Abstract
While Listeria seeligeri and L. monocytogenes contain the main Listeria virulence gene cluster, only L. monocytogenes is considered an intracellular pathogen. Initial evolutionary analyses showed that the virulence genes prfA, hly, and plcA are conserved in L. seeligeri, with specific Hly and PrfA amino acid residues showing evidence for positive selection in L. seeligeri. Our data also show that temperature-dependent transcript patterns for prfA, which encodes a transcriptional regulator of virulence genes, differed between L. monocytogenes and L. seeligeri. To further investigate the divergence of virulence gene function and regulation, L. seeligeri prfA (prfALS), hly (hlyLS), and plcA (plcALS), as well as prfALS constructs with different prfA promoter regions, were introduced into appropriate L. monocytogenes null mutants. Only when prfALS was under the control of the L. monocytogenes prfA promoters (P1- and P2prfA) (P1P2LM prfALS) was prfALS able to fully complement the ΔprfALM deletion. hlyLS introduced into an L. monocytogenes background under its native promoter showed transcript levels similar to those of hlyLM and was able to partially restore L. monocytogenes wild-type-level hemolysis and intracellular growth, even though HlyLM and HlyLS showed distinct patterns of cell- and supernatant-associated hemolytic activities. Our data indicate that (i) regulation of prfA expression differs between L. monocytogenes and L. seeligeri, although hly transcription is temperature dependent in both species, and (ii) PrfA and Hly functions are largely, but not fully, conserved between L. seeligeri and L. monocytogenes. Virulence gene homologues and their expression thus appear to have adapted to distinct but possibly related functions in these two species.
Listeria seeligeri is a putative nonpathogenic bacterial species in the genus Listeria, which includes the hemolytic species L. monocytogenes, L. seeligeri, and L. ivanovii and the nonhemolytic species L. welshimeri, L. innocua, and L. grayi. L. monocytogenes causes disease in a wide range of species, including humans, while L. ivanovii, which affects predominantly sheep, has a narrow host range. Both of these species can also cause disease upon inoculation into laboratory animals (e.g., mice [16]) and can invade and intracellularly multiply in tissue culture cells (35). L. seeligeri, on the other hand, has generally not been found as a natural etiological agent of disease in animals or humans (47), does not cause disease in laboratory animals (25), and does not effectively invade and multiply in tissue culture cells (15, 25). While this species is generally considered nonpathogenic, some possible cases of human disease caused by L. seeligeri have been described, including a human meningitis case (48). L. seeligeri is commonly isolated from natural environments and has been found in some studies (51) to be the most common Listeria species isolated from these environments.
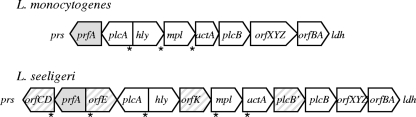
The apparent lack of virulence and pathogenicity for L. seeligeri is intriguing, as most L. seeligeri isolates appear to carry a homologue of the Listeria prfA virulence gene cluster (pVGC) (17, 63), which contains many of the genes required for virulence in both L. monocytogenes and L. ivanovii (29, 60). This virulence gene cluster is absent from the nonhemolytic, nonpathogenic Listeria spp. (i.e., L. welshimeri, L. innocua, and L. grayi) but is located between prs and ldh in L. monocytogenes, L. ivanovii, and L. seeligeri. In L. monocytogenes and L. ivanovii, the pVGC is about 9 kb long and includes six major virulence genes critical for survival and multiplication inside mammalian host cells (60). In all three species this virulence gene cluster contains homologues of the L. monocytogenes virulence genes prfA, plcA, hly, mpl, actA, and plcB (29) (Fig. 1). hly encodes listeriolysin O (LLO) in L. monocytogenes and seeligeriolysin O (LSO) in L. seeligeri; this cholesterol-dependent cytolysin (CDC) is essential for rapid escape of Listeria from the host cell vacuole (1, 44). LSO has 81.5% amino acid (aa) identity to LLO, and similar transcriptional signals (including a PrfA box) upstream of hly in L. monocytogenes and L. seeligeri have been reported (18, 25). The products of plcA (a phosphatidylinositol-specific phospholipase C) and plcB (a broad-range phospholipase C) have accessory roles in escape from the host cell vacuole. plcB, in conjunction with hly, is essential for release of Listeria from the double-membrane vacuole that forms after cell-to-cell spread (44). In one L. seeligeri strain sequenced, the pVGC is 13 kb long and includes, in addition to six main virulence genes, a plcB duplication and several L. seeligeri-specific open reading frames (ORFs) (29). One of these ORFs, orfE, is located between plcA and prfA in L. seeligeri (29) and appears to be PrfA dependent. orfE transcription has been hypothesized to interfere with prfA transcription from the upstream plcA promoter, contributing to the inability of L. seeligeri to escape from the phagosome of mammalian cells (25, 29).
FIG. 1.
Schematic of the prfA virulence gene cluster loci in L. monocytogenes and L. seeligeri. This cluster is located between prs and ldh in both species. prfA, which encodes the transcriptional regulator PrfA, is denoted by gray, and additional ORFs specific to L. seeligeri are denoted as stripes. Asterisks (*) denote the locations of PrfA boxes. Adapted from Kreft et al. (29).
PrfA (positive regulatory factor A), which is also encoded by a gene (i.e., prfA) located in the pVGC, is a transcriptional regulator belonging to the Crp-Fnr family of transcriptional regulators. PrfA interacts with a 14-nucleotide (nt) palindrome DNA sequence known as the “PrfA box,” located upstream of PrfA-regulated genes (14, 30, 55), including the virulence genes located in the pVGC. L. seeligeri PrfA has 73.4% amino acid identity to L. monocytogenes PrfA. With L. monocytogenes, regulation of PrfA has been shown to occur on several molecular levels (12, 13, 33, 46). At the transcriptional level, prfA expression is controlled by three promoter regions, including (i) two promoter regions directly upstream of prfA (the σA-dependent P1prfA promoter as well as P2prfA, which includes overlapping σA- and σB-dependent promoters), and (ii) a PrfA-dependent promoter located upstream of plcA, which produces a bicistronic plcA-prfA transcript (5). Regulation at the posttranscriptional level involves temperature-dependent translation of the P1prfA transcript through a hairpin that masks the Shine-Dalgarno sequence at temperatures of ≤30°C but is relieved at higher temperatures, thus facilitating higher PrfA levels at mammalian and avian body temperatures (13, 14). While it is known that PrfA can exist in active and inactive states, the mechanisms for this posttranslational regulation are still largely unknown (12, 61, 62).
Genome hybridization studies (11) and a recent genome analysis (57) have shown that L. seeligeri does not contain homologues of a number of internalin genes that are present in L. monocytogenes (e.g., inlA and inlB) and encode proteins important for invasion of different mammalian cell lines (11, 19, 39). While the absence of inlA and inlB, in particular, may explain why L. seeligeri has not been found to invade mammalian epithelial cell lines (11, 19, 25), additional differences between L. seeligeri and L. monocytogenes also appear to contribute to the apparent lack of virulence in L. seeligeri. For example, in vitro transcription data (33) in combination with complementation of an L. monocytogenes prfA null mutant with L. seeligeri prfA suggested that L. seeligeri PrfA (PrfALS) has a reduced ability to activate PrfA-dependent transcription in L. monocytogenes (compared to PrfALM). On the other hand, complementation of an L. seeligeri wild-type strain with a plasmid containing the L. monocytogenes plcA-prfA operon allowed expression and activation of L. seeligeri hly, enabling escape from the phagosome of enterocyte- and macrophage-like cells (25). While initial data thus indicate that virulence gene function and regulation differ between L. monocytogenes and L. seeligeri, our overall understanding of the diversification of virulence-associated functions between L. monocytogenes and L. seeligeri has remained limited. The goal of this study was to use a combination of (i) evolutionary analyses of prfA, hly, and plcA, (ii) transcriptional analyses of prfA and hly in L. seeligeri and L. monocytogenes grown under different conditions, and (iii) complementation studies involving introduction of L. seeligeri prfA, hly, and plcA into L. monocytogenes to further characterize diversification of virulence-related functions in the genus Listeria. We specifically focused on complementing an L. monocytogenes strain with selected L. seeligeri genes, as (i) L. monocytogenes contains other critical virulence genes (e.g., inlA and inlB) that are absent in L. seeligeri, thus allowing for evaluation of a wider range of phenotypes in complemented L. monocytogenes strains, and (ii) complementation of L. seeligeri with selected L. monocytogenes genes has previously been attempted (25).
MATERIALS AND METHODS
Sequencing of L. seeligeri prfA, hly, and plcA.
To evaluate conservation and selection patterns, the L. seeligeri virulence genes prfA, hly, and plcA were PCR amplified and sequenced (see Table 1 for primers) from a set of 8 diverse L. seeligeri isolates (see Table 2 for isolates). Sequences were assembled and proofread using Seqman (DNAStar, Madison, WI) and were aligned in Megalign (DNAStar) using the Clustal W algorithm. PESTFind (https://emb1.bcc.univie.ac.at/toolbox/pestfind/pestfind-analysis-webtool.htm [49]) was used to identify PEST sequences in hly.
TABLE 1.
Primers used in this studya
| Primer function or name | Sequence (5′→3′) |
|---|---|
| Amplification of L. seeligeri hly | |
| CRLLShlyF | GGGATCCGCATAGGAAAAATAATGGAGTAAACAGC |
| CRLLShlyR | GCGGCCGCTTATTTTATGGTGTGTGTGTTAAGCG |
| Amplification of L. seeligeri plcA | |
| CRLLSplcAF | GGGATCCGATTCCGAGATTTTTCGGATATATACTAG |
| CRLLSplcAR | GCGGCCGTCTCTCCCCTTCACTTTTTCATTCTTC |
| Amplification of L. seeligeri prfA | |
| CRLLSprfAF | GGGATCCTGAAACAATTAATAAAAAGCGCAAAAG |
| CRLLSprfAR | GCGGCCGCACATATTCCTTAAATTTTGCCTTACAAG |
| Amplification of L. monocytogenes prfA | |
| CRL10LMprfAFpstI | CAACTGCAGCGTACGCGTTCATGAAAATGCT |
| CRL2LMP1P2R | GCGGCCGGTTCGAGGATTAGGCATACTAATCATGG |
| Construction of the ΔprfA P1P2LM356prfALS strainb | |
| CRL7LMP1P2F | GTCTCATCCCCCAATCGTTTTTTATCG |
| CRL8LSprfAR(2) | CGATAAAAAACGATTGGGGGATGATGTGAG |
| CRLLSprfAF | GGGATCCTGAAACAATTAATAAAAAGCGCAAAAG |
| Confirmation of pPL2 integration | |
| NC16 | GTCAAAACATACGCTCTTATC |
| PL95 | ACATAATCAGTCCAAAGTAGATGC |
| 5′ RACE PCR for L. seeligeri prfA | |
| GSP1 CRL11LSprfA | TTATGAAAGCGCCTTTATAGTATTG |
| GSP2 CRL12LSprfA | TTCAGAATATCCCCGCTCTCAC |
| 5′ RACE PCR for L. monocytogenes prfA | |
| GSP1 LM prfART | GCCTGCTCGCTAATGACTTCTA |
| GSP2 LM prfARACE | GGTCCCGTTCTCGCTAATACT |
L. seeligeri primers were designed using ATCC 35967 sequence data, and L. monocytogenes primers were designed using 10403S sequence data.
