Abstract
In damaged or proliferating endothelium, production of nitric oxide (NO) from endothelial nitric oxide synthase (eNOS) is associated with elevated levels of reactive oxygen species (ROS), which are necessary for endothelial migration. We aimed to elucidate the mechanism that mediates NO induction of endothelial migration. NO downregulates expression of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), which positively modulates several genes involved in ROS detoxification. We tested whether NO-induced cell migration requires PGC-1α downregulation and investigated the regulatory pathway involved. PGC-1α negatively regulated NO-dependent endothelial cell migration in vitro, and inactivation of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway, which is activated by NO, reduced NO-mediated downregulation of PGC-1α. Expression of constitutively active Foxo3a, a target for Akt-mediated inactivation, reduced NO-dependent PGC-1α downregulation. Foxo3a is also a direct transcriptional regulator of PGC-1α, and we found that a functional FoxO binding site in the PGC-1α promoter is also a NO response element. These results show that NO-mediated downregulation of PGC-1α is necessary for NO-induced endothelial migration and that NO/protein kinase G (PKG)-dependent downregulation of PGC-1α and the ROS detoxification system in endothelial cells are mediated by the PI3K/Akt signaling pathway and subsequent inactivation of the FoxO transcription factor Foxo3a.
Nitric oxide (NO) produced by endothelial nitric oxide synthase (eNOS) plays critical roles in the physiology of endothelial cells (5), regulating vascular tone (19), endothelial cell growth (10), migration (22), survival under stress (4), and the levels of reactive oxygen species (ROS) (32). NO-induced angiogenesis requires a NO-dependent increase in ROS (2, 25), and although the molecular mechanisms linking NO and ROS homeostasis have been a matter of intense research, they are still only partly understood (reviewed in reference 20). NO-dependent induction of angiogenesis requires activation of the soluble guanylyl cyclase (sGC)/protein kinase G (PKG)/phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway (13). However, whether and how this pathway is associated with the increase in ROS levels are still unclear.
It has been proposed that endothelial angiogenesis requires the inactivation of FoxO transcription factors by Akt (11, 26). Akt directly phosphorylates and inactivates members of the FoxO family, which are consequently translocated from the nucleus to the cytoplasm (12). The FoxO factors Foxo3a and Foxo4 protect cells from oxidative stress (14), although the significance of this process in normal vascular homeostasis is still unknown.
Peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) is a master regulator of cellular energy metabolism (8) and a positive and direct regulator of a set of genes whose products protect against oxidative stress (29, 31). PGC-1α is inactivated by phosphorylation by Akt (15), and Akt can also negatively impact PGC-1α expression via inactivation of the FoxO factors Foxo1a and Foxo3a, which are direct transcriptional regulators of PGC-1α expression (3, 23). NO produced by eNOS regulates ROS at least in part by modulating the expression of PGC-1α; NO regulates PGC-1α transcription via sGC/PKG activation, leading to corresponding changes in PGC-1α target genes, including those involved in ROS metabolism (1). However, the molecular pathway and biological impact of NO-dependent downregulation of PGC-1α expression have not been investigated.
We have investigated the potential role of PGC-1α in NO-induced endothelial cell migration. Our results suggest that NO downregulates PGC-1α via activation of sGC/PKG/PI3K/Akt signaling and subsequent inactivation of Foxo3a. PGC-1α downregulation by NO results in increased ROS levels that are necessary for the induction of endothelial cell migration.
MATERIALS AND METHODS
Cell culture.
Bovine aortic endothelial cells (BAEC) and mouse lung endothelial cells (MLEC) were isolated and cultured as described previously (9, 16). Aortas were obtained from an authorized slaughterhouse. Cells were used from passages 4 to 6.
Adenoviral vectors and infection.
Ad-TM-Foxo3a, Ad Foxo3a (27), and Ad-DN-Akt (30) were as described previously. BAEC were infected at a multiplicity of infection (MOI) of 10 to 25 for 9 h.
siRNA.
Small interfering RNAs (siRNAs) were synthesized with the Silencer siRNA construction kit (Ambion, Austin, TX). BAEC were transfected with Lipofectamine 2000 and harvested at 24 h posttransfection.
Immunofluorescence.
BAEC were grown on coverslips, fixed with 3.7% formaldehyde, permeabilized with 0.1% Triton, and then incubated consecutively with primary antibody (anti-Foxo3a) and secondary antibody (anti-IgG rabbit Alexa 488 conjugate). Cells were counterstained with TO-PRO-3, mounted, and examined by confocal microscopy (Leica TCS SP5).
NO assays.
The pharmacological PI3K inhibitor LY294002 (10 μM) and the Akt inhibitor Akt IV (0.5 μM) were added to BAEC cultures 1 h before addition of the NO donor DETA-NO (62 μM), the inactive control DETA (62 μM), or 8-Br-cyclic GMP (cGMP) (100 μM). Cells were harvested after 12 h.
Chromatin immunoprecipitation (ChIP).
ChIP experiments were carried out as previously described (31). IgG was used as a control for nonspecific binding. ChIP-enriched DNA was analyzed by PCR amplification of a 130-bp region of the PGC-1α promoter spanning the IRS (insulin-responsive site/FoxO binding site). 18S gene amplification was used as an enrichment-negative control.
Mitochondrial superoxide.
Mitochondrial superoxide was measured by labeling cells with MitoSOX Red, essentially as described previously (21). Briefly, MLEC were grown on coverslips and incubated in 2% fetal bovine serum (FBS) with 100 μM 8-Br-cGMP or vehicle for 7 h. Cells were then incubated with 3 μM MitoSOX Red for 10 min, washed, fixed with paraformaldehyde, and analyzed by confocal microscopy (Leica TCS, SP5).
Wound healing and Transwell assays.
