Abstract
Although lignocellulosic sugars have been proposed as the primary feedstock for the biological production of renewable fuels and chemicals, the availability of fatty acid (FA)-rich feedstocks and recent progress in the development of oil-accumulating organisms make FAs an attractive alternative. In addition to their abundance, the metabolism of FAs is very efficient and could support product yields significantly higher than those obtained from lignocellulosic sugars. However, FAs are metabolized only under respiratory conditions, a metabolic mode that does not support the synthesis of fermentation products. In the work reported here we engineered several native and heterologous fermentative pathways to function in Escherichia coli under aerobic conditions, thus creating a respiro-fermentative metabolic mode that enables the efficient synthesis of fuels and chemicals from FAs. Representative biofuels (ethanol and butanol) and biochemicals (acetate, acetone, isopropanol, succinate, and propionate) were chosen as target products to illustrate the feasibility of the proposed platform. The yields of ethanol, acetate, and acetone in the engineered strains exceeded those reported in the literature for their production from sugars, and in the cases of ethanol and acetate they also surpassed the maximum theoretical values that can be achieved from lignocellulosic sugars. Butanol was produced at yields and titers that were between 2- and 3-fold higher than those reported for its production from sugars in previously engineered microorganisms. Moreover, our work demonstrates production of propionate, a compound previously thought to be synthesized only by propionibacteria, in E. coli. Finally, the synthesis of isopropanol and succinate was also demonstrated. The work reported here represents the first effort toward engineering microorganisms for the conversion of FAs to the aforementioned products.
Concerns about climate change and the depletion and cost of petroleum resources have ignited interest in the establishment of a bio-based industry (5, 49, 61), and the conceptual model of a biorefinery has emerged (27, 28, 45). Given its abundance in nature, the carbohydrate portion of edible crops such as sugarcane, sugar beet, maize (corn), and sorghum is currently used as the primary feedstock in the biological production of fuels and chemicals (12, 49, 52). Although the use of nonedible lignocellulosic sugars has been proposed as an efficient and sustainable avenue to the aforementioned processes, the availability of fatty acid (FA)-rich feedstocks and recent progress in the development of oil-accumulating organisms make FAs an attractive alternative. Edible oil-rich crops such as rapeseed, sunflower, soybean, and palm are currently available and widely used as feedstocks for chemical conversion to biodiesel (6), while oleaginous algae and nonedible FA-rich crops along with industrial by-products are receiving greater attention as longer-term alternatives. These nonedible FA-rich feedstocks are presently generated in large amounts and can be exploited for the biological production of fuels and chemicals (14, 22, 51, 56, 57). Unfortunately, microbial platforms to enable this are at present almost absent.
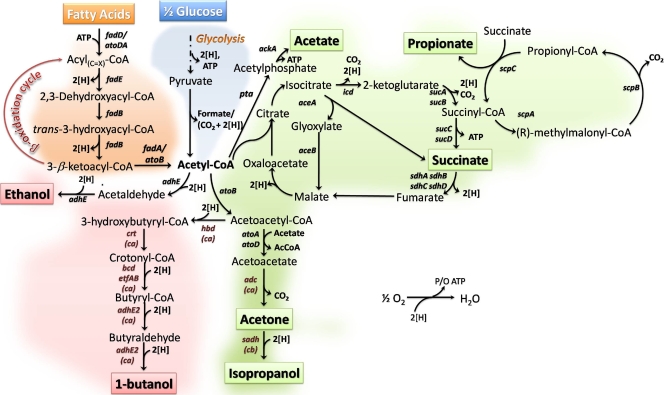
FAs not only are abundant but also offer several advantages when used for fuel and chemical production. For example, their metabolism to the key intermediate metabolite acetyl coenzyme A (acetyl-CoA) is very efficient, as it results in 100% carbon recovery (Fig. 1). Since many fuels and chemicals can be derived from acetyl-CoA, high yields can be realized if FAs are used as the carbon source. In contrast, sugar metabolism generates one molecule of carbon dioxide (or formate) per molecule of acetyl-CoA, limiting the yield of products derived from acetyl-CoA (Fig. 1). The product yield advantage of FAs over sugars is also supported by the more highly reduced nature of their carbon atoms. Table 1 provides a comparison of maximum theoretical yields, on both weight and carbon bases, for the production of biofuels and biochemicals from FAs and lignocellulosic sugars. Maximum theoretical yields have been calculated from stoichiometry based on the pathways shown in Fig. 1 for the utilization of FAs and glucose, the synthesis of products, the tricarboxylic acid (TCA) cycle, and oxidative phosphorylation. The stoichiometric coefficients were obtained by conducting elemental balances on carbon, hydrogen, and oxygen, and an ATP balance was also included in the analysis. As an example, when production of biofuels (e.g., ethanol and butanol) is considered, utilization of FAs (e.g., palmitic acid [C16:0]) as a substrate returns product yields 2.7-fold (wt/wt) or 1.4-fold (C/C) higher than those for sugars (calculations are provided for glucose but are equally valid for other lignocellulosic sugars). Although the current prices of feedstocks on a weight basis are comparable (lower than 20¢/pound), the data reported in Fig. S1a in the supplemental material show that the price per carbon for glucose derived from corn is remarkably higher. Regardless of the basis used for calculations (i.e., weight or carbon basis), when maximum theoretical yields and costs of FA and sugar feedstocks are accounted for, the advantages of using FAs are remarkable (see Fig. S1b in the supplemental material).
FIG. 1.
Pathways engineered in E. coli for the conversion of fatty acids to fuels (red) and chemicals (green). Also shown is the catabolism of fatty acids via the β-oxidation pathway (orange) and of glucose through the Embden-Meyerhof-Parnas pathway (blue). Relevant reactions are represented by the names of the genes coding for the enzymes (E. coli genes unless otherwise specified in parentheses as follows: C. acetobutylicum, ca; C. beijerinckii, cb): aceA, isocitrate lyase; aceB, malate synthase A; adc, acetoacetate decarboxylase (ca); ackA, acetate kinase; adh, secondary alcohol dehydrogenase (cb); adhE, acetaldehyde/alcohol dehydrogenase; adhE2, secondary alcohol dehydrogenase (ca); atoA and atoD, acetyl-CoA:acetoacetyl-CoA transferase; atoB, acetyl-CoA acetyltransferase; bcd, butyryl-CoA dehydrogenase (ca); crt, crotonase (ca); etfAB, electron transfer flavoprotein (ca); fadA, 3-ketoacyl-CoA thiolase; fadB, enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase; fadD, acyl-CoA synthetase; fadE, acyl-CoA dehydrogenase; hbd, β-hydroxybutyryl-CoA dehydrogenase (ca); icd, isocitrate dehydrogenase; pta, phosphate acetyltransferase; sdhABCD, succinate dehydrogenase; scpA, methylmalonyl-CoA mutase; scpB, methylmalonyl-CoA decarboxylase; scpC, propionyl-CoA:succinate CoA transferase; sucA, 2-oxoglutarate dehydrogenase; sucB, dihydrolipoyltranssuccinylase; and sucCD, succinyl-CoA synthetase. Abbreviations: 2[H] = NADH = FADH2 = QH2 = H2; P/O, amount of ATP produced per oxygen consumed in the oxidative phosphorylation.
TABLE 1.
Comparison of maximum theoretical yields for the production of biofuels and biochemicals from fatty acids (palmitic acid) and lignocellulosic sugars (glucose)
| Pathway stoichiometry for the synthesis of the specified product from glucose (C6H12O6) or palmitic acid (C16H32O2)a | Maximum yield (wt basis/C basis) |
|---|---|
| Biofuels | |
| Ethanol (C2H6O) | |
| C6H12O6 → 2C2H6O + 2CO2 | 0.51/0.67 |
| C16H32O2 → 23/3C2H6O + 2/3CO2 | 1.38/0.96 |
| C16H32O2 + 51/7H2O → 53/7C2H6O + 6/7CO2 + 8/7[H]; 8/7[H] + 2/7O2 → 4/7H2O | 1.36/0.95 |
| Butanol (C4H10O) | |
| C6H12O6 → C4H10O + 2CO2 +H2O | 0.41/0.67 |
| C16H32O2 + 7/2H2O → 53/14C4H10O + 6/7CO2 + 8/7[H]; 8/7[H] + 2/7O2 → 4/7H2O | 1.10/0.95 |
| Biochemicals | |
| Acetate (C2H4O2) | |
| C6H12O6 + 2H2O → 3C2H4O2 | 1.00/1.00 |
| C16H32O2 + 7H2O + 7CO2 → 23/2C2H4O2 | 2.70/1.44 |
| Acetone (C3H6O) | |
| C6H12O6 → 3/2C3H6O + 3/2CO2 + 3/2H2O | 0.48/0.75 |
| C16H32O2 + 5/4H2O + 5/4CO2 → 23/4C3H6O | 1.30/1.08 |
| Isopropanol (C3H8O) | |
| C6H12O6 → 4/3C3H8O + 2CO2 + 2/3H2O | 0.44/0.67 |
| C16H32O2 + 40/9H2O → 46/9C3H8O + 2/3CO2 | 1.20/0.96 |
| Succinate (C4H6O4) | |
| C6H12O6 + 6/7CO2 → 12/7C4H6O4 + 6/7H2O | 1.12/1.14 |
| C16H32O2 + 152/17CO2 + 86/17H2O → 106/17C4H6O4 + 80/17[H]; 80/17[H] + 20/17O2 → 40/17H2O | 2.87/1.56 |
| Propionate (C3H6O2) | |
| C6H12O6 → 12/7C3H6O2 + 6/7CO2 + 6/7H2O | 0.70/0.86 |
| C16H32O2 + 262/83CO2 + 370/83H2O → 530/83C3H6O2 + 216/83[H]; 216/83[H] + 54/83O2 → 108/83H2O | 1.81/1.20 |
Stoichiometry is based on the pathways shown in Fig. 1 for the utilization of FAs and glucose, the synthesis of products, the TCA cycle, and oxidative phosphorylation. For the synthesis of biochemicals, CO2 fixation via the Wood-Ljungdahl pathway (50) (2CO2 + ATP + 8[H] → acetyl-CoA) or the carboxylation of phosphoenolpyruvate (54) (phosphoenolpyruvate + CO2 → oxaloacetate + ATP) were also considered (not shown in Fig. 1). The stoichiometric coefficients were obtained by conducting elemental balances on carbon, hydrogen, and oxygen. An ATP balance was also included in the analysis for the reactions shown in italics. All other reactions represent ATP-generating pathways. Every acetyl-CoA oxidized through the TCA cycle generates three NADH, one reduced flavin adenine dinucleotide (FADH2), and one ATP equivalent. Eleven ATPs can be generated from the oxidation of the NADH and FADH2 produced in the TCA cycle (two and three ATPs per FADH2 and NADH, respectively) via coupling between the electron transfer chain and oxidative phosphorylation.
