Abstract
Cronobacter sakazakii is an opportunistic pathogen that actively invades host eukaryotic cells. To identify invasion factors responsible for the intestinal translocation of C. sakazakii, we constructed for the first time outer membrane protein X (OmpX) and A (OmpA) deletion mutants using the lambda Red recombination system. The ompX and ompA deletion mutants showed significantly reduced invasion of human enterocyte-like epithelial Caco-2 and human intestinal epithelial INT-407 cells, and significantly fewer mutant cells were recovered from the livers and spleens of rat pups. Furthermore, compared with intact target cells, the invasion and initial association potentials of the mutants increased at a rate similar to that of the wild type in tight-junction-disrupted target cells, suggesting that OmpX and OmpA are involved in basolateral invasion by C. sakazakii. This is the first report of C. sakazakii virulence determinants that are essential for basolateral invasion and that may be critical for the virulence of C. sakazakii.
Enterobacter sakazakii is an emerging pathogen associated with several outbreaks of meningitis and local necrotizing enterocolitis in premature infants (2, 28, 37). There was considerable diversity among E. sakazakii isolates (13, 14), and the original taxonomic name of E. sakazakii was reclassified as Cronobacter spp., which included Cronobacter sakazakii (13, 14). Therefore, C. sakazakii will be used throughout this paper. Although the incidence of Cronobacter infection is rare, the mortality rate is as high as 33 to 80% (11, 27, 32, 39). Even when infants survive Cronobacter infection, they often experience serious sequelae, including brain abscesses, developmental delay, and impairment of sight and hearing (8). Premature infants, whose immune systems are not fully developed, may be at high risk for Cronobacter infection (26).
Very little is known about the mechanisms of pathogenicity and the virulence determinants of the genus Cronobacter. Adhesion of Cronobacter spp. to eukaryotic cells showed two distinct patterns, i.e., a diffuse pattern and the formation of localized clusters, which was nonfimbrial (21). Pagotto et al. (29) reported that the genus Cronobacter produced enterotoxins and was lethal on intraperitoneal injection into suckling mice at levels as low as 105 CFU per mouse. The genus Cronobacter interacts with and damages intestinal epithelial cells, which results in intestinal injury and villus disruption (12). In addition, the cell-bound zinc-containing metalloprotease encoded by zpx caused rounding of Chinese hamster ovary (CHO) cells (19), which may be important in dissemination of the pathogen into the systemic circulation. Furthermore, Townsend et al. (36) showed that Cronobacter can persist within rat macrophages.
As an oral pathogen causing a systemic infection, C. sakazakii must translocate from the intestinal lumen into the blood circulation. The genus Cronobacter is capable of actively invading various epithelial and endothelial cells of human and animal origin (17, 25, 31). Kim and Loessner (17) reported that the active invasion of human intestinal Caco-2 cells by C. sakazakii requires de novo bacterial protein synthesis and the host cell cytoskeleton and that the invasion efficiency of C. sakazakii was enhanced in the absence of cellular tight junctions. With regard to the virulence determinants related to Cronobacter penetration of the host cells, Mohan Nair and Venkitanarayanan (25) and Singamsetty et al. (31) reported that outer membrane protein A (OmpA) of Cronobacter plays an important role in the invasion of human intestinal epithelial INT-407 cells and human brain microvascular endothelial cells (HBMECs); invasion was dependent on both microfilaments and microtubules in INT-407 cells but only on microtubule condensation in HBMECs. Obviously, bacterial translocation in the intestines is multifactorial, and more detailed studies are needed to gain a better understanding of C. sakazakii pathogenesis.
Outer membrane protein X (OmpX) of C. sakazakii was identified in this study. Previously, OmpX in other bacteria was shown to be involved in the invasion of host cells (7, 18), neutralizing host defense mechanisms, and bacterial defense against the complement systems of the host (10, 38).
In this study, we report for the first time a successful application of the lambda Red recombination system to construct in-frame OmpX and/or OmpA deletion mutants in C. sakazakii. We further report that both outer membrane proteins (OMPs) of C. sakazakii, OmpX and OmpA, play critical roles in its invasion through not only the apical side, but also the basolateral side, of the host cells. We also show that OmpX and OmpA are responsible for C. sakazakii translocation into the deeper organs (i.e., liver and spleen).
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains used in this study are listed in Table 1. C. sakazakii and Escherichia coli were grown in tryptic soy broth (TSB) (Difco, Detroit, MI) and Luria-Bertani (LB) medium, respectively, at 37°C with constant shaking unless otherwise indicated. Strains containing the temperature-sensitive plasmid pKD46 or pCP20 were grown at 30°C. When antibiotics were required, chloramphenicol (Cm) was added at a final concentration of 25 μg/ml and/or ampicillin (Ap), kanamycin (Km), and/or tetracycline (Tc) was added at 50 μg/ml. After the mutants were obtained, the strains were grown at 42°C to induce deletion of the plasmids.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| C. sakazakii | ||
| ATCC 29544 | Wild-type strain | 16 |
| ES1001 | 29544 harboring pKD46 (Apr) | This study |
| ES2004 | ΔompA::kan | This study |
| ES2005 | ΔompA | This study |
| ES2006 | pACYC184-ompA | This study |
| ES2007 | ΔompX::kan | This study |
| ES2008 | ΔompX | This study |
| ES2009 | pACYC184-ompX | This study |
| ES2017 | ΔompX::kan ΔompA | This study |
| ES2018 | ΔompX ΔompA | This study |
| E. coli | ||
| DH5α | λ− φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | 9 |
| TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139ΔΔ(ara-leu)7697 galU galK rpsL (StrR) endA1 nupG | Invitrogen |
| Plasmids | ||
| pKD13 | oriR6Kγ Apr FRT Kanr FRT | 5 |
| pKD46 | oriR101 repA101(Ts) ApraraBADpgam-bet-exo | 5 |
| pCP20 | oripSC101(Ts) Apr Cmr cI857λ PRflp | 5 |
| pACYC184 | Tetr Cmr p15A ori | 5 |
| pBAD202/D-TOPO | Kanr | Invitrogen |
| pBAD202-ompA | Harboring C. sakazakii ompA gene | This study |
| pBAD202-ompX | Harboring C. sakazakii ompX gene | This study |
| pWM1007 | Km; pMW10 ΔlacZ W[(T1)4-Pc-gfp-T1] | 23 |
Apr, ampicillin resistance; Kanr, kanamycin resistance; Cmr, chloramphenicol resistance; Tetr, tetracycline resistance.
Construction of ΔompA, ΔompX, and ΔompX ΔompA mutants using the lambda Red recombination method.