Assays were performed as previously described (9, 17). Migration in wound healing assays was examined by conventional light microscopy (Nikon Eclipse Ti), and migration in Transwell assays was examined by fluorescence microscopy (Leica TCS SP5).
Statistical analysis.
Statistical differences were determined by analysis of variance (ANOVA). Data are expressed as means ± standard deviations. Differences were considered significant at P values of <0.05. All experiments were performed at least three times.
Oligonucleotide sequences and supplementary methodology details will be provided upon request.
RESULTS
cGMP-induced endothelial cell migration requires ROS.
The main ROS normally produced in cells is O2−, which is dismutated by the action of superoxide dismutases to generate H2O2. H2O2 is eliminated by catalase, yielding H2O. It has been proposed that NO-induced cell migration is mediated by a cGMP/PKG-dependent increase in cellular ROS levels. However, this is based mainly on the use of antioxidants that might have pleiotropic activities. EUK-189 is a salen Mn compound that belongs to a new generation of antioxidants with highly specific detoxification activities. EUK-189 has both superoxide dismutase and catalase activities and can enter cell membranes, and its activity has been successfully tested both in vivo and in vitro in oxidative stress models (18, 28). We therefore tested whether EUK-189 prevents cGMP-induced BAEC migration in an in vitro wound healing (scratch) assay. cGMP increased the area recolonized by BAEC by 20 to 50%, and this effect was completely abolished by pretreatment with EUK-189 (Fig. 1 A).
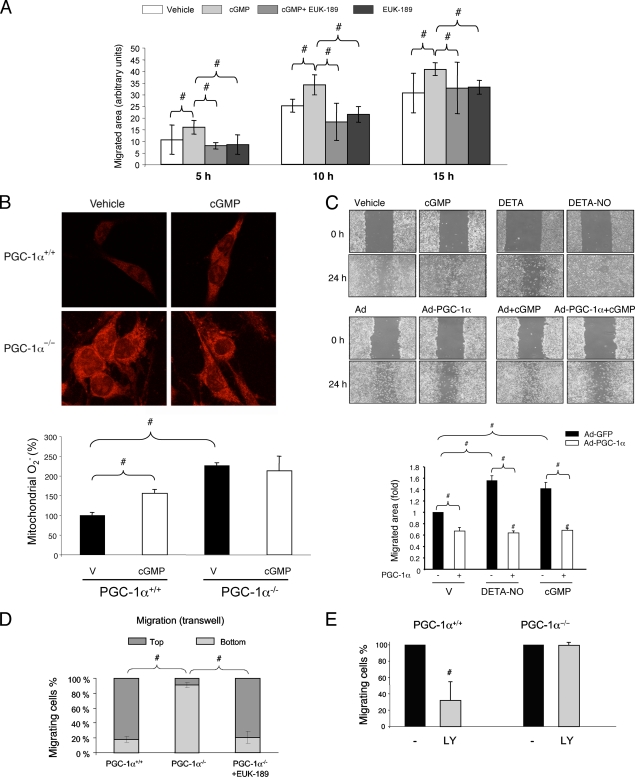
FIG. 1.
PGC-1α inhibits endothelial migration. (A) Quantitative analysis of scratch assays in serum-starved confluent BAEC. Cells were preincubated with EUK-189 (100 μM) for 2 h as indicated and then (right after being scratched) incubated with DETA-NO (62 μM) and 8-Br-cGMP (100 μM) as indicated. Repopulation of the denuded area was monitored by confocal microscopy at the indicated times. (B) MitoSOX Red labeling of fixed MLEC, showing mitochondrial superoxide levels in PGC-1α+/+/PGC-1α−/− cells treated with 100 μM 8-Br-cGMP for 6 h. V, vehicle. (C) Scratch wound healing assays with cells infected with PGC-1α or control adenovirus (MOI of 25) at 24 h before the beginning of the assay. Treatments with DETA-NO and 8-Br-cGMP, as shown in panel A, are shown for comparison. Cell migration was monitored at 18 h. (D and E) A total of 10,000 PGC-1α+/+ or PGC-1α−/− MLEC were seeded in the upper chamber of a Transwell insert and allowed to migrate for 18 h before fixing and labeling for cell nuclei. (D) Quantitative analysis of cross sections (z-axis) of Transwell inserts seeded with PGC-1α+/+ or PGC-1α−/− cells, illustrating the distance migrated. Cells were preincubated with EUK-189 (100 μM) for 2 h as indicated. (E) The chart shows the effect of LY294002 (LY; 10 μM) on the Transwell migration of PGC-1α+/+ and PGC-1α−/− MLEC. #, P < 0.05 versus paired control.
cGMP increases mitochondrial ROS levels.
To date, it has been assumed that cGMP increases cellular ROS levels by activating cytosolic NADPH activity. However, the effect of cGMP on mitochondrial ROS had never been evaluated, although mitochondria are major modulators of cellular ROS. To test the impact of cGMP on mitochondrial ROS, we incubated MLEC with MitoSOX Red, a reagent that labels mitochondrial superoxide. cGMP increased mitochondrial O2− accumulation by about 50%, suggesting that a cGMP-mediated increase in ROS is at least partially dependent on increased mitochondrial O2− accumulation (Fig. 1B). The same result was obtained when cells were treated with a NO donor (DETA-NO) (data not shown).
cGMP-mediated increase in mitochondrial ROS requires PGC-1α.
PGC-1α is a master regulator of the mitochondrial ROS detoxification system and is negatively regulated by NO/cGMP. We found that cGMP was unable to increase mitochondrial O2− levels in PGC-1α-null MLEC, suggesting that PGC-1α is an important mediator of the cGMP effect (Fig. 1B).
PGC-1α inhibits endothelial migration.