Primers used to construct the ΔprfA P1P2LM356 prfALS strain include (i) CRL2LMP1P2R and CRL7LMP1P2F to amplify the L. monocytogenes prfA promoter region and (ii) CRL8LSprfAR(2) and CRLLSprfAF to amplify the L. seeligeri prfA coding sequence.
TABLE 2.
L. seeligeri isolates used for evolutionary analysis of prfA, hly, and plcA
| Isolate no.a | Isolate source | Isolate source location | Sequence information was available and used for: |
Source or reference for DNA sequence data | ||
|---|---|---|---|---|---|---|
| prfA | plcA | hly | ||||
| FSL S4-035 | Vegetation | Syracuse, NY | + | + | +b | This work |
| FSL S4-039 | Soil | Syracuse, NY | + | + | + | This work |
| FSL S4-079 | Vegetation | Adirondack Park, NY | + | + | + | This work |
| FSL S4-116 | Soil | Finger Lakes National Forest, NY | + | + | + | This work |
| FSL S4-200 | Vegetation | Catskills Park, NY | + | + | + | This work |
| FSL S4-212 | Vegetation | Connecticut Hill, NY | + | + | + | This work |
| FSL S4-252 | Soil | Albany, NY | + | + | + | This work |
| FSL S4-307 | Vegetation | Catskills Park, NY | + | + | +b | This work |
| ATCC 35967c | Soil | Germany | + | + | + | 63 |
| SLCC 3379c | Soil | Germany | + | − | + | |
| NRRL 33019c | Soil | Germany | − | − | + | 64 |
Additional isolate information is available under the isolate number at www.pathogentracker.net.
hly sequence was truncated; full gene sequence not used for positive selection analyses.
Sequence data for these isolates were obtained from GenBank.
Positive selection analysis.
Positive Darwinian selection at the DNA sequence level can be evaluated by estimating the ratio (ω) of the rate of nonsynonymous nucleotide substitutions (dN) to that of synonymous substitutions (dS) between homologous protein-coding gene sequences (38). An ω value of >1 suggests that the gene evolved by positive selection. Conversely, an ω value of <1 suggests that the gene evolved by negative selection, and an ω value of 1 indicates neutral evolution of the gene. Model 0 implemented in the program codeml in the software package PAML version 3.15 (66) was used to determine the average dN/dS ratio for hly, prfA, or plcA using the L. seeligeri sequences that were obtained here or were available in GenBank (Table 2).
Positive selection during divergence of L. seeligeri from other Listeria species (i.e., L. monocytogenes and L. ivanovii) was assessed using a branch site test (test 2) implemented in PAML 3.15 (67) as previously detailed (42). This test can detect positive selection affecting a small number of sites along prespecified branches in a phylogeny (67). These positive selection analyses were performed using alignments of hly, plcA, and prfA for L. seeligeri (Table 2), 40 L. monocytogenes isolates (42), and the one L. ivanovii isolate for which sequence data for these genes were available (strain NRRL 33021, GenBank accession no. AY510073 [64]).
Bacterial strains for mutant construction.
L. monocytogenes 10403S (serotype 1/2a) and L. seeligeri FSL S4-039 were used as parent strains for mutant construction (Table 3). FSL S4-039 is an environmental isolate obtained from soil near Syracuse, NY, in 2001 (51). Previously constructed L. monocytogenes 10403S isogenic prfA, hly, and plcA null mutants (Table 3) were used as host strains for complementation experiments.
TABLE 3.
Bacterial strains used in this study
| Strain | Description (strain abbreviation used throughout the text) | Reference or source |
|---|---|---|
| 10403S | L. monocytogenes parent strain | 4 |
| FSL S4-039 | L. seeligeri wild-type strain | 51 |
| FSL B2-046 | 10403S ΔprfAa | 34 |
| FSL R3-003 | 10403S Δhly | 24 |
| FSL R3-004 | 10403S ΔplcA | 5 |
| FSL L5-029 | 10403S ΔplcA tRNAArg::pPL2 plcALS52 (ΔplcA plcALS52 strain) | This work |
| FSL L5-030 | 10403S Δhly tRNAArg::pPL2 hlyLS314 (Δhly hlyLS314 strain) | This work |
| FSL L5-032 | 10403S ΔprfA tRNAArg::pPL2 prfALS157 (ΔprfA prfALS157 strain) | This work |
| FSL L5-162 | 10403S ΔprfA tRNAArg::pPL2 prfALS367 (ΔprfA prfALS367 strain) | This work |
| FSL L5-113 | 10403S ΔprfA tRNAArg::pPL2 prfALM356 (ΔprfA prfALM356 strain) | This work |
| FSL L5-160 | 10403S ΔprfA tRNAArg::pPL2 PplcA prfALM356 (ΔprfA PplcA prfALM356 strain) | This workb |
| FSL L5-112 | 10403S ΔprfA tRNAARG::pPL2 P1P2LM356prfALS (ΔprfA P1P2LM356prfALS strain) | This work |
This strain carries a 339-bp in-frame deletion in prfA.
The plasmid with the PplcA prfALM356 construct that was used to construct the pPL2 plasmid employed to generate this strain was first reported by Wong and Freitag (65).
Construction of L. monocytogenes complementation mutants.
Selected L. seeligeri virulence genes (i.e., prfA, hly, and plcA) were introduced into the appropriate L. monocytogenes 10403S null mutants by cloning these genes and their upstream promoter regions into the pPL2 integration vector, which integrates at tRNAArg-attBB′ on the L. monocytogenes chromosome (31, 65). Briefly, PCR (primers listed in Table 1) was used to amplify the gene of interest and the appropriate promoter region for cloning into pPL2, and the pPL2 constructs were introduced into the appropriate L. monocytogenes mutant strains using electroporation as previously described (31). Strains generated through this approach include the L. monocytogenes hly and plcA null mutants complemented with L. seeligeri hly and plcA (including 314 and 52 nt upstream of the start codon of the respective gene); these strains are designated 10403S Δhly tRNAArg::pPL2 hlyLS314 (Δhly hlyLS314) and 10403S ΔplcA tRNAArg::pPL2 plcALS52 (ΔplcA plcALS52) (Table 3). In addition, we created five strains in which an L. monocytogenes prfA null mutant was complemented with different prfA alleles. Strain 10403S ΔprfA tRNAArg::pPL2 prfALS157 (here denoted as the ΔprfA prfALS157 strain) contains the prfALS ORF and 157 nt upstream of the prfALS start codon, while the ΔprfA tRNAArg::pPL2 prfALS367 (ΔprfA prfALS367) strain contains the prfALS ORF and 367 nt upstream of the prfALS start codon (Table 3); both include the full P1prfA-and-P2prfA (P1P2prfA) promoter region. Two control strains include (i) L. monocytogenes ΔprfA complemented with prfALM and the 356-nt-type upstream promoter region (10403S ΔprfA tRNAArg::pPL2 prfALM356; here denoted as the ΔprfA prfALM356 strain [Table 3]) and (ii) L. monocytogenes ΔprfA complemented with prfALM and its upstream promoter as well as the upstream PplcA promoter region (ΔprfA tRNAArg::pPL2 PplcA prfALM356, here denoted as the ΔprfA PplcA prfALM356 strain [Table 3]). Finally, we also created a pPL2 construct that contained prfALS fused to a 356-nt L. monocytogenes prfA promoter region, which included both the P1prfA and P2prfA promoters. This chimera was constructed using splice overlap extension (SOE) PCR (21) (primers listed in Table 1) and was introduced into L. monocytogenes 10403S ΔprfA yielding the ΔprfA tRNAArg::pPL2 P1P2LM356 prfALS strain (the ΔprfA P1P2LM356 prfALS strain [Table 3]). Correct construction and integration of all pPL2 constructs were confirmed by PCR analysis and sequencing.
Growth conditions.
Unless specified otherwise, all experiments (including RNA studies) were performed using L. monocytogenes or L. seeligeri grown to stationary phase at either 16 or 37°C. An initial overnight culture grown in brain heart infusion (BHI) at 37°C was diluted 1:100 into 5 ml fresh BHI broth and grown at 37°C, with shaking to log phase (optical density at 600 nm [OD600] of 0.4). This log-phase culture was then diluted 1:100 into fresh BHI, incubated with aeration (shaking at 210 rpm) or without shaking at 16 or 37°C until cells reached stationary phase (defined as an OD600 of 1.0 followed by 3 h of incubation for cells grown with shaking and as an OD600 of 0.8 followed by 3 h of incubation for cells grown without shaking).
RNA purification and quantitative reverse transcription-PCR (qRT-PCR).
For RNA purification, 4 ml of culture was added to 8 ml of RNAprotect (Qiagen) and incubated at room temperature for 5 min, and cells were subsequently collected by centrifugation (at 4°C) at 5,000 × g for 5 min. Cell pellets were frozen at −80°C and used for total RNA extraction as previously described (34).
TaqMan qRT-PCR was used to monitor transcript levels for hly, prfA, and plcA in appropriate wild-type and mutant strains. While L. monocytogenes qRT-PCR primers and probes have previously been reported (see Table S1 in the supplemental material), primers and probes for L. seeligeri genes and the prfA chimera construct (i.e., P1P2LM prfALS) were designed using Primer Express (Applied Biosystems, Foster City, CA) (see Table S1). For normalization, transcript levels for the housekeeping genes rpoB and gap were also determined for each RNA sample, as previously described (34). qRT-PCR, including reverse transcriptase negative-control reactions, DNA standard curves, and statistical analysis, was performed as previously described (34). All qRT-PCR experiments were performed on three RNA samples representing independent biological replicates. mRNA transcript levels of target genes were normalized to the geometric mean of the transcript levels for the housekeeping genes rpoB and gap (7, 27), i.e., {[log10 target gene mRNA] − [(log10 rpoB mRNA + log10 gap mRNA)/2]} as previously described (59). While some propose that normalization against transcript levels for at least three housekeeping genes is optimal (59), normalization to one housekeeping gene has been used in a number of studies of L. monocytogenes (20, 37, 58) and normalization to gap and rpoB transcript levels has been used in a number of previous studies (34, 40). The geometric mean of rpoB and gap transcript levels in this study showed limited variation among the different strains grown at 37°C (average 5.53, range 5.21 to 5.83), supporting the approach used here.
5′ RACE PCR.
The L. seeligeri prfA promoter region was mapped with the 5′ rapid amplification of cDNA ends (RACE) system (Invitrogen) according to the manufacturer's protocol as previously described (26). RNA was isolated as described above from cells grown at 37°C with aeration (shaking at 210 rpm) or without aeration. Briefly, RNA was used for gene-specific first-strand cDNA synthesis, dCTP tailing, and subsequent PCR amplification using a nested gene-specific primer and a poly(G/I) primer (primers are listed in Table 1). PCR products of the appropriate size were then purified using a QIAquick gel extraction kit (Qiagen), cloned into pCR2.1 using a TOPO TA cloning kit (Invitrogen), and sequenced to identify transcriptional start sites. As gel extraction will facilitate characterization of a specific transcript, our RACE experiments are expected to map the transcriptional start site for a specific promoter.
Determination of hemolytic activity.
