Abstract
3-Hydroxypropionate (3HP) is an important compound in the chemical industry, and the polymerized 3HP can be used as a bioplastic. In this review, we focus on polyesters consisting of 3HP monomers, including the homopolyester poly(3-hydroxypropionate) and copolyesters poly(3-hydroxybutyrate-co-3-hydroxypropionate), poly(3-hydroxypropionate-co-3-hydroxybutyrate-co-3-hydroxyhexanoate-co-3-hydroxyoctanoate), poly(4-hydroxybutyrate-co-3-hydroxypropionate-co-lactate), and poly(3-hydroxybutyrate-co-3-hydroxypropionate-co-4-hydroxybutyrate-co-lactate). Homopolyesters like poly(3-hydroxybutyrate) are often highly crystalline and brittle, which limits some of their applications. The incorporation of 3HP monomers reduces the glass transition temperature, the crystallinity, and also, at up to 60 to 70 mol% 3HP, the melting point of the copolymer. This review provides a survey of the synthesis and physical properties of different polyesters containing 3HP.
Bacterial polyhydroxyalkanoates (PHAs) are natural biodegradable thermoplastics produced by various microorganisms as intracellular energy and carbon storage compounds. PHAs have attracted increased attention as possible alternatives to petroleum-based polymers. They are biodegradable, insoluble in water, nontoxic, biocompatible, piezoelectric, thermoplastic, and/or elastomeric. These features make PHAs suitable for several applications in the packaging industry, medicine, pharmacy, agriculture, and food industry, as raw materials for the production of enantiomerically pure chemicals, and for the production of paints (2, 44). The best characterized PHA is poly(3-hydroxybutyrate) [poly(3HB)], which is synthesized by many bacteria (28, 31, 38, 41). Unfortunately, poly(3HB) is a highly crystalline and brittle polymer with a low elongation-to-break factor, which has prevented its use in a wide range of applications. To obtain bacterial PHAs with improved physical and mechanical properties, previous studies have demonstrated the biosynthesis of copolyesters consisting of 3-hydroxybutyrate (3HB) and a second constituent. Pathways for the biosynthesis of such PHA copolyesters, like poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [poly(3HB-co-3HV)], poly(3-hydroxybutyrate-co-4-hydroxyvalerate) [poly(3HB-co-4HV)], and poly(3-hydroxybutyrate-co-3-hydroxypropionate) [poly(3HB-co-3HP)], occur naturally in many bacteria or have been engineered (5, 40).
Some of these polyesters exhibit material characteristics comparable to those of petrochemical-derived polymers. However, in contrast to petrochemical-based polymers, PHAs are completely biodegradable to CO2 and water. Another advantage is that they can be produced from renewable resources. Unfortunately, PHA production by bacterial fermentation is costly and, due to inefficient utilization of the resources, not necessarily environmentally convenient in all cases (13).
An example of a bacterium-synthesized copolymer which is competitive with polymers produced from petrochemicals in bulk is poly(3HB-co-3HV). Cupriavidus necator (32), formerly Ralstonia eutropha or Alcaligenes eutrophus, accumulates this copolyester when fed with glucose and propionic acid in a phosphate-depleted batch culture (30). The physical properties of poly(3HB-co-3HV) resemble those of polyethylene and polypropylene (18). Poly(3HB-co-3HV) has been commercially produced through fermentation using a glucose-utilizing mutant of C. necator that requires cofeeding of propionic acid for 3-hydroxyvalerate formation. This polymer was sold by ICI (in 1983) under the trade name Biopol.
In general, the morphology and several physical properties of copolymers strongly depend on their comonomer composition and sequence structure (20). A comonomer lowering the melting temperature, crystallinity, and fragility is 3-hydroxypropionate (3HP). 3HP is an industrially relevant product. The putative applications of 3HP are enormous. Among the possible uses as a monomer for (co)polymerization, it could be used as a precursor for the synthesis of other commercially valuable chemicals, like 1,3-propanediol, acrylic acid, or acrylamide (6).
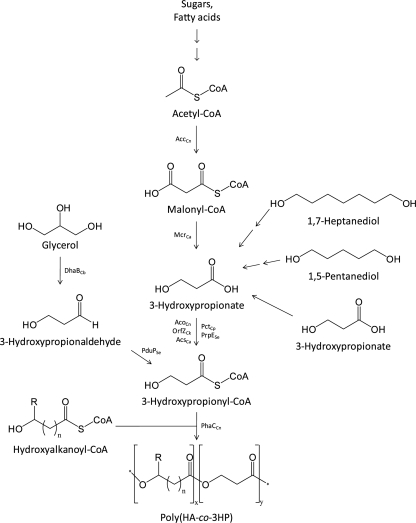
3HP is only produced as a homopolymer from an unrelated carbon source by metabolic engineering in recombinant Escherichia coli (3); alternatively, it is chemically synthesized via ring-opening polymerization of β-propiolactone (4, 15, 49). In this paper, we review the synthesis and physical properties of 3HP-containing copolymers and the preparation of the 3HP units (Fig. 1).
FIG. 1.