Despite the aforementioned advantages, biological conversion of FA-rich feedstocks has been exploited only for the production of polyhydroxyalkanoates (46, 47), with no report to date of organisms engineered for the conversion of FAs to fuels and chemicals (see the text in the supplemental material for more details).
Escherichia coli is one of the most amenable organisms to industrial applications and has been engineered for biofuel production (52). The utilization of FAs in E. coli is mediated by enzymes encoded by the fad regulon and the ato operon (11) (Fig. 1). Products of the fad regulon mediate the transport, acylation, and β-oxidation of medium-chain (C7 to C11) and long-chain (C12 to C18) FAs. Two additional enzymes encoded by the atoD-atoA and atoB genes (part of the atoDAEB operon) are also required for the growth of E. coli on short-chain (C4 to C6) FAs (25). The expression of the fad regulon and ato operon is controlled by FadR (fadR) and AtoC (atoC), respectively (44).
While advantageous, the high degree of reduction of carbon in FAs also poses a metabolic challenge because their average degree of reduction per carbon is higher than in biomass. Therefore, the incorporation of fatty acids into cell mass generates reducing equivalents (Fig. 1) and hence requires the presence of an external electron acceptor. That is, the aforementioned pathways are active only in the respiratory metabolism of FAs, which leads to the synthesis of cell mass and carbon dioxide but no other metabolic product. Therefore, fuel and chemical production from FAs requires the engineering of a respiro-fermentative metabolic mode that would support the synthesis of fermentative products during respiratory metabolism of FAs. To this end, we metabolically engineered native and heterologous pathways for the efficient catabolism of FAs and the synthesis of fuels and chemicals in E. coli. Biofuels, commodity chemicals, and polymer building blocks were chosen as model products to illustrate the feasibility of the proposed approach.
MATERIALS AND METHODS
Strains, plasmids, and genetic methods.
The strains, plasmids, and primers used in this study are listed in Table 2. Wild-type E. coli K-12 strain MG1655 (29) was used as the host to implement metabolic engineering strategies. Gene knockouts were introduced in MG1655 and its derivatives by P1 phage transduction as described elsewhere (39), using as donors single-gene knockout mutants from the National BioResource Project (NIG, Japan) (3). Details of the specific protocol used have been described before (66). An MG1655 fadR* atoC(Con) mutant, which exhibits constitutive expression of the fad regulon (due to fadR*) and the ato operon [due to atoC(Con)], was obtained as follows. Strain MG1655 fadR* was isolated as a spontaneous mutant able to grow on MOPS (morpholinepropanesulfonic acid) minimal medium (41) plates containing 0.2% (wt/vol) decanoic acid (C10:0) as the sole carbon source. While able to metabolize medium-chain FAs (e.g., C10:0), strain fadR* was unable to grow on short-chain FAs (e.g., C6:0) (see Fig. S2 in the supplemental material). The fadR* atoC(Con) strain was then obtained by transducing fadR* with a phage lysate from strain LS5218, which is a FadR* AtoC(Con) mutant (25, 59). Successful transduction of the atoC(Con) mutation into the fadR strain* was identified by growth on MOPS minimal medium plates containing 0.2% (wt/vol) hexanoic acid (C6:0). Further details on the growth of strain MG1655 and the fadR* and fadR*atoC(Con) mutants on FAs of different chain lengths are provided in Fig. S2 in the supplemental material.
TABLE 2.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Description, genotype, or sequence | Reference |
|---|---|---|
| Strains | ||
| MG1655 | F− λ−ilvG rfb-50 rph-1 | 29 |
| MG1655 ΔadhE | MG1655 ΔadhE::FRT-Kan-FRT; adhE deletion mutant in MG1655 | This study |
| MG1655 ΔsdhB | MG1655 ΔsdhB::FRT-Kan-FRT; sdhB deletion mutant of MG1655 | This study |
| LS5218 | fadR601 atoC(Con)2 | 59 |
| MG1655 fadR* | MG1655 evolved for rapid growth on decanoic acid | This study |
| MG1655 fadR* atoC(Con) | MG1655 fadR* transduced with the atoC(Con) gene of LS5218 | This study |
| MG1655 fadR* atoC(Con) ΔadhE | MG1655 fadR* atoC(Con) ΔadhE::FRT-Kan-FRT; adhE deletion mutant of MG1655 fadR* atoC(Con) | This study |
| MG1655 fadR* atoC(Con) ΔsucA | MG1655 fadR* atoC(Con) ΔsucA::FRT-Kan-FRT; sucA deletion mutant of MG1655 fadR* atoC(Con) | This study |
| MG1655 fadR* atoC(Con) ΔsdhB | MG1655 fadR* atoC(Con) ΔsdhB::FRT-Kan-FRT; sdhB deletion mutant of MG1655 fadR* atoC(Con) | This study |
| MG1655 fadR* atoC(Con) ΔsucD | MG1655 fadR* atoC(Con) ΔsucD::FRT-Kan-FRT; sucD deletion mutant of MG1655 fadR* atoC(Con) | This study |
| Plasmids | ||
| pTH.adhE* | E. coli adhE mutant (E568K) under the control of Ptrc (Ampr, oriR pBR322) | This study |
| pZS.crt.bcd.etfAB.hbd | C. acetobutylicum butyryl-CoA synthesis operon (crt, bcd, etfAB, hbd) under the control of PLtetO-1 (Tetr, oriR SC101*, cat) | This study |
| pTH.atoB.adhE2 | E. coli atoB gene and C. acetobutylicum adhE2 gene under the control of Ptrc (Ampr, oriR pBR322) | This study |
| pTH.atoDAB.adc | E. coli atoD, atoA, and atoB genes and C. acetobutylicum adc gene under the control of Ptrc (Ampr, oriR pBR322) | This study |
| pZS.sadh | C. acetobutylicum sadH gene under the control of PLtetO-1 (Tetr, oriR SC101*, cat) | This study |
| pZS.ackA.pta | E. coli ackA-pta operon under the control of PLtetO-1 (Tetr, oriR SC101*, cat) | This study |
| pZS.ackA.pta.sadh | E. coli ackA-pta operon and C. acetobutylicum sadH gene under the control of PLtetO-1 (Tetr, oriR SC101*, cat) | This study |
| pTrc.scpABC | E. coli scpA, scpB, and scpC genes under the control of PLtetO-1 (Ampr, oriR pBR322) | This study |
| Primersa | ||
| v-adhE | GTTTAACATTATCAGGAG; GTCAACTAATCCTTAAC | This study |
| v-sucA | CACATCACTGTGCGTGGTAGTATCC; CAGGTCAGGGACCAGAATATCTACG | This study |
| v-sdhB | CTTCCGTACCGAAAGCCGTG; ACCACGCACAGTGATGTGCG | This study |
| v-sucD | GACAGCGGCCTGAATATTATTGCAG; CATCGCGATAAGCACAAAAAAGGCC | This study |
| m-adhE | CATCCGGAAACTCACTTCGAAAAGCTGGCGCTG; CAGCGCCAGCTTTTCGAAGTGAGTTTCCGGA | This study |
| c-adhE | CATTAAAGAGGAGAAAGGTACCATGGCTGTTACTAATG; GATGCCTCTAGCACGCGTTTAAGCGGATTTTTTCG | This study |
| c-ackA.pta | CATTAAAGAGGAGAAAGGTACCATGTCGAGTAAGTTAG; GATGCCTCTAGCACGCGTTTACTGCTGCTGTGC | This study |
| c-ackA.pta.sadh | CTGCACAGCAGCAGTAAACGCGTGAGGAATGAAAGGCTTTGCG; GATGCCTCTAGCACGCGTTTACAGAATCACCACCGC | This study |
| c-sadh | CATTAAAGAGGAGAAAGGTACCATGAAAGGCTTTGCGATGCTG; GATGCCTCTAGCACGCGTTTACAGAATCACCACCGC | This study |
| c-crt.bcd.etfAB.hbd | GATGGTACCATGGAACTAAACAATGTCATCCTTG; GATCACGCGTTTATTTTGAATAATCGTAGAAACC | This study |
| c-atoB | GAGATCTGCAGCTGGTACCATGAAAAATTGTGTCATC; CTTTTTGATTTGTAACTTTCATTTAATTCAACCGTTCAATCACC | This study |
| c-adhE2 | GGTGATTGAACGGTTGAATTAAATGAAAGTTACAAATCAAAAAG; CGGGCCCAAGCTTCGAATTCTTAAAATGATTTTATATAGATATCC | This study |
| c-atoB.adhE2 | GAGATCTGCAGCTGGTACCATGAAAAATTGTGTCATC; CGGGCCCAAGCTTCGAATTCTTAAAATGATTTTATATAGATATCC | This study |
| c-scpABC | GCTCTAGAATGTCTTATCAGTATGTTAAGG; GCTCTAGATTAATCATGATGCTGGC | This study |
| c-scpAargKscpBC | CGGAATTCATGTCTAACGTGCAGGAGTGG; GACAAGCTTTTAACCCAGCATCGAGCCG | This study |
| c-atoDA | CGGGATCCATGAAAACAAAATTGATGAC; GAGGTACCTCATAAATCACCCCGTTG | This study |
| c-adc | GCGGTACCATGTTAAAGGATGAAG; CGGAATTCTTAAGATAATCATATATAAC | This study |
| c-atoB | GACGGTACCAGGAGGAAATGAAAAATTGTGTCATCGTCAGTGC; GACGGTACCTTCCTCCTTTAATTCAACCGTTCAATCACCATCGC | This study |
v, primer sequences that were used for verification purposes during the creation of disruption mutants by phage transduction. m, primers used for site-directed mutagenesis of adhE. c, primers used for cloning purposes. Sequences are in the 5′-to-3′ direction, and the forward follows the reverse sequence in each case, separated by a semicolon. Genes or operons deleted or cloned are apparent from the primer names.