Both supernatant- and cell-associated hemolysin activities for selected strains were determined as previously described (44), with minor modifications. Briefly, a dilution series (in phosphate-buffered saline [PBS] with 0.5 mM dithiothreitol, pH 5.8) of the supernatant fraction or a cell suspension of bacteria grown in LB-salt for 5 h at 37°C (with shaking) were prepared and incubated for 30 min at 37°C. These dilutions were then mixed with washed sheep red blood cells (RBCs) resuspended in PBS and incubated for 60 min at 37°C. To quantify the lysis of RBCs, hemoglobin release was determined by measuring the optical density (at 420 nm) of the supernatant using a Fusion Universal microplate analyzer (Packard, Meriden, CT). A hemolytic unit was defined as the reciprocal of the supernatant dilution at which 50% of the sheep red blood cells were lysed; the supernatant OD420 values for 50% lysis were determined as the mean of the OD420 values for 100% lysis (using a positive control of RBC lysed with 1% Triton X-100) and spontaneous lysis (supernatant of control RBC without Triton). Three biological replicates were performed for each assay.
Intracellular growth in macrophage-like J774 cells.
Intracellular growth assays using stimulated J774 cells were performed to assess Listeria cell-to-cell spread in J774 cells as previously described (8), with minor modifications. Briefly, 48 h prior to the assay, J774 cells were seeded into 24-well plates at a density of 2 × 105 cells/well, using Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) and without antibiotics. At 24 h prior to infection, J774 cells were stimulated by addition of fresh medium containing 10 μg/ml of Escherichia coli 055:B5 lipopolysaccharide (Sigma). Infection was performed by adding approximately 1 × 106 CFU of Listeria to each well, yielding a multiplicity of infection (MOI) of approximately 1. At 30 min postinfection (p.i.), J774 cells were washed with PBS, and fresh medium containing 50 μg/ml gentamicin was added to each well to kill any extracellular bacteria. J774 cells in separate wells were lysed by addition of ice-cold sterile water at 1.5, 5, and 7 h postinfection. Intracellular Listeria cells were then enumerated by the plating of lysed J774 cells on BHI agar. All experiments were repeated three times.
Intracellular growth in mouse-derived bone marrow macrophage cells.
For selected strains, we also performed intracellular growth assays in bone marrow macrophages (BMMØ) derived from BALB/c mice as previously described (43). Cells were maintained for 10 days in 100-mm petri dishes in DMEM supplemented with 10% fetal calf serum (FCS), 5% horse serum, and 20% L cell-conditioned medium and cultured with penicillin and streptomycin. Intracellular growth assays of these cells were performed as described above for J774 cells.
Statistical analysis.
t tests or one-way analysis of variance (ANOVA) with Tukey-Kramer multiple-comparison correction was performed for comparisons of means for two or more strains. All statistical analyses were performed in JMP 6 (SAS, Inc.), with P values of <0.05 considered significant. Exact P values were reported, except when P was <0.0001.
Nucleotide sequence accession numbers.
Sequences have been deposited in GenBank under accession numbers EU755300 to EU755321.
RESULTS
PrfA, Hly, and PlcA are conserved among L. seeligeri isolates, but prfALS and hlyLS show evidence of positive selection at specific codon sites.
In order to initially characterize the diversification of hly, prfA, and plcA in L. seeligeri, these three genes were sequenced for 8 L. seeligeri strains. These sequences were analyzed together with additional L. seeligeri sequences available in GenBank (Table 2). PrfA, Hly (LSO), and PlcA amino acid sequences were 97 to 99% identical among L. seeligeri strains; the sequences showed between 3 (prfA) and 17 (plcA) nonsynonymous changes (Table 4). To further characterize the evolution of these virulence genes in L. seeligeri, a branch site analysis using test 2 (67) was used to determine if individual amino acids in L. seeligeri PrfA, Hly, and PlcA evolved by positive selection during the divergence of L. seeligeri from other Listeria species, which would provide evidence that a given gene may have adapted to divergent function in L. seeligeri. While no significant evidence for positive selection was found for plcALS and prfALS (Table 4), PrfALS aa 107 showed a high probability of having evolved by positive selection (P of >95%[Table 4]). PrfA aa 107 is located in the β-sheet flanking the β-roll fold, a domain similar to the domain containing the cyclic AMP (cAMP) binding site in Crp regulators (62).
TABLE 4.
Results for PAML positive selection analyses for prfA, plcA, and hly
| Gene (no. of aa in L. seeligeri) | No. of syn. substitutions in L. seeligeri (πNSS)a | No. of nonsyn. substitutions in L. seeligeri (πNNS)b | dN/dSc | P valued | ω | pe | aa sites with BEB of >95%f |
|---|---|---|---|---|---|---|---|
| prfA (237) | 18 (0.042) | 3 (0.001) | 0.038 | 0.179 | 56.720 | 0.015 | 107 |
| plcA (320) | 29 (0.054) | 17 (0.009) | 0.146 | 1 | 1.000 | 0.150 | |
| hly (530) | 14 (0.018) | 11 (0.005) | 0.184 | 0.004 | 5.050 | 0.048 | 5, 39, 67, 117 |
πNSS, nucleotide diversity (average pairwise differences per site) for synonymous (syn.) substitutions.
πNNS, nucleotide diversity (average pairwise differences per site) for nonsynonymous (nonsyn.) substitutions.
dN/dS, number of nonsynonymous changes per nonsynonymous site/number of synonymous changes per synonymous site for the entire gene; calculated based on 10 L. seeligeri isolates for prfA and 9 isolates for hly and plcA.
P values for analyses of positive selection during divergence of L. seeligeri from L. monocytogenes and L. ivanovii.
Proportion of all amino acids falling into the class with the shown ω value of ≥1.
BEB, Bayesian empirical Bayes analysis of positive selection. Identifies amino acid sites with high probability (>95%) of having evolved by positive selection during divergence of L. seeligeri from other Listeria species.
hlyLS showed strong evidence of having evolved by positive selection during the divergence of L. seeligeri from other Listeria species (P = 0.004). Four amino acid sites (positions 5, 39, 67, and 117) were identified as having a significant probability (P of >95%) of having evolved by positive selection in HlyLS (Table 4). One of these amino acid residues (aa 39) was located in the PEST-like region of L. seeligeri Hly; a putative PEST motif has also been described for the N-terminal region of L. monocytogenes Hly (32, 49). A PEST motif is a region, rich in proline (P), glutamic acid (E), serine (S), and threonine (T), which, in general, can facilitate proteolysis of a protein, even though this region has been hypothesized to be important in LLO synthesis during cytosolic growth of L. monocytogenes (52, 53). Only 10 of the 19 aa in the L. seeligeri PEST-like motif were homologous to L. monocytogenes. The L. seeligeri PEST-like motif showed a considerably higher PESTfind score (15.48) than that of L. monocytogenes (4.74), and contains more PEST residues than the L. monocytogenes PEST motif. While one of the amino acid residues under positive selection (aa 5) was located in the Hly signal sequence, SignalP 3.0 (3) showed clear evidence for the presence of a signal sequence in HlyLS and HlyLM, with probabilities of 0.994 and 1.0, respectively.
prfALS transcription in L. seeligeri FSL S4-039 originates from a homologue of the L. monocytogenes P2prfA promoter region.
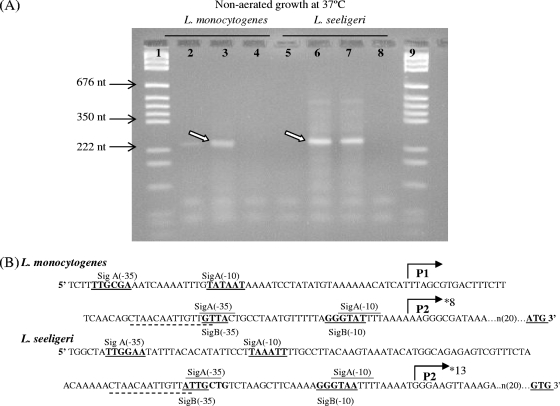
RACE PCR mapping of the L. seeligeri FSL S4-039 prfA promoter region using RNA extracted from bacteria grown at 37°C without aeration showed that prfALS transcription initiated from a promoter site corresponding to the L. monocytogenes P2prfA region (Fig. 2); 13 of the 14 cloned prfALS RACE PCR products mapped to a transcriptional start site that is 10 nt upstream of the −10 region in P2prfA (Fig. 2). prfA transcripts in L. monocytogenes 10403S grown without aeration also mapped to the P2prfA region (in all 8 clones sequenced) (Fig. 2). RACE PCR experiments performed using RNA extracted from L. monocytogenes 10403S grown at 37°C with aeration showed that transcription of prfALM under these conditions initiated predominantly from the P1prfA promoter (only a RACE PCR product corresponding, by size, to initiation from P1prfA was observed, and all 4 clones sequenced mapped to an initiation site 10 nt upstream of the P1prfA −10 site; see Fig. S1 in the supplemental material). This finding is consistent with previous RACE PCR data for L. monocytogenes grown at 37°C to stationary phase with aeration; these data also showed that prfA transcripts initiated predominantly from the P1prfA region (27). On the other hand, RACE PCR results for L. seeligeri grown at 37°C with aeration showed that even under these conditions, prfA transcription predominantly initiates from the L. seeligeri P2prfA promoter (only a RACE PCR product corresponding, by size, to initiation from P2prfA was observed, and all 3 clones sequenced mapped to an initiation site 10 nt upstream of the P2prfA −10 site; see Fig. S1 in the supplemental material). Overall, these results indicate that (i) in L. seeligeri grown at 37°C to stationary phase, prfA transcription originates predominantly from the P2prfA region, regardless of whether cells are grown with or without aeration, and (ii) in L. monocytogenes grown at 37°C to stationary phase, prfA transcription originates predominantly from the P2prfA region in cells grown without aeration, while originating predominantly from P1prfA in cells grown with aeration, suggesting that oxygen tension affects prfA transcription initiation in L. monocytogenes but not in L. seeligeri.
FIG. 2.
Mapping of the L. seeligeri and L. monocytogenes prfA transcriptional start sites through 5′ RACE PCR on RNA isolated from bacteria grown without aeration at 37°C. (A) Agarose gel electrophoresis of 5′ RACE PCR products generated using RNA from stationary-phase L. monocytogenes (lanes 2 to 4) and L. seeligeri (lanes 5 to 8) and prfA-specific primers. Lanes 1 and 9, DNA marker; lanes 2 and 5, PCR on untailed L. monocytogenes and L. seeligeri cDNA, respectively (included to identify unspecific PCR products which would show up in this reaction); lane 3, PCR on tailed L. monocytogenes cDNA; lanes 6 and 7, PCR on tailed L. seeligeri cDNA (run in duplicate); lanes 4 and 8, negative PCR controls (no template). Arrows mark PCR product that was excised for cloning and sequencing to determine transcriptional start sites; the weak larger product found in L. seeligeri RACE PCR did not map to any apparent promoter site. (B) DNA sequence of the prfA promoter region in L. monocytogenes (strain 10403S; GenBank accession no. NZ_AARZ00000000) and L. seeligeri (strain FSL S4-039; determined in this study). The first (5′) nt shown here is 154 and 162 nt upstream of the start codon for L. monocytogenes and L. seeligeri, respectively; the fragments used for complementation all start upstream of the first nt shown here. The L. monocytogenes P1- and P2prfA promoters, including the σA- and σB-dependent promoters, are indicated as previously reported (13, 14, 45); −10 and −35 sequences are marked in bold and underlined; the PrfA binding box (55) is marked by a broken line (—) beneath the sequence; homologous sequences in L. seeligeri are also indicated in the same way. Translational start sites (ATG for L. monocytogenes and GTG for L. seeligeri) are indicated in bold and underlined. Transcriptional start sites mapped by RACE PCR in L. seeligeri and L. monocytogenes grown without aeration are indicated by an asterisk (*) (for L. monocytogenes grown without aeration, the P2prfA start site was identified in all 8 RACE PCR clones sequenced; for L. seeligeri grown without aeration, the P2prfA start site was identified in 13 of 14 RACE PCR clones sequenced).