The whole genome sequence of C. sakazakii BAA-894 was obtained from GenBank (accession number NC_009778) and used in this study (20). In-frame deletion mutants of C. sakazakii ATCC 29544 were generated by the lambda Red recombination method, as described by Datsenko and Wanner (5). Briefly, the kanamycin resistance (Kmr) cassette from plasmid pKD13 was amplified using the following primers: for ompA deletion mutant construction, ompA-F (5′-GTG AAG GAT TTA ACC GTG AAC TTT TCC CAA GGA AAA GCG C TG TAG GCT GGA GCT GCT TCG-3′) and ompA-R (5′-AAA AAA CCC CGC CGT GGC GGG GTT TTT CTT AAC GTT TAA C AT TCC GGG GAT CCG TCG ACC-3′), and for ompX deletion mutant construction, ompX-F (5′-TTA AAT CTT AGG ACT TAC TTG AAG CAC ATT TGA GGT GGT T TG TAG GCT GGA GCT GCT TCG-3′) and ompX-R (5′-AAA ATC CGC CCG TGG GCG GAT TTT TTC ATC ACC GAA GTG A AT TCC GGG GAT CCG TCG ACC-3′). (The nucleotide sequences originating from the C. sakazakii gene of interest are shown in italics, and those from pKD13 are underlined.) PCR was performed in a 50-μl reaction mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 3 mM MgCl2, 200 μM deoxynucleoside triphosphates (dNTPs), 2 U of DNA polymerase (rTaq; Takara, Otsu, Japan), 0.25 μM each primer, and 2.5 μl of template DNA. The amplification reactions were performed using a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA). The thermal-cycling conditions consisted of a hold at 94°C for 10 min, followed by amplification for 30 cycles at 94°C for 30 s, 66°C for 30 s, and 72°C for 90 s and a final extension at 72°C for 5 min. The PCR products were analyzed by electrophoresis in 1% agarose gels with 0.5× TAE buffer (10× TAE is 0.4 M Tris acetate, 0.01 M EDTA, pH 8.0). The gels were stained for 30 min in 0.5× TAE buffer containing 1 μg/ml ethidium bromide and examined on a UV transilluminator.
The PCR products were transformed into the wild-type (WT) strain, C. sakazakii ATCC 29544, harboring the pKD46 plasmid, by electroporation (Gene Pulser; 2.5 V, 200 Ω, 25-μF capacity; Bio-Rad, Hercules, CA) as described previously (17), and cells were selected for Km-resistant transformants, ompA::Km and ompX::Km. Finally, the kanamycin resistance cassette was removed using the pCP20 plasmid, as described by Datsenko and Wanner (5). The ΔompX ΔompA double mutant was constructed using the ΔompA mutant and the primers ompX-F and ompX-R.
Complementation study.
To generate complementation strains, the complete ompA and ompX open reading frames (ORF) were amplified using the primer pairs ompA HindIII-F (5′-GCA TGG TGC ATT AAG CTT TTT TAG-3′) and ompA SphI-R (5′-AGA GGG GCA TGC GGT TCT T-3′) and ompX HindIII-F (5′-ACG GGG TAA GCT TGG CAT CCT-3′) and ompX SphI-R (5′-AAT ACG GCG CAT GCG ATG CG-3′), respectively (nucleotide sequences in italics are artificially added restriction enzyme recognition sites). The thermal-cycling conditions consisted of a hold at 94°C for 10 min, followed by amplification for 30 cycles at 94°C for 30 s, 56°C for 30 s, and 72°C for 60 s, with a final extension at 72°C for 5 min. The PCR products were digested with HindIII and SphI and cloned into a pACYC184 vector that had been digested with the same enzymes (3). The plasmid was then transformed into the mutant cells.
Cell culture and EGTA treatment.
Human enterocyte-like epithelial Caco-2 (ATCC, Manassas, VA) and human epithelial INT-407 (ATCC) cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM) (Invitrogen, Grand Island, NY) containing 10% fetal bovine serum (FBS) (Invitrogen), unless otherwise indicated. Trypsin-treated cells from up to six passages were seeded (approximately 5 × 104 cells per well) into 24-well cell culture plates (Sarstedt, Newton, NC) and grown at 37°C in the presence of 5% CO2. The cells formed monolayers after 1 to 2 days and were cultured for up to 21 days, as needed. The medium was replaced every 2 days, and cell viability was determined by trypan blue straining.
For treatment with EGTA, Caco-2 monolayers were preincubated for 1 h with DMEM containing 3 mM EGTA. The cells were washed once with phosphate-buffered saline (PBS) (pH 7.4) and used for further studies.
Invasion assay.
To determine bacterial invasion of mammalian Caco-2 and INT-407 cells, a gentamicin protection assay was performed as described previously (17). Briefly, bacteria were prepared by transferring 2% inoculum from overnight cultures into fresh, prewarmed TSB, followed by incubation for 2 h at 37°C with constant shaking. C. sakazakii cells were collected by centrifugation at 13,000 × g, washed with PBS (pH 7.4), and resuspended in 1 ml of PBS for infection. Monolayer cells were infected with bacteria at a multiplicity of infection (MOI) of 100 and incubated for 1.5 h without subsequent centrifugation. After the cells were washed four times with PBS, fresh medium containing gentamicin (100 μg/ml; Sigma, St. Louis, MO) was added, and the plates were further incubated for 1.5 h, followed by five washes with PBS. Then, 1 ml of Triton X-100 (0.4% in PBS) was added, and the plates were incubated for a further 30 min before the bacteria were collected and plated on tryptic soy agar (TSA) in decimal dilutions. Bacterial invasion was expressed as the relative percentage of invasion, which was calculated using the following formula: (number of surviving bacteria/number of surviving WT) × 100.
Adhesion assay.
Bacterial adhesion to mammalian cells was performed as described previously (21). Briefly, C. sakazakii was prepared as described for the invasion assay and added to a mammalian cell monolayer at an MOI of 100. After 30 min of incubation, the cells were washed five times with PBS and treated with Triton X-100. Adherent C. sakazakii cells were counted.
Inhibition assay using purified OMPs. (i) Overexpression of C. sakazakii OmpA and OmpX in E. coli.
To overexpress OMPs of C. sakazakii, the complete ompA gene was amplified using the primers ompA-TOPO-F (5′-C ACC ATG GCC TTT TTG GAT GAT AAC G-3′) and ompA-TOPO-R (5′-AGC CTG CGG CTG AGT TAC AAC G-3′), and the complete ompX gene was amplified using ompX-TOPO-F (5′-C ACC ATG AAA AAA ATT GCA TGT CTT TCA G-3′) and ompX-TOPO-R (5′-GAA GCG GTA ACC CAC ACC TGC-3′). The thermal-cycling conditions consisted of a hold at 94°C for 10 min, followed by amplification for 30 cycles at 94°C for 30 s, 56°C for 30 s, and 72°C for 80 s, with a final extension at 72°C for 5 min. The PCR products were inserted into the TOPO recognition site of pBAD202/D-TOPO by a TOPO cloning reaction using E. coli TOP10 following the manufacturer's instructions (Invitrogen, Carlsbad, CA). The recombinant Omp proteins have His-Patch thioredoxin at the N-terminal end and a His6 tag at the C-terminal end. Insertion of the complete gene was confirmed by sequencing using a BigDye terminator cycle-sequencing kit (Applied Biosystems) and an ABI Prism 3700 DNA analyzer (PerkinElmer, Wellesley, MA) at the National Instrumentation Center for Environmental Management (Seoul, South Korea). The activities of the recombinant Omp proteins were confirmed by complementation tests.