Standard recombinant DNA procedures were used for gene cloning, plasmid isolation, and electroporation. Manufacturer protocols and standard methods (39, 53) were followed for DNA purification (Qiagen, CA), restriction endonuclease digestion (New England Biolabs, MA), and DNA amplification (Stratagene, CA, and Invitrogen, CA). The strains were kept in 32.5% glycerol stocks at −80°C. Plates were prepared using Luria-Bertani (LB) medium containing 1.5% agar, and appropriate antibiotics were included at the following concentrations: 100 μg/ml ampicillin, 50 μg/ml kanamycin, 34 μg/ml chloramphenicol, and 12.5 μg/ml tetracycline. All strains created were confirmed by PCR using the verification primers listed in Table 2 and appropriate phenotypic tests, if suitable.
The overexpression of an aerotolerant E. coli alcohol/acetaldehyde dehydrogenase mutant (i.e., AdhE*) was facilitated by the construction of expression vector pTHadhE*. The adhE coding region was PCR amplified using genomic DNA of E. coli MG1655 as the template and c-adhE primers (Table 2). The resulting PCR product was cloned into vector pUC19 (New England BioLabs). A QuikChange site-directed mutagenesis kit from Stratagene Inc. (La Jolla, CA) was then used to generate a Glu568Lys (E568K) mutation in the adhE coding sequence using primers m-adhE (Table 2). The E568K adhE mutation (adhE*), encoding an aerobically active AdhE protein, was then subcloned into the vector pTrc His 2A from Invitrogen Corp. (Carlsbad, CA) using c-adhE primers (Table 2).
The butanol pathway from Clostridium acetobutylicum ATCC 824 was reconstructed in E. coli using plasmids pZS.crt.bcd.etfAB.hbd and pTH.atoB.adhE2. pZS.crt.bcd.etfAB.hbd was constructed by first amplifying the crt-bcd-etfAB-hbd operon from C. acetobutylicum ATCC 824 genomic DNA with primers c-crt.bcd.etfAB.hbd (Table 2) and then ligating the resulting PCR product into the KpnI and MluI sites of pZSKLM (66). The pTrc.atoB.adhE2 plasmid was constructed by using overlap PCR to fuse the PCR-amplified E. coli atoB gene with the PCR-amplified C. acetobutylicum adhE2 gene. The fused atoB-adhE2 genes were then cloned into pTrc-His2A (Invitrogen, Carlsbad, CA) using the In-Fusion PCR cloning system from Clontech Laboratories, Inc. (Mountain View, CA). Details about the primers used along with promoters and antibiotic markers are provided in Table 2.
The phosphotransacetylase (PTA)-acetate kinase (ACK) pathway for the synthesis of acetate from acetyl-CoA (11) (Fig. 1) was amplified through overexpression of the ackA-pta operon (pZS.ackA.pta). The ackA-pta operon was PCR amplified using genomic DNA from strain MG1655 and primers c-ackA.pta (Table 2). The resulting PCR product was cloned into pZSKLM (66) via In-Fusion PCR cloning (Clontech Laboratories, Inc., Mountain View, CA).
The production of acetone was evaluated using plasmid pTrc.atoDAB.adc, which was constructed as follow. The E. coli atoDA genes were amplified using primers c-atoDA (Table 2) and the PCR product directionally cloned into the BamHI and KpnI restriction sites of pTrc His 2A (Invitrogen Corp., Carlsbad, CA). The resulting plasmid was named pTrc.atoDA. The C. acetobutylicum ATCC 824 adc gene was then cloned into pTrc.atoDA within the KpnI and EcoRI restriction sites (c-adc) (Table 2), yielding pTrc.atoDA.adc. Lastly, the E. coli atoB gene was amplified (c-atoB) (Table 2) and subsequently cloned within the KpnI restriction sites of pTrc.atoDA.adc, yielding the final vector pTrc.atoDAB.adc. Multiple colonies were screened by restriction analysis to assess the directionality of the atoB insertion.
To facilitate the conversion of acetone to isopropanol, the sadH gene from C. acetobutylicum was custom synthesized by GenScript Corp. (Piscataway, NJ), PCR amplified with primers c-sadh (Table 2), and cloned into pZSKLM (66) using the In-Fusion PCR cloning system from Clontech Laboratories, Inc. (Mountain View, CA). The same approach was used to clone sadH into pZS.ackA.pta, thus generating pZS.ackA.pta.sadh (Table 2).
The production of propionate from FAs was tested by cloning the E. coli genes scpA, scpB, and scpC into pTrc99a (Invitrogen, Carlsbad, CA). To this effect, the scpA-argK-scpB-scpC operon from E. coli MG1655 was PCR amplified using primers c-scpAargKscpBscpC (Table 2) and cloned into pTrc99a, both digested with EcoRI and HindIII. PCR was used to amplify this construct with the exception of argK (primers c-scpABC) (Table 2), adding a flanking XbaI site downstream of scpA and upstream of scpB. XbaI digestion and religation of this PCR product and cloning into pTrc-His2A rendered pTrc.scpABC.
Culture medium and cultivation conditions.
MOPS minimal medium (41) supplemented with 0.5% (wt/vol) palmitic acid and 0.2% (wt/vol) Brij 58 (Fluka Chemie AG, Buchs, Switzerland) was used. The oxygen transfer rate (kLa) was estimated from previous reports on gas-liquid mass transfer in shake flask systems (37, 63) and further confirmed by in-vessel measurements conducted by the conventional dynamic gassing-out technique (14).
Unless otherwise stated, all chemicals for culture media were obtained from Fisher Scientific (Pittsburgh, PA) and Sigma-Aldrich Co. (St. Louis, MO). MOPS minimal medium, as designed by Neidhardt et al. (41), supplemented with 0.5% (wt/vol) palmitic acid and 0.2% (wt/vol) Brij 58 (Fluka Chemie AG, Buchs, Switzerland) was used, unless otherwise stated. When required the medium was supplemented with 100 μg/ml ampicillin, 50 μg/ml kanamycin, 34 μg/ml chloramphenicol, and 12.5 μg/ml tetracycline. Isopropyl-beta-d-thiogalactopyranoside (IPTG) (0.1 mM) and anhydrotetracycline (100 ng/ml) were used to induce gene expression from constructed plasmids.
Prior to use, cultures (stored as glycerol stocks at −80°C) were streaked onto LB plates (with appropriate antibiotics if required) and incubated overnight at 37°C. A single colony was used to inoculate 10 ml of LB broth in 15-ml test tubes (GeneMate, Kaysville, UT), which were incubated at 37°C until an optical density at 550 nm (OD550) of ∼0.5 was reached. An appropriate volume of this actively growing preculture was centrifuged and the pellet washed and used to inoculate 25 ml of medium in 50-ml shake flasks to an initial OD550 of 0.05. Fifty-milliliter shake flasks (Corning Glass Works, Corning, NY) with four baffles and plastic foam stoppers were used for aerobic cultures. Flasks were incubated for 72 h at 37°C in a C24 rotary incubator shaker (New Brunswick Scientific, NJ). Samples were centrifuged to pellet cells, while the aqueous supernatant was collected for metabolite analysis.
For ethanol and butanol production by high-cell-density cultures, 10 ml LB liquid medium in a test tube was inoculated with E. coli strains and incubated at 37°C in a rotator (Glas-Col Inc., Terre Haute, IN) until an OD550 of 0.7 was reached. This 10 ml culture was used to inoculate 50 ml of LB medium in a 125-ml conical flask (Corning Glass Works, Corning, NY) and incubated at 37°C in a C24 Rotary Incubator Shaker until an OD550 of 0.7 was reached. Cells were collected by centrifugation at 8,000 × g and 4°C for 20 min and washed twice with MOPS minimal medium. The collected cells were resuspended in MOPS minimal medium to a final OD550 of 10. The cell suspension (25 ml) was transferred to a 50-ml baffled shake flask, and palmitic acid (C16:0) was added to a final concentration of 0.5% (wt/vol). Additional palmitic acid (0.5%, wt/vol) was added to the medium at the indicated time intervals.
