Abstract
Infection with monkeypox virus (MPXV) causes disease manifestations in humans that are similar, although usually less severe, than those of smallpox. Since routine vaccination for smallpox ceased more than 30 years ago, there is concern that MPXV could be used for bioterrorism. Thus, there is a need to develop animal models to study MPXV infection. Accordingly, we screened 38 inbred mouse strains for susceptibility to MPXV. Three highly susceptible wild-derived inbred strains were identified, of which CAST/EiJ was further developed as a model. Using an intranasal route of infection with an isolate of the Congo Basin clade of MPXV, CAST/EiJ mice exhibited weight loss, morbidity, and death in a dose-dependent manner with a calculated 50% lethal dose (LD50) of 680 PFU, whereas there were no deaths of BALB/c mice at a 10,000-fold higher dose. CAST/EiJ mice exhibited greater MPXV sensitivity when infected via the intraperitoneal route, with an LD50 of 14 PFU. Both routes resulted in MPXV replication in the lung, spleen, and liver. Intranasal infection with an isolate of the less-pathogenic West African clade yielded an LD50 of 7,600 PFU. The immune competence of CAST/EiJ mice was established by immunization with vaccinia virus, which induced antigen-specific T- and B-lymphocyte responses and fully protected mice from lethal doses of MPXV. The new mouse model has the following advantages for studying pathogenesis of MPXV, as well as for evaluation of potential vaccines and therapeutics: relative sensitivity to MPXV through multiple routes, genetic homogeneity, available immunological reagents, and commercial production.
Monkeypox virus (MPXV), a member of the orthopoxvirus genus of the Chordopoxvirinae subfamily of the Poxviridae, was isolated in 1958 from lesions in a cynomolgous monkey that had been imported from Africa (27). The first human infections with MPXV were reported in 1972, and since then more than two thousand cases have been recorded, most in the Democratic Republic of the Congo and lesser numbers in West African countries (reviewed by Parker et al. [18]). The mortality from human monkeypox in the Congo is estimated to be 10% of infected individuals with clinical symptoms that mimic smallpox, which is caused by another member of the orthopoxvirus genus: variola virus. However, whereas the host range of variola virus is restricted to humans, serological studies indicate that MPXV naturally infects a large number of animal species, particularly squirrels and nonhuman primates. The sporadic occurrence of human monkeypox is thought to arise from close proximity and handling of infected animals. In this respect, a self-limited outbreak in the United States was traced to a shipment of West African rodents (19). Although monkeypox is a minor public health problem when compared historically to smallpox, the potential for expansion of the MPXV host range and adaptations to enhance human transmission make it prudent to continue careful surveillance. Moreover, the potential use of MPXV for bioterrorism has led to its inclusion as a select agent in the United States (http://www.selectagents.gov).
Animal models are crucial for studying virus pathogenesis, and MXPV is no exception. Ground squirrels (22, 26), black-tailed prairie dogs (9, 11, 13, 30), and African dormice (23) are highly susceptible to MPXV. However, as experimental systems, each has limitations with regard to unavailability of commercial breeding, genetic heterogeneity and absence of immunological and other reagents. Laboratory mice, including BALB/c, C57BL/6, and several other mouse strains tested, were found to be resistant to MPXV disease unless impaired in innate or acquired immunity (10, 17, 24). In the present study, we tested a large group of distinct inbred strains of mice chosen for genetic diversity, inclusion of classical and wild-derived strains, and commercial availability. Of 38 inbred mouse strains tested, three wild-derived strains were highly susceptible to MPXV. One of these, CAST/EiJ, was further characterized with regard to MPXV strain sensitivity, route of inoculation, virus dissemination, immune response, and protection by vaccination and drug treatment.
MATERIALS AND METHODS
Cells.
BS-C-1 cells were maintained at 37°C and 5% CO2 in modified Eagle minimal essential medium (Quality Biologicals, Inc., Gaithersburg, MD) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 10 U of penicillin/ml, and 10 μg of streptomycin/ml.
Viruses.