Sequence analysis revealed both a putative σB- and a putative σA-dependent promoter in the L. seeligeri P2prfA region; the putative L. seeligeri σB promoter differs from the L. monocytogenes P2prfA σB promoter by 1 nt in the −10 region and 2 nt in the −35 region (Fig. 2). The putative L. seeligeri P2prfA σA promoter differs from the L. monocytogenes P2prfA σA promoter by 1 nt in the −10 region and 1 nt in the −35 region (Fig. 2). The L. seeligeri region corresponding to the previously reported L. monocytogenes P1prfA promoter shows a putative σA-dependent promoter that differs from the L. monocytogenes P1prfA σA promoter by 1 nt in the −10 region and 2 nt in the −35 region. The putative PrfA box upstream of the L. monocytogenes P1prfA is also largely conserved between L. monocytogenes 10403S and L. seeligeri FSL S4-039; there is only a 1-nt difference in the 3′ end of the PrfA box between these two strains (Fig. 2). Diversification in the prfA promoter regions thus may contribute to differences in transcriptional regulation of prfA between L. monocytogenes 10403S and L. seeligeri FSL S4-039.
L. monocytogenes and L. seeligeri prfA promoter regions differ in their ability to activate transcription of prfALS in an L. monocytogenes ΔprfA strain.
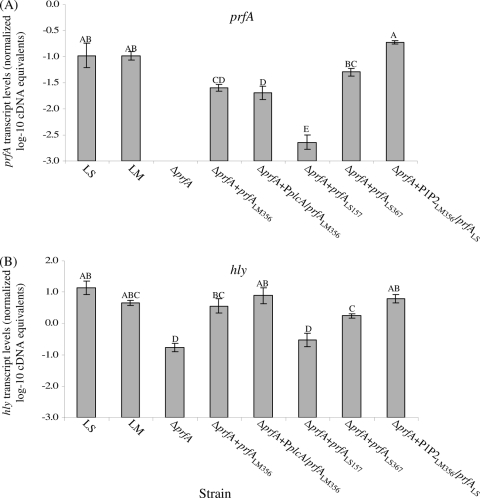
A series of constructs with prfALS and prfALM under the control of different L. monocytogenes and L. seeligeri prfA promoter regions was introduced into an L. monocytogenes 10403S ΔprfA strain (Table 3) to quantify the ability of L. monocytogenes and L. seeligeri prfA promoters to activate prfA transcription. In the control construct in which prfALM is preceded by a 356-nt L. monocytogenes promoter region containing P1- and P2prfA (ΔprfA prfALM356 strain [Table 3]), prfA transcript levels (as determined by qRT-PCR) were significantly lower (0.62 log) (Fig. 3) than prfA transcript levels in the L. monocytogenes wild-type strain. When prfALM was fused to a promoter construct containing P1- and P2prfA as well as the upstream PplcA promoter (which has been suggested to contribute to L. monocytogenes prfA transcription [5, 6, 36]), prfA transcript levels were also significantly lower (0.85 log) (Fig. 3) than prfA transcript levels in the L. monocytogenes wild-type strain. This finding is consistent with qRT-PCR data for a wild-type L. monocytogenes strain, which also indicated that transcription from the PplcA promoter has limited contributions to total prfA transcript levels (27). Overall, these data show that prfA transcription can be activated when prfA (with its native promoter) is inserted in trans in an L. monocytogenes ΔprfA strain, even though prfA transcript levels are slightly lower in the constructs with prfA in trans than in a strain with wild-type prfA.
FIG. 3.
Transcript levels for prfA (A) and hly (B) in L. monocytogenes 10403S (LM) and L. seeligeri FSL S4-039 (LS) wild-type strains, the L. monocytogenes ΔprfA strain, and the L. monocytogenes ΔprfA strain complemented with different pPL2 constructs carrying prfALM and prfALS under the control of different prfA promoters (strains are indicated on the x axis; strain designations are as detailed in Table 3 and in Materials and Methods). Transcript levels were determined by qRT-PCR and are expressed as log cDNA copy numbers/geometric mean of cDNA copy numbers for the housekeeping genes rpoB and gap (i.e., log10 target gene − [(log10 rpoB + log10 gap)/2]), indicated as “normalized log10 cDNA equivalents” on the y axis. All strains were grown at 37°C to stationary phase. Values shown represent the averages of qRT-PCR assays performed on three independent RNA collections; error bars show standard deviations of three independent biological replicates. One-way ANOVA comparison with Tukey-Kramer multiple comparison correction was used to determine whether the transcript levels differed among strains; statistical groupings are shown as letters above the transcript level bars; bars that do not share a letter represent significantly different transcript levels. One-way ANOVA for both prfA and hly transcript levels showed a highly significant effect of the factor strain on transcript levels (P < 0.0001, for prfA and hly).
In order to evaluate transcription of L. seeligeri prfA in an isogenic background, two pPL2 constructs, containing prfALS as well as 157 nt or 367 nt upstream of the prfALS start codon, were introduced into the L. monocytogenes ΔprfA strain (yielding the ΔprfA prfALS157 and ΔprfA prfALS367 strains, respectively); both of these constructs included the P1P2prfA promoters. Both the ΔprfA prfALS157 and ΔprfA prfALS367 strains showed lower prfA transcript levels than either wild-type L. seeligeri or L. monocytogenes grown to the same growth phase (i.e., stationary phase) (Fig. 3). Interestingly, the ΔprfA prfALS367 strain showed significantly higher prfA transcript levels than the ΔprfA prfALS157 strain. These findings suggest that regions upstream of the L. seeligeri P1P2prfA region contribute to transcriptional activation of prfALS.
To further investigate differences between the L. seeligeri and L. monocytogenes prfA promoters and their abilities to activate prfA transcription, we also introduced a fusion between the L. monocytogenes P1prfA-and-P2prfA promoter region and prfALS into the L. monocytogenes ΔprfA strain (yielding the ΔprfA P1P2LM356 prfALS strain). Interestingly, the ΔprfA P1P2LM356 prfALS strain showed prfA transcript levels that were significantly higher than those in the ΔprfA prfALS367 strain (Fig. 3) and not significantly different from the transcript levels for either the L. monocytogenes or L. seeligeri wild-type strain. These data suggest that, in an L. monocytogenes background, the P1P2LM region activates transcription of prfALS more effectively than the wild-type PprfALS region, further supporting that regulation of prfA transcription differs between L. seeligeri and L. monocytogenes. These observed differences cannot be due to differences in qRT-PCR amplification efficiencies for prfALS and prfALM (which were −3.48 and −3.33, respectively), as gene-specific primers and gene-specific standard curves were used to generate absolute cDNA levels for both genes (this procedure takes into account differences in amplification efficiencies).
L. monocytogenes strains with prfALS controlled by different upstream promoter regions differ in their ability to activate hly transcription and in their hemolytic capability.
To evaluate the ability of L. seeligeri and L. monocytogenes PrfA, generated from the different prfA constructs, to activate transcription of PrfA-dependent genes, qRT-PCR was used to measure transcript levels for the PrfA-dependent gene hly. The L. monocytogenes ΔprfA strain showed detectable hly transcript levels, suggesting some PrfA-independent hly transcription and consistent with previous reports that showed PrfA-independent hly transcription (10). Complementation of the ΔprfA strain with prfALM either under the control of the P1P2prfA promoter region (ΔprfA prfALM356 strain) or under the control of both the PplcA and P1P2prfA promoter regions (the ΔprfA PplcA prfALM356 strain) yielded strains that showed hly transcript levels not statistically different from the L. monocytogenes parent strain hly transcript levels (Fig. 3), even though both of these strains showed lower prfA transcript levels than the L. monocytogenes parent strain.
Consistent with the observation that prfALS transcript levels were lowest in the ΔprfA prfALS157 strain, higher in the ΔprfA prfALS367 strain, and even higher in L. monocytogenes with prfALS under the control of the P1P2LM promoter region (the ΔprfA P1P2LM356 prfALS strain) (Fig. 3), hlyLM transcript levels in these three strains followed the same trend. Therefore, higher levels of prfALS transcript correlate to higher levels of hlyLM, even though the relative changes in hlyLM transcript levels are smaller (compared to the changes in prfA transcript levels), most likely because hlyLM is also transcribed from a PrfA-independent promoter.
To further characterize the different prfA complementation mutants, we also performed semiquantitative hemolysis assays. While the L. monocytogenes ΔprfA strain showed no detectable cell-associated hemolysis, it showed low levels of supernatant-associated hemolysis (Table 5), further supporting some PrfA-independent hly transcription. Complementation of the ΔprfA strain with prfALM under the control of either the P1P2prfA promoter region (the ΔprfA prfALM356 strain) or under the control of both the PplcA and P1P2prfA promoter regions (the ΔprfA PplcA prfALM356 strain) yielded strains that showed cell- and supernatant-associated hemolysis similar to that of wild-type L. monocytogenes (Table 5), consistent with hly transcript-level data. Also consistent with the hly transcript-level data, hemolytic activities were lowest for the ΔprfA prfALS157 strain, higher for the ΔprfA prfALS367 strain, and highest for the ΔprfA P1P2LM356 prfALS strain (Table 5). Hemolysis levels for the ΔprfA P1P2LM356 prfALS strain were similar to hemolysis levels in L. monocytogenes 10403S (Table 5), further supporting that PrfALS can upregulate hlyLM.
TABLE 5.
Hemolytic activity of whole-cell suspension and supernatant fractions of L. monocytogenes and L. seeligeri strains
| Strain | Avg no. of hemolytic units for:a |
|
|---|---|---|
| Cell-associated fraction | Supernatant-associated fraction | |
| L. monocytogenes 10403S | 74.7 ± 33 | 37.3 ± 13 |
| L. seeligeri FSL S4-039 | 16.0 ± 0 | 2.0 ± 0 |
| 10403S ΔprfA prfALS157 | <0.5 ± 0 | 3.3 ± 1 |
| 10403S ΔprfA prfALS367 | 5.33 ± 2.3 | 5.3 ± 2 |
| 10403S ΔprfA P1P2LMprfALS | 85.3 ± 37 | 32.0 ± 0 |
| 10403S ΔprfA prfALM | 64.0 ± 0 | 26.7 ± 9 |
| 10403S ΔprfA PplcA prfALM | 85.3 ± 37 | 32.0 ± 0 |
| 10403S Δhly hlyLS | 64 ± 0 | <0.5 ± 0 |
| 10403S ΔplcA plcALM | 85.3 ± 37 | 42.7 ± 18 |
| 10403S ΔprfA | <0.5 ± 0 | 2.7 ± 1 |
| 10403S Δhly | <0.5 ± 0 | <0.5 ± 0 |
| 10403S ΔplcA | 64 ± 0 | 32.0 ± 18 |
Hemolytic unit is defined as the reciprocal of the dilution at which 50% lysis of sheep red blood cells occurred; data represent the average and standard deviations for 3 independent biological replicates.