Since ROS are positive regulators of endothelial migration, we hypothesized that PGC-1α might be a negative regulator of endothelial migration. We therefore tested the effect of PGC-1α overexpression on BAEC migration in the scratch assay. Exogenous PGC-1α severely impaired endothelial migration, reducing the total migrated area by about 40% (Fig. 1C). Consistently, PGC-1α−/− MLEC migrated faster in Transwell cell migration assays than PGC-1α+/+ cells, with most PGC-1α−/− cells reaching the bottom of the well, while PGC-1α+/+MLEC were retained mostly at the top. Importantly, pretreatment of PGC-1α−/− cells with EUK-189 reverted the fast-migrating phenotype (Fig. 1D).
NO/cGMP-induced migration of BAEC cells is reduced by PGC-1α overexpression.
We previously showed that NO and cGMP downregulate PGC-1α gene expression, resulting in an overall inhibition of the cellular ROS detoxification systems. Taken together with the results shown in Fig. 1, this suggests that PGC-1α downregulation might be required for NO/cGMP-induced endothelial migration. Using the scratch assay, we found that overexpression of PGC-1α reduced the stimulatory effects on migration of cGMP (Fig. 1C) and DETA-NO (data not shown), supporting the notion that PGC-1α downregulation is required for NO/cGMP induction of endothelial migration.
PI3K inhibition does not modulate endothelial migration in the absence of PGC-1α.
It has been previously shown that cGMP-mediated activation of endothelial migration occurs via activation of the PI3K/AKT pathway. We therefore tested the effect of the PI3K inhibitor LY294002 on the migration of PGC-1α+/+ and PGC-1α−/− MLEC in Transwell assays (Fig. 1E). LY294002 reduced the number of PGC-1α+/+ MLEC reaching the bottom chamber by 60% but did not inhibit migration by PGC-1α−/− cells, further supporting the hypothesis that the NO/cGMP/PI3K/Akt pathway modulates endothelial cell migration via downregulation of PGC-1α.
Nitric oxide-induced downregulation of endothelial PGC-1α mRNA expression is mediated by PI3K/Akt signaling.
To directly test the involvement of downregulated PGC-1α expression in NO/cGMP/PI3K/Akt-induced endothelial cell migration, we first examined the effect of PI3K inhibitors on NO-dependent downregulation of PGC-1α mRNA levels in BAEC. When BAEC were preincubated with the PI3K inhibitor LY294002, it abolished DETA-NO- and cGMP-induced downregulation of transcript and protein expression of PGC-1α and its target genes, though the inhibitor by itself increased PGC-1α expression (Fig. 2 A and B). These results suggest that PI3K activity is required for the DETA-NO- and cGMP-induced downregulation of PGC-1α expression.
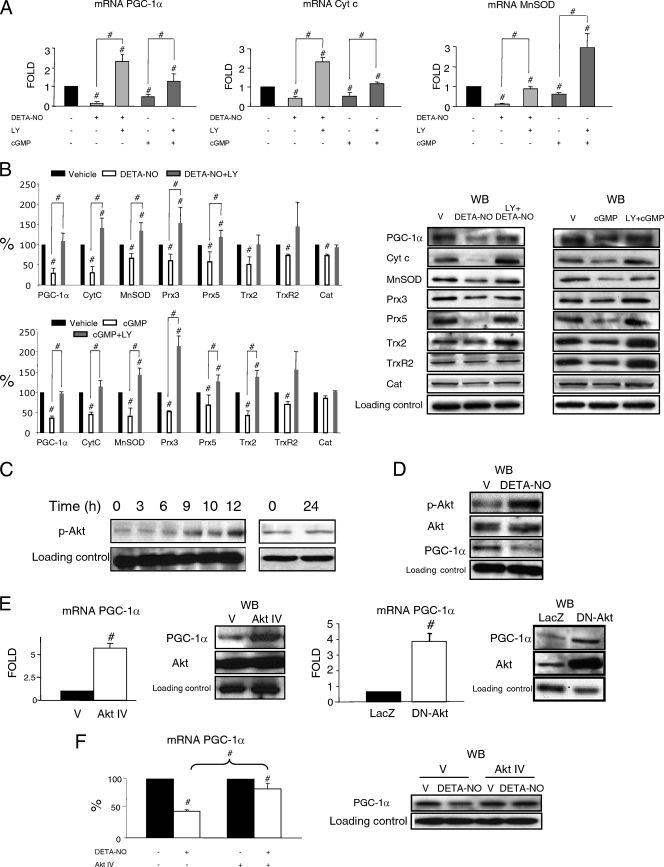
FIG. 2.
(A and B) NO downregulates PGC-1α and the mitochondrial ROS detoxification system via PKG and PI3K. BAEC were stimulated for 12 h with DETA-NO (62 μM) or 8-Br-cGMP (100 μM) with or without pretreatment with 10 μM LY294002 (LY), as indicated. V, vehicle. (A) mRNA expression levels (quantitative PCR [qPCR]) of PGC-1α and its target genes cytochrome c (CytC) and Mn superoxide dismutase (MnSOD). (B) Western blotting (WB) and band intensity quantification of protein levels of PGC-1α, CytC, MnSOD, peroxiredoxins 3 and 5 (Prx3 and Prx5), thioredoxin 2 (Trx2), thioredoxin reductase 2 (TrxR2), and catalase (Cat). (C to F) Akt is required for NO-induced downregulation of PGC-1α. (C) BAEC were incubated with DETA-NO (62 μM) for 3 to 24 h. Protein levels of phosphorylated Akt (p-Akt) were determined by WB. Zero hours corresponds to untreated cells. (D) WB of Akt, phosphorylated Akt (p-Akt), and PGC-1α in BAEC treated with 62 μM DETA-NO or DETA (V) for 12 h. (E) PGC-1α mRNA expression (quantitative reverse transcription-PCR [qRT-PCR]) and protein levels of PGC-1α (WB) are shown. (Left panels) BAEC were treated with Akt IV (0.5 μM) or vehicle (V) for 12 h. (Right panels) BAEC were infected with dominant negative Akt (DN-Akt) adenovirus or a control (LacZ) at an MOI of 25. (F) BAEC were treated with vehicle (V), DETA-NO (62 μM), Akt IV (0.5 μM), and Akt IV plus DETA-NO for 12 h. PGC-1α mRNA expression (qRT-PCR) and protein levels of PGC-1α (WB) are shown. Control samples were assigned the value of 1. #, P < 0.05 versus paired control.