(ii) Purification of C. sakazakii OMPs expressed in E. coli.
The overnight cultures of E. coli TOP10 harboring a plasmid expressing OmpA or OmpX were transferred to new LB medium, and 0.2% l-arabinose was added to the culture when the optical density at 600 nm (OD600) reached 1.0. The induced bacterial cultures were further incubated for 2 h, recovered by centrifugation at 15,000 × g for 5 min at 4°C, and suspended in Tris buffer (20 mM Tris-Cl, pH 7.4, 300 mM NaCl), which was then sonicated with an Ultrasonic Processor GE 130PB (Hielscher Systems, Teltow, Germany) with a 30-s pulse and a 30-s pause for 10 min. After centrifugation of the bacterial lysates at 16,000 × g for 10 min at 4°C, the supernatants were decanted. The pellets were washed three times using Tris buffer, and OMPs were resolved using buffer A (50 mM CAPS [N-cyclohexyl-3-aminopropanesulfonic acid], pH 10.5). After centrifugation at 16,000 × g for 10 min at 4°C, the supernatant was loaded onto an Ni-NTA superflow affinity column (Qiagen, Valencia, CA) and eluted using a gradient of combinations of buffer A and buffer B (50 mM CAPS, pH 10.5, 300 mM imidazole). Peak fractions were confirmed by SDS-PAGE, pooled, and dialyzed against buffer A. The protein concentration was determined by the Bradford method (1).
(iii) Inhibition assay.
Various concentrations (5, 25, and 50 μg/well) of the Omp preparation were preincubated with Caco-2 cell monolayers for 1 h, followed by three washes with PBS. C. sakazakii adhesion and invasion were assayed as described above.
Preparation of crude membrane proteins of C. sakazakii.
Outer membrane proteins were prepared as described previously (6), with modifications. Bacteria from overnight cultures were transferred to new LB medium and then grown until their OD600 reached 4.0. The bacterial cultures were recovered by centrifugation at 6,000 × g for 10 min at 4°C, suspended in 1 ml of PBS (pH 7.4), and sonicated with an Ultrasonic Processor GE 130PB (Hielscher Systems, Teltow, Germany) with a 30-s pulse and a 30-s pause for 10 min. Cell debris was removed by centrifugation as described above, and 10 ml of the supernatant was added to 1 ml of 2% N-lauroylsarcosine (Sarkosyl; Sigma), followed by incubation for 1 h at room temperature. Crude outer membrane proteins were prepared by centrifugation of the mixture at 100,000 × g for 1 h. The pelleted protein was resuspended in 0.2 ml of PBS and stored at −80°C for further experiments. The protein concentration was determined by the Bradford method (1).
SDS-PAGE.
Purified OMPs or crude membrane proteins of C. sakazakii were obtained as described above. Aliquots of 1 μg of purified OMPs or 10 μg of crude membrane proteins of C. sakazakii were prepared in a solution containing 0.05 M Tris-HCl (pH 8), 1.6% SDS, 25% glycerol, 5% 2-mercaptoethanol, and 0.003% bromophenol blue (Sigma), and the proteins were separated by SDS-PAGE in 5% stacking and 12% separating gels. The proteins were visualized by staining them with Coomassie brilliant blue R250 (Bio-Rad, Hercules, CA) and destaining them with a solution of 30% methanol and 10% acetic acid.
Confocal laser fluorescence microscopy.
For the expression of green fluorescent protein (GFP), the plasmid pWM1007 (23) was transformed into C. sakazakii strains by electroporation. The presence of the plasmid and cytosolic GFP did not affect the invasion properties of C. sakazakii (data not shown). For confocal laser scanning microscopy, round coverslips (diameter, 12 mm) were placed in the wells of a 24-well tissue culture plate and seeded with 104 Caco-2 cells, which were incubated for 24 h. After infection with C. sakazakii carrying pWM1007 for 30 min and four washes with PBS, the coverslips were removed, and the cells were fixed with formaldehyde (3.7% in PBS; 15 min), washed three times with PBS, and permeabilized for 3 min with 0.1% Triton X-100. Rhodamine-labeled phalloidin (1:200 dilution of 0.1-mg/ml stock solution; Sigma) was added, and the cells were incubated for 20 min, followed by extensive washing with PBS. Fluorescence signals were recorded using an inverted confocal laser scanning microscope combined with fluorescence correlation spectroscopy (Carl Zeiss Microscopy, Jena, Germany). Different series of images from 0.4-μm x-y-z sections were captured, analyzed, and stacked using appropriate software.
In vivo animal study.
Sprague-Dawley rat pups (SD rat), 2 to 3 days old, were purchased from the Institute of Laboratory Animal Resources, Seoul National University (Seoul, South Korea). Overnight cultures of C. sakazakii strains were collected and resuspended in PBS. Rat pups in each group (n = 5) were fed by oral gavage with 5 × 109 CFU of bacteria. For analysis of bacterial colonization in organs, all rats were euthanized with a mixture of ketamine and xylamine at 24 h postinfection. The spleens and livers were removed aseptically, homogenized in 1 ml of ice-cold PBS, and serially diluted. Bacterial loads were determined by plating on LB agar plates.
Statistical analysis.
All experiments were performed at least in triplicate. Data from cell culture experiments were analyzed by unpaired t tests or one-way analysis of variance with Duncan's posttest at a 95% confidence interval using SAS software (version 9.1.3; SAS Institute, Cary, NC). Values of P below 0.05 were considered significant.
RESULTS
The lambda Red recombination method was successfully applied to C. sakazakii.
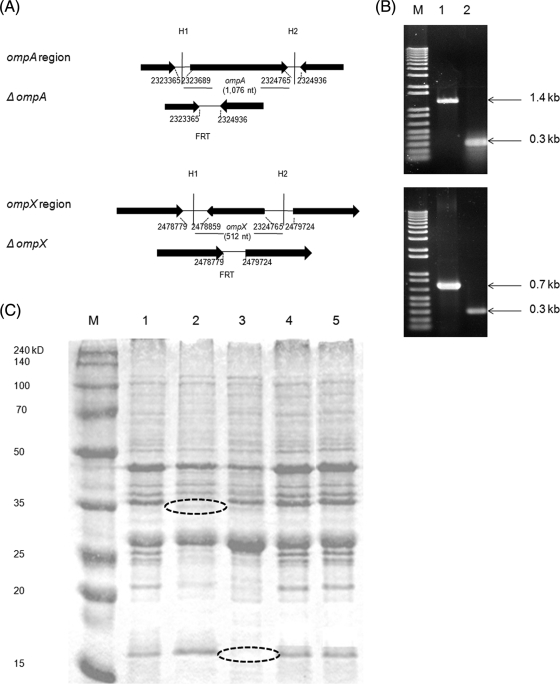
To determine whether OmpA and/or OmpX expression is required for the invasion of mammalian cells by C. sakazakii, we used lambda Red and FLP/FLP recombination target (FRT)-mediated site-specific recombination to construct a series of deletion mutants in C. sakazakii ATCC 29544: ΔompA, ΔompX, and a ΔompX ΔompA double deletion (Fig. 1A). The deletions in the target genes were confirmed by PCR with specific primers, which showed no amplification of the 1.4-kb ompA or 0.7-kb ompX genes in the respective mutants (Fig. 1B). In-frame deletion was also confirmed by nucleotide sequencing of the PCR product (data not shown). Furthermore, SDS-PAGE revealed lack of expression of OmpA (38-kDa) and OmpX (18-kDa) proteins, corresponding to the wild type, in the mutant strains (Fig. 1C). OmpA and OmpX were restored in complemented strains, as observed in SDS-PAGE profiles (Fig. 1C). The ΔompX ΔompA double mutant was constructed using the ΔompA mutant as the parental strain and ompX-F and ompX-R primers for recombination and confirmed by PCR (data not shown).
