Abstract
The effect of UV exposure on Toxoplasma gondii oocysts has not been completely defined for use in water disinfection. This study evaluated UV-irradiated oocysts by three assays: a SCID mouse bioassay, an in vitro T. gondii oocyst plaque (TOP) assay, and a quantitative reverse transcriptase real-time PCR (RT-qPCR) assay. The results from the animal bioassay show that 1- and 3-log10 inactivation is achieved with 4 mJ/cm2 UV and 10 mJ/cm2 low-pressure UV, respectively. TOP assay results, but not RT-qPCR results, correlate well with bioassay results. In conclusion, a 3-log10 inactivation of T. gondii oocysts is achieved by 10-mJ/cm2 low-pressure UV, and the in vitro TOP assay is a promising alternative to the mouse bioassay.
Toxoplasma gondii is an obligate intracellular protozoan parasite that commonly infects humans (19). Its human prevalence ranges worldwide by country from 10% to over 80% of the population (4, 38). Infection with T. gondii is typically asymptomatic in healthy individuals but results in a lifelong infection that can reactivate, causing toxoplasmic encephalitis and death if the individual becomes immunocompromised (49). In addition, if T. gondii is acquired during pregnancy, there is risk of transplacental transmission to the fetus, causing fetal death, abortion, malformation, or mental retardation. Recent studies have reported that acquired acute toxoplasmosis can occur in healthy individuals with symptoms of acute retinitis, prolonged fever, elevated liver enzyme levels, and respiratory distress, which resulted in one patient succumbing to acute disease (11, 12, 16). An outbreak of acquired acute T. gondii retinitis in healthy individuals has also been reported (10). These recent increases in the incidence of acute toxoplasmosis suggest the emergence or reemergence of a T. gondii strain(s) that is more pathogenic and poses greater human health risks than was previously assessed (37).
Typically, T. gondii outbreaks are food borne and associated with tissue cyst-contaminated meat from pigs, sheep, goats, and poultry (58). However, improved animal husbandry and hygiene management practices have significantly reduced the prevalence of T. gondii cysts in meat (20, 58). Despite these significant changes, the seroprevalence in humans remains relatively high, suggesting that other sources of exposure to T. gondii, such as oocysts in water or soil, are likely. Indeed, worldwide, several waterborne outbreaks associated with public water supplies have been documented (4, 5, 7, 17, 30). In the 1994 waterborne toxoplasmosis outbreak in Victoria, British Columbia, the likely source of T. gondii was traced back to a municipal water supplier that provided unfiltered chloraminated drinking water to residents of the Greater Victoria region (7). The cases of toxoplasmosis reported coincided with recent rainfall events but not with any breakdown in treatment processes. Also, no increases in other pathogen levels were reported. An epidemiological survey of wild felid species in the surrounding area further revealed that cougars found living near the watershed were seropositive for T. gondii antibodies, with one cougar actively shedding T. gondii oocysts in its feces, suggesting that the surface water sources were likely contaminated with T. gondii oocysts (2, 3, 7). Recent studies report that toxoplasmosis in California sea otters can be attributed to surface water runoff containing T. gondii oocysts that have contaminated shellfish and other near-shore-dwelling marine animals (35, 45-47). In addition, the oocyst can survive for a long time period and is highly resistant to chemical disinfection (18, 26, 27, 63). Taken together, all of these factors and studies suggest that T. gondii oocysts may pose a significant risk to drinking and recreational water quality worldwide.
Current drinking water regulations for protozoan pathogens focus on Cryptosporidium oocysts and Giardia cysts. No specific regulations, guidelines, or approved methods exist to monitor or control T. gondii oocysts in drinking and recreational water systems. Research efforts aimed at developing methods to monitor for T. gondii oocysts in water are limited and have not been used routinely to monitor source and recreational waters (23, 24, 32, 53, 61, 64). Specific recommendations on disinfection treatment practices for publicly owned treatment works (POTW) or drinking water utilities to inactivate waterborne T. gondii oocysts also do not exist (1).
UV disinfection is a relatively old technology but has grown in popularity and use in drinking water industries because it forms few disinfection by-products (DBP) (31, 59). Cryptosporidium is also highly resistant to chemical inactivation, but several studies show that Cryptosporidium parvum oocysts are inactivated by UV radiation at fluences applicable to drinking water treatment. These studies report that UV fluences of <25 mJ/cm2 achieve at least a 3-log10 inactivation as determined by either animal infectivity or cell culture (9, 13, 14, 51, 55). Similar results are observed with Cryptosporidium hominis oocysts (34). In addition, results from cell culture and animal infectivity studies are not statistically different (51). The USEPA Long-Term 2 Enhanced Surface Water Treatment Rule (LT2) has defined that a 2-, 3-, and 4-log10 inactivation of C. parvum is achieved with UV doses of 5.8, 12, and 22 mJ/cm2, respectively (59). To date, there are only two proceedings documents demonstrating the potential for UV irradiation of T. gondii oocysts (54, 56) and two published studies evaluating UV irradiation and T. gondii oocysts (26, 62). The reports by Sobsey and colleagues suggested that a 2-log10 reduction of T. gondii oocyst infectivity was achieved at 40 mJ/cm2 (54, 56). Dumètre et al. reported a 4-log10 inactivation after a UV dose of 20 mJ/cm2 (26), whereas Wainwright et al. reported that some oocysts remained infectious to mice after UV doses of >500 mJ/cm2 (62). These results report a wide range of UV exposures that are required to inactivate oocysts. To better assess UV inactivation efficacies, a more comprehensive analysis of the effect of UV on T. gondii oocysts is warranted.
This study examines the effect of UV exposure on the viability of T. gondii oocysts and determines the UV fluence required for a 3-log10 inactivation using three analytical approaches established in our laboratory: (i) a SCID mouse bioassay, (ii) a quantitative reverse transcriptase real-time PCR (RT-qPCR) assay, and (iii) an in vitro T. gondii oocyst plaque (TOP) assay.
MATERIALS AND METHODS
T. gondii oocysts.
Partially purified, sporulated T. gondii (VEG strain) oocysts were obtained from the feces of laboratory-infected cats as previously described (22). The oocysts were further purified on a cesium chloride gradient as described, except that the centrifugation was at 12,000 × g (25, 57). The purified oocysts were resuspended in 2% H2SO4 and stored at 4°C. Prior to use, the oocyst storage buffer was neutralized by addition of three-fifths of the original volume (vol/vol) of 1 N NaOH. The oocysts were then washed with phosphate-buffered saline (PBS). Oocysts used for mouse bioassays ranged in age from 1 month to 1 year, with no observed differences in infectivity, while oocysts that were less than 3 months old were used for the TOP assay (18, 26, 27, 63).
Oocyst enumeration.
Oocyst doses were enumerated by flow cytometry. All of the studies evaluating the 4-, 40-, and 100-mJ/cm2-irradiated oocysts and their respective controls were sorted with a FACS Vantage SE (Becton-Dickinson, San Jose, CA) equipped with CloneCyt as previously described for C. parvum, except that the sorting gate was set by autofluorescence isolating sporulated oocysts (42, 48). The remaining studies were sorted with a FACS Aria (Becton-Dickinson), and the oocysts were gated by forward- and side-scatter profiles, as the autofluorescence could not be detected with the laser configuration. The sort total was increased by 10% to normalize for the sporulated oocysts because 10% of the total oocysts were unsporulated. For both flow cytometers, oocyst doses were prepared from 5 to 25,000 oocysts per mouse in PBS by preparing a vial for each dose group.