Low-passage stocks of MPXV-Z79-I-005 and MPXV-USA-2003-044 were obtained from I. Damon, CDC, Atlanta, GA. MPXV-Z79-I-005 was isolated in 1979 from a fatal human case in Zaire (1). MPXV-USA-2003-044 was isolated from a prairie dog infected in the United States during the 2003 outbreak (14). MPXV-Z79 and MPXV-USA are representative of the Congo Basin and West African clades, respectively. Viruses were grown and titered in BS-C-1 cells as described previously (6). All procedures were performed in a registered BSL-3 laboratory.
Virus purification.
Viruses were purified by using a modification of the protocols of I. Damon (unpublished data) and Earl and Moss (6) as follows. Monolayers of BS-C-1 cells were infected with 0.5 PFU/cell. After 4 days, cells were collected by centrifugation for 1 h at 14,900 × g, suspended in 10 mM Tris-HCl (pH 9) and lysed by three freeze-thaw cycles, followed by sonication in ice water for three 1-min intervals. Cell debris was removed by centrifugation at 1,200 × g for 10 min, and the supernatant was removed and kept on ice. Additional virus was extracted from the pellets by suspension in 10 mM Tris-HCl (pH 9), followed by sonication and centrifugation as described above. Supernatants from both centrifugation steps were pooled, and an equal volume of Genetron (1,1,2-trichlorotrifluoroethane; Chemsavers, Powhatan, VA) added. The samples were vortexed and then centrifuged at 1,200 × g for 5 min. The aqueous phase was removed, extracted again with Genentron, and then cushioned through 36% sucrose as previously described (6).
One-step growth curve.
BS-C-1 cells were infected with 3 PFU/cell in 12-well plates. After 1 h, monolayers were washed twice and overlaid with fresh medium. At 0, 2, 5, 8, 12, 48, and 72 h postinfection, cells from triplicate wells were harvested individually and stored at −80°C. The cells were then lysed and sonicated, and cell-associated virus yields were determined by plaque assay on BSC-1 cells (6).
Animals.
African dormice (Graphiurus kelleni), originally obtained from R. M. Buller (Saint Louis University, St. Louis, MO), were bred and maintained in large, ventilated microisolator cages by the Comparative Medicine Branch, NIAID. Four- to eight-month-old males were used. Five- to six-week-old female BALB/c mice were obtained from Taconic Biotechnology (Germantown, NY). Other strains of mice were 5- to 11-week-old females obtained from Jackson Laboratories (Bar Harbor, ME).
Inoculation of animals.
On the day of infection, MPXV was diluted in phosphate-buffered saline containing 0.05% bovine serum albumin, and the virus concentration of each dilution used in animals was verified by plaque assay. Animals were anesthetized by inhalation of isoflurane prior to infection. Intranasal (i.n.) infections were performed by introduction of 10 μl of virus into one nostril. Virus in a volume of 100 to 200 μl was used for intraperitoneal (i.p.) infections. Mock-infected control animals were similarly inoculated with an equivalent volume of diluent. Animals were weighed and observed three to seven times per week for up to 45 days postinfection. Animals that lost 30% of their starting weight were euthanized in accordance with NIAID Animal Care and Use protocols. All MPXV experiments were performed in an ABSL-3 facility with approval of the NIAID Animal Care and Use Committee and the Centers for Disease Control.
Titration of virus from organs.
Lungs, livers, spleens, and ovaries were removed from CAST/EiJ mice on the day of death and placed in 2 to 3 ml of balanced salt solution supplemented with 0.1% bovine serum albumin and stored at −80°C until use. After thawing, tissues were homogenized with a GLH-1 mechanical grinder equipped with hard-tissue disposable probes and aerosol-proof caps (Omni International, Kennesaw, GA). Homogenates were sonicated for three 45-s intervals in ice water and then centrifuged for 10 s at 400 × g in an Eppendorf 5415 centrifuge (Eppendorf, Hauppauge, NY). Supernatants were transferred to fresh tubes, and virus titers were determined by plaque assay on BS-C-1 cells.
Vaccine and antiviral studies.