FIG. 1.
Construction of ΔompA and ΔompX mutants with the lambda Red system. (A) Gene arrangement near ompA and ompX in C. sakazakii WT and the mutants. H1 and H2 indicate 40-nucleotide (nt) homology extensions. The nucleotide numbers are adopted from the C. sakazakii whole-genome database. (B) PCR confirmation of the mutants. Lane M, nucleotide size markers; lane 1, WT; lane 2, mutant. (C) Confirmation of mutant construction by SDS-PAGE of crude outer membrane proteins. Lane M, protein marker; lane 1, C. sakazakii ATCC 29544; lane 2, ompA deletion mutant; lane 3, ompX deletion mutant; lane 4, ompA complementation; lane5, ompX complementation. The dashed ovals indicate the approximate positions of OmpA (38 kDa) and OmpX (18 kDa).
OmpA and OmpX are required for invasion of host eukaryotic cells.
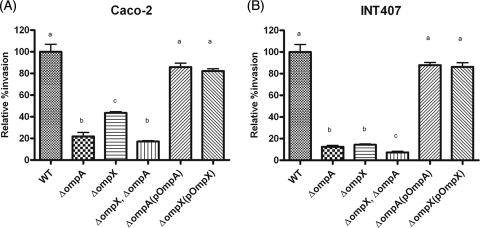
The abilities of the WT, mutants, and complemented strains to invade Caco-2 and INT-407 cells were compared in a gentamicin protection assay (Fig. 2). Compared with the WT, approximately 20% (1.6 × 104 ± 4.9 × 104 CFU/well) of the ΔompA mutant, 40% (3.2 × 104 ± 1.7 × 104 CFU/well) of the ΔompX mutant, and 17% (1.26 × 104 ± 1.4 × 104 CFU/well) of the double mutant were able to penetrate Caco-2 cells. The complementation strains showed restored invasion ability similar to that of the WT strain (Fig. 2A). The differences in invasion efficiency between the ΔompA and ΔompX mutants and between the ΔompX ΔompA double mutant and the ΔompX mutant were statistically significant, whereas there was no significant difference between the ΔompX ΔompA double mutant and the ΔompA mutant (Fig. 2A). These observations suggest that OmpA and OmpX play important roles in the invasion of Caco-2 cells but that OmpA and OmpX do not act synergistically in Caco-2 cells.
FIG. 2.
Invasion by C. sakazakii in Caco-2 (A) and INT-407 (B) cells. Confluent monolayers of eukaryotic cells were infected with C. sakazakii at an MOI of 100 and incubated for 1.5 h, followed by gentamicin treatment (100 μg/ml) for 1.5 h. The cells were then treated with 0.4% Triton X-100 to obtain the intracellular bacteria. The bars represent means plus standard deviations (SD) from three independent experiments performed in triplicate. Statistical differences are indicated by lowercase letters at P values of <0.05.
In the case of INT-407 cells, only 12.5% (8.0 × 102 ± 0.9 × 102 CFU/well) of the ΔompA mutant, 19.1% (1.2 × 103 ± 1.5 × 103 CFU/well) of the ΔompX mutant, and 8.5% (5.4 × 102 ± 0.4 × 102 CFU/well) of the double mutant invaded the cells compared to the WT. The difference in invasion efficiency between the ΔompA and ΔompX mutants was not statistically significant, whereas those between either the ΔompA or ΔompX single mutant and the ΔompX ΔompA double mutant were statistically significant (Fig. 2B). These observations indicate that OmpA and OmpX act synergistically to invade INT-407 cells. The addition of an extrachromosomal copy of the deleted gene restored the invasion potential of the mutants (Fig. 2B). Taken together, these results suggest that OmpA and OmpX are required for invasion of host cells.
OmpA and OmpX proteins can block Caco-2 cell invasion by C. sakazakii.
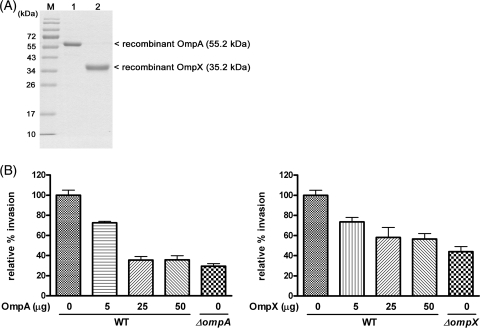
To determine whether C. sakazakii OmpA and OmpX proteins interact directly with Caco-2 cells, we performed an inhibition assay using purified OmpA and OmpX proteins. Each protein was overexpressed in E. coli TOP10 by l-arabinose induction, and the OMPs were isolated using metal affinity resin (Fig. 3A). Preincubation of Caco-2 cells with purified OmpA or OmpX decreased the invasion of Caco-2 cells by C. sakazakii ATCC 29544 in a dose-dependent manner (Fig. 3B) (P < 0.05). These observations suggest that preincubated OmpA and OmpX may occupy an unknown host receptor(s) on Caco-2 cells, thereby inhibiting the invasive ability of C. sakazakii.
FIG. 3.
Inhibition of C. sakazakii ATCC 29544 (WT) invasion of Caco-2 cells by pretreatment with purified proteins. (A) C. sakazakii recombinant OmpA and OmpX were overexpressed in E. coli TOP10 and isolated using metal affinity resin. The purified proteins were resolved by SDS-PAGE. Lane 1, size marker proteins; lane 2, recombinant OmpA; lane 3, recombinant OmpX. (B) Various concentrations (0.5, 25, and 50 μg) of purified outer membrane proteins were added to confluent monolayers of Caco-2 cells and incubated for 1 h at 37°C, followed by PBS washing and a gentamicin protection assay with C. sakazakii ATCC 29544 or the ompA deletion mutant. The experiments were performed at least three times in triplicate and are expressed as means plus SD. Statistical differences are indicated by lowercase letters at P values of <0.05.
To validate the function of these recombinant OMPs as the original OMPs in C. sakazakii ATCC 29544, an invasion assay was conducted using ΔompA and ΔompX mutants harboring pBAD202-ompA and pBAD202-ompX, respectively. Complementation by pBAD202-ompA and pBAD202-ompX could recover the defective phenotype of ΔompA and ΔompX mutants in their invasion ability (data not shown).