Mice.
For each study, 20 to 85 male IcrTac:ICR-Prkdcscid mice aged 3 to 4 weeks were obtained from Taconic (Hudson, NY) and acclimated for at least 1 week prior to the start of experiments. They were housed in groups of 3 to 5 under barrier conditions, including sterile cages with 0.22-μm cage isolator filters, sterile corn cob bedding, sterile water, and irradiated Pico Lab mouse diet ad libitum. The cages were housed in an animal isolator (NuAire model 602-400; Plymouth, MN). All animal and tissue manipulations were performed in an animal cage station (NuAire model 650-600). All animal studies were approved and overseen by the Cincinnati USEPA Institutional Animal Care and Use Committee.
UV inactivation.
A low-pressure collimated beam apparatus was used to deliver UV fluences ranging from 4 mJ/cm2 to 100 mJ/cm2 as previously described (29). The irradiance was measured using a factory-calibrated, U.S. NIST-traceable, model IL-1700 radiometer (International Light, Inc., Newburyport, MA) as described previously (29). The UV fluences were calculated as described by Bolton and Linden (6).
SCID mouse bioassay and necropsy.
The mice were exposed to the oocysts by vortexing the oocyst suspension and exposing each mouse to a 0.2-ml intraperitoneal (i.p.) injection with a 27-gauge needle. In a preliminary study, the mice were exposed by cage to various numbers of T. gondii oocyst doses to establish the baseline 50% infective dose (ID50). This curve was then used to evaluate the log inactivation by UV by comparing the ID50 of exposed oocysts to that of control oocysts by using a modification of the approach taken by Korich et al. (40). Each study also included a negative control group in which the mice were exposed to 0.2 ml PBS. Mice were observed twice daily for signs of being moribund, such as weight loss, ruffled fur, reduced activity, and shivering. Moribund mice were euthanized and then necropsied. In necropsy, the brains, spleens, and lungs were aseptically removed, placed in individual cryovials, flash frozen in liquid nitrogen, and stored at −20°C. Mice which did not become moribund were euthanized at 42 days postinfection and necropsied as described above.
Duplex qPCR tissue confirmation.
Tissue samples were partially thawed and divided by scalpel, and approximately 20% of the spleen and 10% of the lung and brain from the center of the tissue were used to isolate genomic DNA. These tissue portions were weighed, and the DNA was isolated using the Qiagen QiaAmp DNA minikit (Qiagen, Valencia, CA) by following the manufacturer's protocol, except that RNase A was not used and the centrifugations of 6,000 × g were increased to 10,000 × g. Approximately 200 mg of brain and lung tissue and 100 mg of spleen tissue were processed for each sample.
The tissue samples were used to confirm the observed T. gondii infection data using a duplex qPCR assay as previously described (44). All control results were also confirmed by this assay. Sample amplification was performed in triplicate with a duplex reaction using an Applied Biosystems 7900 real-time PCR detection system (Applied Biosystems, Foster City, CA). The primers, probes, and GenBank accession numbers for the 35-fold B1 gene of T. gondii and the mouse glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH) are listed in Table 1 (39, 44). Each PCR assay consisted of 20 nM both probes, 20 nM GAPDH primers, 200 nM B1 primers, 3.5 mM MgCl2, 200 μM deoxynucleoside triphosphate (dNTP), 1× ROX reference dye (Invitrogen), and 2.5 U AmpliTaq gold (Applied Biosystems). Amplification conditions were denaturation for 5 min at 95°C followed by 40 cycles of denaturation at 95°C for 30 s and annealing and extension at 60°C for 1 min.
TABLE 1.
Real-time PCR primers for mouse GAPDH and the T. gondii B1 genes
| Genea | Primer/probe name | Sequence (5′→3′) | Nucleotide position | Amplicon size (bp) |
|---|---|---|---|---|
| Mouse GAPDH | GAPDH-ENV for | TCA TCT CCG CCC CTT CTG | 2575-2592 | 54 |
| GAPDH-ENV rev | TCG TGG TTC ACA CCC ATC AC | 2610-2629 | ||
| VIC-GAPDH-MGB | CGA TGC CCC CAT GTT | 2594-2608 | ||
| T. gondii B1b | Tg-TX2F for | CTA GTA TCG TGC GGC AAT GTG | 531-551 | 62 |
| Tg-TX2R rev | GGC AGC GTC TCT TCC TCT TTT | 572-592 | ||
| FAM-TGTX2M1-MGB | CCA CCT CGC CTC TTG G | 552-567 |
The cycle threshold (CT) values for each tissue sample were averaged by gene. In those cases in which qPCRs failed to amplify within 40 cycles, the sample was assigned a CT value of 40.1. This allowed undetermined values to be included in the calculations while ensuring that the resulting average was a conservative estimate of the CT value. Brain tissues were used for primary classifications, and mice were considered positive for T. gondii if they became moribund and the mean B1 CT was ≤34. Samples were considered negative for T. gondii if the mouse was asymptomatic and the mean B1 CT was ≥38.7. Spleen and then lung tissues were analyzed using the same criteria if the B1 CT was not definitive or the animal became moribund without detectable T. gondii DNA. The GAPDH reaction was used both to serve as a PCR control and to confirm the presence of mouse tissue. The qPCR results were not normalized, and total parasite burden was not determined.
Calculation of ID50 and statistical methodology.
Dose-response curves and associated ID50s were based on the logistic regression of the likelihood of infection with a log10 dose. Odds of infection were considered dependent on UV irradiation level, taken as a categorical effect. Based on a lack of a statistically significant (P > 0.05) difference in their odds ratios, the data from UV irradiations that were ≥15 mJ/cm2 were combined. Analysis of the results from the TOP assays was based on a hierarchical Bayes model that assumed the plaque counts to have a Poisson distribution, with the mean density being lognormally distributed from experiment to experiment and constant log reduction at 4 mJ/cm2 UV irradiation. SAS version 9.2 (SAS Institute, Cary, NC) and WinBUGS version 1.4 were used for analysis (43).
Total RNA extraction.
RNA extraction was performed using a QIAgen RNeasy microkit according to the manufacturer's suggestions. Briefly, 3 × 104 to 1 × 105 oocysts were resuspended in 150 μl of RLT buffer and subjected to five freeze-thaw cycles in liquid nitrogen and a 55°C water bath. Proteinase K (30 μl of 2 mg/ml) and 270 μl diethyl pyrocarbonate (DEPC) water were added to each sample, vortexed, and incubated for 1 h at 55°C. Total oocyst lysates were then further processed according to the manufacturer's protocol. Briefly, RNA was precipitated with absolute ethanol and 5 μl (4 mg/ml) carrier RNA. The RNA was washed with RNeasy wash solutions. The genomic DNA was removed by adding DNase I (1 U) to each sample and incubating the sample for 15 min at ambient temperature. Total RNA was then eluted with 35 μl of RNase-free water and stored at −80°C until use.
RT-qPCR.