Dryvax vaccine, obtained from the Centers for Disease Control and Prevention, was reconstituted according to the manufacturer's specifications and stored in aliquots at −80°C until use. A 10-μl droplet of Dryvax containing 9 × 105 PFU of vaccinia virus (VACV) was applied to the shaved backs of four CAST/EiJ mice and scarification was achieved by scratching with a 25-gauge needle. A separate group of mice was similarly scarified with phosphate buffered saline. All animals that received Dryvax developed a lesion at the site of inoculation that resolved within 11 days. At 4 weeks postvaccination, mice were challenged i.n. with either 7 × 103 (10 LD50) or 7 × 104 PFU (100 LD50) of MPXV-Z79-CB2. Animals were observed and weighed three times per week. Cidofovir (Gilead Sciences), obtained from the NIH Pharmacy, was diluted according to the manufacturer's specifications. Groups of six 11- to 13-week-old CAST/EiJ mice were infected with 104 PFU of MPXV-Z79-CB2 (14 LD50) by the i.n. route. At 4 and 24 h postinfection, mice received either 1.3 mg of cidofovir or phosphate-buffered saline by i.p. injection. Animals were observed and weighed three times per week.
Intracellular cytokine staining of splenocytes.
The procedures for preparation and staining of splenocytes were modified from the method previously described (7) as follows. Splenocytes, prepared from individual mice 2 weeks post-Dryvax vaccination, were infected with either VACV strain WR or modified VACV Ankara at a multiplicity of infection of 5. After 6 h at 37°C, brefeldin A (Sigma, St. Louis, MO) was added to a concentration of 10 μg/ml, and incubation was continued for 10 h. Cells were incubated for 10 min with anti-CD16/32, clone 2.4G2 (a gift from J. Bennink, Laboratory of Viral Diseases), and then stained with peridinin chlorophyll a protein conjugated anti-CD8 and phycoerythrin-conjugated anti-CD8 for 30 min at room temperature. After fixation and permeabilization, the cells were stained with allophycocyanin-conjugated anti-gamma interferon (anti-IFN-γ) and fluorescein isothiocyanate-conjugated tumor necrosis factor (FITC-TNF) for 1 h. Cells were then washed and resuspended in 2% paraformaldehyde. All staining reagents were purchased from BD Pharmingen (San Jose, CA). At least 100,000 cells were acquired on a FACSCalibur cytometer using CellQuest software (BD Biosciences, San Jose, CA) and analyzed by using FlowJo software (TreeStar, Cupertino, CA).
VACV enzyme-linked immunosorbent assay (ELISA).
Serum IgG titers were determined from mice 4 weeks after vaccination with Dryvax as previously described (4). Peroxidase-conjugated anti-mouse IgG (Roche Applied Science, Indianapolis, IN) was used.
RESULTS
Isolation and characterization of MPXV clones.
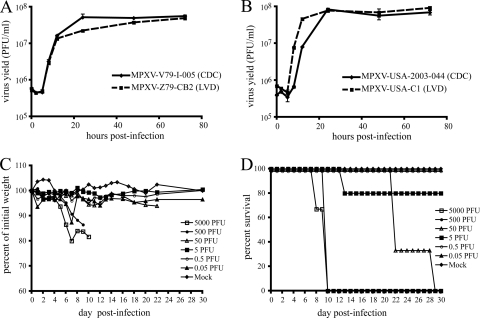
Four independent clones were isolated from low-passage stocks of MPXV-Z79-I-005 (Congo Basin clade) and MPXV-USA-2003-044 (West African clade) by four rounds of plaque purification in BS-C-1 cells. After amplification, plaque and comet morphology, as well as intracellular and extracellular virus production, were compared to the parental stock. No differences between clones and the parental stock were noted (not shown), so one isolate from each strain was chosen for further characterization. One-step growth curves were performed in BS-C-1 cells, and virus titers were determined at various times after infection. Growth kinetics and total yield of virus for both of the selected clones (MPXV-Z79-CB2 and MPXV-USA-C1) mimicked that of the parental stock (Fig. 1 A and B). These clones were then used for further experimentation.
FIG. 1.