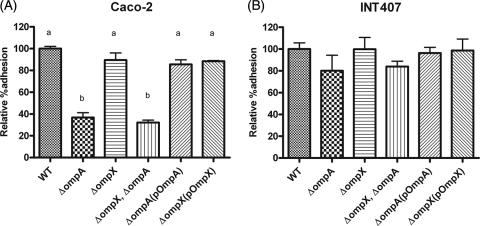
OmpA, but not OmpX, is required for adhesion to Caco-2 cells.
Compared with the WT, only approximately 30% of the ΔompA mutants or the ΔompX ΔompA double mutants were able to adhere to Caco-2 cell monolayers in an adhesion assay (Fig. 4A) (P < 0.005). The ompA complement restored adhesion capability to a level similar to that of the parental strain, C. sakazakii ATCC 29544. On the other hand, deletion of ompX did not affect adhesion efficiency. In addition, there were no significant differences in adhesion to INT-407 cells among the WT, the ΔompA mutant, the ΔompX mutant, and the ΔompX ΔompA double mutant (Fig. 4B).
FIG. 4.
Adhesion of C. sakazakii strains to Caco-2 (A) and INT-407 (B) cells. C. sakazakii was incubated with confluent monolayers of the host cells for 30 min, followed by PBS washing and Triton X-100 treatment for bacterial enumeration. The bars represent means plus SD from three independent experiments performed in triplicate.
OmpA and OmpX are essential for basolateral invasion by C. sakazakii.
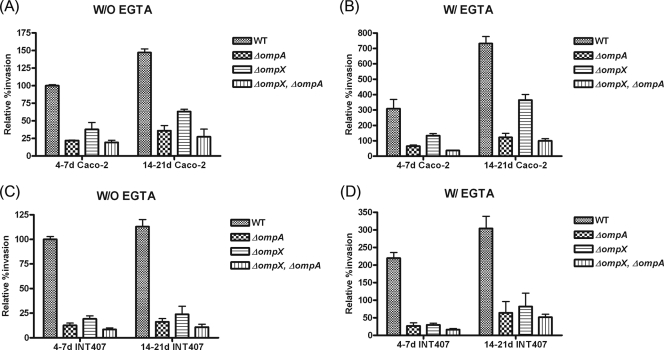
We examined whether the invasion of Caco-2 or INT-407 cells by the mutant strains was affected by tight junctions or cell age (Fig. 5). First, with 14- to 21-day-old Caco-2 cells, the invasion potential increased approximately 1.5-fold (150.1%, 35.9%, 63.1%, and 27.2% for the WT, ΔompA and ΔompX mutants, and ΔompX ΔompA double mutant, respectively) compared with the invasion of 4- to 7-day-old Caco-2 cells (100%, 20%, 40%, and 17%, respectively) (Fig. 5A). Second, when 14- to 21-day-old Caco-2 cells were pretreated with EGTA before bacterial infection, the invasion potential of the WT increased approximately 7.3-fold (Fig. 5B), compared with the invasion of untreated 4- to 7-day-old Caco-2 cells (Fig. 5A). Similarly, 6.0-, 9.0-, and 5.8-fold increases were found for the ΔompA, ΔompX, and ΔompX ΔompA mutants, respectively (Fig. 5A and B). Third, when 14- to 21-day-old INT-407 cells were used, the invasion capability of the C. sakazakii strains was increased by 1.2-fold (Fig. 5C) compared with invasion of 4- to 7-day-old INT-407 cells. Fourth, when EGTA-pretreated 14- to 21-day-old INT407 cells were used, an approximately 3-fold increase in invasion potential was observed, regardless of the C. sakazakii strain, compared with the invasion of untreated 4- to 7-day-old INT-407 cells (Fig. 5D). These data indicate that OmpA and OmpX are required for basolateral invasion of Caco-2 cells by C. sakazakii.
FIG. 5.
Invasion of C. sakazakii into Caco-2 and INT-407 cells after pretreatment with EGTA. The monolayers were either 4 to 7 or 14 to 21 days old and were pretreated (W/ EGTA) or not pretreated (W/O EGTA) with 3 mM EGTA. Internalized bacteria were determined as described in the legend to Fig. 2 using Caco-2 (A and B) and INT-407 (C and D) cells. Percent invasion was determined by comparison to the non-EGTA-treated WT. Note that the scales in the y axes are different.
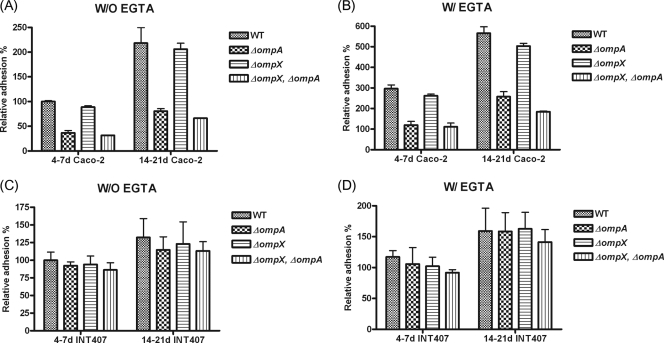
Basolateral adhesion of C. sakazakii to Caco-2 cells is dependent on OmpA, but not OmpX.
To examine whether OmpA or OmpX is required for basolateral adhesion to Caco-2 or INT-407 cells, we measured the initial association of C. sakazakii strains with Caco-2 and INT-407 cells. The association of the WT was increased slightly, by about 2-fold (218.2%), when 14- to 21-day-old Caco-2 cells were used (Fig. 6A) compared with 4- to 7-day-old Caco-2 cells. Similarly, the adhesion of the ΔompA, ΔompX, and ΔompX ΔompA mutants to 14- to 21-day-old Caco-2 cells was also increased, by about 2.2-fold (80.6%), 2.6-fold (178.8%), and 2.1-fold (66.1%), respectively (Fig. 6A), compared with adhesion to 4- to 7-day-old Caco-2 cells (36.5%, 88.7%, and 31.3%, respectively). When the tight junctions of polarized Caco-2 cells were disrupted by EGTA pretreatment, the associations of the WT and ΔompA, ΔompX, and ΔompX ΔompA mutants were significantly increased, by about 5.6-fold (565.6%), 8.6-fold (257.7%), 5-fold (503.3%), and 6-fold (184.4%), respectively, compared with adhesion to non-EGTA-treated Caco-2 cells (100%, 36.5%, 88.7%, and 31.3%, respectively) (Fig. 6B). These data indicate that OmpA, but not OmpX, is required for basolateral adhesion of C. sakazakii to Caco-2 cells.
FIG. 6.
Association of C. sakazakii with Caco-2 and INT-407 cells after pretreatment with EGTA. The monolayers were either 4 to 7 or 14 to 21 days old and were or were not pretreated with 3 mM EGTA. An adhesion assay was performed as described in Materials and Methods. The data are the means and standard deviations of three independent experiments.
For INT-407 cells, adhesion of all of the C. sakazakii strains was increased slightly, by 1.2-fold, when 14- to 21-day-old INT-407 cells were used (Fig. 6C) compared with 4- to 7-day-old cells. When the tight junctions of polarized INT-407 cells were disrupted, the initial association of all C. sakazakii strains tested showed an increase of about 1.6-fold (Fig. 6C). These data suggest that neither OmpA nor OmpX is required for the initial basolateral association of C. sakazakii with INT-407 cells.