Temperature-dependent patterns of prfA and hly transcription differ between L. seeligeri and L. monocytogenes.
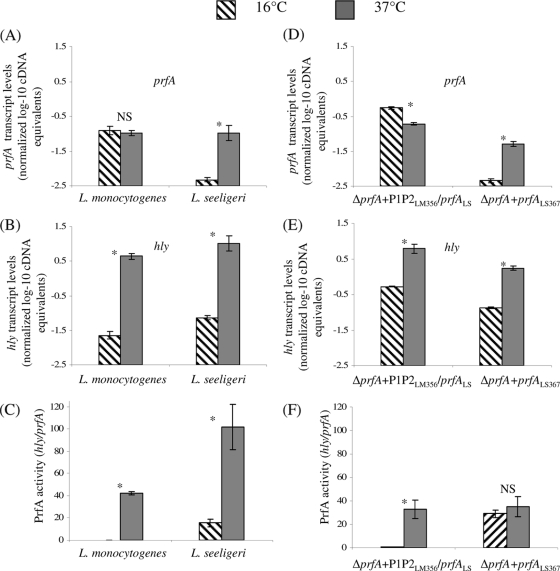
To probe temperature-dependent transcription of prfA and hly, transcript levels for these genes were initially determined, using qRT-PCR, in L. monocytogenes 10403S and L. seeligeri FSL S4-039, both grown to stationary phase at 16 or 37°C. While L. monocytogenes prfA transcript levels at 16 and 37°C were not different (P = 0.6494), hly transcript levels were 2.3 log higher in L. monocytogenes grown at 37°C than at 16°C (P = 0.0001) (Fig. 4). These observations are consistent with posttranscriptional upregulation of PrfA in L. monocytogenes grown at 37°C, leading to increased transcription of PrfA-dependent hly (23, 28). In L. seeligeri, prfA transcript levels were significantly higher in bacteria grown at 37°C than in those at 16°C (1.3 log difference; P = 0.0059) (Fig. 4A). L. seeligeri hly transcripts were also significantly higher in bacteria grown at 37°C than in those at 16°C (2.3 log difference; P = 0.0024) (Fig. 4B). To compare levels of active PrfA in L. monocytogenes and L. seeligeri grown at different temperatures, we also calculated PrfA activity, which we defined as the ratio of normalized hly transcript levels to normalized prfA transcript levels (Fig. 4C). These data showed that PrfA activity in L. monocytogenes grown at 37°C is approximately 42 times higher than that in L. monocytogenes grown at 16°C, while PrfA activity in L. seeligeri grown at 37°C was only about 7-fold higher than that in L. seeligeri grown at 16°C. L. seeligeri thus shows reduced temperature-dependent activation of PrfA, while showing greater absolute PrfA activity at both 16 and 37°C than L. monocytogenes at the same temperatures. While PrfA activity is higher at 37°C than at 16°C in both L. seeligeri and L. monocytogenes, only L. seeligeri shows temperature-dependent transcription of prfA (with higher prfA transcript levels for bacteria grown at 37°C than at 16°C), suggesting differences in temperature-dependent regulation of virulence gene expression between the L. monocytogenes and L. seeligeri strains tested here.
FIG. 4.
Normalized, log-transformed prfA (A) and hly (B) transcript levels and PrfA activity levels (C) in wild-type L. monocytogenes 10403S and L. seeligeri FSL S4-039, and normalized, log-transformed prfA (D) and hly (E) transcript levels and PrfA activity levels (F) in L. monocytogenes ΔprfA P1P2LM356 prfALS and ΔprfA prfALS367, all grown to stationary phase at both 16 and 37°C. Transcript levels were determined by qRT-PCR and are expressed as log cDNA copy numbers/geometric mean of cDNA copy numbers for the housekeeping genes rpoB and gap (i.e., log10 target gene − [(log10 rpoB + log10 gap)/2]; indicated as “normalized log-10 cDNA equivalents” on the y axis). Values shown represent the averages of qRT-PCR assays performed on three independent RNA collections; error bars show standard deviations of three independent biological replicates. “PrfA activity” is defined as the ratio of normalized hly transcript levels/prfA transcript levels, which indicates the activity of PrfA (measured as transcripts of the PrfA-dependent gene hly) normalized to the number of prfA transcripts; higher values thus indicate either enhanced translation or enhanced PrfA activity. Bars labeled with an asterisk (*) indicate transcript levels or PrfA activity levels that differed significantly between 16 and 37°C; NS indicates that no significant difference was found.
Temperature-dependent patterns of L. seeligeri and L. monocytogenes prfA and hly transcription are due to differences in the prfA promoter sequences in these two species.
To further explore temperature-dependent transcription of L. seeligeri and L. monocytogenes prfA and hly, transcript levels for these two genes were measured in two isogenic L. monocytogenes strains transcribing prfALS under the control of either (i) the L. seeligeri prfA promoter region (the ΔprfA prfALS367 strain) or (ii) the L. monocytogenes prfA promoter region (the ΔprfA P1P2LM356 prfALS strain); transcript levels were measured in these strains grown to stationary phase at 16°C or 37°C (without aeration). Overall, prfA and hly transcript patterns in these isogenic mutants were similar to transcript patterns in the corresponding wild-type strains that served as the source of the promoter in these constructs, e.g., transcript patterns for the strain transcribing prfALM under the control of the L. seeligeri prfA promoter (Fig. 4D) were similar to the transcript patterns in the L. seeligeri wild-type strain (Fig. 4A). prfA transcript levels were lower at 37°C than at 16°C (0.45 log difference; P < 0.0001, two-sided t test) in the strain transcribing prfALS from the L. monocytogenes prfA promoter. In the strain transcribing prfALS from the L. seeligeri prfA promoter, prfA transcript levels were higher at 37°C than at 16°C (1.05 log difference; P < 0.0001, two-sided t test), further supporting that the L. seeligeri prfA promoter may have evolved to facilitate temperature-dependent transcription of prfA. For both strains, hly transcript levels were significantly higher in bacteria grown at 37°C than in those grown at 16°C (Fig. 4E).
For the strain transcribing prfALS from the L. monocytogenes prfA promoter, PrfA activity was significantly higher for bacteria grown at 37°C than for those grown at 16°C (Fig. 4F), similar to the trend seen with the L. monocytogenes wild-type strain (Fig. 4C and F). These findings are consistent with previously reported posttranscriptional activation of PrfA in L. monocytogenes, i.e., through increased translation of the prfA transcript at 37°C compared to <30°C, due to temperature-dependent secondary structures in the region upstream of the start codon (23, 28). In the L. monocytogenes strain transcribing prfALS from the L. seeligeri prfA promoter, there was no significant temperature-dependent PrfA activation, even though temperature dependence of PrfA activity was observed with the L. seeligeri wild-type strain, suggesting that the temperature-dependent activation of PrfA in L. seeligeri may depend on genetic elements outside the prfA fragment introduced into L. monocytogenes (e.g., noncoding RNAs).
prfALS must be transcribed from the L. monocytogenes prfA promoter region to fully complement an L. monocytogenes ΔprfA strain in an intracellular growth assay in activated J774 cells.
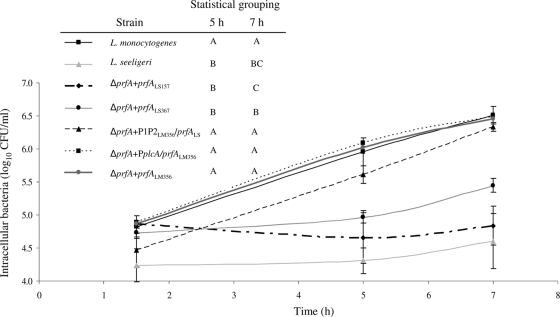
To investigate the ability of L. seeligeri prfA to regulate transcription of virulence genes during intracellular infection, we evaluated the ability of the different prfA complementation mutants (Table 3) to grow intracellularly in activated J774 cells. While the L. monocytogenes parent strain was clearly able to grow intracellularly (Fig. 5), consistent with previous reports (17, 25), the L. seeligeri parent strain showed no significant growth over time (Fig. 6), also consistent with previous data (25). These findings are also consistent with a previous study that showed a lack of proliferation of L. seeligeri in another macrophage cell line (9). The L. monocytogenes ΔprfA strains expressing prfALM under the control of the P1prfA-and-P2prfA promoter region (the ΔprfA prfALM356 strain) or under the control of the P1P2prfA region and the upstream plcA promoter (the ΔprfA PplcA prfALM strain) showed intracellular growth at 5 and 7 h that did not differ significantly from intracellular growth of the L. monocytogenes parent strain (Fig. 5).
FIG. 5.
Intracellular growth, in activated J774 cells, of L. monocytogenes, L. seeligeri, and L. monocytogenes isogenic mutants expressing prfALS and prfALM from different promoters (see Table 3 for strain designations). The graph shows intracellular bacterial numbers (log10 CFU/ml) at 1.5 h, 5 h, and 7 h postinfection; values shown represent the averages of three independent biological replicates; error bars show standard deviations of these replicates. These data were also used to calculate intracellular growth between (i) 1.5 and 5 h (i.e., [CFU/ml at 5 h] − [CFU/ml at 1.5 h]) and (ii) 1.5 and 7 h, which were used for statistical analyses; statistical analyses were performed on intracellular growth data rather than absolute intracellular bacterial numbers at different time points, as there was a significant variation between intracellular bacterial numbers for the different strains at 1.5 h p.i. Results from the statistical analyses (one-way ANOVA comparison with Tukey-Kramer multiple-comparison correction) of growth between 1.5 and 5 h, and 1.5 and 7 h are shown in the inserted table; strains that do not share a letter (e.g., “A”) for a given time point show a significant difference in their intracellular growth levels between 1.5 and 5 h or 1.5 and 7 h.
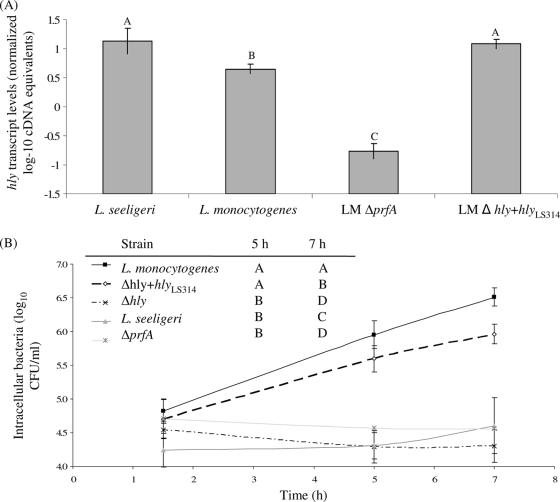
FIG. 6.
hly transcript levels (A) and intracellular growth (B) in activated J774 cells of L. monocytogenes, L. seeligeri, and L. monocytogenes Δhly hlyLS314. hly transcript levels were determined using qRT-PCR on RNA extracted from bacteria grown at 37°C to stationary phase; transcript levels are expressed as log cDNA copy numbers/geometric mean of cDNA copy numbers for the housekeeping genes rpoB and gap (i.e., log10 target gene − [(log10 rpoB + log10 gap)/2], indicated as “normalized log-10 cDNA equivalents” on the y axis. Values shown represent the averages of qRT-PCR assays performed on three independent RNA collections; error bars show standard deviations of these replicates. Transcript levels and intracellular growth for L. monocytogenes ΔprfA grown under the same conditions are included as a control (these data are also shown in Fig. 4). One-way ANOVA for hly transcript levels showed a significant effect of the factor strain on transcript levels (P < 0.0001). Statistical groupings are shown as letters above the transcript level bars. Results from the statistical analyses (one-way ANOVA comparison with Tukey-Kramer multiple-comparison correction) of growth between 1.5 and 5 h, and 1.5 and 7 h are shown in the inserted table; strains that do not share a letter (e.g., “A”) for a given time point show a significant difference in their intracellular growth levels (e.g., between 1.5 and 5 h or 1.5 and 7 h).