Artificial pathways for poly(HA-co-3HP) accumulation from different carbon sources. AccCn, acetyl-CoA carboxylase (C. necator); AcoCn, acetyl-CoA synthase (C. necator); AcsCa, 3HP-CoA synthase domain of propionyl-CoA synthase (C. aurantiacus); DhaBCb, glycerol dehydratase (C. butyricum); McrCa, malonyl-CoA reductase (C. aurantiacus); OrfZCk, acetyl-CoA:4-hydroxybutyrate-CoA transferase (C. kluyveri); PctCp, propionyl-CoA transferase (C. propionicum); PduPSe, propionaldehyde dehydrogenase (Salmonella enterica serovar Typhimurium LT2); PhaCCn, PHA synthase (C. necator); PrpESe, propionyl-CoA synthetase (S. enterica). The indices (n) at the hydroxyalkanoate moieties indicate the presence of lactate (n = 0), 3-hydroxyalkanoates (n = 1), and 4-hydroxybutyrate (n = 4).
CHEMICAL SYNTHESIS OF POLY(3-HYDROXYPROPIONATE)
For the synthesis of 3HP-containing polyesters, a 3HP supply is required. There are several chemical approaches to provide 3HP for copolymer synthesis (45).
Polymerization of β-propiolactone is a well-known method for the preparation of poly(3HP). Gresham et al. (15) observed the polymerization of β-propiolactone by heating (150°C) in the presence of different acids, bases, and salts. The most effective catalysts were ferric chloride, stannic chloride, sulfuric acid, and sodium hydroxide. Ring-opening polymerization with lanthanide alkoxides such as decamethylsamaroceneethylate diethyl ether [SmOEt(C5Me5)2(Et2O)], di-decamethylyttrocenemethylate ([YOMe(C5H5)2]2), and decamethylyttrocenemethylate tetrahydrofuran [YOMe-(C5Me5)2(THF)] at 0°C is very effective to obtain high-molecular-weight poly(3HP)s. They have average molecular numbers of up to 63,000 and were formed with conversion rates from 86 to 91% (49).
As β-propiolactone is a human carcinogen, other methods of synthesis were attempted. Based on the commercially available and noncarcinogenic 3HP, poly(3HP) was synthesized by Nanba et al. (35). The polymer was prepared by condensation of the corresponding hydroxycarboxylic acid in the presence of a transesterification catalyst at 70°C. In comparison to the ring-opening reaction, the condensation process has a lower degree of control over molecular weight, comonomer incorporation, and end-group definition. To combine the benefits of the ring-opening reaction with the advantages of 3HP condensation, poly(3HP) was polymerized from macrocyclic esters constructed from 3HP (51). The macrocyclic 3HP polyesters were obtained by acid-catalyzed self-condensation with concomitant removal of water.
To the best of our knowledge, chemical synthesis of 3HP-containing copolyesters has not yet been described.
BACTERIAL SYNTHESIS OF POLY(3HB-co-3HP) COPOLYMERS
The best characterized copolymer containing 3HP constituents is poly(3HB-co-3HP). The homopolymer of 3-hydroxybutyrate (3HB) is synthesized by various bacteria, including C. necator, Bacillus megaterium, and Azotobacter vinelandii (28).
The 3HB-based copolyesters containing an additional monomer unit without a side chain, poly(3HB-co-3HP), could be accumulated by several (natural or artificial) pathways (Table 1). For example, it was synthesized via the phaC1AB operon of C. necator (phaC1ABCn) with 3-hydroxypropionic acid, 1,5-pentanediol, or 1,7-heptanediol as the sole carbon source (Fig. 1) and accumulated only up to 7 mol% of the copolymer. In the case of the diols, the authors postulated the formation of 3-hydroxypropionyl-coenzyme A (CoA) via an oxidative cycle which was not defined in detail (34).
TABLE 1.
Physical properties of 3-hydroxypropionate-containing polyesters and their natural or artificial synthesis from different carbon sources
| Polymer | Carbon substrate(s)a | Polyester content (% CDW) | Monomer composition (mol% [range]) | Mn (10−5) | Mw/Mn | Organism | Gene(s) involved | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| Poly(3HP) | Glycerol | 5-12 | 3HP (100) | E. coli | dhaB1B2Cb, pduPSe, phaC1Cn | 3 | ||
| Poly(3HB-co-3HP) | 1,5-Pentanediol | 1-42 | 3HP (1-3), 3HB (97-99) | 0.4-2.1 | 2-6.5 | C. necator | phaC1ABCn, acoECn | 34 |
| 1,7-Heptanediol | 10-13 | 3HP (2-4), 3HB (96-98) | 1.4-1.8 | 2.4-2.6 | C. necator | phaC1ABCn, acoECn | 34 | |
| 3-Hydroxypropionate | 11-50 | 3HP (13-22), 3HB (12-87) | 1.1-4.0 | 2.1-4.5 | A. lata | phaC1ABCn | 19, 39 | |
| 3-Hydroxypropionate | 1-23 | 3HP (4-7), 3HB (93-96) | 5.9-14 | 1.6-2.5 | C. necator | phaC1ABCn, acoECn | 34 | |
| 3-Hydroxypropionate | 12-16 | 3HP (13-27), 3HB (73-97) | E. coli | phaC1ABCn, prpESe | 43 | |||
| 3-Hydroxypropionate | 10-14 | 3HP (17-21), 3HB (79-83) | E. coli | phaC1ABCn, orfZCk | 43 | |||
| 3-Hydroxypropionate | 3.9-11.8 | 3HP (4.3-10.5), 3HB (89.5-95.7) | Methylobacterium sp. strain KCTC 0048 | phaC1ABCn | 23 | |||
| Sugars, fatty acids | 31-53 | 3HP (0.4-2.1), 3HB (97.9-99.6) | C. necator | phaC1ABCn, mcrCa, acsCa | 16 | |||
| Poly(4HB-co-3HP-co-2HP) | 3-Hydroxypropionate | E. coli | pctCp, ptbCa, bukCa | 37 | ||||
| Poly(3HB-co-3HP-co-4HB-co-2HP) | 3-Hydroxypropionate | E. coli | pctCp, ptbCa, bukCa | 37 | ||||
| Poly(3HP-co-3HB-co-3HH-co-3HO) | Octanoate, acrylic acid | 11.8 | 3HP (1.4-6.5), 3HB (81.7-95.9), 3HH (2.1-10.2), 3HO (0.6-1.6) | 6.3-8.0 | 2.3-2.5 | C. necator | phaC1ABCn, phaC1Pa | 14 |
Carbon substrate converted into the 3HP monomer.
