenced by the presence of toluene.
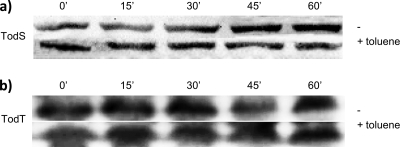
As discussed above, the activation of PtodX is dependent on the presence of TodS, TodT, and an effector. Effector binding to TodS alters the phosphorylation of TodS, resulting in the phosphorylation of TodT and the activation of transcription from PtodX (10). We hypothesized that the protein stability of either TodS or TodT could be influenced by the level of phosphorylation. To test this hypothesis, polyclonal antibodies against TodS and TodT were prepared. The total proteins from cultures grown with and without toluene were separated in polyacrylamide gels and then submitted to Western blotting to quantify the levels of TodS and TodT. Densitometric analyses revealed no significant differences in the protein levels of TodS (Fig. 3 a) and TodT (Fig. 3b) in cells growing in the presence or absence of effectors. In addition, their stability over time was apparently not affected by the presence of toluene.
FIG. 3.
Stability assays of TodS and TodT. Pseudomonas putida DOT-T1E cells were grown on minimal medium with glucose. When cells reached a turbidity of 0.6, the culture was separated into two flasks of equal volume, to one of which toluene was added in the vapor phase. Protein production was inhibited with 30 μg/ml of tetracycline after an additional 30 min. Aliquots of 200 μl were removed at different time points after inhibition of protein production, to monitor TodS or TodT stability in the presence or absence of toluene. Ten-microliter samples were boiled for 5 min in Laemmli buffer, separated by SDS-PAGE, electroblotted to polyvinylidene difluoride (PVDF) membranes (Millipore, Madrid, Spain), and probed with polyclonal antibodies against TodS or TodT, generated in rabbits. The specific bands were detected by chemiluminescence [goat anti-rabbit IgG (H+L)-horseradish peroxidase conjugate; Caltag Labs, Burlingame, CA] and quantified with Quantity One software (Perkin Elmer).
This set of results indicated that control of expression from PtodX is essentially a posttranslational event in which phosphorylation of TodT is of critical importance. We previously generated a TodT mutant in which the phosphorylatable D57 residue was mutated to alanine. As expected, in vitro transphosphorylation assays showed that the TodTD57A mutant was not transphosphorylated by TodS. Furthermore, in a reconstituted system in strain KT2440 with PtodX::lacZ, the TodS/TodT system induced high levels of β-galactosidase in response to toluene, whereas no induction was seen when TodTD57A was used instead of TodT.
The PtodS promoter is under catabolite repression control.
Catabolite repression control refers to the ability of an organism to preferentially metabolize one carbon source over another when both carbon sources are present in the growth medium (1, 4, 5, 18). Our previous studies on the degradation of toluene mediated by the TOL plasmid revealed that when P. putida KT2440(pWW0) was grown in the presence of glucose and toluene, both carbon sources were simultaneously metabolized through a kind of crossed catabolite repression (6). This led us to test if the chromosomally encoded TOD pathway is subject to catabolite repression. In order to do this, we performed a series of assays in which P. putida DOT-T1E was transformed with the pTodS or pMIR77 plasmid, bearing a transcriptional fusion of the PtodS or PtodX promoter to ′lacZ. Transformed cells were grown with glucose, toluene, and a mixture of glucose and toluene. Our results revealed that in the presence of glucose, the expression levels from both PtodS and PtodX were low, while in cells growing with toluene the expression levels from both promoters were high (Table 2). Furthermore, when glucose was consumed, the expression levels increased to about 60 Miller units from the PtodS promoter and about 800 Miller units from the PtodX promoter. These results suggest that glucose represses the TOD catabolic pathway and that this effect is mediated by regulating expression from PtodS. Expression from PtodS became partially derepressed in genetic backgrounds deficient in Crc, PtsN, and CyoB, suggesting that utilization of toluene can be influenced by more than one global regulatory system, which emphasizes the interplay required by bacteria to thrive with certain nutrients.
TABLE 2.