Since Akt mediates many of the biological actions of PI3K, including cell migration, we next evaluated the role of Akt in NO-induced downregulation of PGC-1α. In response to treatment with DETA-NO, phosphorylation of Akt in BAEC increased in a time-dependent manner for up to 12 h (Fig. 2C), coinciding with maximal inhibition of PGC-1α expression (Fig. 2D) (1). This result suggests that Akt activity is increased in response to DETA-NO treatment.
Akt has been proposed to downregulate PGC-1α expression (3). Treatment of BAEC with the Akt inhibitor Akt IV significantly increased PGC-1α expression (Fig. 2E). PGC-1α was similarly upregulated by expression of a dominant negative form of Akt (DN-Akt) (Fig. 2E), confirming that Akt is a negative regulator of endothelial PGC-1α. To evaluate whether Akt activation mediates the effect of NO on PGC-1α, we pretreated BAEC for 1 h with Akt IV before treatment with DETA-NO. We observed that Akt IV reduced DETA-NO downregulation of PGC-1α (Fig. 2F), supporting the hypothesis that Akt activation is required for NO downregulation of PGC-1α.
DETA-NO inactivates Foxo3a.
Foxo3a is a direct target of Akt and has been shown to play a role in angiogenesis and ROS homeostasis; however, there has been no reported link between FoxO factors and NO function. To test whether FoxO activity was inhibited in response to DETA-NO, we transfected BAEC with a luciferase reporter driven by a promoter harboring three copies of a canonical FoxO binding site (PrFx3X). Treatment with DETA-NO (12 h) reduced luciferase activity by 50% relative to control cells (Fig. 3A), suggesting that NO is a negative regulator of FoxO transcriptional activity. We next investigated the effect of DETA-NO on Akt-mediated phosphorylation of Foxo3a, using an antibody that recognizes Foxo3a phosphorylated at specific Akt target residues. As predicted, treatment of BAEC with DETA-NO increased Foxo3a phosphorylation (Fig. 3B), suggesting that DETA-NO induces Akt-mediated phosphorylation of Foxo3a. Moreover, this effect was concomitant with the decrease in PGC-1α expression. Phosphorylation of Foxo3a normally results in its nuclear exclusion and, hence, inactivation. However, alternative activation pathways can override Akt-mediated inactivation (7). We therefore examined the subcellular distribution of Foxo3a by immunofluorescence. Both DETA-NO and cGMP significantly increased the cytosolic/nuclear ratio of Foxo3a (Fig. 3C and D), supporting the hypothesis that DETA-NO treatment inactivates Foxo3a.
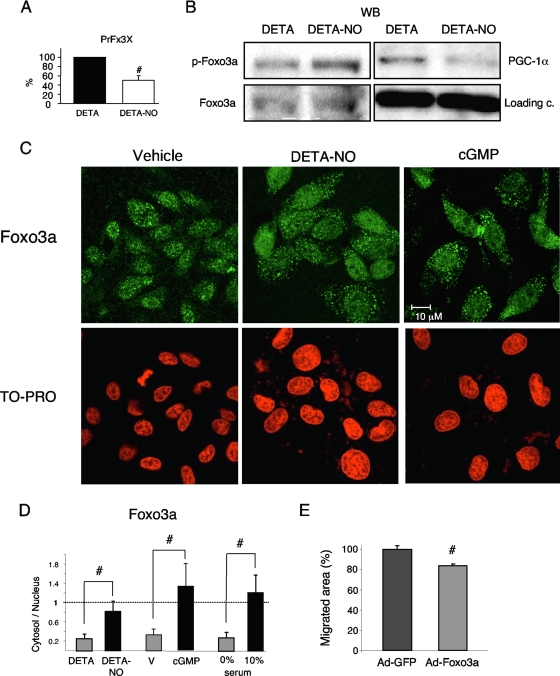
FIG. 3.
NO inactivates Foxo3a. (A) BAEC were transfected with 200 ng (PrFx3X) and, 24 h later, treated with DETA (V) or DETA-NO (62 μM) for 12 h. Control samples were assigned the value 100%. (B) BAEC were incubated with DETA (V) or DETA-NO (62 μM) for 12 h. WB of Foxo3a, phosphorylated Foxo3a (p-Foxo3a) and PGC-1α. c, control. (C and D) Immunofluorescence analysis of the cellular localization of Foxo3a. Serum-starved BAEC were incubated with DETA, DETA-NO (62 μM), or cGMP (100 μM) for 12 h. Cells in 10% FBS were used as a positive control. Nuclei were labeled with TO-PRO-3. (D, left) Distribution of TO-PRO-3 and Foxo3a signal intensity along cross sections of representative cells. (Right) Foxo3a cytosol/nuclear ratio. Averaged staining intensities were calculated from cross-sectional traces. (E) Scratch wound healing assays with cells infected with Foxo3a or control adenovirus (MOI, 10) 24 h before the beginning of the assay. Cell migration was monitored every 2 h for 20 h. #, P < 0.05 versus paired control.
Foxo3a inhibits endothelial migration.
Next, we directly tested Foxo3a activity on endothelial migration in a scratch assay. We found that moderate overexpression of Foxo3a, driven by a recombinant adenovirus in BAEC cells, resulted in a 20% reduction of the migrated area per hour over a 20-h period (Fig. 3E).