In vitro and in vivo comparisons of low passage and cloned MPXV-Z79. (A and B) Growth curves. BS-C-1 cells were infected with 3 PFU per cell of the parental stock (CDC) or clonal isolate (LVD) of Congo Basin (A) or West African (B) clades of MPXV. At the indicated times after infection, cells from triplicate wells were individually harvested and lysed, and titers were determined by plaque assay on BS-C-1 cells. (C and D) Infection of African dormice. Groups of 4- to 8-month-old male dormice were infected i.n. with doses of MPXV-Z79-CB2 between 0.05 and 5,000 PFU. Animals were monitored for weight loss (C) and death (D) for 30 days. Control mice were mock infected with virus diluent. Group size was three to five animals.
Since dormice have been shown to be highly susceptible to MPXV infection (23), we tested our clonal isolates using that model. Groups of three to five male dormice aged 4 to 8 months were infected by the i.n. route with MPXV-Z79-CB2 with log-increasing doses from 0.05 to 5,000 PFU. Animals were monitored for weight loss and death (Fig. 1C and D, respectively) for 45 days. All animals infected with 500 or 5,000 PFU died by 10 days postinfection with maximum average weight losses of 14 and 18%, respectively. Animals in the 50-PFU group also succumbed to MPXV infection with a time to death of 22 to 29 days postinfection and only 6% weight loss. One animal in the 5 PFU group died on day 12 postinfection, but none of the others showed any outward signs of disease. Likewise, none of the animals in the 0.5 and 0.05 PFU groups lost weight or became lethargic during the 45-day follow-up. The calculated LD50 for this virus isolate was 12 PFU, in good agreement with that determined by Schultz et al. for the low-passage MPXV-Z79 stock in dormice (23). We concluded that our isolate, MPXV-Z79-CB2, was representative of the parental stock received from the Centers for Disease Control and Prevention.
Susceptibility of inbred mouse strains to MPXV.
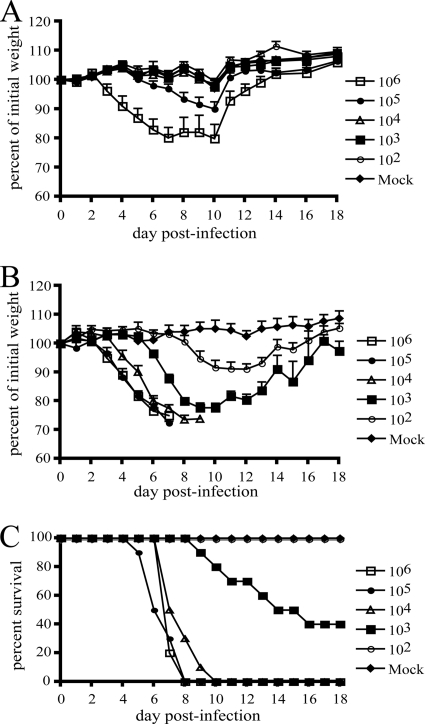
Animal models using inbred strains of mice have many advantages, including commercial availability, uniformity of responses due to genetic homogeneity, information on genotype, and a large variety of immunological reagents. Thus, we decided to search for a strain of mouse that would be most suitable for use as a model for MPXV infection. First, we tested the commonly used BALB/c strain. Seven-week-old female mice were infected with doses from 102 to 106 PFU of MPXV-Z79-CB2 and monitored for weight loss, changes in activity, and death. The results of weight loss over a follow-up period of 18 days are shown in Fig. 2 A. Mice infected with 106 PFU exhibited hunched posture, ruffled fur, and maximum weight loss of 20% of their initial weight. However, they rebounded after day 10 postinfection and recovered the lost weight. Animals that received 105 PFU showed weight loss of only 10% and also recovered rapidly. All animals infected with 104 PFU or less showed no signs of disease. In a separate experiment, three BALB/c mice were infected i.n. with 107 PFU of MPXV-Z79. Although they suffered weight loss of 20 to 25% by day 8, recovery was complete by day 12 postinfection. Since no animals died in these experiments, the LD50 was >107, a value too high to be practical for studies on pathogenicity of MPXV.