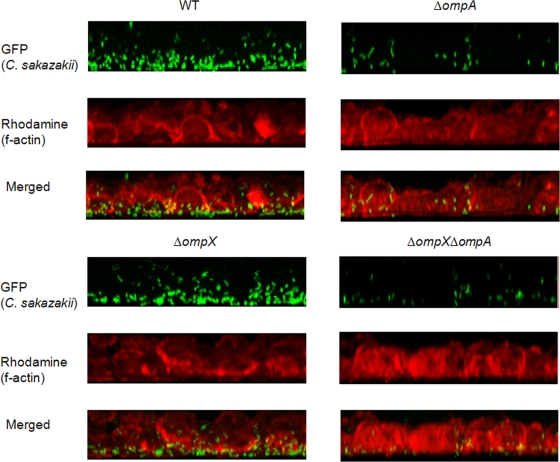
To further examine the roles of OmpA and OmpX, we used confocal fluorescence microscopy to visualize the initial association of the C. sakazakii strains with 14- to 21-day-old Caco-2 cells pretreated with EGTA (Fig. 7). The C. sakazakii strains associated mainly with the basolateral region of Caco-2 cells, and this association was dependent on OmpA, but not OmpX.
FIG. 7.
Confocal fluorescence microscopy of Caco-2 cells that were EGTA treated and infected with C. sakazakii strains containing pWM1007 for 30 min. The f-actin molecules were stained with rhodamine-labeled phalloidin. Different series of images from 1-μm x-y-z sections were obtained, analyzed, and stacked using the recommended software (LSM-FCS).
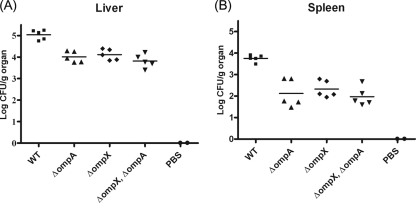
OmpA and OmpX are essential for invasion into deeper tissue in rat pups.
To examine whether the expression of OmpA and/or OmpX is required for C. sakazakii translocation in rat pups, we assessed the virulence of the WT and its isogenic ΔompA, ΔompX, and ΔompX ΔompA mutant strains in vivo. After 24 h of incubation, the number of WT cells in the rat liver was 5.1 log CFU/g, whereas the numbers of ΔompA, ΔompX, and ΔompX ΔompA mutant cells were 4.01, 4.12, and 3.80 log CFU/g, respectively (Fig. 8A). In the rat spleen, the number of recovered WT cells was 3.85 log CFU/g, whereas the numbers of ΔompA, ΔompX, and ΔompX ΔompA mutant cells were 2.12, 2.32, and 1.97 log CFU/g, respectively (Fig. 8B). C. sakazakii was not detected in the PBS-treated group (Fig. 8). There were no significant differences among the mutants (Fig. 8). Therefore, this experiment demonstrated that both OmpA and OmpX contribute to C. sakazakii virulence in rat pups.
FIG. 8.
In vivo animal study. Isogenic C. sakazakii strains grown aerobically were used to infect 2- or 3-day-old rat pups. A group of five rats were orally inoculated with 5 × 109 CFU of C. sakazakii. To analyze bacterial colonization in different organs, the rat pups were anesthetized 24 h postinfection. The spleens and livers were removed, homogenized, and then plated on LB agar plates.
DISCUSSION
The pathogenesis of Cronobacter spp., opportunistic pathogens that cause systemic infection, is virtually unknown. This may be partly because of the lack of appropriate animal models and genetic tools applicable to the genus Cronobacter. Nevertheless, as an oral pathogen, C. sakazakii is thought to be equipped with the means to cross the intestinal barrier (17). In this study, we constructed, for the first time in C. sakazakii, in-frame deletion mutants in the outer membrane proteins, OmpA and OmpX, using the lambda Red recombination system. Cronobacter OmpA was shown previously to play a role in the invasion of various mammalian host cells and is important for Cronobacter resistance to blood and serum killing in newborn rats (24, 25, 31). However, C. sakazakii ompX was first identified by a BLAST search of Enterobacter cloacae ompX against the C. sakazakii BAA-894 genome (locations 2478859 to 2479371) database (81% identity with E. cloacae OmpX) in this study. We report here that OmpX and OmpA are critical for the basolateral invasion of host cells by C. sakazakii and for its movement into deeper organs, such as the liver and spleen.
Few genetic tools applicable to Cronobacter spp. have been reported to date. Only two different ΔompA insertion mutants, generated by double-crossover gene replacement, have been reported (25, 31). In the present study, we applied the lambda Red recombination system to construct ΔompA and ΔompX deletion mutants. This site-specific recombination can generate chromosomal mutations using PCR products with as little as 30 bp of homology at the ends of the gene of interest (5). This system has been used to construct mutant strains in E. coli (5), Salmonella (4, 33), and Klebsiella (15). Here, we successfully constructed ΔompA, ΔompX, and ΔompX ΔompA deletion mutant strains, as confirmed by PCR and the disappearance of the target protein on SDS-PAGE. Given that the whole genome sequence of C. sakazakii BAA-894 is now available (http://www.ncbi.nlm.nih.gov/nuccore/156932229?ordinalpos=1&itool=EntrezSystem2.PEntrez.Sequence.Sequence_ResultsPanel.Sequence_RVDocSum), this mutation system will be useful for generating in-frame deletions in nonessential genes of C. sakazakii, which will facilitate the study of the pathogen.
Invasion (Fig. 2 and 5) and inhibition (blocking) (Fig. 3) studies clearly demonstrated that the OmpX and OmpA proteins are essential for the invasion of Caco-2 and INT-407 cells by C. sakazakii. However, these proteins may have different roles in other host cells. In Caco-2 cells, the invasion rate of the ΔompX mutant was higher than that of either the ΔompA or ΔompX ΔompA mutant, and there was no significant difference between the ΔompA and ΔompX ΔompA mutants (Fig. 2A). This suggests that OmpA plays a more dominant role than OmpX in Caco-2 cell invasion by C. sakazakii. On the other hand, when INT-407 cells were used, a significant difference in the invasion rate was observed between either of the single mutants (ΔompA or ΔompX) and the double mutant (Fig. 2B), suggesting that the two Omp proteins act in a synergistic/additive manner.
OmpX was shown to be involved in the invasion of rabbit ileal tissue by E. cloacae, and Yersinia pestis KIM OmpX is known to be required for adhesion to and invasion of Hep-2 cells (7, 18). In addition, OmpX appears to play an important role in virulence by neutralizing host defense mechanisms and is involved in bacterial defense against the complement systems of the host (10, 38). OmpX has the same β-sheet topology (eight antiparallel amphipathic β-sheets) as the structurally related OmpA. However, their barrel structures differ with respect to the shear numbers (8 in OmpX and 10 in OmpA) and the internal hydrogen-bonding networks. At present, it is not clear whether C. sakazakii OmpA and OmpX share a host receptor for invasion. These data suggest that the receptors for C. sakazakii OmpA and OmpX may be different, as the invasion potentials of the single mutants (ΔompA and ΔompX) were different in the different host cells (Caco-2 and INT-407).