Foxo3a directly regulates PGC-1α expression in BAEC.
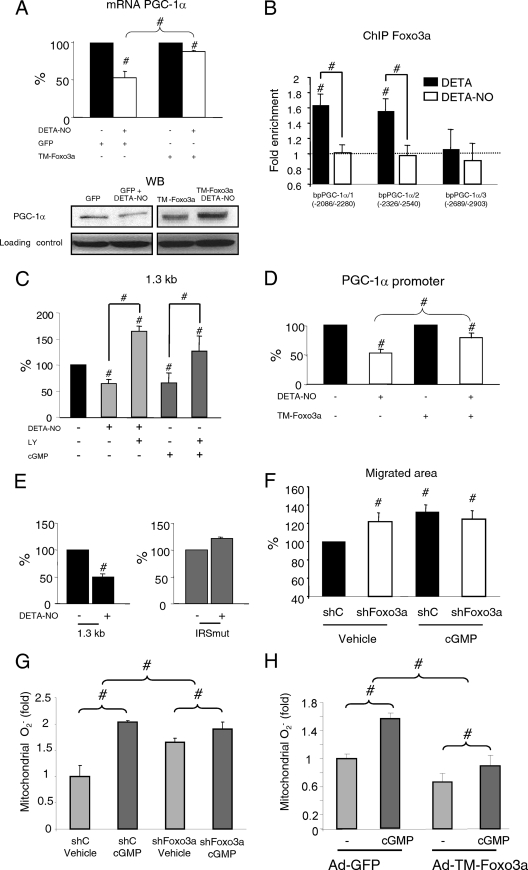
We recently showed that Foxo3a is a direct transcriptional regulator of PGC-1α expression in human umbilical vein endothelial cells (HUVEC) (23) and, therefore, would be expected to also be involved in PGC-1α transcriptional regulation in BAEC. Transfection of BAEC with a constitutively active form of Foxo3a (TM-Foxo3a), which is not susceptible to inactivation by Akt phosphorylation (TM-Foxo3a), increased PGC-1α levels (Fig. 4 A), indicating that Foxo3a is a positive regulator of PGC-1α. In ChIP experiments, Foxo3a coprecipitated with the PGC-1α promoter (Fig. 4B), thus indicating that the PGC-1α promoter is a direct target of Foxo3a. siRNA silencing of Foxo3a downregulated PGC-1α expression, correlating closely with the downregulation of Foxo3a itself (Fig. 4C) and confirming that Foxo3a plays a role in the modulation of PGC-1α levels.
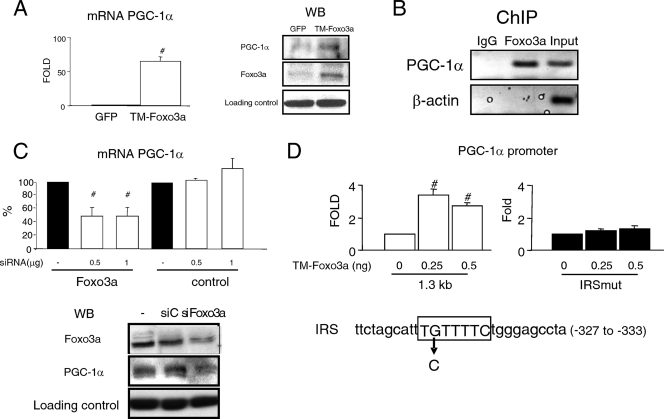
FIG. 4.
Foxo3a regulates PGC-1α expression. (A) BAEC were infected with TM-Foxo3a adenovirus (MOI, 10). (Left) PGC-1α mRNA (qPCR); (right) WB of PGC-1α and Foxo3a. GFP, green fluorescent protein. (B) Foxo3a binding to the PGC-1α promoter was determined by ChIP in BAEC. The 18S RNA gene was used as negative control (qPCR; not shown). (C) BAEC were transfected with the indicated amounts of Foxo3a siRNA (siFoxo3a) or control siRNA (siC; GAPDH [glyceraldehyde-3-phosphate dehydrogenase]). (Top) PGC-1α mRNA; (bottom) WB of PGC-1α and Foxo3a. (D) BAEC were cotransfected with 500 ng of the PGC-1α promoter (1.3 kb) or the IRS mutant vector (IRSmut) and the indicated amounts of the TM-Foxo3a vector or the corresponding control. Control samples were assigned the value of 100%. #, P < 0.05 versus paired control.
Foxo3a regulates the PGC-1α promoter fragment via a functional IRS.
To further characterize the regulation of PGC-1α by Foxo3a, we tested the activity of TM-Foxo3a on a 1.3-kb fragment of the mouse PGC-1α promoter. Foxo3a increased PGC-1α promoter activity. Examination of the promoter-fragment sequence identified a putative IRS (insulin-responsive site/FoxO binding site). Introduction of a point mutation abolished upregulation by TM-Foxo3a, suggesting that this is a functional IRS (Fig. 4D).
NO-induced downregulation of PGC-1α expression is mediated by inactivation of Foxo3a.
Taking into account the observations that Foxo3a is inactivated by DETA-NO treatment and that FoxO3a regulates PGC-1α expression, we next examined whether Foxo3a inactivation is required for NO-dependent downregulation of PGC-1α. In cells expressing TM-Foxo3a, DETA-NO-induced downregulation of PGC-1α was abolished (Fig. 5 A), suggesting that Foxo3a inactivation is required for NO-induced inhibition of PGC-1α expression. DETA-NO treatment significantly reduced Foxo3a localization at the PGC-1α promoter (Fig. 5B).
FIG. 5.