Another attempt to assemble poly(3HB-co-3HP) was described by Kang et al. (23). The authors cultivated Methylobacterium sp. strain KCTC 0048 with methanol and 3HP and obtained poly(3HB-co-3HP) yields which were comparable to those achieved by Nakamura et al. (34). The copolymer contents ranged between 3.9 and 11.8% (wt/wt) of the cell dry weight (CDW), with 3HP fractions between 4.3 and 10.5 mol%.
Higher copolymer contents in wild-type strains were obtained by using Azohydromonas lata (formerly Alcaligenes latus [47]). Poly(3HB-co-3HP) copolyesters with a wide range of compositions, varying from 0 to 88 mol% 3HP, were produced by A. lata from mixed carbon substrates consisting of 3-hydroxypropionic acid and sucrose or 3-hydroxybutyric acid (19, 39, 46). A. lata did not grow on 3-hydroxypropionic acid as sole carbon source. The cell densities and polyester contents of the cells decreased as the 3-hydroxypropionic acid fraction in the carbon source mix (3-hydroxypropionic acid/sucrose) increased. In contrast, the 3HP fraction in the copolymer increased to 29 mol%. With 3-hydroxybutyric acid and 3-hydroxypropionic acid, the 3HP content rose from 25 to 76 mol%. The chirality of the carbon source has a direct influence on the 3HP molecules incorporated and the copolymer content. When (R)-(−)-3-hydroxybutyric acid was used instead of (S)-(+)-3-hydroxybutyric acid, the 3HP fraction increased up to 88 mol%, but the cells' polyester content decreased to 6 weight percent (wt%).
The first production of poly(3HB-co-3HP) in recombinant E. coli was reported by Valentin et al. (43). When E. coli cells harboring the phaC1ABCn operon of C. necator and expressing the propionyl-CoA synthase PrpESe from Salmonella enterica serovar Typhimurium LT2 or the acetyl-CoA:4-hydroxybutyrate CoA transferase OrfZCk from Clostridium kluyveri were grown in LB medium containing 1% 3HP, polyesters containing approximately 90 mol% 3HP were accumulated. Cultivation in the presence of 1% (wt/vol) mannitol plus 1% (wt/vol) 3HP resulted in a similar accumulation of 3HP. Without mannitol, the 3HP fraction decreased to only 20 to 25 mol%. The expression of the acetyl-CoA synthase AcoECn from C. necator under such growth conditions resulted in significantly lower levels of 3HP in the polyester. The total amount of polyesters accumulated in these strains did not vary significantly (12 to 14% CDW).
Fukui et al. (12) showed the microbial synthesis of poly(3HB-co-3HP) from an unrelated carbon source, such as fructose, by a metabolically engineered C. necator strain. For this, the authors established an artificial 3HP biosynthesis pathway. The genes for malonyl-CoA reductase (mcrCa) and the N-terminal 3HP-CoA synthase domain of propionyl-CoA synthase (acsCa) from Chloroflexus aurantiacus were tandemly organized and inserted downstream of the pBAD promoter of the pBBad broad-host-range vector (16). The expression of the respective genes in C. necator was induced with l-arabinose. When mcrCa and acsCa were coexpressed in the recombinant C. necator H16, 3HP units were detected by gas chromatography analysis after methanolysis and subsequent O-trimethylsilylation of the dried cells. While a small fraction (0.2 mol%) of 3HP was observed even in the absence of an inducer, the 3HP content in the copolymer increased from 0.2 to 1.0 mol% concomitantly with the rise from 0.001 to 0.1% (vol/vol) l-arabinose. The cell densities and PHA contents of the induced cultures (1.8 to 1.9 g/liter and 57 to 59 wt%, respectively) were comparable to those of the strain harboring only the vector. Supplementation with gluconate instead of fructose also resulted in copolyester biosynthesis despite a lower productivity. With octanoate as the sole carbon source, poly(3HB-co-3-HP) was accumulated up to 76 wt%, with a 3HP content of 0.6 mol%. With longer alkyl chains, dodecanoate, or soybean oil, no or only trace amounts of 3HP units were incorporated into the polymer.
BACTERIAL SYNTHESIS OF POLY(3HP) HOMOPOLYMERS
The first biotechnological approach accumulating the poly(3HP) homopolymer and producing a 3HP-containing polyester independently from 3HP as a precursor was recently established in our laboratory by engineering a nonnatural pathway (3). For construction of the novel pathway, we expressed the genes for the glycerol dehydratase (dhaB1Cb) of Clostridium butyricum, the propionaldehyde dehydrogenase (pduPSe) of Salmonella enterica serovar Typhimurium LT2, and the PHA synthase (phaC1Cn) of C. necator in a recombinant E. coli strain. Poly(3HP) was accumulated up to 12% of the CDW in two-step fed-batch fermentations.