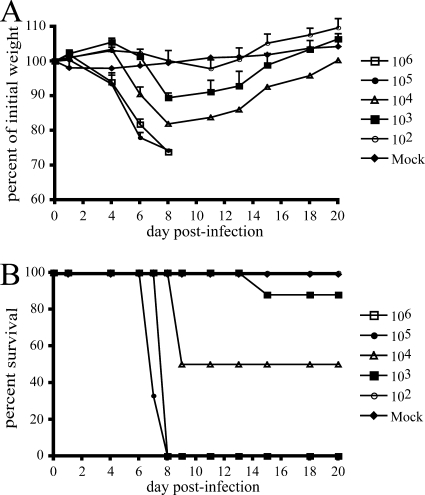
FIG. 2.
i.n. infection of BALB/c and CAST/EiJ mice with MPXV-Z79-CB2. Groups of five to ten 5- to 6-week-old female mice were infected i.n. with doses of MPXV-Z79-CB2 ranging from 102 to 106 PFU. Another group was mock infected. Animals were monitored daily for weight loss and death for 18 days. (A) Weight loss in BALB/c mice; (B) weight loss in CAST/EiJ mice; (C) survival of CAST/EiJ mice.
We then tested 37 additional strains of mouse recommended by Jackson Laboratories for their genetic diversity and availability (http://www.jax.org/phenome). Five female animals aged 5 to 11 weeks from each strain were infected i.n. with 2 × 104 PFU of uncloned MPXV-V79-I-005 or our clonal isolate MPXV-Z79-CB2. Another three animals from each strain served as mock-infected controls. Weight loss, changes in activity or appearance, and death were monitored for 17 to 23 days postinfection. Groups of 6 to 10 different strains were tested simultaneously. As an in vivo control, five BALB/c mice infected with a dose of 106 PFU were included with each group. Weight loss in the BALB/c animals was virtually identical to that shown in Fig. 2A confirming the consistency in preparation of virus inocula. The results for weight loss and lethality of the 38 strains of mouse are shown in Table 1. Twenty-two strains appeared healthy and active and exhibited no clinical signs of disease. Twelve strains exhibited loss of 3 to 14% of their initial weights, but these animals recovered. In contrast, three strains—CAST/EiJ, MOLF/EiJ, and PERA/EiJ—were more susceptible to MPXV infection at the dose of 2 × 104 PFU. The maximum weight losses were 23.6, 21.3, and 24.8% for CAST/EiJ, MOLF/EiJ, and PERA/EiJ, respectively, and the lethalities were 100, 75, and 40%, respectively. Because the CAST/EiJ mice were highly susceptible and more readily available than MOLF/EiJ, and PERA/EiJ, we chose to continue studies with this strain.
TABLE 1.
Lethality and weight loss in different strains of mice infected intranasally with 2 × 104 PFU of MPXV-Z79
| Mouse straina | % Lethality | % of initial wt |
|---|---|---|
| 129S1/SvlmJ | 0 | 5.2 |
| A/J | 0 | 5.4 |
| BALB/cByJ | 0 | 0 |
| C3H/HeJ | 0 | 0 |
| C57BL/6J | 0 | 3.6 |
| CAST/EiJ (WD) | 100 | 23.6 |
| DBA/2J | 0 | 0 |
| FVB/NJ | 0 | 0 |
| SJL/J | 0 | 0 |
| SPRET/EiJ (WD) | 0 | 0 |
| AKR/J | 0 | 0 |
| C57L/J | 0 | 0 |
| C58/J | 0 | 14.4 |
| MOLF/EiJ (WD) | 75 | 21.3 |
| NOD/ShiLtJ | 0 | 0 |
| NZB/BINJ | 0 | 0 |
| PERA/EiJ (WD) | 40 | 24.8 |
| PL/J | 0 | 9.1 |
| SM/J | 0 | 0 |
| SWR/J | 0 | 3.5 |
| BUB/BnJ | 0 | 0 |
| C57BL/10J | 0 | 3.1 |
| C57BLKS/J | 0 | 8.5 |
| CBA/J | 0 | 0 |
| CZECHII/EiJ (WD) | 0 | 2.8 |
| LP/J | 0 | 0 |
| RIIIS/J | 0 | 0 |
| WSB/EiJ (WD) | 0 | 0 |
| BTBR T+ tf/J | 0 | 0 |
| C57BR/cdJ | 0 | 0 |
| CE/J | 0 | 0 |
| I/LnJ | 0 | 0 |
| MA/MyJ | 0 | 0 |
| NON/ShiLtJ | 0 | 0 |
| NZW/LacJ | 0 | 14.1 |
| PWK/PhJ (WD) | 0 | 7.7 |
| SEA/GnJ | 0 | 2.6 |
| BALB/c | 0 | 0 |
WD (in parentheses), wild derived.