NO-induced downregulation of PGC-1α is mediated by Foxo3a. (A) BAEC were infected with TM-Foxo3a (MOI, 10) or control adenovirus and, 24 h later, were incubated with DETA or DETA-NO (62 μM, 12 h) as indicated. (Top) PGC-1α mRNA expression. Control samples (DETA) were assigned the value of 100%. (Bottom) PGC-1α WB. (B) BAEC were incubated with DETA or DETA-NO (62 μM, 12 h), and Foxo3a binding to three positions within the PGC-1α promoter was analyzed by ChIP. IP DNA was analyzed by qPCR. The 18S RNA gene was used as a negative control. (C) BAEC were transfected with 500 ng of the PGC-1α promoter vector and, 24 h later, incubated as indicated with DETA-NO (62 μM), 8-Br-cGMP (100 μM), and LY294002 (10 μM) for 12 h. (D) BAEC were cotransfected with the PGC-1α promoter vector (500 ng) and TM-Foxo3a (1 ng) and treated as indicated with DETA-NO. (E) BAEC were transfected with the PGC-1α promoter or with the IRS mutant vector (500 ng). Control samples (DETA) were assigned the value of 100%. (F) Scratch assay using serum-starved confluent BAEC. Cells were infected with Foxo3a shRNA (shFoxo3a) or control adenovirus (MOI, 25) at 24 h before the beginning of the assay and then treated with 100 μM 8-Br-cGMP as indicated. Cells were allowed to migrate for 18 h. Control samples (control shRNA [shC] plus vehicle) were assigned the value of 100%. (G, H) MitoSOX Red labeling of fixed BAEC infected with shFoxo3a (G), TM-Foxo3a (H), or the corresponding control adenovirus and treated with 100 μM 8-Br-cGMP for 6 h. #, P < 0.05 versus paired control.
To further investigate the role of Foxo3a in NO-induced PGC-1α downregulation, we looked for NO-responsive elements in the PGC-1α promoter. We first tested if DETA-NO/8-Br-cGMP could modulate the transcriptional activity of the 1.3-kb PGC-1α promoter construct in BAEC (Fig. 5C). Both DETA-NO and 8-Br-cGMP inhibited promoter activity, and this inhibition was prevented by pretreatment with LY294002 (Fig. 5C), suggesting that there is at least one NO-responsive element in this promoter fragment. TM-Foxo3a impaired NO inhibition of PGC-1α promoter activity (Fig. 5D), indicating that Foxo3a inactivation is important for the NO effect. Furthermore, the PGC-1α promoter bearing the point mutation in the IRS was unresponsive to DETA-NO (Fig. 5E), supporting the view that inhibition of Foxo3a is an important step in NO downregulation of PGC-1α.
These results implied that Foxo3a might be involved in NO/cGMP-dependent induction of endothelial migration. To test this, we analyzed the effect of Foxo3a knockdown on cGMP-induced endothelial migration in scratch assays. Adenovirus-mediated expression of Foxo3a short hairpin RNA (shRNA) increased the speed of endothelial cell migration, and treatment with cGMP was unable to further increase endothelial migration in this context, suggesting that Foxo3a inactivation is a crucial step in NO/cGMP-induced endothelial migration (Fig. 5F).
A cGMP-mediated increase in mitochondrial ROS is reduced by Foxo3a.
Foxo3a has been previously shown to positively regulate the cellular ROS detoxification capacity. Therefore, we decided to test if a cGMP-dependent increase in mitochondrial O2− required Foxo3a. We found that, while knockdown of Foxo3a increased mitochondrial O2− and expression of TM-Foxo3a reduced it, both knockdown and expression of the constitutively active form of Foxo3a significantly reduced the capacity of cGMP to regulate mitochondrial O2−, suggesting that Foxo3a is an important mediator of the effect of cGMP on mitochondrial O2− levels (Fig. 5G and H).
DISCUSSION
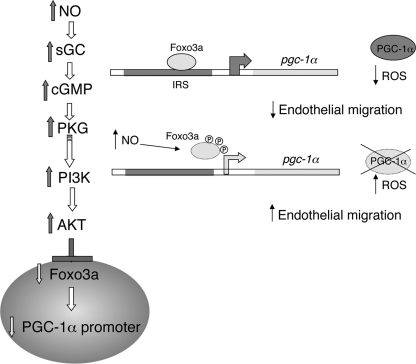
The mechanism through which NO/cGMP induces endothelial cell migration has so far remained unknown beyond the observation that it required PI3K/AKT activation and high levels of ROS. Our results show that NO/cGMP-mediated downregulation of PGC-1α is a necessary step for NO/cGMP-dependent induction of endothelial migration. We show that cGMP treatment of endothelial cells results in increased mitochondrial ROS levels and that this cGMP-dependent increase in ROS does not occur in the absence PGC-1α. Furthermore, we show that the cGMP-dependent increase in ROS is necessary for endothelial migration and that the cGMP effect is reduced when PGC-1α levels are kept artificially high. The idea that PGC-1α negatively regulates endothelial migration is further supported by the observation that endothelial cells obtained from PGC-1α−/− mice migrate faster than those obtained from PGC-1+/+ mice. Our results also show that NO/cGMP downregulation of PGC-1α is mediated by sGC/PKG-dependent activation of the PI3K/Akt pathway. The relevance of PI3K-dependent downregulation of PGC-1α on endothelial migration is supported by the finding that PI3K inhibition does not modulate endothelial migration in the absence of PGC-1α. Finally, we show that cGMP induction of migration also relies on AKT inactivation of Foxo3a and that cGMP does not further increase migration in cells where Foxo3a is knocked down. Importantly, cGMP downregulation of PGC-1α expression also depends on Foxo3a inactivation, indicating that Foxo3a activity is largely dependent on its role as a regulator of PGC-1α expression (Fig. 6).
FIG. 6.