Glycerol dehydratases are mainly present in a few Clostridia and enteric bacteria. They were also used for the conversion of glycerol to 1,3-propanediol, which can be chemically polymerized with terephthalic acid or dimethyl terephthalate (26). The resulting polymer is distributed by DuPont as Sorona.
IN VITRO SYNTHESIS OF POLY(3HB-co-3HP)
The copolymer of 3HB and 3HP was not only synthesized in vivo. Han et al. (17) used an improved two-phase reaction system (TPRS) for chemoenzymatic poly(3HB-co-3HP) synthesis. For this, they constructed a fusion protein of the PHA synthase (PhaC1Cn) from C. necator and the propionyl-CoA transferase (PctCp) from Clostridium propionicum JCM1430. The TPRS contained a sodium phosphate-buffered water phase with 3HB and 3HP as substrates, the purified fusion protein, and coenzyme A. Hexane and acetyl thioester of ethyl thioglycolate (AcETG) constituted the organic solvent phase. An ester exchange reaction between AcETG in the organic solvent phase and coenzyme A in the water phase was performed on the interface to synthesize acetyl-CoA. 3HB-CoA and 3HP-CoA were generated by transfer reactions between acetyl-CoA and a free molecule of 3HB or 3HP, respectively, catalyzed by PctCp. The hydroxyacyl moieties of 3HB-CoA and 3HP-CoA were then polymerized sequentially by PhaCCn. The molar ratio of 3HP in the copolyester increased with increased 3HP concentrations in the reaction mixture. It was possible to control the monomer composition of the polymer thereby. Due to the higher affinity toward 3HP than for 3HB, as indicated by a lower Michaelis-Menten constant of PctCp for 3HP, the molar ratios of 3HP units in the polymers were significantly higher than those in the corresponding reaction mixture (17).
BACTERIAL SYNTHESIS OF OTHER COPOLYESTERS CONTAINING 3HP
Green et al. (14) showed that C. necator synthesizes poly(3-hydroxypropionate-co-3-hydroxybutyrate-co-3-hydroxyhexanoate-co-3-hydroxyoctanoate) [poly(3HP-co-3HB-co-3HH-co-3HO)] copolyesters in the presence of acrylic acid and sodium octanoate as the sole carbon source. It is known that acrylic acid inhibits fatty acid β-oxidation and that the incubation of C. necator in the presence of this inhibitor leads to the incorporation of medium-chain-length 3-hydroxyalkanoates (3HAs) into the PHA. Since sodium acrylate is toxic to bacteria, no growth occurred if its concentration exceeded 5 mM in the mineral salt medium. To test the effects of higher levels of acrylate, Green et al. (14) established a two-stage cultivation and PHA production protocol. The bacteria were first grown to high cell densities in LB medium and then harvested and suspended in mineral salt medium containing different concentrations of sodium acrylate. The ratios of 3HP, 3HH, and 3HO in the copolymer increased as the concentration of acrylate increased, while the 3HB content decreased. When C. necator was incubated with 10.6 mM acrylate, the copolyesters contained 1.4 mol% 3HP, 95.9 mol% 3HB, 2.1 mol% 3HH, and 0.6 mol% 3HO. If the acrylate concentration was increased to 29.3 mM, the 3HB fraction decreased to 81.7 mol% and the fractions of 3HP, 3HH, and 3HO in the copolyesters increased to 6.5, 10.2, and 1.6 mol%, respectively (Table 1). Unlike for poly(3HB-co-3HP), the melting point decreased concomitantly with the decline of the 3HB content. In contrast to this, the glass transition temperature of poly(3HB-co-3HP) was only marginally influenced.
Park et al. (37) described a method to synthesize different polymers containing 3HP, namely, poly(4-HB-co-3-HP-co-lactate), poly(4HB-co-3HP-co-2HP), poly(3-HB-co-3-HP-co-4-HB-co-lactate), and poly(3HB-co-3HP-co-4HB-co-2HP). They incubated a recombinant E. coli TOP10 strain harboring the propionyl-CoA transferase (PctCp) from Clostridium propionicum, the phosphate butyryltransferase (PtbCa) and the butyrate kinase (BukCa) from Clostridium acetobutyricum, and the PHA synthase from Pseudomonas sp. strain 6-19 in the presence of lactate, 4HB, and 3HP or lactate, 4HB, 3HP, and 3HB, respectively, as substrates (Table 1). This process was related to a process previously described by Liu and Steinbüchel (27) and Lütke-Eversloh et al. (29).
THERMAL AND PHYSICAL PROPERTIES OF POLY(3HB-co-3HP)
It is well known that the physical properties of the given bacterial copolyesters will, in principle, be regulated by factors such as comonomer structure, average comonomer composition, comonomer compositional distribution, etc.