The two L. monocytogenes strains expressing prfALS under the control of L. seeligeri upstream promoter regions of different lengths (i.e., the ΔprfA prfALS157 and ΔprfA prfALS367 strains) both had significantly reduced intracellular growth compared to the L. monocytogenes parent strain. Growth of the ΔprfA prfALS157 strain was not significantly different from the intracellular growth of the L. monocytogenes ΔprfA strain, while the ΔprfA prfALS367 strain had significantly higher levels of intracellular growth at 5 and 7 h postinfection than the ΔprfA strain, indicating partial complementation (Fig. 5). The ΔprfA P1P2LM356 prfALS strain, which expresses prfALS under the control of the L. monocytogenes P1P2prfA region, showed intracellular growth that was not significantly different from that of the L. monocytogenes parent strain (Fig. 5), suggesting that PrfALS, when transcribed from the L. monocytogenes prfA promoter, can appropriately regulate L. monocytogenes virulence genes essential for intracellular growth. Overall, our data suggest that the L. seeligeri prfA promoter region does not allow for appropriate regulation of prfA transcription during intracellular growth of L. monocytogenes.
While hlyLS is expressed and retains hemolytic capability when introduced into an L. monocytogenes Δhly strain, HlyLS and HlyLM differ in their associations with cell and supernatant fractions.
In order to evaluate the functional conservation of L. monocytogenes and L. seeligeri hemolysins, hlyLS (under the control of its native L. seeligeri promoter region) was introduced into an L. monocytogenes 10403S Δhly strain, resulting in the Δhly hlyLS314 strain. hlyLS transcript levels in Δhly hlyLS314 strain cells grown at 37°C to stationary phase were not significantly different from hlyLS transcript levels found in L. seeligeri but were significantly higher than hlyLM transcript levels found in L. monocytogenes 10403S grown under the same conditions (Fig. 6). These results suggest that PrfALM can activate transcription of hlyLS utilizing its native upstream PrfA box, consistent with previous in vitro studies (33).
Cell-associated hemolytic activity in the Δhly hlyLS314 strain (64 hemolytic units [HU]) was similar to that in the L. monocytogenes parent strain (74.7 HU) but considerably higher than cell-associated hemolytic activity observed for the L. seeligeri parent strain (16.0 HU [Table 5]). The supernatant-associated hemolytic activities for the Δhly hlyLS314 strain (<0.5 HU) and the L. seeligeri parent strain (2.0 HU) were considerably lower than the supernatant-associated hemolytic activity for the L. monocytogenes parent strain (37.3 HU) (Table 5). The L. monocytogenes parent strain showed similar levels of cell- and supernatant-associated hemolysis (74.3 and 37.3 HU, respectively [Table 5]; HU are expressed as a reciprocal of the serial dilution for which 50% hemolysis was observed, with 32 and 64 being sequential dilutions), while both the L. seeligeri parent strain and the L. monocytogenes strain expressing hlyLS showed considerably higher cell-associated hemolytic activities than supernatant-associated hemolytic activities (Table 5). These data suggest differences in cell association between L. seeligeri and L. monocytogenes Hly.
To further evaluate the functional conservation of L. monocytogenes and L. seeligeri Hly, intracellular growth assays with the L. monocytogenes Δhly hlyLS314 strain were also performed (Fig. 6). While the L. monocytogenes Δhly strain showed no intracellular growth, the L. monocytogenes Δhly hlyLS314 strain showed considerable intracellular growth that is significantly higher than growth of either the L. monocytogenes Δhly or ΔprfA strain or L. seeligeri (Fig. 6). While the intracellular growth pattern of the Δhly hlyLS314 strain was similar to the growth pattern for the L. monocytogenes parent strain, overall growth at 5 h and 7 h postinfection was numerically slightly lower for the Δhly hlyLS314 strain compared to the L. monocytogenes parent strain (Fig. 6); the difference in growth at 7 h p.i. was statistically significant. These findings suggest that hlyLS can largely, but not completely, complement a ΔhlyLM mutant. Partial complementation may be related to the observation that L. seeligeri Hly shows lower supernatant-associated hemolytic activities than L. monocytogenes Hly.
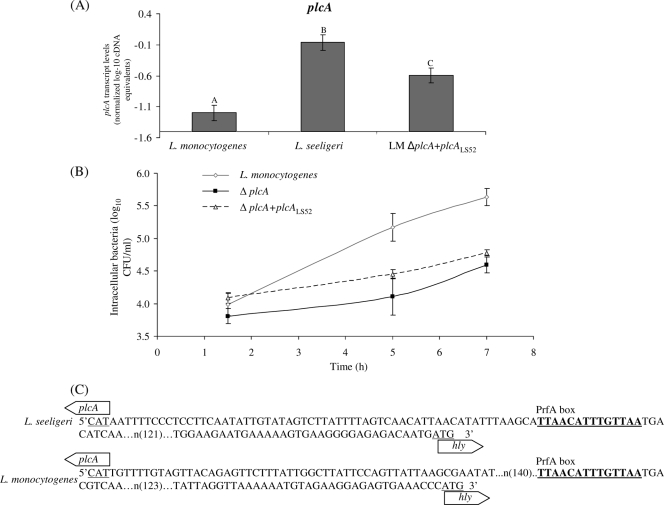
plcALS cannot complement an L. monocytogenes 10403S ΔplcA strain.
To evaluate the conservation of additional L. seeligeri virulence genes, plcALS (with its native promoter) was introduced into an L. monocytogenes ΔplcA strain, yielding the ΔplcA plcALS52 strain (Table 3). qRT-PCR analysis revealed slightly but significantly lower plcALS transcript levels in the ΔplcA plcALS52 strain (grown at 37°C to stationary phase) than in the L. seeligeri parent strain; these transcript levels were higher than the plcA transcript levels in the L. monocytogenes parent strain (Fig. 7). Evaluation of the ΔplcA plcALS52 strain in an intracellular growth assay in primary mouse macrophage cells showed an intracellular growth pattern that did not differ significantly from that of the 10403S ΔplcA strain (Fig. 7). These data suggest that plcALS cannot functionally complement a plcALM deletion, although it cannot be excluded that complementation could occur if plcALS were transcribed at higher levels in L. monocytogenes.
FIG. 7.
plcA transcript levels (A) and intracellular growth (B) in primary bone marrow-derived mouse macrophage cells of L. monocytogenes, L. monocytogenes ΔplcA, and L. monocytogenes ΔplcA plcALS52 grown at 37°C. (C) DNA sequence of the intergenic region between plcA and hly in L. seeligeri and L. monocytogenes. plcA transcript levels were determined using qRT-PCR on RNA from bacteria grown at 37°C to stationary phase; transcript levels are expressed as log cDNA copy numbers/geometric mean of cDNA copy numbers for the housekeeping genes rpoB and gap (i.e., log10 target gene − [(log10 rpoB + log10 gap)/2], indicated as “normalized log-10 cDNA equivalents” on the y axis). Values shown represent the averages of qRT-PCR assays performed on three independent RNA collections; error bars show standard deviations of these replicates. One-way ANOVA for plcA transcript levels showed a significant effect of the factor strain on transcript levels (P < 0.0001). Statistical groupings are shown as letters above the transcript level bars.
DISCUSSION
Comparative sequence analyses of L. seeligeri prfA, hly, and plcA and heterologous complementation studies of L. monocytogenes revealed that (i) the virulence genes prfA, hly, and plcA are conserved among L. seeligeri isolates but are distinct from the virulence gene homologues in L. monocytogenes, (ii) while regulation of prfA transcription has diversified between L. monocytogenes and L. seeligeri, prfA and hly transcript levels are higher in L. seeligeri grown at 37°C (than in those grown at 16°C), suggesting a function of L. seeligeri virulence genes in warm-blooded hosts, and (iii) PrfA and Hly functions are largely, but not fully, conserved between L. seeligeri and L. monocytogenes. Overall, our findings suggest that while prfA and hly have diversified in L. seeligeri, including in their transcriptional regulation, they have maintained similar functions, possibly involving pathogenic or commensal interactions with warm-blooded hosts.
The virulence genes prfA, hly, and plcA are conserved among L. seeligeri isolates but are distinct from the virulence gene homologues in L. monocytogenes.
While previous studies have shown that some L. seeligeri strains lack the Listeria prfA virulence gene cluster (63), our data show that prfA, hly, and plcA are fairly conserved among the L. seeligeri strains that contain this virulence gene cluster, providing initial evidence that these genes are not pseudogenes and/or that these genes are not under neutral selection. Interestingly, patterns of conservation of the protein sequences encoded by these three genes were very similar between L. monocytogenes and L. seeligeri, with PrfA being the most conserved protein, followed by Hly, and PlcA (which represented the most diverse protein among both the L. seeligeri sequences analyzed here and L. monocytogenes sequences analyzed previously [41]). These findings are also consistent with functional studies, discussed in more detail below, which show that L. seeligeri prfA and hly can largely complement the L. monocytogenes ΔprfA and Δhly null mutants, supporting considerable functional conservation of PrfA and Hly.
While considerable diversification of the overall Listeria prfA virulence gene cluster and the specific coding regions within this cluster has previously been reported (e.g., reference 25), no formal evolutionary analyses of the selection of virulence gene homologues during the divergence of L. seeligeri from other Listeria species have previously been reported. Our analyses showed significant evidence for positive selection in hlyLS. Two HlyLS amino acid residues with evidence for positive selection (aa 67 and 117) are located, respectively, within putative domains 1 and 2 (50, 53), which have no clearly assigned functions. The N-terminal region of Hly, which shows the least conservation between L. seeligeri and L. monocytogenes, contains the other two amino acid residues with evidence for positive selection; aa 5 falls into the N-terminal secretion signal sequence, while aa 39 falls within a PEST-like region (32, 52), a region hypothesized to be important in LLO synthesis during cytosolic growth of L. monocytogenes (52, 53). Overall, our evolutionary analyses, combined with the finding that a smaller fraction of HlyLS (than of HlyLM) is secreted, suggest some functional differences between the L. seeligeri and L. monocytogenes Hly proteins and a possible functional adaptation of HlyLS to a distinct niche.
While regulation of prfA transcription has diversified between L. monocytogenes and L. seeligeri, prfA and hly transcription in L. seeligeri is higher at 37°C, suggesting a function of L. seeligeri virulence genes in warm-blooded hosts or additional environments.