For acetate production by high-cell-density cultures, 12.5 ml LB liquid medium in a test tube was inoculated with E. coli strains and incubated at 37°C in a rotator (Glas-Col Inc., Terre Haute, IN) until an OD550 of 0.8 was reached. This 12.5-ml culture was used to inoculate 250 ml of LB medium in a 500-ml conical flask (Corning Glass Works, Corning, NY) and incubated at 37°C in a C24 rotary incubator shaker until an OD550 of 0.8 was reached. Cells were collected by centrifugation at 8,000 × g and 4°C for 20 min and washed twice with MOPS minimal medium. The collected cells were resuspended in MOPS minimal medium to a final OD550 of 10. The cell suspension (50 ml) was transferred to a 500-ml fermenter in a SixFors multifermentation system (Infors HT, Bottmingen, Switzerland) with independent control of temperature (37°C), pH (controlled at 7.0 with NaOH 10 M), stirrer speed (500 rpm), and dissolved oxygen (not controlled) (64). Palmitic acid (C16:0) was added to a final concentration of 0.5% (wt/vol). Additional palmitic acid (0.5% wt/vol) was added to the medium at the indicated time intervals. Microaerobic conditions were maintained by sparging the medium with air at 0.05 liter/min.
Enzyme activities.
The activity of alcohol dehydrogenase (AdhE) was measured by the procedure reported by Kessler and coworkers (30). E. coli cells from anaerobic or aerobic cultures (OD550 of ∼0.7) were harvested by centrifugation (2 min, 10,000 × g), washed twice with a solution of NaCl (9 g/liter), and stored as cell pellets at −20°C. For assays, cell pellets were resuspended in 0.2 ml of 0.1 M MOPS-KOH and permeabilized by vortex mixing with chloroform. Alcohol dehydrogenase activity was assayed by measuring the change in absorbance at 340 nm and 30°C in a 1-ml reaction mixture containing 0.1 M MOPS-KOH buffer (pH 7.5), 6 mM dithiothreitol (DTT), 5 mM MgSO4, 0.3 mM Fe(NH4)2(SO4)2, 0.4 mM NADH, 10 mM acetaldehyde, and 30 μl crude cell extract (30). For the anaerobic assay, the above-described sample preparation and assay were carried out in a Bactron I anaerobic chamber (Sheldon Manufacturing Inc., Cornelius, OR). Linearity of the reactions (protein concentration and time) was established for all preparations. All spectrophotometric measurements were conducted in a BioMate 5 spectrophotometer (Thermo Scientific, MA). The nonenzymatic rates were subtracted from the observed initial reaction rates. Enzyme activities are reported as millimoles of substrate/minute/milligram of cell protein and represent averages for at least three cell preparations.
Analytical methods.
Cell growth was monitored by measuring total protein concentration using the method of Lowry (35) and a predetermined correlation between cell dry weight (CDW) and total protein concentration. The CDW was determined by collecting 5 ml of a cell suspension on a membrane filter (diameter, 47 mm; pore size, 0.45 mm) (Sartorius, Gottingen, Germany) and applying suction. The filter was washed twice with distilled water, dried to a constant weight, and desiccated before weighting.
The identity of all metabolic products was determined through one-dimensional (1D) proton nuclear magnetic resonance (NMR) spectroscopy as previously described (40). Organic acids, ethanol, and isopropanol were quantified by high-pressure liquid chromatography (HPLC) as previously reported (13).
Butanol was quantified in a Varian CP-3800 gas chromatograph (Varian Associates, Inc., Palo Alto, CA) equipped with a flame ionization detector (FID) following a modification of the method reported by Atsumi and coworkers (2). The separation of alcohol compounds was carried out using a VF-5ht column (15 m, 0.32-mm internal diameter, 0.10-μm film thickness; Varian Associates, Inc., Palo Alto, CA). The oven temperature was initially held at 40°C for 2 min and then raised with a gradient of 5°C/min to 45°C and held for 4 min. The temperature was then raised with a gradient of 15°C/min to 230°C and held for 4 min. Helium (Matheson Tri-Gas, Longmont, CO) was used as the carrier gas with a 14-lb/in2 inlet pressure. The injector and detector were maintained at 225°C. A 0.5-μl sample was injected in splitless injection mode.
The analysis of FAs was carried out in a Varian CP-3800 gas chromatograph (Varian Associates, Inc., Palo Alto, CA) after hexane-methyl tertiary butyl ether (MTBE) extraction and FA transesterification with methanol (32), according to the following method: 50°C held for 1 min, 30°C/min to 160°C, 15°C/min to 200°C, 200°C held for 1.5 min, 10°C/min to 225°C, and 225°C held for 15 min.
Sample preparation (i.e., MTBE extraction and transesterification) was conducted as follows. One-milliliter samples were transferred to 5-ml serum bottles (Supelco, Bellefonte, PA) containing 2 ml of hexane-MTBE (1:1). Eighty microliters of 50% H2SO4 and 0.05 g NaCl were added for pH and ionic strength adjustment, respectively (31). Triplicate calibration standards for FA analysis of 1, 2, 5, 10, 20, 30, 40, 50, and 100 mg/liter were prepared in a 1:1 mixture of chloroform-methanol using a 1,500-mg/liter stock solution of each FA. The bottles were sealed with Teflon-lined septa (Fisher Scientific Co., Fair Lawn, NJ), secured with caps, and shaken using an orbital shaker at 200 rpm for 15 min. The samples were then centrifuged for 5 min at 1,750 × g to separate the aqueous and organic layers. After centrifugation, the top organic layer was separated from the aqueous layer. A 0.5-ml aliquot of the top layer was transferred carefully using glass pipettes to 1.0-ml Supelco Reacti-Vials (Sigma-Aldrich, St. Louis, MO) and dried under a stream of nitrogen in an N-EVAP evaporator (Organomation Associates, Inc., Berlin, MA).
A fresh solution of the transesterification reaction mix (methanol-hydrochloric acid-chloroform [10:1:1, vol/vol/vol; 3 ml]) was added to the dried lipid extract in Reacti-Vials (Sigma-Aldrich, St. Louis, MO), which were capped tightly and vortexed. These vials were placed in a heater block (AccuBlock digital dry bath; LabNet, Woodbridge, NJ) and heated at 90°C for 15 min.
The transesterification reaction tubes were cooled to room temperature. Water (1 ml) was added to each tube, and the fatty acid methyl esters (FAMEs) were extracted with hexane and chloroform (4:1, vol/vol; three times with 2 ml). The tubes were vortexed for 30 s and the upper organic phase collected with a Pasteur pipette. This extraction procedure was repeated three times.
The combined hexane-chloroform solution was evaporated under N2 to dryness, and the dry residue was redissolved in 60 μl of hexane, transferred to GC vials (Fisher Scientific Co., Fair Lawn, NJ), and capped under N2. A 2.0-μl aliquot of FAME solution was injected (injection temperature, 300°C) into the chromatograph. The carrier gas flow rate was 2.0 ml/min, with a split ratio of 1:25.
Stearic (C18:0), palmitic (C16:0), myristic (C14:0), lauric (C12:0), capric (C10:0), caprylic (C8:0), and caproic (C6:0) acids (Sigma Chemical Co., St. Louis, MO) were used to calibrate the gas chromatograph. The carrier gas used was helium (Matheson Tri-Gas, Longmont, CO). Hexane, chloroform, diethyl ether, and MTBE were HPLC grade (Sigma Chemical Co., St. Louis, MO). Sodium chloride and concentrated sulfuric acid were reagent grade (VWR International, West Chester, PA).
Calculation of fermentation parameters.
Growth and product yields (gram of product per gram of substrate consumed) were calculated as the amount of cell mass or product synthesized per amount of palmitic acid (C16:0) consumed. Production of caproic acid (C6:0) and biomass was accounted for in product yield calculations. An average molecular mass for an E. coli cell of 24.7 g/C-mol, which corresponds to an average cell of a molecular formula CH1.9O0.5N0.2 (43), was used in these calculations.
RESULTS
Engineering fermentative pathways in E. coli for biofuel production from fatty acids. (i) Ethanol.
Although ethanol is only a minor product of fermentative metabolism in E. coli (55), this bacterium has been engineered for the production of ethanol from sugars (24) and glycerol (14, 66). The maximum theoretical yield for ethanol synthesis from glucose is 0.51 g ethanol/g glucose, or 0.67 on C mole basis (Table 1). The low ethanol yield on glucose is due to the low metabolic efficiency of the pathway that converts this sugar into ethanol, which generates two molecules each of ethanol and CO2 from one molecule of glucose (Fig. 1 and Table 1). Given their higher energy content and reduced state, the use of FAs represents a more efficient alternative, as each β-oxidation cycle yields one molecule of acetyl-CoA, which can be converted to ethanol without any carbon loss (Fig. 1). When the fact that the last 2-C fragment of a FA molecule converted to acetyl-CoA does not generate reducing equivalents is taken into account, a maximum theoretical yield of 1.38 (wt/wt) is obtained from a 16-C FA molecule (i.e., palmitic acid [C16:0], the most abundant FA in oil-rich feedstocks) (also see the text in the supplemental material) (Table 1). This value is 2.71-fold higher than the yield on glucose. On a carbon basis, the ethanol yield on C16:0 is 1.44-fold higher (Table 1). These calculations, however, do not account for the 2 ATP equivalents required to drive the β-oxidation pathway, which when accounted for would slightly reduce the maximum theoretical value (Table 1). While maximum theoretical yields on both weight and carbon bases are presented in Table 1, in the remainder of this paper only the weight basis will be used (also see the text in the supplemental material for rationale and further details).