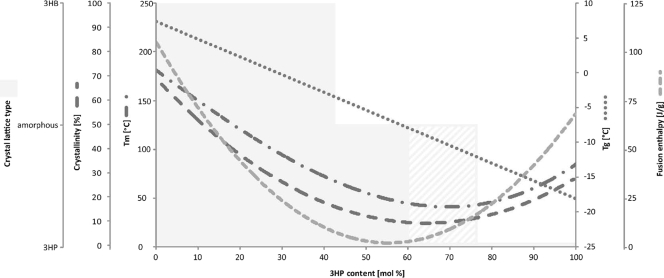
The influence of varying the 3HP content in poly(3HB-co-3HP) on the physical and thermal properties of the polyester has been investigated in detail. The glass transition temperature decreased from +4 to −19°C as the 3HP fraction increased from 0 to 100 mol%. This effect also occurred in other copolyesters in which 3HB was partially replaced by a different hydroxyalkanoic acid constituent. Poly(3HB-co-3HH) and poly(3HB-co-4HB) behave in a similar manner (27, 36). The alteration of the melting temperature shows a parabolic behavior. Poly(3HB) has a melting temperature of 177°C. The incorporation of 3HP units up to 67 mol% led to a decrease to 44°C, whereas further increasing the 3HP content raised it again, to 77°C [100 mol% 3HP]. The melting temperatures of other copolyesters similar to poly(3HB-co-3HH) and poly(3HB-co-4HB) showed the same tendency. This obviously implies that the reduction of the melting temperature value is not solely influenced by the chemical structure, due to the side chains, but is also an effect of the secondary structure and the crystallinity (11). The enthalpy of fusion showed a similar behavior. First, it decreased from 97 J/g (0 mol% 3HP) to 2 J/g (43 mol% 3HP) and then increased up to 74 J/g (100 mol% 3HP). Thermal degradation of poly(3HB-co-3HP) increased with the fraction of 3HP (19, 39). It was shown by Wang et al. (46) that poly(3HB-co-3HP) with 3HP contents below 42 mol% formed only the poly(3HB)-type crystalline structure, while samples with 3HP contents higher than 56 mol% formed only the poly(3HP)-type crystalline structure. Both types of crystals occurred with a 3HP content of 48.8 mol%.
The secondary structures of poly(3HB-co-3HP) also differ with the 3HP fraction. Cao et al. (9) have reported, based on wide-angle X-ray diffraction of poly(3HB-co-3HP)s, that 3HB-rich (0 to 38.3 mol% 3HP) and 3HP-rich (77.9 to 100 mol% 3HP) copolyesters form distinct helix structures, while copolyesters with 3HP contents ranging from about 45 to 75 mol% appear in the amorphous state. The results of 13C nuclear magnetic resonance (NMR) experiments by Cao et al. (7) confirmed that 3HP units are essential to comprise the poly(3HP) lattice-type crystallites for the 3HP-rich semicrystalline copolyesters. Isomorphism, a phenomenon of cocrystallization observed in the bacterial poly(3HB-co-3HV) system, does not occur in the poly(3HB-co-3HP) system. The 3HB or 3HP units were entirely excluded from the respective poly(3HB)- or poly(3HP)-type crystallites and constituted the amorphous regions with another structural unit. In addition, the 3HB units showed more significantly suppressing effects on the crystallization in the 3HP-rich semicrystalline copolyesters. A possible reason for that could be the large difference in molecular mobility between the 3HB and 3HP units.
Different from poly(3HB-co-3HV) copolymers, the degree of crystallinity of poly(3HB-co-3HP) copolymers decreased from about 68% to less than 10% when the 3HP fraction of the copolymer increased from 0 to 48.8 mol%. It remained nearly constant at 3HP contents ranging from 48.8 to 74.2 mol% and increased with the increase of the 3HP fraction from 74.2 to 100 mol% (46). The main influences of the 3HP fraction in poly(3HB-co-3HP) copolyesters are summarized in Fig. 2.
FIG. 2.
Changes in the physical properties of the copolymer poly(3HB-co-3HP) by varying the 3HP content. The glass transition temperatures (Tg) and melting temperatures (Tm), crystallinity, crystal lattice type, and fusion enthalpy are plotted against the 3HP content. The figure compiles the data of Shimamura et al. (39), Ischikawa et al. (19), Cao et al. (7, 8, 9), Na et al. (33), Wang et al. (46), and Feng et al. (11). For the hatched area of the crystal lattice type, the data are contrary. Both the amorphous and the 3HP crystal lattice type have been reported.
ENZYMATIC DEGRADATION OF 3HP- CONTAINING POLYESTERS
The ability to utilize PHAs as a sole carbon source for growth is widely distributed among bacteria, as well as eukaryotic microorganisms. For this, they have evolved several carboxyesterases, also referred to as PHA depolymerases, which are secreted into the medium. All PHA depolymerase proteins (PhaZ) have composite domain structures and consist of a signal peptide segment, a large catalytic domain at the N terminus, a C-terminal substrate-binding domain, and a linking domain between the catalytic and binding domains. Three strictly conserved amino acids, serine, aspartate, and histidine, constitute the active center of the catalytic domain. The extracellular PHA depolymerases are classified into four types by differences in the linker domain structure or in the position of the lipase box in the catalytic domain (21, 22). The enzymatic hydrolysis of polymers has been studied on solution cast films (10) and melt-crystallized films (25, 42), which demonstrated that the enzymatic hydrolysis occurred first at the amorphous region and subsequently at the crystal region. For poly(3HB) films with similar degrees of crystallinity, the rate of enzymatic hydrolysis is influenced by spherulite and crystal sizes.
Kasuya et al. (24) described the degradation of poly(3HP), which was synthesized by ring-opening polymerization of β-propiolactone, by the extracellular PHB depolymerases from Ralstonia pickettii (previously referred to as Alcaligenes faecalis [48]) (PhaZRp), Pseudomonas stutzeri (PhaZPs), and Comamonas acidovorans (PhaZCa). The rates of erosion occurring in solid specimens of the material ranged between 0.1 (PhaZRp) and nearly 0.3 mg/h/cm2 (PhaZPs). Surprisingly, the corresponding values for the actual substrate poly(3HB) of all three depolymerases were lower (24).