Adhesion studies (Fig. 4 and 6) showed that OmpA was important for binding to intestinal epithelial Caco-2 cells. However, OmpA was not required for binding to INT-407 cells, consistent with the results of previous studies (25). On the other hand, OmpX did not play a significant role in binding to the eukaryotic cells examined within the incubation time used here (30 min). There are several implications of these findings. First, adhesion of C. sakazakii to Caco-2 cells is mediated by OmpA, but adhesion to INT-407 cells is not. Second, the initial association of C. sakazakii with Caco-2 and INT-407 cells is independent of OmpX. Third, the adhesion mediated by OmpA is not an absolute prerequisite for invasion within the incubation time used here, given that the association/dissociation constants between the reacting molecules were not considered. Fourth, C. sakazakii OmpA interacts with more than one cellular molecule.
A previous study showed that when the cellular tight junctions of Caco-2 cell monolayers were removed, the invasion efficiency of C. sakazakii increased markedly, depending on the age of the cells (4 to 7 days versus 17 to 20 days) (17). Considering the importance of tight junctions in protection against bacterial pathogens, it was suggested that the enhanced ability of C. sakazakii to penetrate the host cells in the absence of this physical barrier may be important for the pathogenesis of infection by the organism in premature neonates (25). In this study, we found that the invasion potential of the mutant strains was affected by the presence of tight junctions; OmpA and OmpX mediated basolateral invasion of both Caco-2 and INT-407 cells by C. sakazakii. These data also suggest that the receptor for C. sakazakii invasin is located not only on the apical side, but also on the basolateral side, of intestinal cells.
The present study did not elucidate the precise mechanisms underlying the involvement of OmpA and OmpX in the penetration of host cells by C. sakazakii, and further studies are required to address this issue. Previously, it was shown that E. coli OmpA binds to Ecgp on the surface of HBMECs during invasion (30) and causes a temporary breakdown of the tight junctions and an increase in vascular permeability (34). It will be of interest to investigate whether C. sakazakii OmpA is also able to induce the breakdown of tight junctions. To our knowledge, there have been no previous reports regarding the interaction of OmpX with specific host molecules, although Salmonella enterica serovar Typhimurium pagC, which shows 81% nucleotide identity with C. sakazakii ompX, was reported to be involved in survival in macrophages (22).
The neonatal-rat model was used to assess bacterial translocation from the intestinal tract to deeper tissues following endotoxin administration, inducing apoptosis and intracranial infection (12, 35, 36). We also examined the translocation efficiency of ΔompA and ΔompX mutant strains in rat pups. The absence of ompA and ompX expression resulted in attenuated virulence in orally infected rat pups, as indicated by the decreased bacterial loads in the spleen and liver.
In conclusion, we have shown that OmpA and OmpX expression in C. sakazakii is required for not only apical, but also basolateral, adhesion to and invasion of mammalian cells. OmpA and OmpX show some additive effects in facilitating the invasion of host cells, suggesting that these two outer membrane proteins have different host cell receptors. Further investigations are required to determine the specific mechanisms of the interactions between C. sakazakii outer membrane proteins and host cell receptors.
Acknowledgments
This work was supported by a National Research Foundation of Korea grant funded by the South Korean government (KRF-2008-314-F00061). K.K. and J.-A.L. were the recipients of graduate fellowships provided by the Ministry of Education, Science and Technology through the Brain Korea 21 Project.
Footnotes
Published ahead of print on 11 June 2010.
REFERENCES
- 1.Bollag, D. M., and S. J. Edelstein. 1991. Protein methods. Wiley-Liss, Inc., New York, NY.
- 2.Caubilla-Barron, J., E. Hurrell, S. Townsend, P. Cheetham, C. Loc-Carrillo, O. Fayet, M. F. Prere, and S. J. Forsythe. 2007. Genotypic and phenotypic analysis of Enterobacter sakazakii strains from an outbreak resulting in fatalities in a neonatal intensive care unit in France. J. Clin. Microbiol. 45:3979-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi, J., D. Shin, and S. Ryu. 2007. Implication of quorum sensing in Salmonella enterica serovar Typhimurium virulence: the luxS gene is necessary for expression of genes in pathogenicity island 1. Infect. Immun. 75:4885-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debroy, C., J. Yealy, R. A. Wilson, M. K. Bhan, and R. Kumar. 1995. Antibodies raised against the outer membrane protein interrupt adherence of enteroaggregative Escherichia coli. Infect. Immun. 63:2873-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Kort, G., A. Bolton, G. Martin, J. Stephen, and J. A. van de Klundert. 1994. Invasion of rabbit ileal tissue by Enterobacter cloacae varies with the concentration of OmpX in the outer membrane. Infect. Immun. 62:4722-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurtler, J. B., J. L. Kornacki, and L. R. Beuchat. 2005. Enterobacter sakazakii: a coliform of increased concern to infant health. Int. J. Food Microbiol. 104:1-34. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 10.Heffernan, E. J., L. Wu, J. Louie, S. Okamoto, J. Fierer, and D. G. Guiney. 1994. Specificity of the complement resistance and cell association phenotypes encoded by the outer membrane protein genes rck from Salmonella typhimurium and ail from Yersinia enterocolitica. Infect. Immun. 62:5183-5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter, C. J., M. Petrosyan, H. R. Ford, and N. V. Prasadarao. 2008. Enterobacter sakazakii: an emerging pathogen in infants and neonates. Surg. Infect. 9:533-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter, C. J., V. K. Singamsetty, N. K. Chokshi, P. Boyle, V. Camerini, A. V. Grishin, J. S. Upperman, H. R. Ford, and N. V. Prasadarao. 2008. Enterobacter sakazakii enhances epithelial cell injury by inducing apoptosis in a rat model of necrotizing enterocolitis. J. Infect. Dis. 198:586-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iversen, C., A. Lehner, N. Mullane, E. Bidlas, I. Cleenwerck, J. Marugg, S. Fanning, R. Stephan, and H. Joosten. 2007. The taxonomy of Enterobacter sakazakii: proposal of a new genus Cronobacter gen. nov. and descriptions of Cronobacter sakazakii comb. nov., Cronobacter sakazakii subsp. sakazakii comb. nov., Cronobacter sakazakii subsp. malonaticus subsp. nov., Cronobacter turicensis sp. nov., Cronobacter muytjensii sp. nov., Cronobacter dublinensis sp. nov. and Cronobacter genomospecies 1. BMC Evol. Biol. 7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iversen, C., A. Lehner, N. Mullane, J. Marugg, S. Fanning, R. Stephan, and H. Joosten. 2007. Identification of “Cronobacter” spp. (Enterobacter sakazakii). J. Clin. Microbiol. 45:3814-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janes, B. K., P. J. Pomposiello, A. Perez-Matos, D. J. Najarian, T. J. Goss, and R. A. Bender. 2001. Growth inhibition caused by overexpression of the structural gene for glutamate dehydrogenase (gdhA) from Klebsiella aerogenes. J. Bacteriol. 183:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, K., S. S. Jang, S. K. Kim, J. H. Park, S. Heu, and S. Ryu. 2008. Prevalence and genetic diversity of Enterobacter sakazakii in ingredients of infant foods. Int. J. Food Microbiol. 122:196-203. [DOI] [PubMed] [Google Scholar]