Despite the aforementioned theoretical advantages of FAs, no ethanol was produced by wild-type E. coli strain MG1655 during the respiratory metabolism of palmitic acid (Fig. 2a ). Efforts to engineer homoethanol production in E. coli have focused on diverting carbon at the pyruvate node by manipulating native (pyruvate dehydrogenase) or heterologous (Zymomonas mobilis pyruvate decarboxylase) pathways (see reference 22 and references therein). These strategies, however, are not applicable to the production of ethanol from FAs, because pyruvate is not an intermediate in the β-oxidation pathway (Fig. 1). Instead, we focus on diverting carbon to ethanol synthesis at the acetyl-CoA node by manipulating the enzyme acetaldehyde/alcohol dehydrogenase (AdhE) (Fig. 1). Since AdhE is oxygen sensitive and adhE expression is very low in the presence of oxygen (20), we hypothesized that the lack of ethanol production in MG1655 could be related to the low activity/expression of this enzyme under aerobic conditions. We then constructed an aerotolerant mutant of AdhE (AdhE*) and overexpressed it from the oxygen-independent trc promoter. AdhE* was obtained by site-directed mutagenesis of AdhE to replace the residue at position 568 (Glu568Lys) (20). Enzyme assays of cells overexpressing AdhE* confirmed its high activity under both anaerobic and aerobic conditions (see Fig. S3 in the supplemental material). Overexpression of AdhE* in MG1655 resulted in the production of ethanol at a yield of 0.48 g/g C16:0 (Fig. 2a, strain MG1655 [adhE*+]). To investigate a potential competition between the ethanol and biosynthetic and respiratory pathways for reducing equivalents, we decreased the respiration rate by lowering the volumetric oxygen transfer coefficient (kLa) from 14.5 h−1 to 6.7 h−1. This modification led to a 2-fold increase in ethanol yield (0.92 g ethanol/g C16:0) (Fig. 2a).
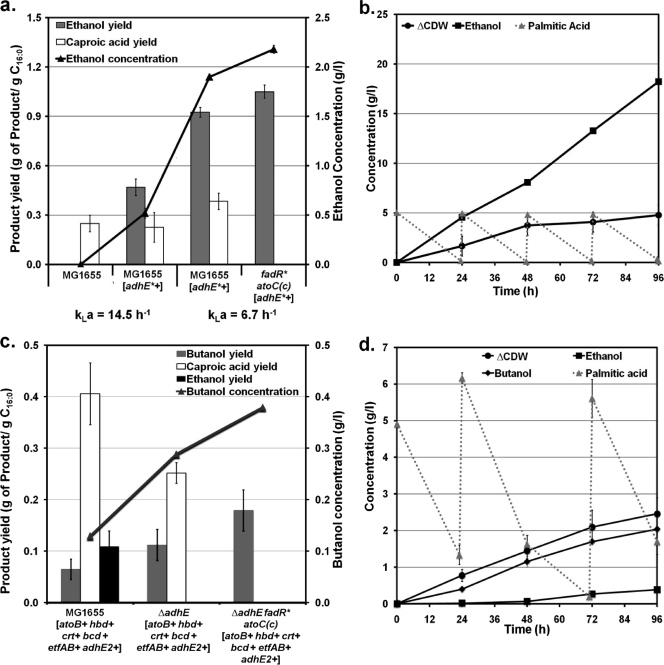
FIG. 2.
Engineering E. coli for the production of ethanol (a and b) and butanol (c and d) from fatty acids. Values represent the means and standard deviations for triplicate cultures. Gene overexpression and deletion are indicated by + and Δ, respectively, next to the corresponding gene or operon. Details about the pathways can be found in Fig. 1. (a) Ethanol concentration (line) and ethanol and caproic acid yields (bars) for 72-hour cultures of wild-type MG1655 and strains containing engineered pathways for ethanol production (oxygen-tolerant acetaldehyde/alcohol dehydrogenase, adhE*) and efficient catabolism of FAs [fadR* atoC(Con)]. kLa, volumetric oxygen transfer coefficient (h−1). (b) Fermentation profile for strain MG1655 ΔadhE fadR*atoC(Con) [adhE*+] in minimal medium with 5 g/liter palmitic acid (C16:0) and using a kLa of 6.7 h−1. Additions of palmitic acid (5 g/liter each) were made every 24 h. ΔCDW, increase in cell dry weight with respect to the initial value. (c) Butanol concentration (line) and butanol, ethanol, and caproic acid yields (bars) for 72-hour cultures of strains engineered by (i) expression of the C. acetobutylicum pathway for the synthesis of butanol from acetoacetyl-CoA (hbd, crt, bcd, etfAB, and adhE2), (ii) overexpression of the E. coli acetyl-CoA acetyltransferase (atoB) for the conversion of acetyl-CoA to acetoacetyl-CoA, (iii) elimination of the E. coli native ethanol pathway (adhE), and (iv) engineering the β-oxidation pathway for efficient catabolism of FAs [fadR*atoC(Con)]. A kLa of 6.7 h−1 was used. (d) Fermentation profile for strain MG1655 ΔadhE fadR*atoC(Con) [atoB+ hbd+ crt+ bcd+ etfAB+ adhE2+] in minimal medium with 5 g/liter palmitic acid (C16:0) and a kLa of 6.7 h−1. Two additions of palmitic acid (5 g/liter each) were made at 24 and 72 h. ΔCDW, increase in cell dry weight with respect to initial value.
While the ethanol yield was very high in strain MG1655 [adhE*+], oxidation of palmitic acid was incomplete resulting in the accumulation of caproic acid (C6:0) (Fig. 2a). The metabolism of short-chain FAs (C4 to C6) in E. coli requires expression of both the fad regulon (regulated by FadR) and two degradative enzymes encoded by the atoDAEB operon (regulated by AtoC) (11) (Fig. 1). Previous studies have shown that mutants exhibiting constitutive expression of the fad regulon and ato operon [referred to here as fadR* and atoC(Con), respectively] catabolize both long- and short-chain FAs (25, 44). However, such mutants have not been tested for their ability to completely oxidize FAs without the generation of short-chain FAs as by-products, which is the specific problem observed in strain MG1655 [adhE*+]. Therefore, we engineered the fadR*atoC(Con) phenotype in MG1655 (see Fig. S2 in the supplemental material) and evaluated its ability to prevent the generation of by-product caproic acid. Overexpression of AdhE* in strain MG1655 fadR*atoC(Con) resulted in efficient metabolism of palmitic acid without the accumulation of caproic acid and also in a high ethanol yield (Fig. 2a). It is noteworthy that the ethanol yield in this strain is twice the maximum theoretical value from sugars (wt/wt basis) and already represents 77% of the maximum achievable from palmitic acid (Fig. 2a). We also performed studies to illustrate the production of ethanol at higher titers. For this purpose, experiments were conducted using a higher concentration of palmitic acid and a higher cell density (Fig. 2b). Under these conditions, strain MG1655 fadR*atoC(Con) [adhE*+] produced ethanol as the only fermentation product at concentrations close to 20 g/liter, demonstrating that high yield and titers can be achieved.
(ii) Butanol.
While microbial production of butanol traditionally utilizes Clostridium acetobutylicum (36, 38), the limitations of this organism have triggered efforts to engineer E. coli, Pseudomonas putida, Bacillus subtilis, and Saccharomyces cerevisiae for butanol production, reaching titers and yields of up to 1.2 g/liter and 6% (wt/wt), respectively (2, 23, 42, 60).
Production of butanol from FAs would provide a 2.7-fold yield advantage over sugars (wt/wt), as can be seen in Table 1. Our strategy to produce butanol from FAs in E. coli is based on the engineering of a synthetic pathway composed of the genes required for butanol synthesis from acetoacetyl-CoA in C. acetobutylicum (crt, bcd, etfAB, hbd, and adhE2) in combination with the E. coli gene responsible for the conversion of acetyl-CoA to acetoacetyl-CoA (atoB) (Fig. 1). When these pathways were expressed in E. coli cells metabolizing palmitic acid, we observed significant production of butanol (Fig. 2c, strain MG1655 [atoB+ hbd+ crt+ bcd+ etfAB+ adhE2+]). We hypothesized that the relatively low yields and titers observed originate in part from the competition between the ethanol and butanol pathways for acetyl-CoA and reducing equivalents (Fig. 1). In agreement with this hypothesis, deletion of the native ethanol pathway (ΔadhE) resulted in a 2-fold increase in butanol yield (Fig. 2c, strain MG1655 ΔadhE [atoB+ hbd+ crt+ bcd+ etfAB+ adhE2+]). As in ethanol-producing strains (see above), the engineering of the β-oxidation pathway for more efficient utilization of FAs [fadR*atoC(Con)] also led to an increase in product yield {Fig. 2c, strain MG1655 ΔadhE fadR*atoC(Con) [atoB+ hbd+ crt+ bcd+ etfAB+ adhE2+]}. Overall, our approach resulted in butanol yields that were up to 3-fold higher than those currently reported in the literature (2, 23, 42, 60) and represent 16.4% of the maximum achievable from palmitic acid.