Our data provide clear evidence that regulation of prfA has diversified between L. seeligeri and L. monocytogenes, including (i) that prfALS transcript levels (in an L. monocytogenes genetic background) when prfALS is transcribed from an L. monocytogenes prfA promoter are higher than those when prfALS is transcribed from the native L. seeligeri promoter, (ii) temperature-dependent prfA transcription in L. seeligeri but not L. monocytogenes, (iii) differential utilization of the P1- and P2prfA promoters depending on aeration in L. monocytogenes but not in L. seeligeri, and (iv) considerably more-pronounced temperature-dependent apparent PrfA activity in L. monocytogenes than in L. seeligeri. The observation that prfA transcript levels are higher in L. monocytogenes 10403S carrying prfALS under the P1P2LM region than in the strain carrying prfALM under the same promoter also suggests the higher transcript stability of prfALS (i.e., slower transcript turnover) than of prfALM. While it has been well established that L. monocytogenes prfA transcript originating from the P1prfA promoter includes a thermosensor that allows for temperature-dependent prfA translation and facilitates induction of translation at temperatures >30°C (23), no evidence for temperature-dependent expression of virulence genes has previously been reported for L. seeligeri. Interestingly, the mechanism of temperature-dependent virulence gene expression in L. seeligeri includes increased transcription of prfA itself at 37°C, which is not observed with L. monocytogenes. L. seeligeri also appears to have a mechanism for posttranscriptional temperature-dependent regulation of PrfA activity (mediated by either increased translation or activation of the translated PrfA), even though posttranscriptional temperature-dependent regulation of PrfA activity in L. seeligeri is less pronounced than in L. monocytogenes. As temperature changes can act as a signal for transition into a mammalian or avian host environment (13, 14, 23), our findings, overall, suggest that L. seeligeri virulence genes may play a role in facilitating interactions with a warm-blooded host.
Differences in regulation of prfA transcription between L. monocytogenes and L. seeligeri suggest that L. seeligeri may have adapted to interact with hosts other than those infected by L. monocytogenes. Furthermore, the observed differences in prfA transcriptional initiation between L. monocytogenes grown with and without aeration may be related to the enhanced virulence of L. monocytogenes grown in microaerophilic growth conditions (2), possibly as prfA transcription originates predominantly from P2prfA in cells grown without aeration, with this promoter allowing for temperature-independent translation of prfA transcript. The critical nature of differences in prfA transcription between L. monocytogenes and L. seeligeri is also supported by the observation that introduction of L. monocytogenes prfA (including the upstream plcA promoter) into L. seeligeri facilitated LSO synthesis and escape from the phagosome, a phenotype that was not observed with wild-type L. seeligeri (25).
PrfA and Hly functions are largely, but not fully, conserved between L. seeligeri and L. monocytogenes.
Our data showed that expression of prfALS from the L. monocytogenes prfA promoter was able to functionally complement an L. monocytogenes ΔprfA mutant. These findings suggest that PrfALS can interact with L. monocytogenes PrfA boxes and upregulate L. monocytogenes virulence genes needed for intracellular growth and survival. Interestingly, Mauder et al. (33) found, using in vitro transcription assays, that PrfALS has decreased binding affinity (compared to PrfALM) to homologous and heterologous PrfA boxes and that chromosomal replacement of prfALM by prfALS (also placing prfALS under the control of the L. monocytogenes prfA promoters) only partially restores hemolytic activity and virulence in a mouse model. Apparent differences in our findings may possibly be explained by the use of different prfALS genes in our studies (while the full prfA sequence used by Mauder et al. [33] is not available in GenBank, evaluation of primer sequences used for mutant construction suggests at least 2 aa differences between the prfALS alleles used here and by Mauder et al. [33]). It is important to note, though, that in our studies, prfALS transcribed from the L. monocytogenes promoters showed transcript levels numerically (but not statistically significantly) higher than prfA transcript levels in the L. monocytogenes wild-type strain, which could possibly help to overcome a reduced promoter affinity of PrfALS. Our L. monocytogenes strain transcribing prfALS from an L. monocytogenes promoter also showed numerically (but not significantly) reduced intracellular numbers compared to those of the L. monocytogenes parent strain in the intracellular growth assay. Combined with our data that suggest positive selection of at least one PrfA residue in L. seeligeri and the observation that the L. monocytogenes and L. seeligeri PrfA proteins differ by 63 aa residues, it thus appears likely that PrfALS shows some functional differences from PrfALM, including somewhat reduced affinity to PrfA promoters.
Complementation of an L. monocytogenes Δhly strain with hlyLS (under the control of its native L. seeligeri promoter) indicated effective recognition of the L. seeligeri hly promoter by PrfALM, consistent with previous in vitro transcription (33) and L. seeligeri complementation studies (25). However, expression of HlyLS in L. monocytogenes showed a distinct pattern of cell- and supernatant-associated hemolysis, including reduced supernatant-associated hemolysis activity compared to that of HlyLM, possibly due to diversification of the signal peptide sequence. Combined with clear evidence for positive selection in hlyLS, our functional data suggest that HlyLS has diverged functionally from HlyLM, while maintaining the ability to lyse the host cell vacuole and interact with mammalian host cells. Some level of functional conservation of Hly is supported by other studies that showed that HlyLS could facilitate vacuolar escape in L. seeligeri if hlyLS transcription is activated (25) and that HlyLS and HlyLM have identical abilities to induce gamma interferon in mouse spleen cells (22).
Conclusions.
Combined with previous studies (e.g., references 25 and 33), our data suggest that virulence differences between L. seeligeri and L. monocytogenes do not simply reflect a loss of function in the L. seeligeri pVGC genes but rather reflect adaptation of L. seeligeri to a specific niche and/or hosts. This adaptation appears to have occurred at multiple levels, including gene loss or acquisition and diversification of virulence gene regulation as well as allelic variation and diversification of effector protein functions (e.g., hemolysin). Interestingly, the L. seeligeri strain tested not only showed higher virulence gene expression when grown at temperatures typical of mammalian and avian hosts but also showed a mechanism for temperature-dependent regulation (i.e., temperature-dependent regulation of prfA transcription) different than that for L. monocytogenes. While it has been suggested that maintenance of virulence genes in environmental microorganisms in general (54, 56, 68) and in L. seeligeri in particular may occur as a result of interaction with nonmammalian hosts (e.g., amoebae, nematodes, and insects), our data suggest that the pVGC virulence genes in L. seeligeri have adapted to function in warm-blooded hosts. Additional experiments that characterize regulation of virulence gene expression during temperature shifts will be needed, though, to provide further insight into virulence gene regulation in L. seeligeri. Confirmation of our findings with other L. seeligeri strains will also be necessary to ensure that these results are not strain specific, but pertain to L. seeligeri as a species.
Supplementary Material
Acknowledgments
We thank Laura Goodman for initial sequencing of selected L. seeligeri virulence genes and Robin Yates of the David Russell lab at Cornell for help with the primary mouse macrophage work.
This project was supported by USDA Special Research grants (2003-34459-12999, 2004-34459-14296, and 2005-34459-15625).
Footnotes
Published ahead of print on 11 June 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Alouf, J. E. 2001. Pore-forming bacterial protein toxins: an overview. Curr. Top. Microbiol. Immunol. 257:1-14. [PubMed] [Google Scholar]
- 2.Andersen, J. B., B. B. Roldgaard, B. B. Christensen, and T. R. Licht. 2007. Oxygen restriction increases the infective potential of Listeria monocytogenes in vitro in Caco-2 cells and in vivo in guinea pigs. BMC Microbiol. 7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 4.Bishop, D. K., and D. J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005-2009. [PubMed] [Google Scholar]
- 5.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty, T., M. Leimeister-Wachter, E. Domann, M. Hartl, W. Goebel, T. Nichterlein, and S. Notermans. 1992. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J. Bacteriol. 174:568-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturongakul, S., and K. J. Boor. 2006. SigmaB activation under environmental and energy stress conditions in Listeria monocytogenes. Appl. Environ. Microbiol. 72:5197-5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conte, M. P., G. Petrone, A. M. Di Biase, M. G. Ammendolia, F. Superti, and L. Seganti. 2000. Acid tolerance in Listeria monocytogenes influences invasiveness of enterocyte-like cells and macrophage-like cells. Microb. Pathog. 29:137-144. [DOI] [PubMed] [Google Scholar]
- 9.Dallas, H. L., D. P. Thomas, and A. D. Hitchins. 1996. Virulence of Listeria monocytogenes, Listeria seeligeri, and Listeria innocua assayed with in vitro murine macrophagocytosis. J. Food Prot. 59:24-27. [DOI] [PubMed] [Google Scholar]
- 10.Domann, E., J. Wehland, K. Niebuhr, C. Haffner, M. Leimeister-Wachter, and T. Chakraborty. 1993. Detection of a prfA-independent promoter responsible for listeriolysin gene expression in mutant Listeria monocytogenes strains lacking the PrfA regulator. Infect. Immun. 61:3073-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doumith, M., C. Cazalet, N. Simoes, L. Frangeul, C. Jacquet, F. Kunst, P. Martin, P. Cossart, P. Glaser, and C. Buchrieser. 2004. New aspects regarding evolution and virulence of Listeria monocytogenes revealed by comparative genomics and DNA arrays. Infect. Immun. 72:1072-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ermolaeva, S., S. Novella, Y. Vega, M. T. Ripio, M. Scortti, and J. A. Vazquez-Boland. 2004. Negative control of Listeria monocytogenes virulence genes by a diffusible autorepressor. Mol. Microbiol. 52:601-611. [DOI] [PubMed] [Google Scholar]
- 13.Freitag, N. E., and D. A. Portnoy. 1994. Dual promoters of the Listeria monocytogenes prfA transcriptional activator appear essential in vitro but are redundant in vivo. Mol. Microbiol. 12:845-853. [DOI] [PubMed] [Google Scholar]
- 14.Freitag, N. E., L. Rong, and D. A. Portnoy. 1993. Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect. Immun. 61:2537-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaillard, J. L., P. Berche, J. Mounier, S. Richard, and P. Sansonetti. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gellin, B. G., and C. V. Broome. 1989. Listeriosis. JAMA 261:1313-1320. [PubMed] [Google Scholar]
- 17.Gouin, E., J. Mengaud, and P. Cossart. 1994. The virulence gene cluster of Listeria monocytogenes is also present in Listeria ivanovii, an animal pathogen, and Listeria seeligeri, a nonpathogenic species. Infect. Immun. 62:3550-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haas, A., M. Dumbsky, and J. Kreft. 1992. Listeriolysin genes: complete sequence of ilo from Listeria ivanovii and of lso from Listeria seeligeri. Biochim. Biophys. Acta 1130:81-84. [DOI] [PubMed] [Google Scholar]