Morbidity and mortality of CAST/EiJ mice after i.n. infection with MPXV-Z79-CB2.
We performed a dose escalation experiment in 5- to 6-week-old female CAST/EiJ mice. Groups of 5 to10 animals were infected i.n. with MPXV-Z79-CB2 with doses between 102 and 106 PFU and were monitored for 18 days postinfection. Figure 2B and C show results for weight loss and survival. Doses of 104 PFU and higher resulted in rapid weight loss (up to 28% of the starting weight) and uniform lethality. All animals that succumbed to the infection exhibited ruffled fur, hunched posture, and lethargy. At doses of 105 and 106 PFU, deaths occurred between days 5 and 8 postinfection. Disease and death were somewhat delayed in the 104 PFU challenge group compared to the higher doses with deaths occurring between days 8 and 10 postinfection. After inoculation with 103 PFU, weight loss was delayed by several more days, and 40% of the animals recovered. After the 102 PFU challenge all animals survived, and the weight loss was even more delayed and did not exceed 8% of the starting weight. This experiment demonstrated the high sensitivity of CAST/EiJ mice for MPXV-Z79-CB2 with a calculated LD50 of 680 PFU. The LD50 for male CAST/EiJ mice was similar to that of female mice (not shown).
Morbidity and mortality of CAST/EiJ after i.p. infection with MPXV-Z79-CB2.
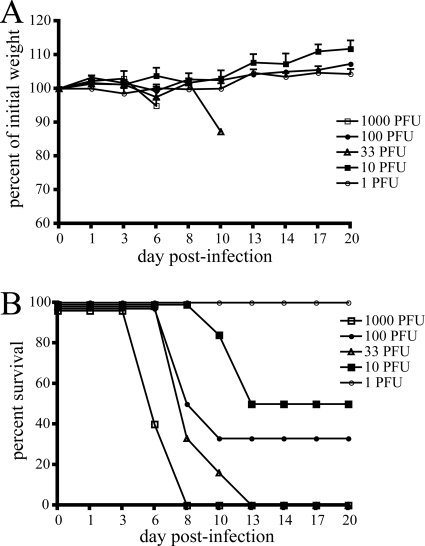
The susceptibility of CAST/EiJ mice to MPXV by the i.p. route was also determined. Initial experiments in a few animals had shown that a dose of 103 PFU was uniformly lethal. Thus, groups of mice (five to six animals/group) were infected with between 1 and 103 PFU. Figure 3 A and B shows weight loss and survival results, respectively. In contrast to i.n. infection, little if any change in weight was observed in mice infected by the i.p. route. Administration of 103 PFU of MPXV resulted in deaths between 6 and 8 days postinfection. Lower doses of 100 and 33 PFU resulted in 35 and 0% survival, respectively, and delayed time-to-death (8 to 10 days postinfection). That more animals died at a dose of 33 PFU than at a dose of 100 PFU is likely due to experimental variation and the narrow dose range. At a dose of 10 PFU, 50% of the animals died, with a time to death of 10 to 13 days postinfection. The calculated LD50 for mice infected by the i.p. route was 14 PFU. Thus, CAST/EiJ mice are highly susceptible to infection by this route, even more so than via the i.n. route.
FIG. 3.
i.p. infection of CAST/EiJ mice with MPXV-Z79-CB2. Groups of five or six 9- to 11-week-old female CAST/EiJ mice were infected i.p. with doses of MPXV-Z79-CB2 ranging from 1 to 1,000 PFU. A separate group was mock infected. Animals were monitored three times per week for weight loss (A) and daily for death (B).