To further ascertain the potential of the engineered strains for butanol production, we performed experiments with strain MG1655 ΔadhE fadR*atoC(Con) [atoB+ hbd+ crt+ bcd+ etfAB+ adhE2+] at a higher palmitic acid concentration and a higher cell density (Fig. 2d). Butanol was produced as the main fermentation product at a concentration of 2.05 g/liter, along with very small amounts of ethanol (Fig. 2d). The synthesis of ethanol could be due to the ability of clostridial secondary alcohol dehydrogenase to catalyze the conversion of acetyl-CoA to ethanol (48, 67). Even though the butanol pathway has yet to be optimized, the butanol yield and titer achieved by our strategy are superior to those reported with strains engineered to produce butanol from other carbon sources (2, 23, 42, 60).
Engineered strains for the production of biochemicals from fatty acids.
Industrial by-product streams with high FA content, such as palm fatty acid distillate or palm oil mill effluent (62, 65), can be exploited for biological production of chemicals (see the text in the supplemental material for details). In the next sections we present the engineering of E. coli to enable the respiro-fermentative production of commodity chemicals, organic solvents, and polymer building blocks from FAs.
(i) Acetic acid (acetate).
Acetate is an important industrial chemical (1) and a native product of sugar fermentation in E. coli, where it is produced primarily through the phosphotransacetylase (PTA)-acetate kinase (ACK) pathway (11) (Fig. 1). Although the maximum theoretical yield for acetate production from glucose is 1.00 (wt/wt) (Table 1), the biological process used in the commercial production of acetate from sugars (9) and that developed by engineering E. coli (7) are both based on a maximum theoretical yield of 0.67 g of acetate/g glucose. The synthesis of acetate from FAs contributes to CO2 fixation and can be achieved at a much higher yield; e.g., a yield of 2.70 (wt/wt) can be realized with palmitic acid (Table 1).
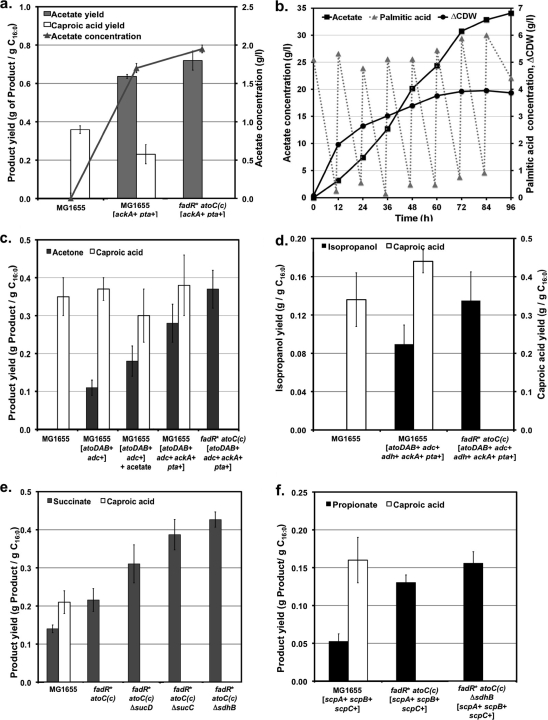
Unlike metabolism of sugars, utilization of FAs by E. coli does not lead to the synthesis of acetate (Fig. 3a). Since acetyl-CoA is generated in the β-oxidation of FAs, we reasoned that overexpression of the ACK-PTA pathway (Fig. 1) should lead to significant production of acetate. Indeed, strain MG1655 [ackA+ pta+] produced acetate at a yield of 0.65 g acetate/g C16:0 (Fig. 3a). As in the case of other products discussed in previous sections, a strain with constitutive expression of the fad regulon and ato operon {i.e., strain MG1655 fadR*atoC(Con) [ackA+ pta+]} not only utilized palmitic acid more efficiently but improved acetate production as well (Fig. 3a), exhibiting yields that surpassed those reported for E. coli strains engineered to produce acetate from glucose (6). An experiment was conducted to assess the production of acetate at higher titers. As shown in Fig. 3b, 35 g/liter of acetate was produced at a yield of 1.10 g acetate/g C16:0 (40.7% of the maximum achievable from palmitic acid), demonstrating the feasibility of achieving high yields and titers. It is noteworthy that this yield is in excess of the maximum theoretical yield on glucose (Table 1) and about 2-fold higher than the best yield reported to date from an E. coli strain engineered to produce acetate (6).
FIG. 3.
Production of acetate (a and b), acetone (c), isopropanol (d), succinate (e), and propionate (f) from FAs in engineered E. coli strains. Details about the engineered pathways are shown in Fig. 1. Gene overexpression and deletion are indicated by + and Δ, respectively, next to the corresponding gene or operon. A kLa of 14.5 h−1 was used in all experiments. Values represent the means and standard deviations for 72-hour triplicate cultures. (a) Acetate concentration (line) and acetate and caproic acid yields (bars) in wild-type MG1655 and recombinant strains constructed by overexpressing the native phopshoacetyltransferase (pta)-acetate kinase (ackA) pathway for conversion of acetyl-CoA to acetate and modifying the β-oxidation pathway for efficient catabolism of FAs [fadR*atoC(Con)]. (b) Fermentation profile for strain MG1655 fadR*atoC(Con) [ackA+ pta+] in minimal medium with 5 g/liter palmitic acid (C16:0). Additions of palmitic acid (5 g/liter each) were made every 12 h. ΔCDW, increase in cell dry weight with respect to initial value. (c) Acetone and caproic acid yields in strains constructed by overexpressing E. coli acetyl-CoA and acetoacetyl-CoA transferases (atoDAB) and C. acetobutylicum acetoacetate decarboxylase (adc) along with amplification of the native acetate pathway (ackA-pta). These modifications were implemented in wild-type MG1655 and the fadR*atoC(Con) derivative. (d) Isopropanol and caproic acid yields in strains engineered for acetone production and overexpressing a secondary alcohol dehydrogenase from C. butyricum (adh), which converts acetone to isopropanol. (e) Effects of fadR*atoC(Con) genotype and deletions of genes encoding TCA cycle enzymes on succinate and caproic acid yields. (f) Propionate and caproic acid yields in strains overexpressing a metabolic cycle that catalyzes the decarboxylation of succinate to propionate (scpA, scpB, scpC). The effect of amplification of this cycle, along with the deletion of succinate dehydrogenase (ΔsdhB), was evaluated in wild-type MG1655 and the fadR*atoC(Con) derivative.
(ii) Acetone.
Acetone is a product of the acetone-butanol fermentation by Clostridium spp. (36) and an industrially relevant chemical. Low product selectivity and titers are key factors that represent a driver for heterologous expression of this solventogenic pathway in industrial organisms such as E. coli (4). The acetone pathway involves the conversion of acetyl-CoA to acetone via reactions catalyzed by three different enzymes (26), as shown in Fig. 1. Conversion of sugars to acetone is also inefficient in terms of C recovery, with a maximum theoretical yield on glucose limited to 0.48 (wt/wt) (Table 1). Utilization of palmitic acid would support a yield 2.71-fold higher than for sugars (Table 1).
Since acetone is not a native product of E. coli metabolism, a synthetic pathway containing genes from C. acetobutylicum and E. coli was required to convert the acetyl-CoA produced by the β-oxidation of FAs to acetone (Fig. 1). While significant acetone production was achieved with this strategy, the resulting yield was low (0.11 g acetone/g C16:0) (Fig. 3c, strain MG1655 [atoDAB+ adc+]). We hypothesized that acetone production may be limited by the low levels of acetate, a metabolite required in the second step of the acetone pathway (i.e., the conversion of acetoacetyl-CoA to acetoacetate [Fig. 1]). In agreement with this hypothesis, supplementation of the growth medium with acetate increased the acetone yield by more than 60% (Fig. 3c). Simultaneous overexpression of the acetate-producing ACK-PTA pathway, along with the synthetic acetone pathway, enabled the synthesis of acetone in the absence of added acetate at a yield of 0.28 g acetone/g C16:0 (Fig. 3c, strain MG1655 [atoDAB+ adc+ ackA+ pta+]). When this strategy was implemented in a strain with constitutive expression of the fad regulon and ato operon [i.e., strain MG1655 fadR*atoC(Con)], efficient utilization of palmitic acid along with further improvement in acetone production was observed (Fig. 3c). The acetone yield on this strain (0.37 g acetone/g C16:0) surpassed the yields previously reported using engineered E. coli (4, 18) and corresponds to 28.5% of the maximum achievable from palmitic acid.
(iii) Isopropanol.
Isopropanol is a secondary alcohol with applications as both a chemical intermediate and a solvent (34). Isopropanol is produced in Clostridium beijerinckii (8, 15) from acetyl-CoA via the acetone pathway (Fig. 1) as part of a mixed-product fermentation. Limitations in the utilization of native strains have increased interest in engineering the pathway in industrially relevant hosts (18). Isopropanol production from glucose is limited to a yield of 0.44 (wt/wt) (Table 1). Compared to sugars, the use of FAs offers a 2.73-fold higher yield (Table 1). Since isopropanol is a nonnative product of E. coli metabolism, we introduced the clostridial route, which produces this alcohol from acetyl-CoA, as shown in Fig. 1. For this purpose, three different pathways were assembled in strain MG1655 (Fig. 1): (i) the C. acetobutylicum acetone pathway, which converts acetyl-CoA to acetone (see previous section); (ii) a secondary alcohol dehydrogenase from C. beijerinckii that converts acetone to isopropanol; and (iii) the E. coli pathway that converts acetyl-CoA to acetate. The resulting strain, MG1655 [atoDAB+ adc+ ackA+ pta+ adh+], produced isopropanol at a yield of 0.09 g/g C16:0 (Fig. 3d). When these pathways were assembled in the fadR*atoC(Con) strain, efficient metabolism of FAs and a further increase in isopropanol yield were observed (0.13 g of isopropanol/g C16:0, 10.8% of the maximum achievable from palmitic acid) (Fig. 3d).