RT-qPCR was performed to detect the ACT1 and SporoSAG mRNA as previously described (60). Each sample analyzed contained total RNA extracted from 1,000 oocysts as described above. The RT reaction mixture contained 1 μl of murine leukemia virus (MULV) RT (Promega, Madison, WI), 2.5 μl 10× PCR buffer II (Applied Biosystems), 1.5 μl 2.5 mM magnesium chloride, 1 μl 10 μM reverse primer (Table 2), 2.5 μl 25 μM dNTPs (Promega), and 0.75 μl RNasin (Promega) in a total volume of 25 μl. The RT reaction was performed using a DMJ PTC-200 thermal cycler (MJ Research, Inc., Watertown, MA) at 43°C for 1 h and then at 94°C for 5 min. Immediately following the RT reaction, a qPCR was performed in DEPC-treated water containing 25 μl RT reaction mixture, 2.5 μl 10× PCR buffer II, 5.5 μl 25 mM magnesium chloride, 1 μl ROX dye (Invitrogen), 1 μl 10 μM forward primer, 1 μl 10 μM probe, 0.5 μl AmpliTaq gold (Applied Biosystems) in a total volume of 50 μl. The qPCR was performed using ABI 7900HT (Applied Biosystems) consisting of 50°C for 2 min, 95°C for 10 min, and 40 cycles at 95°C for 15 s and then 60°C for 1 min. All RT-qPCRs were done in triplicate. ACT1 and SporoSAG RT-qPCR primers and probes were designed to specifically amplify the mRNA species and not genomic DNA (Table 2). The ACT1 gene was selected to detect all forms of T. gondii and the SporoSAG gene to detect infectious sporozoites. To verify that endogenous DNA was destroyed, RT-qPCRs were performed without the addition of reverse transcriptase in all experiments performed. No amplification occurred in these samples.
TABLE 2.
Primer and probe sequences used for RT-qPCRa
| Gene | Primer/probe | Sequence (5′→3′) | Amplicon size (bp) |
|---|---|---|---|
| ACT1 | TgACT1 for | ACA TCA AGG AGA AGC TTT GCT ACA | 60 |
| TgACT1 rev | TCA GCC GCC TTC ATT TCC | ||
| VIC-ACT1-MGB | CGC CCT CGA CTT C | ||
| SporoSAG | SporoSAG for | GCG GAG ACA AGC GTT TTT TT | 124 |
| SporoSAG rev | AGC CTG TGG CTG CGC TTT | ||
| FAM-SporoSAG-MGB | CCT ATG CCA AAG AAC |
Reference 60.
Oocyst excystation.
Oocyst excystation as reported by Villegas et al. was used for this study (60). Briefly, oocysts at a concentration of 2 × 106 in 1 ml of 1× PBS were added to 2-ml microcentrifuge screw cap conical tubes containing 0.5 g of 0.5-mm glass beads (Biospec Products, Inc., Bartlesville, OK) and mechanically disrupted using a mini-BeadBeater (BioSpec Products, Inc.) for 20 s at 250 rpm. Bovine bile (Sigma; 500 μl, 10%) was then added to the sample and incubated at 37°C for 1.5 h. The solution was then transferred to a new tube and centrifuged at 21,000 × g for 10 min at 4°C. The supernatant was then aspirated and discarded, and the pellet was washed once with 1× PBS. The pellet was then resuspended in 1 ml 10% Dulbecco modified Eagle medium (DMEM) (Gibco, Gaithersburg, MD) in preparation for the TOP assay (see below).
TOP assay.
Human foreskin fibroblasts (HFF) were purchased from the American Type Culture Collection (ATCC; Manassas, VA; no. CRL-1634) and grown in DMEM (Gibco) containing 10% fetal bovine serum (HyClone, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin, and 25 g/ml amphotericin B (Gibco) at 37°C with 5% CO2. HFF cells were maintained as monolayers in 75-cm2 cell culture flasks or seeded onto six-well tissue culture plates (Becton Dickinson Labware, Franklin Lakes, NJ) and grown to confluence for TOP assays. The cell monolayers were exposed to various dilutions (1 to 1 × 106) of excysted T. gondii oocysts and incubated at 37°C and in 5% CO2 for 10 days. At the end of the 10-day period, the cell monolayers were stained with crystal violet for 30 min, washed, and analyzed microscopically by counting of plaques as previously described (52, 60).
RESULTS
SCID mouse bioassay.
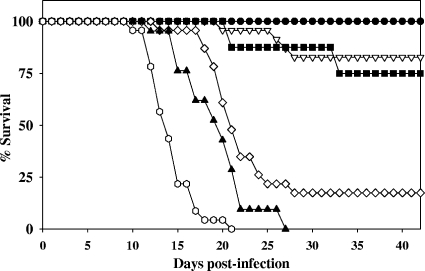
The SCID mouse bioassay was developed and evaluated by determining the ID50 for male SCID mice via i.p. exposure. Figure 1 shows the effects on mice, with times postexposure and at different oocyst doses, showing an ID50 between 10 and 50 oocysts. The date of the onset of symptoms is related to the number of oocysts used to infect the animal. Animals exposed to ≥500 oocysts became moribund approximately 2 weeks after exposure, while animals exposed to <500 oocysts became moribund approximately 3 weeks postexposure. No animals developed signs of infection after day 33, even though the study continued for 42 days postinfection. Results were confirmed by tissue analysis. A similar survival curve was observed with SCID mice exposed by oral gavage (44; data not shown).
FIG. 1.
T. gondii oocyst infection by intraperitoneal exposure in male SCID mice. Mouse survival by percentage of cohort after exposure to T. gondii oocysts plotted against day of death postexposure by oocyst dose. Uninfected (•) mice or mice exposed to 5 (▿), 10 (▪), 50 (⋄), 500 (▴), and 5,000 (○) oocysts were monitored for moribundity for 42 days.
Effects of UV irradiation on T. gondii oocysts by SCID mouse bioassay.
The SCID mouse bioassay was used to estimate the effects of UV irradiation on sporulated T. gondii oocysts as shown in Table 3. Mice not exposed to oocysts were asymptomatic. The control group exposed to untreated oocysts showed that the ID50 was <50 oocysts because 83% of the mice exposed to 50 oocysts and all of the mice exposed to ≥500 oocysts became moribund. The 4-mJ/cm2 group were asymptomatic when exposed to <50 oocysts, 48% became moribund when exposed to 500 oocysts, and all mice exposed to >5,000 oocysts became moribund. In contrast, the 10-mJ/cm2 group of mice were asymptomatic when dosed with ≤5,000 oocysts, and 60% of the mice exposed to >5,000 oocysts became moribund. In oocysts exposed to ≥15-mJ/cm2 UV, all mice were asymptomatic when exposed to ≤5,000 oocysts, and 20 to 33% of the mice exposed to >5,000 oocysts became moribund. The data presented in Table 3 were confirmed by the detection of T. gondii B1 DNA by using a duplex qPCR assay.
TABLE 3.
SCID mouse bioassay results by T. gondii oocyst and UV dose
| UV dose (mJ/cm2) | No. of mice infected/no. of mice exposed to indicated no. of oocysts per animala |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 50 | 500 | 5,000 | >5,000 | |
| 0 | 0/15 | 1/17 | 2/8** | 13/17 | 15/15** | 17/17 | ND |
| 4 | ND | 0/15 | 0/5 | 4/18 | 10/21 | 13/13 | 5/5 |
| 10 | ND | ND | ND | ND | 0/10 | 0/9** | 6/10 |
| 15 | ND | ND | ND | ND | 0/10 | 0/10 | 2/10 |
| 20 | ND | ND | ND | ND | 0/9** | 0/10 | 2/10 |
| 40 | ND | 0/8 | 0/5 | 0/15 | 0/15 | 0/20 | 2/6 |
| 100 | ND | ND | ND | 0/9 | 0/15 | 0/29 | ND |
**, mouse/mice removed from study because tissue from moribund animal(s) was negative for T. gondii B1 gene in all tissues (data not shown). ND, not done.