- 17.Kim, K. P., and M. J. Loessner. 2008. Enterobacter sakazakii invasion in human intestinal Caco-2 cells requires the host cell cytoskeleton and is enhanced by disruption of tight junction. Infect. Immun. 76:562-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolodziejek, A. M., D. J. Sinclair, K. S. Seo, D. R. Schnider, C. F. Deobald, H. N. Rohde, A. K. Viall, S. S. Minnich, C. J. Hovde, S. A. Minnich, and G. A. Bohach. 2007. Phenotypic characterization of OmpX, an Ail homologue of Yersinia pestis KIM. Microbiology 153:2941-2951. [DOI] [PubMed] [Google Scholar]
- 19.Kothary, M. H., B. A. McCardell, C. D. Frazar, D. Deer, and B. D. Tall. 2007. Characterization of the zinc-containing metalloprotease encoded by zpx and development of a species-specific detection method for Enterobacter sakazakii. Appl. Environ. Microbiol. 73:4142-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kucerova, E., S. W. Clifton, X. Q. Xia, F. Long, S. Porwollik, L. Fulton, C. Fronick, P. Minx, K. Kyung, W. Warren, R. Fulton, D. Feng, A. Wollam, N. Shah, V. Bhonagiri, W. E. Nash, K. Hallsworth-Pepin, R. K. Wilson, M. McClelland, and S. J. Forsythe. 2010. Genome sequence of Cronobacter sakazakii BAA-894 and comparative genomic hybridization analysis with other Cronobacter species. PLoS One 5:e9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mange, J. P., R. Stephan, N. Borel, P. Wild, K. S. Kim, A. Pospischil, and A. Lehner. 2006. Adhesive properties of Enterobacter sakazakii to human epithelial and brain microvascular endothelial cells. BMC Microbiol. 6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mecsas, J., R. Welch, J. W. Erickson, and C. A. Gross. 1995. Identification and characterization of an outer membrane protein, OmpX, in Escherichia coli that is homologous to a family of outer membrane proteins including Ail of Yersinia enterocolitica. J. Bacteriol. 177:799-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, W. G., A. H. Bates, S. T. Horn, M. T. Brandl, M. R. Wachtel, and R. E. Mandrell. 2000. Detection on surfaces and in Caco-2 cells of Campylobacter jejuni cells transformed with new gfp, yfp, and cfp marker plasmids. Appl. Environ. Microbiol. 66:5426-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittal, R., Y. Wang, C. J. Hunter, I. Gonzalez-Gomez, and N. V. Prasadarao. 2009. Brain damage in newborn rat model of meningitis by Enterobacter sakazakii: a role for outer membrane protein A. Lab. Invest. 89:263-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohan Nair, M. K., and K. Venkitanarayanan. 2007. Role of bacterial OmpA and host cytoskeleton in the invasion of human intestinal epithelial cells by Enterobacter sakazakii. Pediatr. Res. 62:664-669. [DOI] [PubMed] [Google Scholar]
- 26.Mullane, N. R., D. Drudy, P. Whyte, M. O'Mahony, A. G. M. Scannell, P. G. Wall, and S. Faning. 2006. Enterobacter sakazakii biological properties and significance in dried infant milk formula (IMF) powder. Int. J. Dairy Technol. 59:102-111. [Google Scholar]
- 27.Mullane, N. R., C. Iversen, B. Healy, C. Walsh, P. Whyte, P. G. Wall, T. Quinn, and S. Fanning. 2007. Enterobacter sakazakii an emerging bacterial pathogen with implications for infant health. Minerva Pediatr. 59:137-148. [PubMed] [Google Scholar]
- 28.Nazarowec-White, M., and J. M. Farber. 1997. Enterobacter sakazakii: a review. Int. J. Food Microbiol. 34:103-113. [DOI] [PubMed] [Google Scholar]
- 29.Pagotto, F. J., M. Nazarowec-White, S. Bidawid, and J. M. Farber. 2003. Enterobacter sakazakii: infectivity and enterotoxin production in vitro and in vivo. J. Food Prot. 66:370-375. [DOI] [PubMed] [Google Scholar]
- 30.Prasadarao, N. V., P. K. Srivastava, R. S. Rudrabhatla, K. S. Kim, S. H. Huang, and S. K. Sukumaran. 2003. Cloning and expression of the Escherichia coli K1 outer membrane protein A receptor, a gp96 homologue. Infect. Immun. 71:1680-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singamsetty, V. K., Y. Wang, H. Shimada, and N. V. Prasadarao. 2008. Outer membrane protein A expression in Enterobacter sakazakii is required to induce microtubule condensation in human brain microvascular endothelial cells for invasion. Microb. Pathog. 45:181-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skovgaard, N. 2007. New trends in emerging pathogens. Int. J. Food Microbiol. 120:217-224. [DOI] [PubMed] [Google Scholar]
- 33.Stanley, T. L., C. D. Ellermeier, and J. M. Slauch. 2000. Tissue-specific gene expression identifies a gene in the lysogenic phage Gifsy-1 that affects Salmonella enterica serovar typhimurium survival in Peyer's patches. J. Bacteriol. 182:4406-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sukumaran, S. K., and N. V. Prasadarao. 2003. Escherichia coli K1 invasion increases human brain microvascular endothelial cell monolayer permeability by disassembling vascular-endothelial cadherins at tight junctions. J. Infect. Dis. 188:1295-1309. [DOI] [PubMed] [Google Scholar]
- 35.Townsend, S., J. Caubilla Barron, C. Loc-Carrillo, and S. Forsythe. 2007. The presence of endotoxin in powdered infant formula milk and the influence of endotoxin and Enterobacter sakazakii on bacterial translocation in the infant rat. Food Microbiol. 24:67-74. [DOI] [PubMed] [Google Scholar]
- 36.Townsend, S. M., E. Hurrell, I. Gonzalez-Gomez, J. Lowe, J. G. Frye, S. Forsythe, and J. L. Badger. 2007. Enterobacter sakazakii invades brain capillary endothelial cells, persists in human macrophages influencing cytokine secretion and induces severe brain pathology in the neonatal rat. Microbiology 153:3538-3547. [DOI] [PubMed] [Google Scholar]
- 37.van Acker, J., F. de Smet, G. Muyldermans, A. Bougatef, A. Naessens, and S. Lauwers. 2001. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J. Clin. Microbiol. 39:293-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogt, J., and G. E. Schulz. 1999. The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence. Structure 7:1301-1309. [DOI] [PubMed] [Google Scholar]
- 39.Willis, J., and J. E. Robinson. 1988. Enterobacter sakazakii meningitis in neonates. Pediatr. Infect. Dis. J. 7:196-199. [DOI] [PubMed] [Google Scholar]