Expression from PtodS and PtodX fused to ′lacZ in the wild-type genetic background and with todS/todT cloned into a medium-copy-number vectora
| Growth substrate | β-Galactosidase activity (Miller units) |
||
|---|---|---|---|
| PtodS | PtodX |
||
| Low-copy-number todS/todT | Multicopy todS/todT | ||
| Glucose | 10 ± 3 | 50 ± 5 | 70 ± 3 |
| Toluene | 60 ± 6 | 850 ± 150 | 850 ± 125 |
| Glucose + toluene | 45 ± 5 | 60 ± 3 | 3,200 ± 100 |
Pseudomonas putida DOT-T1E(pTodST) (low-copy-number todS/todT) and P. putida DOT-T1E(pMIR77, pBBRM5:todS/todT) (multicopy todS/todT) cells were grown on M9 minimal medium with 16 mM glucose, toluene (supplied via the gas phase), or 16 mM glucose plus toluene (gas phase) until the cultures reached a turbidity at 660 nm of 0.7 ± 0.05 unit. The β-galactosidase activity was determined in permeabilized cells.
To further provide support that the TodS expression level is critical for catabolite repression, we cloned the todST operon into the pBBRM5 plasmid, which provides cells with at least a 10-fold increase in todS expression. We then measured expression from PtodX::′lacZ in this new background in the absence and presence of glucose. We found that expression from PtodX without toluene was low but that higher levels were achieved in cells growing on toluene or with toluene and glucose. These results suggest that overexpression of PtodX from the plasmid overcame, in part, the effect of the catabolite repression system.
Our results support the finding that the TodS/TodT system does not regulate its own expression; however, this operon is under global catabolite control. This situation is similar to that in other TCS; for example, in Escherichia coli, 4 of 20 TCS are under the control of another transcription factor (16). Therefore, the current results are compatible with the following circuit control. The expression of the todS/todT operon is modulated in response to alternative carbon sources. In the absence of catabolite repressors, the expression of the operon occurs mainly from a single promoter, at a low level that gives rise to a certain level of TodS and TodT proteins. In the presence of toluene, a phosphorylation cascade is initiated at the TodS N-terminal domain and ends in the active regulatory form of TodT, which in turn modulates the expression of PtodX.
Acknowledgments
This study was supported by FEDER-cofinanced grants Consolider Ingenio CSD2007-00005 and BIO2006-05668 (Consolider-C) from the Ministerio de Ciencias e Innovación and by FEDER grants CVI-3010 PO9-RNM-4509 and CVI-1912 from the Consejería de Ciencia y Empresa de la Junta de Andalucía. We also thank the BBVA foundation for support. Hortencia Silva-Jiménez is a recipient of a fellowship from CONACYT-Mexico.
Footnotes
Published ahead of print on 11 June 2010.
REFERENCES
- 1.Aranda-Olmedo, I., J. L. Ramos, and S. Marqués. 2005. Integration of signals through Crc and PtsN in catabolite repression of Pseudomonas putida TOL plasmid pWW0. Appl. Environ. Microbiol. 71:4191-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busch, A., M. E. Guazzaroni, J. Lacal, J. L. Ramos, and T. Krell. 2009. The sensor kinase TodS operates by a phosphorelay mechanism involving two autokinase domains. J. Biol. Chem. 284:10353-10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busch, A., J. Lacal, A. Martos, J. L. Ramos, and T. Krell. 2007. Bacterial sensor kinase TodS interacts with agonistic and antagonistic signals. Proc. Natl. Acad. Sci. U. S. A. 104:13774-13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collier, D. N., P. W. Hager, and P. V. Phibbs, Jr. 1996. Catabolite repression control in the pseudomonads. Res. Microbiol. 14:551-561. [DOI] [PubMed] [Google Scholar]