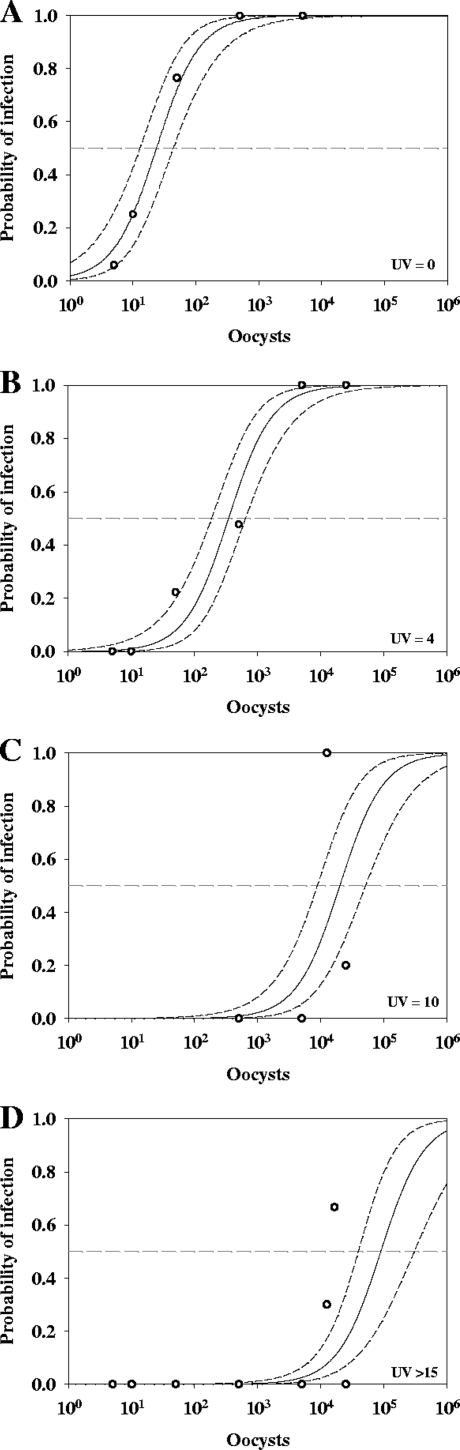
Figure 2 shows the ID50 dose-response curves developed from the bioassay data, with the model shown by solid lines and the dashed lines representing the 95% confidence intervals. Table 4 presents the numerical data shown in Fig. 2 and the log10 inactivation achieved by the corresponding UV exposure. The control group had an ID50 of 24 oocysts (log10 = 1.37) (Fig. 2A and Table 4). The model derived from the 4-mJ/cm2 UV exposure group had an ID50 of 346 oocysts (log10 = 2.54), achieving a 1.17-log10 inactivation (Fig. 2B and Table 4). The model from the 10-mJ/cm2 UV exposure group had an ID50 of 2.0 × 104 oocysts (log10 = 4.31), with a 2.93-log10 inactivation (Fig. 2C and Table 4). All of the data from UV exposures of ≥15 mJ/cm2 were analyzed individually, but the models for all of the groups were not statistically different and therefore combined. The ID50 of this group was 9.0 × 104 oocysts (log10 = 4.96), achieving a 3.58-log10 inactivation (Fig. 2D and Table 4).
FIG. 2.
Dose-response results for SCID mice for unexposed (UV = 0) and UV-exposed (4, 10, and ≥15 mJ/cm2) T. gondii oocysts (A to D, respectively). Open circles are animal infectivity results, and solid lines are the dose-response model results developed from the animal results with 95% confidence intervals. The dashed lines are the ID50s determined by the dose-response model.
TABLE 4.
Mouse bioassay numerical dose-response model and determined log inactivation
| UV exposure (mJ/cm2) | ID50 (oocysts) | Log ID50 | 95% confidence interval | Log inactivationa |
|---|---|---|---|---|
| 0 | 24 | 1.37 | 1.11-1.64 | NA |
| 4 | 346 | 2.54 | 2.27-2.81 | 1.17 |
| 10 | 2.0 × 104 | 4.31 | 3.95-4.71 | 2.94 |
| ≥15 | 9.0 × 104 | 4.96 | 4.60-5.49 | 3.59 |
NA, not applicable.
Duplex qPCR tissue confirmation.
To confirm T. gondii-induced pathology following infection, a duplex qPCR amplifying the T. gondii B1 and mouse GAPDH genes was developed. Results from tissue analysis by this duplex qPCR are shown in Table 5. Tissues from all animals were taken, and the brains were used for primary classification of infection status. The tissues from asymptomatic, unexposed controls were used to determine the B1 gene CT value, ≥38.7, for a T. gondii-negative sample (mean minus 2 standard deviations [SD]). The determined CT values observed in the unexposed controls may be due to duplex qPCR. A mouse was considered positive for T. gondii if the animal became moribund and the average B1 CT value was ≤34, reflecting at least a 1.5 order of magnitude change in the expression of T. gondii B1 DNA between negative and positive tissues.
TABLE 5.
Summary of tissue resultsc
| Tissue type | qPCR parametera | Unexposed mice | Asymptomatic mice | Moribund miceb |
|---|---|---|---|---|
| Brain | B1 mean CT ± SD | 39.7 ± 0.5 | 39.9 ± 0.3 | 28.8 ± 2.2 |
| GAPDH mean CT ± SD | 22.1 ± 0.9 | 22.1 ± 1.5 | 21.9 ± 1.2 | |
| n | 15 | 263 | 88 | |
| Spleen | B1 mean CT ± SD | NA | 40.1 ± 0.1 | 30.9 ± 3.1 |
| GAPDH mean CT ± SD | NA | 19.9 ± 1.3 | 19.0 ± 2.1 | |
| n | NA | 6 | 3 | |
| Lung | B1 mean CT | NA | NA | 28.4 |
| GAPDH mean CT | NA | NA | 19.4 | |
| n | NA | NA | 1 |
Tissue results are averages from triplicate duplex qPCRs. Undetermined results were given a value of 40.1. Moribund mice were positive for T. gondii if they had a mean B1 CT value of ≤34. Asymptomatic mice were negative if they had a mean B1 CT value of ≥38.7. Primary determination was by brain tissue and, if required, by spleen and then by lung.
Seven moribund mice did not have T. gondii and were not included in the analysis (data not shown).
NA, no tissues analyzed.
Brain tissues were used to confirm the results for 366 out of 383 mice used in this study, and 10 were confirmed by spleen and lung tissues. All tissues were positive for the mouse housekeeping gene GAPDH, with the CT averaging approximately 22 for the brain and 19 for the lung and spleen. There were no significant differences in GAPDH CT values for the same tissue type. In contrast to the GAPDH results, the B1 results were significantly different for the moribund mice, with an average B1 CT value of 28.8 ± 2.2 (SD) for brain tissues compared to 39.9 ± 0.3 and 39.7 ± 0.5 for the asymptomatic and unexposed mice, respectively. Spleen and then lung tissue were analyzed if the brain results could not be confirmed using the criteria listed above or if there was a discrepancy between the bioassay and qPCR results. Seven mice became moribund without detectable T. gondii DNA. These animals were not included in the analysis and are <2% of the animals used in the study.