(iv) Succinic acid (succinate).
Succinate is expected to become a future platform chemical (64), and major efforts in recent years have focused on its production by microbial fermentation of sugars (21, 58). Succinate is a minor product of sugar fermentation in E. coli (10), but it is not produced under aerobic conditions because this metabolite is an intermediate of the TCA cycle (Fig. 1). As in the case of other products discussed above, production of succinate from FAs would offer a significant yield advantage (Table 1). Metabolic engineering strategies previously reported for the production of succinate from sugars in E. coli (33, 58) cannot be implemented for its production from FAs because of the significant differences in the metabolic pathways. For example, while the synthesis of succinate from sugars is limited by the availability of both reducing equivalents and intermediate phosphoenolpyruvate (21), its production from FAs is a redox-generating process that relies on the availability of acetyl-CoA (Table 1 and Fig. 1). Since fadR* mutants are known to exhibit higher expression of the glyoxylate shunt enzymes (16) and succinate is generated in this pathway (Fig. 1), we evaluated succinate production in the fadR*atoC(Con) strain and observed a significant increase in yield (Fig. 3e). Succinate production was further increased by minimizing its conversion to fumarate (ΔsdhB mutation) and decreasing the oxidation of acetyl-CoA to CO2 via the TCA cycle (ΔsucC and ΔsucD mutations) (Fig. 1 and Fig. 3e). These engineering strategies resulted in up to a 2.3-fold increase in succinate yield with respect to the wild type (Fig. 3e), which represents 14.6% of the maximum achievable from palmitic acid.
(v) Propionic acid (propionate).
There is a high interest in producing propionate from sugars via fermentation with propionibacteria, but this process suffers from relatively low product yield and concentration (19). While a propionate yield on glucose of 0.70 (wt/wt) can be realized, the use of FAs would support yields approximately 2.59-fold higher (Table 1). Three of the genes located in the E. coli scpA-argK-scpB-scpC operon encode the enzymes of a postulated metabolic cycle that can catalyze the decarboxylation of succinate to propionate (Fig. 1) (17). However, propionate has not been reported as a product of E. coli metabolism. By overexpressing the aforementioned genes, we achieved for the first time the production of propionate in this organism (Fig. 3f). Since succinate is the precursor of propionate in this pathway, the same modifications that led to increased production of succinate (Fig. 3e) also resulted in higher propionate yields (Fig. 3f), with a maximum yield of 8.8% of the maximum achievable from palmitic acid.
DISCUSSION
The use of FA-rich feedstocks for the production of biofuels and biochemicals is a promising avenue to establish biorefineries (see the text in the supplemental material for more details). In addition to their abundance, the metabolism of FAs is highly efficient, as it yields 100% carbon recovery in the key intermediate metabolite acetyl-CoA, from which most biofuels and biochemicals can be derived (Fig. 1). This high metabolic efficiency, along with the highly reduced state of carbon in FAs, could enable the production of fuels and chemicals at yields superior than those obtained with the use of lignocellulosic sugars (Table 1). However, metabolism of FAs requires the presence of an external electron acceptor, which in turn precluded the synthesis of fermentation products (Fig. 2a and Fig. 3a). To overcome this hurdle, we engineered a respiro-fermentative metabolic mode that enables the efficient production of the desired fuels and chemicals in combination with adequate catabolism of FAs.
E. coli was chosen as model organism to illustrate the feasibility of this approach, which was demonstrated by engineering the synthesis of ethanol, butanol, acetate, acetone, isopropanol, succinate, and propionate (Fig. 2 and 3). This work represents the first effort toward engineering microorganisms for the conversion of FAs to the aforementioned products. The yields of ethanol (Fig. 2a and b), acetate (Fig. 3a and b), and acetone (Fig. 3c) in the engineered strains exceeded those reported in the literature for their production from sugars (4, 7, 18, 24). In the cases of ethanol and acetate, the yields also surpassed the maximum theoretical values that can be achieved from lignocellulosic sugars. Butanol (Fig. 2c and d), on the other hand, was produced at yields and titers between 2- and 3-fold higher than those reported for its production from lignocellulosic sugars in engineered E. coli, P. putida, B. subtilis, and S. cerevisiae strains (2, 23, 42, 60). Our work also represents the first report of production of propionate, a compound previously thought to be synthesized only by propionibacteria, in E. coli (Fig. 3f). Finally, the synthesis of isopropanol and succinate was also demonstrated (Fig. 3d and e).
Taken together, the results reported here represent the foundation to establish a new approach for the production of biofuels and biochemicals from renewable feedstocks. The synthetic respiro-fermentative metabolic mode engineered in this work will also serve as the basis for the synthesis of other reduced products in the presence of external electron acceptors (e.g., oxygen), thus taking advantage of the most significant attributes of respiratory and fermentative metabolism.
Supplementary Material
Acknowledgments
We thank B. L. Wanner, F. R. Blattner, B. Erni, and H. Mori for providing research materials and S. Moran and J. M. Clomburg for assistance with NMR techniques.
Footnotes
Published ahead of print on 4 June 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Agreda, V. H., and J. R. Zoeller. 1993. Acetic acid and its derivatives. CRC Press, Boca Raton, FL.
- 2.Atsumi, S., A. F. Cann, M. R. Connor, C. R. Shen, K. M. Smith, M. P. Brynildsen, K. J. Y. Chou, T. Hanai, and J. C. Liao. 2008. Metabolic engineering of Escherichia coli for 1-butanol production. Metab. Eng. 10:305-311. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermejo, L. L., N. E. Welker, and E. T. Papoutsakis. 1998. Expression of Clostridium acetobutylicum ATCC 824 genes in Escherichia coli for acetone production and acetate detoxification. Appl. Environ. Microbiol. 64:1079-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevan, M. W., and M. C. R. Franssen. 2006. Investing in green and white biotech. Nat. Biotechnol. 24:765-767. [DOI] [PubMed] [Google Scholar]
- 6.Carlsson, A. S. 2009. Plant oils as feedstock alternatives to petroleum—a short survey of potential oil crop platforms. Biochimie 91:665-670. [DOI] [PubMed] [Google Scholar]
- 7.Causey, T. B., S. Zhou, K. T. Shanmugam, and L. O. Ingram. 2003. Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: homoacetate production. Proc. Natl. Acad. Sci. U. S. A. 100:825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, J. S., and S. F. Hiu. 1986. Acetone-butanol-isopropanol production by Clostridium beijerinckii (synonym, Clostridium butylicum). Biotechnol. Lett. 8:371-376. [Google Scholar]
- 9.Cheryan, M., S. Parekh, M. Shah, and K. Witjitra. 1997. Production of acetic acid by Clostridium thermoaceticum. Adv. Appl. Microbiol. 43:1-33. [DOI] [PubMed] [Google Scholar]
- 10.Clark, D. P. 1989. The fermentation pathways of Escherichia coli. FEMS Microbiol. Rev. 5:223-234. [DOI] [PubMed] [Google Scholar]
- 11.Clark, D. P., and J. E. Cronan. October 2005, posting date. Chapter 3.4.4, Two-carbon compounds and fatty acids as carbon sources. In R. Curtis III et al. (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. http://www.ecosal.org.
- 12.Dellomonaco, C., F. Fava, and R. Gonzalez. 2010. The path to next generation biofuels: successes and challenges in the era of synthetic biology. Microb. Cell Fact. 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dharmadi, Y., and R. Gonzalez. 2005. A better global resolution function and a novel iterative stochastic search method for optimization of high-performance liquid chromatographic separation. J. Chromatogr. A 1070:89-101. [DOI] [PubMed] [Google Scholar]
- 14.Durnin, G., J. Clomburg, Z. Yeates, P. J. J. Alvarez, K. Zygourakis, P. Campbell, and R. Gonzalez. 2009. Understanding and harnessing the microaerobic metabolism of glycerol in Escherichia coli. Biotechnol. Bioeng. 103:148-161. [DOI] [PubMed] [Google Scholar]
- 15.George, H. A., J. L. Johnson, W. E. C. Moore, L. V. Holdeman, and J. S. Chen. 1983. Acetone, isopropanol, and butanol production by Clostridium beijerickii (syn Clostridium butylicum) and Clostridium aurantibutylicum. Appl. Environ. Microbiol. 45:1160-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gui, L. Z., A. Sunnarborg, and D. C. LaPorte. 1996. Regulated expression of a repressor protein: FadR activates iclR. J. Bacteriol. 178:4704-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haller, T., T. Buckel, J. Retey, and J. A. Gerlt. 2000. Discovering new enzymes and metabolic pathways: conversion of succinate to propionate by Escherichia coli. Biochemistry 39:4622-4629. [DOI] [PubMed] [Google Scholar]
- 18.Hanai, T., S. Atsumi, and J. C. Liao. 2007. Engineered synthetic pathway for isopropanol production in Escherichia coli. Appl. Environ. Microbiol. 73:7814-7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Himmi, E. H., A. Bories, A. Boussaid, and L. Hassani. 2000. Propionic acid fermentation of glycerol and glucose by Propionibacterium acidipropionici and Propionibacterium freudenreichii ssp shermanii. Appl. Microbiol. Biotechnol. 53:435-440. [DOI] [PubMed] [Google Scholar]
- 20.Holland-Staley, C. A., K. Lee, D. P. Clark, and P. R. Cunningham. 2000. Aerobic activity of Escherichia coli alcohol dehydrogenase is determined by a single amino acid. J. Bacteriol. 182:6049-6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong, S. H., and S. Y. Lee. 2002. Importance of redox balance on the production of succinic acid by metabolically engineered Escherichia coli. Appl. Microbiol. Biotechnol. 58:286-290. [DOI] [PubMed] [Google Scholar]
- 22.Hu, Q., M. Sommerfeld, E. Jarvis, M. Ghirardi, M. Posewitz, M. Seibert, and A. Darzins. 2008. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J. 54:621-639. [DOI] [PubMed] [Google Scholar]
- 23.Inui, M., M. Suda, S. Kimura, K. Yasuda, H. Suzuki, H. Toda, S. Yamamoto, S. Okino, N. Suzuki, and H. Yukawa. 2008. Expression of Clostridium acetobutylicum butanol synthetic genes in Escherichia coli. Appl. Microbiol. Biotechnol. 77:1305-1316. [DOI] [PubMed] [Google Scholar]
- 24.Jarboe, L. R., T. B. Grabar, L. P. Yomano, K. T. Shanmugan, and L. O. Ingram. 2007. Development of ethanologenic bacteria. Adv. Biochem. Eng. Biotechnol. 108:237-261. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins, L. S., and W. D. Nunn. 1987. Genetic and molecular characterization of the genes involved in short-chain fatty acid degradation in Escherichia coli: the ato system. J. Bacteriol. 169:42-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, D. T., and D. R. Woods. 1986. Acetone-butanol fermentation revisited. Microbiol. Rev. 50:484-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamm, B., P. R. Gruber, and M. Kamm. 2006. Biorefineries—industrial processes and products: status quo and future directions. Wiley-VCH Verlag GmbH, Weinheim, Germany.