- 5.Daniels, C., P. Godoy, E. Duque, M. A. Molina-Henares, J. de la Torre, J. M. del Arco, C. Herrera, A. Segura, M. E. Guazzaroni, M. Ferrer, and J. L. Ramos. 2010. Global regulation of food supplied by Pseudomonas putida DOT-T1E. J. Bacteriol. 192:2169-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Castillo, T., and J. L. Ramos. 2007. Simultaneous catabolite repression between glucose and toluene metabolism in Pseudomonas putida is channeled through different signaling pathways. J. Bacteriol. 189:6602-6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duque, E., A. Segura, G. Mosqueda, and J. L. Ramos. 2001. Global and cognate regulators control the expression of the organic solvent efflux pumps TtgABC and TtgDEF of Pseudomonas putida. Mol. Microbiol. 39:1100-1106. [DOI] [PubMed] [Google Scholar]
- 8.Georgolius, D., O. Kwon, and E. C. C. Lin. 2001. Quinones as the redox signal for the Arc two-component system of bacteria. Science 292:2314-2316. [DOI] [PubMed] [Google Scholar]
- 9.Krell, T., J. Lacal, A. Busch, H. Silva-Jiménez, M. E. Guazzaroni, and J. L. Ramos. Bacterial sensor kinases: diversity in the recognition of environmental signals. Annu. Rev. Microbiol., in press. [DOI] [PubMed]
- 10.Lacal, J., A. Busch, M. E. Guazzaroni, T. Krell, and J. L. Ramos. 2006. The TodS/TodT two-component regulatory system recognizes a wide range of effectors and works with DNA-bending proteins. Proc. Natl. Acad. Sci. U. S. A. 103:8191-8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacal, J., M. E. Guazzaroni, A. Busch, T. Krell, and J. L. Ramos. 2008. Hierarchical binding of the TodT response regulator to its multiple recognition sites at the tod pathway operon promoter. J. Mol. Biol. 376:325-337. [DOI] [PubMed] [Google Scholar]
- 12.Lacal, J., M. E. Guazzaroni, P. Gutiérrez del Arroyo, A. Busch, M. Vélez, T. Krell, and J. L. Ramos. 2008. Two levels of cooperativeness in the binding of TodT to the tod operon promoter. J. Mol. Biol. 384:1037-1047. [DOI] [PubMed] [Google Scholar]
- 13.Lau, P. C. K., Y. Wang, A. Patel, D. Labbé, H. Bergeron, R. Brousseau, Y. Konishi, and M. Rawlings. 1997. A bacterial basic region leucine zipper histidine kinase regulating toluene degradation. Proc. Natl. Acad. Sci. U. S. A. 94:1453-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marqués, S., J. L. Ramos, and K. N. Timmis. 1993. Analysis of the mRNA structure of the Pseudomonas putida TOL meta-fission pathway operon. Biochim. Biophys. Acta 1216:227-236. [DOI] [PubMed] [Google Scholar]
- 15.Mosqueda, G., M. I. Ramos-González, and J. L. Ramos. 1999. Toluene metabolism by the solvent-tolerant Pseudomonas putida DOT-T1 strain, and its role in solvent impermeabilization. Gene 232:69-76. [DOI] [PubMed] [Google Scholar]
- 16.Olivera, B. C., E. Ugalde, and A. Martínez-Antonio. 2010. Regulatory dynamics of standard two-component systems in bacteria. J. Theor. Biol. 264:560-569. [DOI] [PubMed] [Google Scholar]
- 17.Ramos-González, M. I., M. J. Campos, and J. L. Ramos. 2005. Analysis of Pseudomonas putida KT2440 gene expression in the maize rhizosphere: in vivo expression technology capture and identification of root-activated promoters. J. Bacteriol. 187:4033-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rojo, R. 2010. Carbon catabolite repression in Pseudomonas: optimizing metabolic versatility and interactions with the environment. FEMS Microbiol. Rev. doi: 10.1111/j.1574-6976.2010.00218.x. [DOI] [PubMed]
- 19.Ruiz, N., and T. J. Silhavy. 2005. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 8:122-126. [DOI] [PubMed] [Google Scholar]
- 20.Ulrich, E., E. V. Koonin, and I. B. Zhulin. 2005. One-component systems dominate signal transduction in prokaryotes. Trends Microbiol. 13:52-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zylstra, G. J., and D. T. Gibson. 1989. Toluene degradation by Pseudomonas putida F1. Nucleotide sequence of the todC1C2BADE genes and their expression in Escherichia coli. J. Biol. Chem. 264:14940-14946. [PubMed] [Google Scholar]