Analysis of UV irradiation on T. gondii oocysts by an RT-qPCR assay.
An RT-qPCR assay targeting the ACT1 or SporoSAG gene was also employed as an alternative rapid viability assay to the SCID mice bioassay described above. As shown in Table 6, CT values for ACT1 and SporoSAG in unexposed live T. gondii oocysts were 28.12 ± 3.13 and 31.07 ± 4.45, respectively. Following UV exposure at 4, 40, and 100 mJ/cm2, CT values for ACT1 remained unchanged: 28.21 ± 3.43, 28.16 ± 1.99, and 28.46 ± 1.40, respectively. Similarly, SporoSAG mRNA levels between unexposed and UV-treated oocysts were not significantly different: 4 mJ/cm2 = 30.98 ± 4.93, 40 mJ/cm2 = 31.73 ± 3.38, and 100 mJ/cm2 = 32.64 ± 2.14.
TABLE 6.
RT-qPCR results to determine T. gondii oocyst viability following UV exposure
| UV dose (mJ/cm2) |
CT ± SDa |
|
|---|---|---|
| Act1 | SporoSAG | |
| Unexposed | 28.12 ± 3.13 | 31.07 ± 4.45 |
| 4 | 28.21 ± 3.43 | 30.98 ± 4.93 |
| 40 | 28.16 ± 1.99 | 31.73 ± 3.38 |
| 100 | 28.46 ± 1.40 | 32.64 ± 2.14 |
Average CT values from three independent experiments ± standard deviation. Triplicate RT-qPCRs were performed for each condition in each of the three independent experiments.
Effects of UV irradiation on T. gondii oocysts by TOP assay.
A less-expensive alternative approach to the SCID mouse bioassay described above, the TOP assay was used as a faster means of measuring effects of UV exposure on oocyst infectivity. As shown in Table 7, a 1.8 (± 0.2)-log10 reduction of infectivity (95% interval range of 1.6 to 2.1) was observed with oocysts exposed to 4 mJ/cm2 UV irradiation compared to the unexposed controls. Oocysts exposed to ≥40 mJ/cm2 UV irradiation resulted in at least a 3-log10 reduction of infectivity. No observable differences in excystation efficiencies were detected between unexposed and UV-treated oocysts (data not shown). The TOP assay revealed that at least 1- and 3-log10 reductions in infectivity can be achieved with 4 and ≥40 mJ/cm2 UV exposure, respectively.
TABLE 7.
TOP assay results of UV-irradiated T. gondii oocysts
| Expt no. | UV dose (mJ/cm2) | No. of plaques with indicated no. of oocystsa |
||||||
|---|---|---|---|---|---|---|---|---|
| 100 | 101 | 102 | 103 | 104 | 105 | 106 | ||
| 1 | Unexposed | 0 | 0.33 ± 0.557 | 21.33 ± 4.73 | 171.17 ± 6.51 | * | * | * |
| 4 | 0 | 0 | 0.33 ± 0.56 | 1.67 ± 0.58 | 21.67 ± 3.52 | 180.33 ± 4.51 | * | |
| 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | Unexposed | 0 | 0.33 ± 0.56 | 22.33 ± 4.51 | 167.67 ± 13.61 | * | * | * |
| 4 | 0 | 0 | 0.67 ± 0.57 | 3 ± 1.00 | 16.67 ± 4.04 | 175.00 ± 9.85 | * | |
| 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Data represent mean number of plaques from triplicate wells ± standard deviation. *, too numerous to count.
DISCUSSION
The enhancement of disinfection practices utilized by the water industry has been driven by concerns of DBP and chemically resistant microorganisms, such as T. gondii and Cryptosporidium oocysts (26, 31, 40, 63). UV disinfection has been widely adopted worldwide because it forms few DBPs, and exposure to <12 mJ/cm2 achieves a 3-log10 inactivation of Cryptosporidium oocysts and Giardia cysts (9, 14, 15, 29, 41, 51). Information about the effects of UV on T. gondii oocysts is limited. Sobsey and colleagues reported a 2-log10 reduction of T. gondii oocyst infectivity at 40 mJ/cm2 (55, 56). These studies, however, used a limited number of animals, and so, an accurate dose-response model able to quantify inactivation rates could not be developed. Wainwright et al. demonstrated that oocysts were infectious to mice even after UV exposures of ≥500 mJ/cm2 but did not report log inactivation (62). Dumètre et al. reported an ID50 of 6.2 oocysts and a 4-log10 inactivation by 40 mJ/cm2 UV, the only UV dose evaluated by their bioassay (26).
We report an ID50 of 24 oocysts, and 1- and 3-log10 reductions are achieved with UV fluences of 4 and 10 mJ/cm2, respectively, by mouse bioassay. The differences in the ID50 and maximal log inactivation values observed between these studies may be explained by several factors, including the animal model, confirmation method, oocyst strain, and study size. This study used SCID mice, which are completely susceptible to T. gondii infection, making this model potentially more sensitive for determining oocyst infectivity compared to immunocompetent mouse models (36). This sensitivity leads to a more conservative estimate of the risk of infection. SCID mice become moribund with advanced toxoplasmosis, allowing for a simple preliminary determination of oocyst infectivity compared to asymptomatic immunocompetent models, which may require further analysis, such as serology, to reveal results. Wainwright et al. used a different strain of T. gondii (Type X) which may be more resistant to UV. The different oocyst strains used may not account for the discrepancies since studies using different Cryptosporidium oocyst strains and species showed all to have similar sensitivities to UV (14, 34).
Oocyst infectivity appears to have a nonlinear response to UV fluences above 10 mJ/cm2, and based on the dose-response model shown in Fig. 2, a 4-log10 inactivation could be achieved within error of the model. More importantly, a 3-log10 inactivation of T. gondii oocysts is easily achieved by a UV fluence of ≥10 mJ/cm2 as reported here and by Dumètre et al. (26). Another possible explanation for this nonlinear effect is the unique ability of T. gondii oocyst and sporocyst walls to autofluoresce upon exposure to UV (42). These walls may be protecting the sporozoites from UV radiation by converting UV energy into its autofluorescent properties. It should be noted that ionizing and nonionizing irradiation exposure affects parasite intracellular replication, with a minimal effect on the invasion process (21, 28).
The gold standard for determining T. gondii oocyst viability is through animal infectivity, which is expensive and labor-intensive and requires at least 6 weeks to allow for infection to develop in the SCID mice used in this study. This study determines the mouse infectivity status primarily by animal health followed by a novel confirmation by a duplex qPCR assay instead of serology or immunohistochemistry. The B1 qPCR assay used to detect T. gondii in infected tissues was adopted from an assay developed by Kompalic-Cristo et al. (39). This approach is very reliable and less labor-intensive than serology and immunohistochemistry and resulted in a dual detection of both mouse tissue and the presence or absence of T. gondii. No attempt was made to quantify the parasite load by qPCR since the primary purpose of this assay is to confirm T. gondii-induced pathology and morbidity in SCID mice. The duplex qPCR assay was also able to determine that seven moribund mice were negative for T. gondii. We believe that these immunocompromised SCID mice had an undetermined secondary infection. These results emphasize that mouse survival data must be confirmed by tissue analysis.