- 19.Hain, T., C. Steinweg, and T. Chakraborty. 2006. Comparative and functional genomics of Listeria spp. J. Biotechnol. 126:37-51. [DOI] [PubMed] [Google Scholar]
- 20.Hanna, S. E., and H. H. Wang. 2006. Assessment of environmental factors on Listeria monocytogenes Scott A inlA gene expression by relative quantitative Taqman real-time reverse transcriptase PCR. J. Food Prot. 69:2754-2757. [DOI] [PubMed] [Google Scholar]
- 21.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 8:528-535. [PubMed] [Google Scholar]
- 22.Ito, Y., I. Kawamura, C. Kohda, H. Baba, T. Nomura, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2003. Seeligeriolysin O, a cholesterol-dependent cytolysin of Listeria seeligeri, induces gamma interferon from spleen cells of mice. Infect. Immun. 71:234-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson, J., P. Mandin, A. Renzoni, C. Chiaruttini, M. Springer, and P. Cossart. 2002. An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell 110:551-561. [DOI] [PubMed] [Google Scholar]
- 24.Jones, S., and Portnoy, D. A. 1994. Small plaque mutants. Methods Enzymol. 236:526-531. [DOI] [PubMed] [Google Scholar]
- 25.Karunasagar, I., R. Lampidis, W. Goebel, and J. Kreft. 1997. Complementation of Listeria seeligeri with the plcA-prfA genes from L. monocytogenes activates transcription of seeligerolysin and leads to bacterial escape from the phagosome of infected mammalian cells. FEMS Microbiol. Lett. 146:303-310. [DOI] [PubMed] [Google Scholar]
- 26.Kazmierczak, M. J., S. C. Mithoe, K. J. Boor, and M. Wiedmann. 2003. Listeria monocytogenes sigma B regulates stress response and virulence functions. J. Bacteriol. 185:5722-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazmierczak, M. J., M. Wiedmann, and K. J. Boor. 2006. Contributions of Listeria monocytogenes sigma B and PrfA to expression of virulence and stress response genes during extra- and intracellular growth. Microbiology 152:1827-1838. [DOI] [PubMed] [Google Scholar]
- 28.Kreft, J., and J. A. Vazquez-Boland. 2001. Regulation of virulence genes in Listeria. Int. J. Med. Microbiol. 291:145-157. [DOI] [PubMed] [Google Scholar]
- 29.Kreft, J., J. A. Vazquez-Boland, S. Altrock, G. Dominguez-Bernal, and W. Goebel. 2002. Pathogenicity islands and other virulence elements in Listeria, p. 109-125. In J. Hacker and J. B. Kaper (ed.), Pathogenicity islands and the evolution of pathogenic microbes, vol. 2. Springer, New York, NY. [PubMed] [Google Scholar]
- 30.Lampidis, R., R. Gross, Z. Sokolovic, W. Goebel, and J. Kreft. 1994. The virulence regulator protein of Listeria ivanovii is highly homologous to PrfA from Listeria monocytogenes and both belong to the Crp-Fnr family of transcription regulators. Mol. Microbiol. 13:141-151. [DOI] [PubMed] [Google Scholar]
- 31.Lauer, P., M. Y. Chow, M. J. Loessner, D. A. Portnoy, and R. Calendar. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 184:4177-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lety, M. A., C. Frehel, I. Dubail, J. L. Beretti, S. Kayal, P. Berche, and A. Charbit. 2001. Identification of a PEST-like motif in listeriolysin O required for phagosomal escape and for virulence in Listeria monocytogenes. Mol. Microbiol. 39:1124-1139. [DOI] [PubMed] [Google Scholar]
- 33.Mauder, N., R. S. Ecke, D. I. Mertins, G. Loeffler, M. Seidel, W. Sprehe, W. Hillen, W. Goebel, and S. Muller-Altrock. 2006. Species-specific differences in the activity of PrfA, the key regulator of listerial virulence genes. J. Bacteriol. 22:7941-7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGann, P., R. Ivanek, M. Wiedmann, and K. J. Boor. 2007. Temperature-dependent expression of Listeria monocytogenes internalin and internalin-like genes suggests functional diversity of these proteins among the listeriae. Appl. Environ. Microbiol. 73:2806-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLauchlin, J. 1997. Listeria and listeriosis. Clin. Microbiol. Infect. 3:484-492. [DOI] [PubMed] [Google Scholar]
- 36.Mengaud, J., M. F. Vicente, J. Chenevert, J. M. Pereira, C. Geoffroy, B. Gicquel-Sanzey, F. Baquero, J. C. Perez-Diaz, and P. Cossart. 1988. Expression in Escherichia coli and sequence analysis of the listeriolysin O determinant of Listeria monocytogenes. Infect. Immun. 56:766-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milohanic, E., P. Glaser, J. Y. Coppee, L. Frangeul, Y. Vega, J. A. Vazquez-Boland, F. Kunst, P. Cossart, and C. Buchrieser. 2003. Transcriptome analysis of Listeria monocytogenes identifies three groups of genes differently regulated by PrfA. Mol. Microbiol. 47:1613-1625. [DOI] [PubMed] [Google Scholar]
- 38.Nei, M., and S. Kumar. 2000. Molecular evolution and phylogenetics, Oxford University Press, New York, NY.
- 39.Nelson, K. E., D. E. Fouts, E. F. Mongodin, J. Ravel, R. T. DeBoy, J. F. Kolonay, D. A. Rasko, S. V. Angiuoli, S. R. Gill, I. T. Paulsen, J. Peterson, O. White, W. C. Nelson, W. Nierman, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, R. J. Dodson, A. S. Durkin, R. Madupu, D. H. Haft, J. Selengut, S. Van Aken, H. Khouri, N. Fedorova, H. Forberger, B. Tran, S. Kathariou, L. D. Wonderling, G. A. Uhlich, D. O. Bayles, J. B. Luchansky, and C. M. Fraser. 2004. Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res. 32:2386-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ollinger, J., B. Bowen, M. Wiedmann, K. J. Boor, and T. M. Bergholz. 2009. Listeria monocytogenes σB modulates PrfA-mediated virulence factor expression. Infect. Immun. 77:2113-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orsi, R. H., S. B. Maron, K. K. Nightingale, M. Jerome, H. Tabor, and M. Wiedmann. 2008. Lineage specific recombination and positive selection in coding and intragenic regions contributed to evolution of the main Listeria monocytogenes virulence gene cluster. Infect. Genet. Evol. 8:566-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orsi, R. H., Q. Sun, and M. Wiedmann. 2008. Genome-wide analyses reveal lineage specific contributions of positive selection and recombination to the evolution of Listeria monocytogenes. BMC Evol. Biol. 8:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pethe, K., D. L. Swenson, S. Alonso, J. Anderson, C. Wang, and D. G. Russell. 2004. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc. Natl. Acad. Sci. U. S. A. 101:13642-13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rauch, M., Q. Luo, S. Muller-Altrock, and W. Goebel. 2005. σB-dependent in vitro transcription of prfA and some newly identified genes of Listeria monocytogenes whose expression is affected by PrfA in vivo. J. Bacteriol. 187:800-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ripio, M. T., G. Dominguez-Bernal, M. Suarez, K. Brehm, P. Berche, and J. A. Vazquez-Boland. 1996. Transcriptional activation of virulence genes in wild-type strains of Listeria monocytogenes in response to a change in the extracellular medium composition. Res. Microbiol. 147:371-384. [DOI] [PubMed] [Google Scholar]
- 47.Rocourt, J., and P. A. D. Grimont. 1983. Listeria welshimeri sp. nov. and Listeria seeligeri sp. nov. Int. J. Syst. Bacteriol. 33:866-869. [Google Scholar]
- 48.Rocourt, J., H. Hof, A. Schrettenbrunner, R. Malinverni, and J. Bille. 1986. Acute purulent Listeria seeligeri meningitis in an immunocompetent adult. Schweiz. Med. Wochenschr. 116:248-251. [PubMed] [Google Scholar]
- 49.Rogers, S., R. Wells, and M. Rechsteiner. 1986. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science 234:364-368. [DOI] [PubMed] [Google Scholar]
- 50.Rossjohn, J., S. C. Feil, W. J. McKinstry, R. K. Tweten, and M. W. Parker. 1997. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell 89:685-692. [DOI] [PubMed] [Google Scholar]
- 51.Sauders, B. 2005. Molecular epidemiology, diversity, distribution, and ecology of Listeria. Ph.D. dissertation. Cornell University, Ithaca, NY.
- 52.Schnupf, P., D. A. Portnoy, and A. L. Decatur. 2006. Phosphorylation, ubiquitination and degradation of listeriolysin O in mammalian cells: role of the PEST-like sequence. Cell. Microbiol. 8:353-364. [DOI] [PubMed] [Google Scholar]
- 53.Schnupf, P., and D. A. Portnoy. 2007. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 9:1176-1187. [DOI] [PubMed] [Google Scholar]
- 54.Schulenburg, H., and J. J. Ewbank. 2004. Diversity and specificity in the interaction between Caenorhabditis elegans and the pathogen Serratia marcescens. BMC Evol. Biol. 4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheehan, B., A. Klarsfeld, T. Msadek, and P. Cossart. 1995. Differential activation of virulence gene expression by PrfA, the Listeria monocytogenes virulence regulator. J. Bacteriol. 177:6469-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steenbergen, J. N., and A. Casadevall. 2003. The origin and maintenance of virulence for the human pathogenic fungus Cryptococcus neoformans. Microbes Infect. 5:667-675. [DOI] [PubMed] [Google Scholar]
- 57.Steinweg, C., C. T. Kuenne, A. Billion, M. A. Mraheil, E. Domann, R. Ghai, S. B. Barbuddhe, U. Karst, A. Goesmann, A. Puhler, B. Weisshaar, J. Wehland, R. Lampidis, J. Kreft, W. Goebel, T. Chakraborty, and T. Hain. 2010. Complete genome sequence of Listeria seeligeri, a nonpathogenic member of the genus Listeria. J. Bacteriol. 192:1473-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sue, D., D. Fink, M. Wiedmann, and K. J. Boor. 2004. σB-dependent gene induction and expression in Listeria monocytogenes during osmotic and acid stress conditions simulating the intestinal environment. Microbiology 150:3843-3855. [DOI] [PubMed] [Google Scholar]
- 59.Vandesompele, J., K. De Preter, F. Pattyn, B. Poppe, N. Van Roy, A. De Paepe, and F. Speleman. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:research0034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vázquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vega, Y., C. Dickneite, M. T. Ripio, R. Bockmann, B. Gonzalez-Zorn, S. Novella, G. Dominguez-Bernal, W. Goebel, and J. A. Vazquez-Boland. 1998. Functional similarities between the Listeria monocytogenes virulence regulator PrfA and cyclic AMP receptor protein: the PrfA* (Gly145Ser) mutation increases binding affinity for target DNA. J. Bacteriol. 180:6655-6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vega, Y., M. Rauch, M. J. Banfield, S. Ermolaeva, M. Scortti, W. Goebel, and J. A. Vazquez-Boland. 2004. New Listeria monocytogenes prfA* mutants, transcriptional properties of PrfA* proteins and structure-function of the virulence regulator PrfA. Mol. Microbiol. 52:1553-1565. [DOI] [PubMed] [Google Scholar]
- 63.Volokhov, D., J. George, C. Anderson, R. E. Duvall, and A. D. Hitchins. 2006. Discovery of natural atypical nonhemolytic Listeria seeligeri isolates. Appl. Environ. Microbiol. 72:2439-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ward, T. J., L. Gorski, M. K. Borucki, R. E. Mandrell, J. Hutchins, and K. Pupedis. 2004. Intraspecific phylogeny and lineage group identification based on the prfA virulence gene cluster of Listeria monocytogenes. J. Bacteriol. 186:4994-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong, K., and N. Freitag. 2004. A novel mutation within the central Listeria monocytogenes regulator PrfA that results in constitutive expression of virulence gene products. J. Bacteriol. 186:6265-6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang, Z., R. Nielsen, N. Goldman, and A. M. Pedersen. 2000. Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics 155:431-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, J., R. Nielsen, and Z. Yang. 2005. Evaluation of an improved branch-site likelihood method for detecting positive selection at the molecular level. Mol. Biol. Evol. 22:2472-2479. [DOI] [PubMed] [Google Scholar]
- 68.Zhou, X., J. Elmose, and D. R. Call. 2007. Interactions between the environmental pathogen Listeria monocytogenes and a free-living protozoan (Acanthamoeba castellanii). Environ. Microbiol. 9:913-922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.