- 28.Kamm, B., and M. Kamm. 2004. Principles of biorefineries. Appl. Microbiol. Biotechnol. 64:137-145. [DOI] [PubMed] [Google Scholar]
- 29.Kang, Y. S., T. Durfee, J. D. Glasner, Y. Qiu, D. Frisch, K. M. Winterberg, and F. R. Blattner. 2004. Systematic mutagenesis of the Escherichia coli genome. J. Bacteriol. 186:8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessler, D., I. Leibrecht, and J. Knappe. 1991. Pyruvate-formate-lyase-deactivase and acetyl-CoA reductase activities of Escherichia coli reside on a polymeric protein particle encoded by adhE. FEBS Lett. 281:59-63. [DOI] [PubMed] [Google Scholar]
- 31.Lalman, J. A., and D. M. Bagley. 2004. Extracting long-chain fatty acids from a fermentation medium. J. Am. Oil Chem. Soc. 81:105-110. [Google Scholar]
- 32.Lewis, T., P. D. Nichols, and T. A. McMeekin. 2000. Evaluation of extraction methods for recovery of fatty acids from lipid-producing microheterotrophs. J. Microbiol. Methods 43:107-116. [DOI] [PubMed] [Google Scholar]
- 33.Lin, H., G. N. Bennett, and K. Y. San. 2005. Genetic reconstruction of the aerobic central metabolism in Escherichia coli for the absolute aerobic production of succinate. Biotechnol. Bioeng. 89:148-156. [DOI] [PubMed] [Google Scholar]
- 34.Logsdon, J. E., and R. A. Loke. 2000. Isopropyl alcohol. In Kirk-Othmer encyclopedia of chemical technology. John Wiley & Sons, New York, NY.
- 35.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 36.Maddox, I. S. 1989. The acetone-butanol-ethanol fermentation: recent progress in technology. Biotechnol. Genet. Eng. 7:189-220. [DOI] [PubMed] [Google Scholar]
- 37.Maier, U., M. Losen, and J. Buchs. 2004. Advances in understanding and modeling the gas-liquid mass transfer in shake flasks. Biochem. Eng. J. 17:155-167. [Google Scholar]
- 38.Mermelstein, L. D., N. E. Welker, D. J. Petersen, G. N. Bennett, and E. T. Papoutsakis. 1994. Genetic and metabolic engineering of Clostridium acetobutylicum ATCC-824. Ann. N. Y. Acad. Sci. 721:54-68. [DOI] [PubMed] [Google Scholar]
- 39.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 40.Murarka, A., Y. Dharmadi, S. S. Yazdani, and R. Gonzalez. 2008. Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals. Appl. Environ. Microbiol. 74:1124-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture media for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen, D. R., E. Leonard, S.-H. Yoon, H.-C. Tseng, C. Yuan, and K. L. Jones Prather. 2009. Engineering alternative butanol production platforms in heterologous bacteria. Metab. Eng. 11:262-273. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen, J., J. Villadsen, and G. Lidén. 2003. Bioreaction engineering principles. Kluwer Academic/Plenum Publishers, New York, NY.
- 44.Nunn, W. D. 1986. A molecular view of fatty acid catabolism in Escherichia coli. Microbiol. Rev. 50:179-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Octave, S., and D. Thomas. 2009. Biorefinery: toward an industrial metabolism. Biochimie 91:659-664. [DOI] [PubMed] [Google Scholar]
- 46.Park, S. J., J. I. Choi, and S. Y. Lee. 2005. Engineering of Escherichia coli fatty acid metabolism for the production of polyhydroxyalkanoates. Enzyme Microb. Technol. 36:579-588. [Google Scholar]
- 47.Park, S. J., J. P. Park, and S. Y. Lee. 2002. Metabolic engineering of Escherichia coli for the production of medium-chain-length polyhydroxyalkanoates rich in specific monomers. FEMS Microbiol. Lett. 214:217-222. [DOI] [PubMed] [Google Scholar]
- 48.Petersen, D. J., R. W. Welch, F. B. Rudolph, and G. N. Bennett. 1991. Molecular cloning of an alcohol (butanol) dehydrogenase gene cluster from Clostridium acetobutylicum ATCC-824. J. Bacteriol. 173:1831-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ragauskas, A. J., C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer, and T. Tschaplinski. 2006. The path forward for biofuels and biomaterials. Science 311:484-489. [DOI] [PubMed] [Google Scholar]
- 50.Ragsdale, S. W., and E. Pierce. 2008. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta 1784:1873-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenberg, J. N., G. A. Oyler, L. Wilkinson, and M. J. Betenbaugh. 2008. A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr. Opin. Biotechnol. 19:430-436. [DOI] [PubMed] [Google Scholar]
- 52.Rude, M. A., and A. Schirmer. 2009. New microbial fuels: a biotech perspective. Curr. Opin. Microbiol. 12:274-281. [DOI] [PubMed] [Google Scholar]
- 53.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 54.Sauer, U., and B. J. Eikmanns. 2005. The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol. Rev. 29:765-794. [DOI] [PubMed] [Google Scholar]
- 55.Sawers, R. G., and D. P. Clark. July 2004, posting date. Chapter 3.5.3, Fermentative pyruvate and acetyl-coenzyme A metabolism. In R. Curtis III et al. (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. http://www.ecosal.org. [DOI] [PubMed]
- 56.Schenk, P. M., S. R. Thomas-Hall, E. Stephens, U. C. Marx, J. H. Mussgnug, C. Posten, O. Kruse, and B. Hankamer. 2008. Second generation biofuels: high-efficiency microalgae for biodiesel production. BioEnerg. Res. 1:1939. [Google Scholar]
- 57.Service, R. F. 2009. ExxonMobil fuels Venter's efforts to run vehicles on algae-based oil. Science 325:379. [DOI] [PubMed] [Google Scholar]
- 58.Song, H., and S. Y. Lee. 2006. Production of succinic acid by bacterial fermentation. Enzyme Microb. Technol. 39:352-361. [Google Scholar]
- 59.Spratt, S. K., C. L. Ginsburgh, and W. D. Nunn. 1981. Isolation and genetic characterization of Escherichia coli mutants defective in propionate metabolism. J. Bacteriol. 146:1166-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steen, E. J., R. Chan, N. Prasad, S. Myers, C. J. Petzold, A. Redding, M. Ouellet, and J. D. Keasling. 2008. Metabolic engineering of Saccharomyces cerevisiae for the production of n-butanol. Microb. Cell Fact. 7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stephanopoulos, G. 2007. Challenges in engineering microbes for biofuels production. Science 315:801-804. [DOI] [PubMed] [Google Scholar]
- 62.Sumathi, S., S. P. Chai, and A. R. Mohamed. 2008. Utilization of oil palm as a source of renewable energy in Malaysia. Renew. Sust. Energ. Rev. 12:2404-2421. [Google Scholar]
- 63.Van Suijdam, J. C., N. W. F. Kossen, and A. C. Joha. 1978. Model for oxygen transfer in a shake flask. Biotechnol. Bioeng. 20:1695-1710. [Google Scholar]
- 64.Werpy, T., and G. Petersen. 2004. Results of screening for potential candidates from sugars and synthesis gas. U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Washington, DC.
- 65.Wu, T. Y., A. W. Mohammad, J. M. Jahim, and N. Anuar. 2009. A holistic approach to managing palm oil mill effluent (POME): biotechnological advances in the sustainable reuse of POME. Biotechnol. Adv. 27:40-52. [DOI] [PubMed] [Google Scholar]
- 66.Yazdani, S. S., and R. Gonzalez. 2008. Engineering Escherichia coli for the efficient conversion of glycerol to ethanol and co-products. Metab. Eng. 10:340-351. [DOI] [PubMed] [Google Scholar]
- 67.Youngleson, J. S., J. D. Santangelo, D. T. Jones, and D. R. Woods. 1988. Cloning and expression of a Clostridium acetobutylicum alcohol dehydrogenase gene in Escherichia coli. Appl. Environ. Microbiol. 54:676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.