NO-dependent downregulation of PGC-1α expression requires inactivation of Foxo3a by Akt. Foxo3a is a direct and positive regulator of PGC-1α expression, and its inactivation leads to the transcriptional downregulation of PGC-1α. Foxo3a inactivation and PGC-1α downregulation result in reduced ROS detoxification capacity, increased levels of ROS, and induction of endothelial migration.
These results have important physiological implications, particularly in angiogenesis, where NO-mediated increases in ROS levels are necessary for endothelial migration and proliferation during both physiological angiogenesis (wound healing) (6) and pathological angiogenesis (tumor growth) in response to ischemia (33). There is mounting evidence supporting a role for nitric oxide as a regulator of cellular detoxification capacity and a mediator of angiogenesis (1, 13). The results presented here help to define the molecular mechanism linking the ability of NO to modulate ROS levels with its ability to induce angiogenesis and are likely to be crucial to understanding how activation of PI3K/Akt and inactivation of FoxO regulate angiogenesis (26).
It has been previously suggested that NO promotes endothelial migration by inducing the enzymatic production of ROS by NADPH oxidase. Our present results suggest that NO activity depends not only on induction of cytosolic ROS-producing systems but also on reduction in the mitochondrial catabolism of ROS. These findings thus describe a new pathway through which NO regulates ROS levels to induce endothelial cell migration. NO downregulates endothelial PGC-1α expression through the activation of PI3K/Akt and subsequent inactivation of Foxo3a, which directly modulates PGC-1α promoter activity.
The transcription factor Foxo3a cooperates with PGC-1α to regulate cellular ROS levels through the transcriptional upregulation of detoxification enzymes and is itself a direct regulator of PGC-1α expression (23). Foxo3a has also been shown to regulate endothelial angiogenesis in vivo (24) and to inhibit endothelial migration in vitro (26). However, Foxo3a activity in angiogenesis had not previously been associated with NO-mediated regulation of cell migration or changes in ROS levels. The data presented here support the notion that Foxo3a is inactivated by NO during angiogenesis and that this inactivation allows the necessary increase in ROS.
Our results provide a novel link between the regulation of central metabolic factors, ROS, and vascular function that is likely to be relevant to the elucidation of mechanisms linking metabolic dysfunctions and aberrant angiogenic processes, such as diabetic retinopathy, and may open new possibilities for therapeutic intervention.
Acknowledgments
This work was supported by the Spanish Ministry of Science and Innovation (grants SAF2006-01619 and SAF2009-07599 to M.M., grant CSD 2007-00020 to M.M. and S.L., and grants SAF2006-02410 and SAF2009-07520 to S.L.), the Spanish Society of Nephrology (grant-in-aid to S.L.), a CNIC-Bancaja predoctoral fellowship to I.V., and an Instituto de Salud Carlos III (ISCIII) contrato de apoyo a la investigación to F.M.-G. The CNIC is supported by the Spanish Ministry of Science and Innovation and the Pro-CNIC Foundation.
Editorial support was provided by S. Bartlett. The FoxO reporter pGL3 construct (3×IRE-luc) was a gift from L. del Peso. The Foxo3a expression vector and adenovirus were gifts from K. Walsh. The PGC-1α−/− mice were a gift from B. Spiegelman. We thank Elvira Arza and Raquel Nieto for technical support; Enrique Samper, Mariano Redondo, and Alicia Garcia Arroyo for technical advice; and Ramón Bartroons and Eva Cano for careful reading of the manuscript.
Footnotes
Published ahead of print on 14 June 2010.
REFERENCES
- 1.Borniquel, S., I. Valle, S. Cadenas, S. Lamas, and M. Monsalve. 2006. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1alpha. FASEB J. 20:1889-1891. [DOI] [PubMed] [Google Scholar]
- 2.Bucci, M., F. Roviezzo, I. Posadas, J. Yu, L. Parente, W. C. Sessa, L. J. Ignarro, and G. Cirino. 2005. Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proc. Natl. Acad. Sci. U. S. A. 102:904-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daitoku, H., K. Yamagata, H. Matsuzaki, M. Hatta, and A. Fukamizu. 2003. Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes 52:642-649. [DOI] [PubMed] [Google Scholar]
- 4.Dimmeler, S., and A. M. Zeiher. 1999. Nitric oxide—an endothelial cell survival factor. Cell Death Differ. 6:964-968. [DOI] [PubMed] [Google Scholar]
- 5.Dudzinski, D. M., J. Igarashi, D. Greif, and T. Michel. 2006. The regulation and pharmacology of endothelial nitric oxide synthase. Annu. Rev. Pharmacol. Toxicol. 46:235-276. [DOI] [PubMed] [Google Scholar]
- 6.Duval, C., A. V. Cantero, N. Auge, L. Mabile, J. C. Thiers, A. Negre-Salvayre, and R. Salvayre. 2003. Proliferation and wound healing of vascular cells trigger the generation of extracellular reactive oxygen species and LDL oxidation. Free Radic. Biol. Med. 35:1589-1598. [DOI] [PubMed] [Google Scholar]
- 7.Essers, M. A., S. Weijzen, A. M. de Vries-Smits, I. Saarloos, N. D. de Ruiter, J. L. Bos, and B. M. Burgering. 2004. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 23:4802-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finck, B. N., and D. P. Kelly. 2007. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation 115:2540-2548. [DOI] [PubMed] [Google Scholar]