The best characterized degradation of a 3HP-containing copolymer is the hydrolysis of poly(3HP-co-3HB). Shimamura et al. (39) degraded poly(3HB-co-3HP) films with different 3HP contents with PhaZRp. These results, in addition to those of Cao et al. (8), suggested that the rates of enzymatic degradation were controlled not only by the crystallinity of the polymer but also by the chemical structure of the building blocks and the substrate specificity of PHA depolymerases. The crystalline structure and the degree of crystallinity of poly(3HB-co-3HP) copolymers were shown to change with alterations in the 3HP content. The poly(3HP) homopolymer-type crystalline structure of the copolymer could be degraded at a higher rate than poly(3HB) homopolymer crystallites. Although the erosion rates for the poly(3HP)-type crystalline structures were higher than those of the poly(3HB) type, the maximum degradation rate has been observed for the poly(3HB-co-3HP) copolyester with a 3HP content of about 20 to 30 mol% (8). A similar effect was observed during the degradation of poly(3HB-co-3HP) samples by PhaZ from Acidovorax sp. strain TP4 (PhaZAc). The rate increased in parallel with the increase of the 3HP content from 0 to 56.2 mol%. Samples with 3HP contents from 56.2 to 83.9 mol% showed almost similar degradation rates. When the 3HP content was higher than 84 mol%, the enzymatic hydrolysis decreased with the increase of the copolymer crystallinity. 1H NMR spectra of the degradation products of unfractionated poly(3HB-co-21.0 mol% 3HP) indicated that PhaZAc degrades poly(3HB-co-3HP) films first into trimers or dimers and then into the monomers. In contrast to PhaZRp, PhaZAc depolymerizes poly(3HB-co-3HP) films with lower degrees of crystallinity at higher rates (46). The water-soluble degradation products obtained from poly(3HB-co-3HP) by PhaZAc were monomers (3HB and 3HP), dimers (3HB-3HB, 3HB-3HP, 3HP-3HB, and 3HP-3HP), and trimers (3HB-3HP-3HP, 3HB-3HB-3HP, and 3HP-3HP-3HP) (1).
FUTURE DIRECTIONS AND PERSPECTIVES
This review has focused on our current knowledge of the synthesis of the poly(3HP) homopolymer and 3HP-containing copolymers. Some enzymes and artificial pathways have been identified until now, but via metabolic engineering, new approaches may still be found. One significant challenge is to synthesize poly(3HP) and its copolymers from structurally unrelated carbon sources. For competitiveness with petrochemically synthesized polyesters, there is an urgent need for independence from 3HP as a precursor. Glycerol could be a good candidate for a cheap carbon source due to its occurrence as a by-product of biodiesel production (50).
The studies of Fukui et al. (12) and Andreeßen et al. (3) represent first demonstrations of methods to solve this problem. To achieve higher 3HP fractions in the copolymer, more investigations concerning the accumulation of 3HP are needed. Further research should also aim at synthesizing new copolyesters containing 3HP together with other, hitherto unidentified hydroxyalkanoic acids in the polymer backbone. The structure and physical properties of 3HP exhibit several advantages: (i) the homopolymer exhibits high rigidity, ductility, and exceptional tensile strength in drawn films and, (ii) in copolyesters, it reduces the glass transition temperature and crystallinity but increases the enzymatic degradability, due to the missing methyl groups in the polymer backbone (Figs. 1 and 2).
Acknowledgments
Financial support from the BMVEL/FNR (FKZ 22015806 and 06NR158) is gratefully acknowledged.
Footnotes
Published ahead of print on 11 June 2010.
REFERENCES
- 1.Abe, H., and Y. Doi. 1999. Structural effects on enzymatic degradabilities for poly[(R)-3-hydroxybutyric acid] and its copolymers. Int. J. Biol. Macromol. 25:185-192. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, A. J., and E. A. Dawes. 1990. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 54:450-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreeßen, B., A. B. Lange, H. Robenek, and A. Steinbüchel. 2010. Conversion of glycerol to poly(3-hydroxypropionate) in recombinant Escherichia coli. Appl. Environ. Microbiol. 76:622-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba, H., N. Tanahashi, Y. Kumagai, and Y. Doi. 1992. Effects of molecular structure on enzymatic degradation of polyesters. J. Chem. Soc. Jpn. 5:527-533. [Google Scholar]
- 5.Braunegg, G., G. Lefebvre, and K. F. Genser. 1998. Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J. Biotechnol. 65:127-161. [DOI] [PubMed] [Google Scholar]
- 6.Brown, S. F. 2003. Bioplastic fantastic. Fortune 148:92-97. [PubMed] [Google Scholar]
- 7.Cao, A., N. Asakawa, N. Yoshie, and Y. Inoue. 1999. High-resolution solid-state C-13 n.m.r. study on phase structure of the compositionally fractionated bacterial copolyester poly(3-hydroxybutyric acid-co-3-hydroxypropionic acid)s. Polymer 40:3309-3322. [Google Scholar]
- 8.Cao, A., Y. Arai, N. Yoshie, K. I. Kasuya, Y. Doi, and Y. Inoue. 1999. Solid structure and biodegradation of the compositionally fractionated poly(3-hydroxybutyric acid-co-3-hydroxypropionic acid)s. Polymer 40:6821-6830. [Google Scholar]