There are ethical concerns associated with animal research; thus, a scientifically acceptable alternative is needed. In vitro cell culture results have been correlated with animal infectivity results for Cryptosporidium disinfection studies (51); however, to date there are only limited data for T. gondii oocysts. Dumètre et al. evaluated the UV inactivation of T. gondii by both in vitro and animal infectivity assays, but the relationship between the two assays was not rigorously evaluated, in that only two groups, unexposed and exposed to 40 mJ/cm2, were evaluated. Nevertheless, they report that both assays achieved a 4-log10 inactivation after exposure to 40 mJ/cm2 UV. In addition, they determined that UV fluences of 7.9, 11.8, 15.3, and 17.5 mJ/cm2 were required to achieve 1-, 2-, 3-, and 4-log10 reductions, respectively, only by the in vitro assay (26). Our study also compares a mouse bioassay and TOP assay and demonstrates 1- to 2-log10 inactivation at 4 mJ/cm2 by bioassay and TOP assay and >3-log10 inactivation at ≥40 mJ/cm2 UV by both assays. Taken together, cell culture assays appear to be a promising alternative to animal bioassays; however, a more complete evaluation is needed to determine their ability to assess disinfection efficacies of other inactivation treatment processes.
Cell culture obtains data in a week to 10 days, and this time period would not be rapid enough to assay risk reduction in the case of a water utility contamination event. A potential alternative is RT-qPCR, by which results are obtained in a few hours. Our studies demonstrate that RT-qPCR results after UV exposure do not correlate with the TOP assay or bioassay results. Jenkins et al. compared fluorescence in situ hybridization (FISH), cell culture, and animal bioassay to assess the sensitivity of these methods in detecting the viability and infectivity of C. parvum oocysts in water and found that even when mRNA levels were not detected or minimally detected by RT-PCR, the oocysts were able to effectively infect mice and cell cultures (33). Major unknown factors in the use of RT-PCR analysis of T. gondii oocyst inactivation following UV light exposure are the rate and conditions of decay of the T. gondii ACT1 and SporoSAG mRNA following inactivation. The mRNA decay may be a significant contributing factor to the low sensitivity observed when using this method and should be further elucidated. Although the SporoSAG and ACT1 RT-qPCR assay was not effective at predicting T. gondii viability following UV exposure, it may still provide a rapid and sensitive alternative assay for determining total levels of T. gondii oocysts present in the environment. In addition, an RT-qPCR technique could be combined with the TOP assay to more accurately assess oocyst viability as well as reduce the time in culture required to obtain a result; similar methods have been described for C. parvum (50). Another promising alternative to an integrated cell culture/RT-qPCR assay is the use of propidium monoazide in conjunction with a molecular assay such as PCR, as recently applied to assess viable Cryptosporidium oocysts (8).
This study more fully elucidates the effectiveness of UV irradiation against T. gondii oocysts and shows that 3-log10 inactivation is fully achievable at a 10-mJ/cm2 UV dose, which is applicable to the water industry. Low-pressure UV appears to be effective against T. gondii oocysts under ideal conditions; however, environmental samples have not been evaluated, and research is needed to better understand the true susceptibility of this pathogen in drinking water and wastewater systems. In addition, while the RT-qPCR approach did not appear to be a reliable indicator of infectivity, the TOP assay showed promise as a sensitive alternative to established mice bioassays or the SCID mouse model presented here.
Acknowledgments
We acknowledge the animal care and technical assistance of Sharon Detmer, Paula McCain, Diana Miller, and Katrina Pratt. We also acknowledge Eugene Rice and Frank W. Schaefer III for their critical reviews of the manuscript.
The United States Environmental Protection Agency through its Office of Research and Development funded and collaborated in the research described here under interagency agreement number DW-12-92289801-0 to USDA and contract number EP-D-06-100 to the McConnell Group. It has been subjected to agency review and approved for publication.
Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Author contributions were as follows. E.N.V., S.A.J.A., and M.W.W. conceived and designed the experiments. M.W.W., S.A.J.A., D.O.E., M.J.S., S.L.H., and E.N.V. performed the experiments. M.W.W., S.A.J.A., L.W., and E.N.V. analyzed the data. J.P.D. contributed reagents/materials/analysis tools. E.N.V., M.W.W., and S.A.J.A. wrote the paper.
Footnotes
Published ahead of print on 11 June 2010.
REFERENCES
- 1.American Water Works Association Research Division Microbiological Contaminants Research Committee. 1999. Committee report: emerging pathogens—viruses, protozoa, and algal toxins. J. Am. Water Works Assoc. 91:110-121. [Google Scholar]
- 2.Aramini, J. J., C. Stephen, and J. P. Dubey. 1998. Toxoplasma gondii in Vancouver Island cougars (Felis concolor vancouverensis): serology and oocyst shedding. J. Parasitol. 84:438-440. [PubMed] [Google Scholar]
- 3.Aramini, J. J., C. Stephen, J. P. Dubey, C. Engelstoft, H. Schwantje, and C. S. Ribble. 1999. Potential contamination of drinking water with Toxoplasma gondii oocysts. Epidemiol. Infect. 122:305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahia-Oliveira, L. M., J. L. Jones, J. Azevedo-Silva, C. C. Alves, F. Orefice, and D. G. Addiss. 2003. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg. Infect. Dis. 9:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benenson, M. W., E. T. Takafuji, S. M. Lemon, R. L. Greenup, and A. J. Sulzer. 1982. Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. N. Engl. J. Med. 307:666-669. [DOI] [PubMed] [Google Scholar]
- 6.Bolton, J., and K. Linden. 2003. Standardization of methods for fluence (UV dose) determination in bench scale UV experiments. J. Environ. Eng. 129:209-215. [Google Scholar]
- 7.Bowie, W. R., A. S. King, D. H. Werker, J. L. Isaac-Renton, A. Bell, S. B. Eng, and S. A. Marion. 1997. Outbreak of toxoplasmosis associated with municipal drinking water. The BC Toxoplasma Investigation Team. Lancet 350:173-177. [DOI] [PubMed] [Google Scholar]
- 8.Brescia, C. C., S. M. Griffin, M. W. Ware, E. A. Varughese, A. I. Egorov, and E. N. Villegas. 2009. Cryptosporidium propidium monoazide-PCR, a molecular biology-based technique for genotyping of viable Cryptosporidium oocysts. Appl. Environ. Microbiol. 75:6856-6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukhari, Z., T. M. Hargy, J. R. Bolton, B. Dussert, and J. L. Clancy. 1999. Medium-pressure UV for oocyst inactivation. J. Am. Water Works Assoc. 91:86-94. [Google Scholar]
- 10.Burnett, A. J., S. G. Shortt, J. Isaac-Renton, A. King, D. Werker, and W. R. Bowie. 1998. Multiple cases of acquired toxoplasmosis retinitis presenting in an outbreak. Ophthalmology 105:1032-1037. [DOI] [PubMed] [Google Scholar]
- 11.Carme, B., F. Bissuel, D. Ajzenberg, R. Bouyne, C. Aznar, M. Demar, S. Bichat, D. Louvel, A. M. Bourbigot, C. Peneau, P. Neron, and M. L. Darde. 2002. Severe acquired toxoplasmosis in immunocompetent adult patients in French Guiana. J. Clin. Microbiol. 40:4037-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carme, B., M. Demar, D. Ajzenberg, and M. L. Darde. 2009. Severe acquired toxoplasmosis caused by wild cycle of Toxoplasma gondii, French Guiana. Emerg. Infect. Dis. 15:656-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clancy, J. L., T. M. Hargy, M. M. Marshall, and J. E. Dyksen. 1998. UV light inactivation of Cryptosporidium oocysts. J. Am. Water Works Assoc. 90:92-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clancy, J. L., M. M. Marshall, T. M. Hargy, and D. G. Korich. 2004. Susceptibility of five strains of Cryptosporidium parvum oocysts to UV light. J. Am. Water Works Assoc. 96:84-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craik, S. A., D. Weldon, G. R. Finch, J. R. Bolton, and M. Belosevic. 2001. Inactivation of Cryptosporidium parvum oocysts using medium- and low- pressure ultraviolet radiation. Water Res. 35:1387-1398. [DOI] [PubMed] [Google Scholar]
- 16.Demar, M., D. Ajzenberg, D. Maubon, F. Djossou, D. Panchoe, W. Punwasi, N. Valery, C. Peneau, J. L. Daigre, C. Aznar, B. Cottrelle, L. Terzan, M. L. Darde, and B. Carme. 2007. Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clin. Infect. Dis. 45:e88-e95. [DOI] [PubMed] [Google Scholar]
- 17.de Moura, L., L. M. Bahia-Oliveira, M. Y. Wada, J. L. Jones, S. H. Tuboi, E. H. Carmo, W. M. Ramalho, N. J. Camargo, R. Trevisan, R. M. Graca, A. J. da Silva, I. Moura, J. P. Dubey, and D. O. Garrett. 2006. Waterborne toxoplasmosis, Brazil, from field to gene. Emerg. Infect. Dis. 12:326-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubey, J. P. 1998. Toxoplasma gondii oocyst survival under defined temperatures. J. Parasitol. 84:862-865. [PubMed] [Google Scholar]
- 19.Dubey, J. P. 2009. Toxoplasmosis of animals and humans, 2nd ed. CRC Press, Inc., Boca Raton, FL.