- 9.Genis, L., P. Gonzalo, A. S. Tutor, B. G. Galvez, A. Martinez-Ruiz, C. Zaragoza, S. Lamas, K. Tryggvason, S. S. Apte, and A. G. Arroyo. 2007. Functional interplay between endothelial nitric oxide synthase and membrane type 1 matrix metalloproteinase in migrating endothelial cells. Blood 110:2916-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gooch, K. J., C. A. Dangler, and J. A. Frangos. 1997. Exogenous, basal, and flow-induced nitric oxide production and endothelial cell proliferation. J. Cell. Physiol. 171:252-258. [DOI] [PubMed] [Google Scholar]
- 11.Hosaka, T., W. H. Biggs III, D. Tieu, A. D. Boyer, N. M. Varki, W. K. Cavenee, and K. C. Arden. 2004. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc. Natl. Acad. Sci. U. S. A. 101:2975-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, H., and D. J. Tindall. 2007. Dynamic FoxO transcription factors. J. Cell Sci. 120:2479-2487. [DOI] [PubMed] [Google Scholar]
- 13.Kawasaki, K., R. S. Smith, Jr., C. M. Hsieh, J. Sun, J. Chao, and J. K. Liao. 2003. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol. Cell. Biol. 23:5726-5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kops, G. J., T. B. Dansen, P. E. Polderman, I. Saarloos, K. W. Wirtz, P. J. Coffer, T. T. Huang, J. L. Bos, R. H. Medema, and B. M. Burgering. 2002. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419:316-321. [DOI] [PubMed] [Google Scholar]
- 15.Li, X., B. Monks, Q. Ge, and M. J. Birnbaum. 2007. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature 447:1012-1016. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Ongil, S., O. Hernandez-Perera, J. Navarro-Antolin, G. Perez de Lema, M. Rodriguez-Puyol, S. Lamas, and D. Rodriguez-Puyol. 1998. Role of reactive oxygen species in the signalling cascade of cyclosporine A-mediated up-regulation of eNOS in vascular endothelial cells. Br. J. Pharmacol. 124:447-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Rivera, E., T. R. Lizarbe, M. Martinez-Moreno, J. M. Lopez-Novoa, A. Rodriguez-Barbero, J. Rodrigo, A. P. Fernandez, A. Alvarez-Barrientos, S. Lamas, and C. Zaragoza. 2005. Matrix metalloproteinase 13 mediates nitric oxide activation of endothelial cell migration. Proc. Natl. Acad. Sci. U. S. A. 102:3685-3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melov, S., S. R. Doctrow, J. A. Schneider, J. Haberson, M. Patel, P. E. Coskun, K. Huffman, D. C. Wallace, and B. Malfroy. 2001. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J. Neurosci. 21:8348-8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moncada, S., M. W. Radomski, and R. M. Palmer. 1988. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem. Pharmacol. 37:2495-2501. [DOI] [PubMed] [Google Scholar]
- 20.Monsalve, M., S. Borniquel, I. Valle, and S. Lamas. 2007. Mitochondrial dysfunction in human pathologies. Front. Biosci. 12:1131-1153. [DOI] [PubMed] [Google Scholar]
- 21.Mukhopadhyay, P., M. Rajesh, G. Hasko, B. J. Hawkins, M. Madesh, and P. Pacher. 2007. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat. Protoc. 2:2295-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murohara, T., B. Witzenbichler, I. Spyridopoulos, T. Asahara, B. Ding, A. Sullivan, D. W. Losordo, and J. M. Isner. 1999. Role of endothelial nitric oxide synthase in endothelial cell migration. Arterioscler. Thromb. Vasc. Biol. 19:1156-1161. [DOI] [PubMed] [Google Scholar]
- 23.Olmos, Y., I. Valle, S. Borniquel, A. Tierrez, E. Soria, S. Lamas, and M. Monsalve. 2009. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J. Biol. Chem. 284:14476-14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik, J. H., R. Kollipara, G. Chu, H. Ji, Y. Xiao, Z. Ding, L. Miao, Z. Tothova, J. W. Horner, D. R. Carrasco, S. Jiang, D. G. Gilliland, L. Chin, W. H. Wong, D. H. Castrillon, and R. A. DePinho. 2007. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128:309-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polytarchou, C., and E. Papadimitriou. 2005. Antioxidants inhibit human endothelial cell functions through down-regulation of endothelial nitric oxide synthase activity. Eur. J. Pharmacol. 510:31-38. [DOI] [PubMed] [Google Scholar]
- 26.Potente, M., C. Urbich, K. Sasaki, W. K. Hofmann, C. Heeschen, A. Aicher, R. Kollipara, R. A. DePinho, A. M. Zeiher, and S. Dimmeler. 2005. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J. Clin. Invest. 115:2382-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skurk, C., H. Maatz, H. S. Kim, J. Yang, M. R. Abid, W. C. Aird, and K. Walsh. 2004. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J. Biol. Chem. 279:1513-1525. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasan, V., S. Doctrow, V. K. Singh, and M. H. Whitnall. 2008. Evaluation of EUK-189, a synthetic superoxide dismutase/catalase mimetic as a radiation countermeasure. Immunopharmacol. Immunotoxicol. 30:271-290. [DOI] [PubMed] [Google Scholar]
- 29.St-Pierre, J., S. Drori, M. Uldry, J. M. Silvaggi, J. Rhee, S. Jager, C. Handschin, K. Zheng, J. Lin, W. Yang, D. K. Simon, R. Bachoo, and B. M. Spiegelman. 2006. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397-408. [DOI] [PubMed] [Google Scholar]
- 30.Ueki, K., R. Yamamoto-Honda, Y. Kaburagi, T. Yamauchi, K. Tobe, B. M. Burgering, P. J. Coffer, I. Komuro, Y. Akanuma, Y. Yazaki, and T. Kadowaki. 1998. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J. Biol. Chem. 273:5315-5322. [DOI] [PubMed] [Google Scholar]
- 31.Valle, I., A. Alvarez-Barrientos, E. Arza, S. Lamas, and M. Monsalve. 2005. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 66:562-573. [DOI] [PubMed] [Google Scholar]
- 32.Wassmann, S., K. Wassmann, and G. Nickenig. 2004. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension 44:381-386. [DOI] [PubMed] [Google Scholar]
- 33.Wu, W. S. 2006. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 25:695-705. [DOI] [PubMed] [Google Scholar]