- 9.Cao, A., K. Kasuya, H. Abe, Y. Doi, and Y. Inoue. 1998. Studies on comonomer compositional distribution of the bacterial poly(3-hydroxybutyric acid-co-3-hydroxypropionic acid)s and crystal and thermal characteristics of their fractionated component copolyesters. Polymer 39:4801-4816. [Google Scholar]
- 10.Doi, Y., S. Kitamura, and H. Abe. 1995. Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 28:4822-4828. [Google Scholar]
- 11.Feng, L., N. Yoshie, N. Asakawa, and Y. Inoue. 2004. Comonomer-unit compositions, physical properties and biodegradability of bacterial copolyhydroxyalkanoates. Macromol. Biosci. 4:186-198. [DOI] [PubMed] [Google Scholar]
- 12.Fukui, T., M. Suzuki, T. Tsuge, and S. Nakamura. 2009. Microbial synthesis of poly((R)-3-hydroxybutyrate-co-3-hydroxypropionate) from unrelated carbon sources by engineered Cupriavidus necator. Biomacromolecules 10:700-706. [DOI] [PubMed] [Google Scholar]
- 13.Gerngross, T. U. 1999. Can biotechnology move us toward a sustainable society? Nat. Biotechnol. 17:541-544. [DOI] [PubMed] [Google Scholar]
- 14.Green, P. R., J. Kemper, L. Schechtman, L. Guo, M. Satkowski, S. Fiedler, A. Steinbüchel, and B. H. A. Rehm. 2002. Formation of short chain length/medium chain length polyhydroxyalkanoate copolymers by fatty acid β-oxidation inhibited Ralstonia eutropha. Biomacromolecules 3:208-213. [DOI] [PubMed] [Google Scholar]
- 15.Gresham, T. L., J. E. Jansen, and F. W. Shaver. 1948. β-Propiolactone. I. Polymerization reactions. J. Am. Chem. Soc. 70:998-999. [Google Scholar]
- 16.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han, X., Y. Satoh, K. Tajima, T. Matsushima, and M. Munekata. 2009. Chemo-enzymatic synthesis of polyhydroxyalkanoate by an improved two-phase reaction system (TPRS). J. Biosci. Bioeng. 108:517-523. [DOI] [PubMed] [Google Scholar]
- 18.Holmes, P. A. 1985. Applications of PHB—a microbially produced biodegradable thermoplastic. Phys. Technol. 16:32-36. [Google Scholar]
- 19.Ichikawa, M., K. Nakamura, N. Yoshie, N. Asakawa, and Y. Inoue. 1996. Morphological study of bacterial poly(3-hydroxybutyrate-co-3-hydroxypropionate). Macromol. Chem. Phys. 197:2467-2480. [Google Scholar]
- 20.Inoue, Y. 1998. Solid-state structure and properties of bacterial copolyesters. J. Mol. Struct. 441:119-127. [Google Scholar]
- 21.Jendrossek, D., and R. Handrick. 2002. Microbial degradation of polyhydroxyalkanoates. Annu. Rev. Microbiol. 56:403-432. [DOI] [PubMed] [Google Scholar]
- 22.Jendrossek, D., A. Schirmer, and H. G. Schlegel. 1996. Biodegradation of polyhydroxyalkanoic acids. Appl. Microbiol. Biotechnol. 46:451-463. [DOI] [PubMed] [Google Scholar]
- 23.Kang, C. K., H. S. Lee, and J. H. Kim. 1993. Accumulation of PHA and its copolyesters by Methylobacterium sp. KCTC 0048. Biotechnol. Lett. 15:1017-1020. [Google Scholar]
- 24.Kasuya, K., T. Ohura, K. Masuda, and Y. Doi. 1999. Substrate and binding specificities of bacterial polyhydroxybutyrate depolymerases. Int. J. Biol. Macromol. 24:329-336. [DOI] [PubMed] [Google Scholar]
- 25.Koyama, N., and Y. Doi. 1997. Effects of solid-state structures on the enzymatic degradability of bacterial poly(hydroxyalkanoic acids). Macromolecules 30:826-832. [Google Scholar]
- 26.Laffend, L. A., V. Nagarajan, and C. E. Nakamura. November 2006. Processes for the bioconversion of a fermentable carbon source to 1,3-propanediol by a single microorganism. U.S. patent 7,135,309.
- 27.Liu, S. J., and A. Steinbüchel. 2000. A novel genetically engineered pathway for synthesis of poly(hydroxyalkanoic acids) in Escherichia coli. Appl. Environ. Microbiol. 66:739-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundgren, D. G., R. Alper, C. Schnaitman, and R. H. Marchessault. 1965. Characterization of poly-β-hydroxybutyrate extracted from different bacteria. J. Bacteriol. 89:245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lütke-Eversloh, T., A. Fischer, U. Remminghorst, J. Kawada, R. H. Marchessault, A. Bogershausen, M. Kalwei, H. Eckert, R. Reichelt, S. J. Liu, and A. Steinbüchel. 2002. Biosynthesis of novel thermoplastic polythioesters by engineered Escherichia coli. Nat. Mater. 1:236-240. [DOI] [PubMed] [Google Scholar]
- 30.Madden, L. A., A. J. Anderson, and J. Asrar. 1998. Synthesis and characterization of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) polymer mixtures produced in high-density fed-batch cultures of Ralstonia eutropha (Alcaligenes eutrophus). Macromolecules 31:5660-5667. [Google Scholar]
- 31.Madison, L. L., and G. W. Huisman. 1999. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63:21-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makkar, N. S., and L. E. Casida. 1987. Cupriavidus necator gen. nov., sp. nov.; a nonobligate bacterial predator of bacteria in soil. Int. J. Syst. Evol. Microbiol. 37:323-326. [Google Scholar]
- 33.Na, Y. H., Y. He, N. Asakawa, N. Yoshie, and Y. Inoue. 2002. Miscibility and phase structure of blends of poly(ethylene oxide) with poly(3-hydroxybutyrate), poly(3-hydroxypropionate), and their copolymers. Macromolecules 35:727-735. [Google Scholar]
- 34.Nakamura, S., M. Kunioka, and Y. Doi. 1991. Biosynthesis and characterization of bacterial poly(3-hydroxybutyrate-co-3-hydroxypropionate). J. Macromol. Sci. A 28:15-24. [Google Scholar]
- 35.Nanba, T., H. Ito, H. Kobayashi, and T. Hayashi. 1994. Production process of poly(hydroxyalkanoate). JP patent 6329774.