- 20.Dubey, J. P., D. E. Hill, J. L. Jones, A. W. Hightower, E. Kirkland, J. M. Roberts, P. L. Marcet, T. Lehmann, M. C. B. Vianna, K. Miska, C. Sreekumar, O. C. H. Kwok, S. K. Shen, and H. R. Gamble. 2005. Prevalence of viable Toxoplasma gondii in beef, chicken, and pork from retail meat stores in the United States: risk assessment to consumers. J. Parasitol. 91:1082-1093. [DOI] [PubMed] [Google Scholar]
- 21.Dubey, J. P., M. C. Jenkins, D. W. Thayer, O. C. Kwok, and S. K. Shen. 1996. Killing of Toxoplasma gondii oocysts by irradiation and protective immunity induced by vaccination with irradiated oocysts. J. Parasitol. 82:724-727. [PubMed] [Google Scholar]
- 22.Dubey, J. P., G. V. Swan, and J. K. Frenkel. 1972. A simplified method for isolation of Toxoplasma gondii from the feces of cats. J. Parasitol. 58:1005-1006. [PubMed] [Google Scholar]
- 23.Dumètre, A., and M. L. Darde. 2007. Detection of Toxoplasma gondii in water by an immunomagnetic separation method targeting the sporocysts. Parasitol. Res. 101:989-996. [DOI] [PubMed] [Google Scholar]
- 24.Dumètre, A., and M. L. Darde. 2003. How to detect Toxoplasma gondii oocysts in environmental samples? FEMS Microbiol. Rev. 27:651-661. [DOI] [PubMed] [Google Scholar]
- 25.Dumètre, A., and M. L. Darde. 2004. Purification of Toxoplasma gondii oocysts by cesium chloride gradient. J. Microbiol. Methods 56:427-430. [DOI] [PubMed] [Google Scholar]
- 26.Dumètre, A., C. Le Bras, M. Baffet, P. Meneceur, J. P. Dubey, F. Derouin, J. P. Duguet, M. Joyeux, and L. Moulin. 2008. Effects of ozone and ultraviolet radiation treatments on the infectivity of Toxoplasma gondii oocysts. Vet. Parasitol. 153:209-213. [DOI] [PubMed] [Google Scholar]
- 27.Frenkel, J. K., A. Ruiz, and M. Chinchilla. 1975. Soil survival of Toxoplasma oocysts in Kansas and Costa Rica. Am. J. Trop. Med. Hyg. 24:439-443. [DOI] [PubMed] [Google Scholar]
- 28.Grimwood, B. G. 1980. Infective Toxoplasma gondii trophozoites attenuated by ultraviolet irradiation. Infect. Immun. 28:532-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes, S. L., E. W. Rice, M. W. Ware, and F. W. Schaefer III. 2003. Low pressure ultraviolet studies for inactivation of Giardia muris cysts. J. Appl. Microbiol. 94:54-59. [DOI] [PubMed] [Google Scholar]
- 30.Heukelbach, J., V. Meyer-Cirkel, R. C. Moura, M. Gomide, J. A. Queiroz, P. Saweljew, and O. Liesenfeld. 2007. Waterborne toxoplasmosis, northeastern Brazil. Emerg. Infect. Dis. 13:287-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hijnen, W. A., E. F. Beerendonk, and G. J. Medema. 2006. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Res. 40:3-22. [DOI] [PubMed] [Google Scholar]
- 32.Isaac-Renton, J., W. R. Bowie, A. King, G. S. Irwin, C. S. Ong, C. P. Fung, M. O. Shokeir, and J. P. Dubey. 1998. Detection of Toxoplasma gondii oocysts in drinking water. Appl. Environ. Microbiol. 64:2278-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins, M. C., J. Trout, M. S. Abrahamsen, C. A. Lancto, J. Higgins, and R. Fayer. 2000. Estimating viability of Cryptosporidium parvum oocysts using reverse transcriptase-polymerase chain reaction (RT-PCR) directed at mRNA encoding amyloglucosidase. J. Microbiol. Methods 43:97-106. [DOI] [PubMed] [Google Scholar]
- 34.Johnson, A. M., K. Linden, K. M. Ciociola, R. De Leon, G. Widmer, and P. A. Rochelle. 2005. UV inactivation of Cryptosporidium hominis as measured in cell culture. Appl. Environ. Microbiol. 71:2800-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson, C. K., M. T. Tinker, J. A. Estes, P. A. Conrad, M. Staedler, M. A. Miller, D. A. Jessup, and J. A. Mazet. 2009. Prey choice and habitat use drive sea otter pathogen exposure in a resource-limited coastal system. Proc. Natl. Acad. Sci. U. S. A. 106:2242-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson, L. L. 1992. SCID mouse models of acute and relapsing chronic Toxoplasma gondii infections. Infect. Immun. 60:3719-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones, J. L., and J. P. Dubey. 2010. Waterborne toxoplasmosis—recent developments. Exp. Parasitol. 124:10-25. [DOI] [PubMed] [Google Scholar]
- 38.Jones, J. L., D. Kruszon-Moran, M. Wilson, G. McQuillan, T. Navin, and J. B. McAuley. 2001. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am. J. Epidemiol. 154:357-365. [DOI] [PubMed] [Google Scholar]
- 39.Kompalic-Cristo, A., C. Frotta, M. Suarez-Mutis, O. Fernandes, and C. Britto. 2007. Evaluation of a real-time PCR assay based on the repetitive B1 gene for the detection of Toxoplasma gondii in human peripheral blood. Parasitol. Res. 101:619-625. [DOI] [PubMed] [Google Scholar]
- 40.Korich, D. G., J. R. Mead, M. S. Madore, N. A. Sinclair, and C. R. Sterling. 1990. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 56:1423-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, D., S. A. Craik, D. W. Smith, and M. Belosevic. 2009. Infectivity of Giardia lamblia cysts obtained from wastewater treated with ultraviolet light. Water Res. 43:3037-3046. [DOI] [PubMed] [Google Scholar]
- 42.Lindquist, H. D., J. W. Bennett, J. D. Hester, M. W. Ware, J. P. Dubey, and W. V. Everson. 2003. Autofluorescence of Toxoplasma gondii and related coccidian oocysts. J. Parasitol. 89:865-867. [DOI] [PubMed] [Google Scholar]
- 43.Lunn, D. J., A. Thomas, N. Best, and D. Spiegelhalter. 2000. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat. Comput. 10:325-337. [Google Scholar]
- 44.Massie, G. N., M. W. Ware, E. N. Villegas, and M. W. Black. 2010. Uptake and transmission of Toxoplasma gondii oocysts by migratory, filter-feeding fish. Vet. Parasitol. 169:296-303. [DOI] [PubMed] [Google Scholar]
- 45.Miller, M. A., I. A. Gardner, C. Kreuder, D. M. Paradies, K. R. Worcester, D. A. Jessup, E. Dodd, M. D. Harris, J. A. Ames, A. E. Packham, and P. A. Conrad. 2002. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis). Int. J. Parasitol. 32:997-1006. [DOI] [PubMed] [Google Scholar]
- 46.Miller, M. A., M. E. Grigg, C. Kreuder, E. R. James, A. C. Melli, P. R. Crosbie, D. A. Jessup, J. C. Boothroyd, D. Brownstein, and P. A. Conrad. 2004. An unusual genotype of Toxoplasma gondii is common in California sea otters (Enhydra lutris nereis) and is a cause of mortality. Int. J. Parasitol. 34:275-284. [DOI] [PubMed] [Google Scholar]
- 47.Miller, M. A., W. A. Miller, P. A. Conrad, E. R. James, A. C. Melli, C. M. Leutenegger, H. A. Dabritz, A. E. Packham, D. Paradies, M. Harris, J. Ames, D. A. Jessup, K. Worcester, and M. E. Grigg. 2008. Type X Toxoplasma gondii in a wild mussel and terrestrial carnivores from coastal California: new linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters. Int. J. Parasitol. 38:1319-1328. [DOI] [PubMed] [Google Scholar]
- 48.Miller, T. A., M. W. Ware, L. J. Wymer, and F. W. Schaefer III. 2007. Chemically and genetically immunocompromised mice are not more susceptible than immunocompetent mice to infection with Cryptosporidium muris. Vet. Parasitol. 143:99-105. [DOI] [PubMed] [Google Scholar]
- 49.Montoya, J. G., and O. Liesenfeld. 2004. Toxoplasmosis. Lancet 363:1965-1976. [DOI] [PubMed] [Google Scholar]
- 50.Rochelle, P., D. Ferguson, T. Handojo, R. De Leon, M. Stewart, and R. Wolfe. 1997. An assay combining cell culture with reverse transcriptase PCR to detect and determine the infectivity of waterborne Cryptosporidium parvum. Appl. Environ. Microbiol. 63:2029-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rochelle, P. A., M. M. Marshall, J. R. Mead, A. M. Johnson, D. G. Korich, J. S. Rosen, and R. De Leon. 2002. Comparison of in vitro cell culture and a mouse assay for measuring infectivity of Cryptosporidium parvum. Appl. Environ. Microbiol. 68:3809-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roos, D. S., R. G. Donald, N. S. Morrissette, and A. L. Moulton. 1994. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45:27-63. [DOI] [PubMed] [Google Scholar]
- 53.Schwab, K. J., and J. J. McDevitt. 2003. Development of a PCR-enzyme immunoassay oligoprobe detection method for Toxoplasma gondii oocysts, incorporating PCR controls. Appl. Environ. Microbiol. 69:5819-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin, G., K. Linden, J. Dubey, and M. Sobsey. 2003. Inactivation of Toxoplasma gondii oocysts by UV irradiation. In Proceedings of the International Ultraviolet Association Second International Congress on UV Technologies. International Ultraviolet Association, Vienna, Austria.
- 55.Shin, G. A., K. G. Linden, M. J. Arrowood, and M. D. Sobsey. 2001. Low-pressure UV inactivation and DNA repair potential of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 67:3029-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simmons, O. D., K. G. Linden, M. D. Sobsey, and J. P. Dubey. 2006. UV inactivation of Toxoplasma gondii oocysts. In Proceedings of the Water Quality Technology Conference. American Water Works Association, Denver, CO.
- 57.Staggs, S. E., M. J. See, J. P. Dubey, and E. N. Villegas. 2009. Obtaining highly purified Toxoplasma gondii oocysts using a discontinuous cesium chloride gradient. J. Vis. Exp. 33:1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tenter, A. M., A. R. Heckeroth, and L. M. Weiss. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30:1217-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.U.S. Environmental Protection Agency. 2006. National primary drinking water regulations: long term 2 enhanced surface water treatment rule. 40 CFR, parts 9, 141, and 142. Fed. Regist. 71:654-786. [Google Scholar]
- 60.Villegas, E. N., S. A. Augustine, L. F. Villegas, M. W. Ware, M. J. See, H. D. Lindquist, F. W. Schaefer III, and J. P. Dubey. 2010. Using quantitative reverse transcriptase PCR and cell culture plaque assays to determine resistance of Toxoplasma gondii oocysts to chemical sanitizers. J. Microbiol. Methods 81:219-225. [DOI] [PubMed] [Google Scholar]
- 61.Villena, I., D. Aubert, P. Gomis, H. Ferte, J. C. Inglard, H. Denis-Bisiaux, J. M. Dondon, E. Pisano, N. Ortis, and J. M. Pinon. 2004. Evaluation of a strategy for Toxoplasma gondii oocyst detection in water. Appl. Environ. Microbiol. 70:4035-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wainwright, K. E., M. Lagunas-Solar, M. A. Miller, B. C. Barr, I. A. Gardner, C. Pina, A. C. Melli, A. E. Packham, N. Zeng, T. Truong, and P. A. Conrad. 2007. Physical inactivation of Toxoplasma gondii oocysts in water. Appl. Environ. Microbiol. 73:5663-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wainwright, K. E., M. A. Miller, B. C. Barr, I. A. Gardner, A. C. Melli, T. Essert, A. E. Packham, T. Truong, M. Lagunas-Solar, and P. A. Conrad. 2007. Chemical inactivation of Toxoplasma gondii oocysts in water. J. Parasitol. 93:925-931. [DOI] [PubMed] [Google Scholar]
- 64.Yang, W., H. D. A. Lindquist, V. Cama, F. W. Schaefer III, E. Villegas, R. Fayer, E. J. Lewis, Y. Feng, and L. Xiao. 2009. Detection of Toxoplasma gondii oocysts in water sample concentrates by real-time PCR. Appl. Environ. Microbiol. 75:3477-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]