- 36.Pan, P., and Y. Inoue. 2009. Polymorphism and isomorphism in biodegradable polyesters. Prog. Polym. Sci. 34:605-640. [Google Scholar]
- 37.Park, S. J., S. H. Lee, E. J. Lee, H. O. Kang, T. W. Kim, T. H. Yang, and S. Y. Lee. 2008. Copolymer comprising 4-hydroxybutyrate unit and lactate unit and its manufacturing method. KR patent WO 2008/062995 A1.
- 38.Philip, S., T. Keshavarz, and I. Roy. 2007. Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 82:233-247. [Google Scholar]
- 39.Shimamura, E., M. Scandola, and Y. Doi. 1994. Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxypropionate). Macromolecules 27:4429-4435. [Google Scholar]
- 40.Steinbüchel, A. 2001. Perspectives for biotechnological production and utilization of biopolymers: metabolic engineering of polyhydroxyalkanoate biosynthesis pathways as a successful example. Macromol. Biosci. 1:1-24. [Google Scholar]
- 41.Sudesh, K., H. Abe, and Y. Doi. 2000. Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog. Polym. Sci. 25:1503-1555. [Google Scholar]
- 42.Tomasi, G., and M. Scandola. 1996. Enzymatic degradation of bacterial poly(3-hydroxybutyrate) by a depolymerase from Pseudomonas lemoignei. Macromolecules 29:507-513. [Google Scholar]
- 43.Valentin, H. E., T. A. Mitsky, D. A. Mahadeo, M. Tran, and K. J. Gruys. 2000. Application of a propionyl coenzyme A synthetase for poly(3-hydroxypropionate-co-3-hydroxybutyrate) accumulation in recombinant Escherichia coli. Appl. Environ. Microbiol. 66:5253-5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Walle, G. A., G. J. de Koning, R. A. Weusthuis, and G. Eggink. 2001. Properties, modifications and applications of biopolyesters. Adv. Biochem. Eng. Biotechnol. 71:263-291. [DOI] [PubMed] [Google Scholar]
- 45.van Maris, A. J. A., W. N. Konings, J. P. van Dijken, and J. T. Pronk. 2004. Microbial export of lactic and 3-hydroxypropanoic acid: implications for industrial fermentation processes. Metabol. Eng. 6:245-255. [DOI] [PubMed] [Google Scholar]
- 46.Wang, Y., Y. Inagawa, T. Saito, K. Kasuya, Y. Doi, and Y. Inoue. 2002. Enzymatic hydrolysis of bacterial poly(3-hydroxybutyrate-co-3-hydroxypropionate)s by poly(3-hydroxyalkanoate) depolymerase from Acidovorax sp. TP4. Biomacromolecules 3:828-834. [DOI] [PubMed] [Google Scholar]
- 47.Xie, C. H., and A. Yokota. 2005. Reclassification of Alcaligenes latus strains IAM 12599(T) and IAM 12664 and Pseudomonas saccharophila as Azohydromonas lata gen. nov., comb. nov., Azohydromonas australica sp. nov. and Pelomonas saccharophila gen. nov., comb. nov., respectively. Int. J. Syst. Evol. Microbiol. 55:2419-2425. [DOI] [PubMed] [Google Scholar]
- 48.Yabuuchi, E., Y. Kosako, I. Yano, H. Hotta, and Y. Nishiuchi. 1995. Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen. nov.: proposal of Ralstonia ettii (Ralston, Palleroni and Doudoroff 1973) comb. nov., Ralstonia solanacearum (Smith 1896) comb. nov. and Ralstonia eutropha (Davis 1969) comb. nov. Microbiol. Immunol. 39:897-904. [DOI] [PubMed] [Google Scholar]
- 49.Yamashita, M., Y. Takemoto, E. Ihara, and H. Yasuda. 1996. Organolanthanide-initiated living polymerizations of ɛ-caprolactone, δ-valerolactone, and β-propiolactone. Macromolecules 29:1798-1806. [Google Scholar]
- 50.Yazdani, S. S., and R. Gonzalez. 2008. Engineering Escherichia coli for the efficient conversion of glycerol to ethanol and co-products. Metabol. Eng. 10:340-351. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, D., M. A. Hillmyer, and W. B. Tolman. 2004. A new synthetic route to poly[3-hydroxypropionic acid] (P[3-HP]): ring-opening polymerization of 3-HP macrocyclic esters. Macromolecules 37:8198-8200. [Google Scholar]