In order to test other routes of infection, groups of four CAST/EiJ mice were infected with either 2 × 103 or 2 × 104 PFU of MPXV-Z79-CB2 either by scarification on the shaved back or by footpad inoculation. Neither route resulted in weight loss, ruffled fur, or hunched posture. Mice that were scarified developed noticeable lesions at the site of infection but did not show any other outward signs of disease. Footpad inoculation resulted in swelling of the lower part of the leg lasting for several days and somewhat slower movement and limping during that time period. One of four animals infected by footpad injection with 2 × 104 PFU died on day 14 postinfection. MPXV was recovered from the lungs, liver, and spleen from this animal on the day of death (results not shown). All of the mice infected by scarification or footpad developed antibodies to MPXV (not shown).
Virus replication in organs of CAST/EiJ mice infected with MPXV-Z79-CB2.
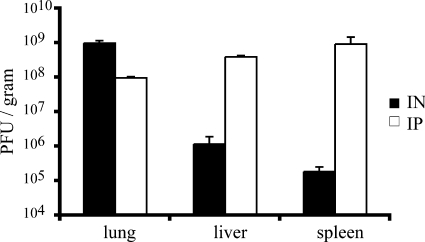
On the day of death, we removed the lungs, livers, and spleens from animals infected with 1 × 104 or 2 × 104 PFU of MPXV-Z79-CB2 by the i.n. or i.p. route. Entire organs were ground up, and virus titers were determined. Results, expressed as the virus yield per gram of tissue, are shown in Fig. 4. Robust MPXV replication was found in the three organs, but the patterns differed, depending on the route of inoculation. The i.n. route yielded ∼3 logs more virus in the lungs (9.9 × 108 PFU) than in either the liver (1.2 × 106 PFU) or the spleen (1.9 × 105 PFU). In contrast, virus recovery from the lung, liver, and spleen was more uniform from animals infected by the i.p. route, although it was somewhat higher in the spleen (9.3 × 108 PFU) and liver (3.9 × 108 PFU) than in the lungs (9.6 × 107 PFU). The relatively high lung titer after i.n. administration of MPXV suggested a direct spread from the site of inoculation.
FIG. 4.
Virus titers in organs of CAST/EiJ mice infected with MPXV-Z79-CB2.On the day of death the lungs, livers, and spleens were removed from mice infected with 1 × 104 PFU or 2 × 104 PFU of MPXV-Z79-CB2 by the i.n. (n = 4) or i.p. (n = 3) route. Organs were weighed and stored frozen. After thawing and homogenization, titers were determined by plaque assay on BS-C-1 cells.
Morbidity and mortality of CAST/EiJ mice after i.n. infection with MPXV-USA-C1.
Because the West African MPXV has been reported to be less virulent in humans (2) and other animal models (11, 17, 22), we assessed the lethality of MPXV-USA-C1 in CAST/EiJ mice. Groups of 5- to 9-week-old mice (five to eight animals/group) were infected with escalating doses from 102 to 106 PFU by the i.n. route. Weight loss and death were monitored, and the results are shown in Fig. 5. All animals infected with 106 or 105 PFU rapidly lost weight and died by day 8 postinfection. Mice infected with 104 PFU lost an average of 18% of their starting weights, and 50% of the animals in this group recovered from the infection. Weight loss in the 103 PFU group averaged 10%, and all but one fully recovered. The dose of 102 PFU resulted in no visible signs of disease. The calculated LD50 for this virus was 7,600 PFU, more than 1 log higher than that for MPXV-Z79-CB2. Comparisons of weight loss and death in animals infected with the two viruses are given in Table 2. Although the LD50 experiments with MPXV-Z79-CB2 and MPXV-USA-C1 were performed separately and with different batches of mice, the groups were age matched, and titers for each inoculum were verified on the day of infection. There was a notable difference in the effects of the two viruses at 103 and 104 PFU. At 103 PFU, 87% of the MPXV-USA-C1-infected animals survived compared to only 40% for MPXV-Z79-CB2. The higher dose of 104 was 100% lethal for animals infected with MPXV-Z79-CB2 but only 50% lethal for animals infected with MPXV-USA-C1. A more severe disease course was also shown by the differences in weight loss exhibited by mice infected with the two viruses. Infection with MPXV-Z79-CB2 and MPXV-USA-C1 caused loss of 22.4 to 26.4% and 10.5 to 18.1%, respectively. Because of the difference in pathogenicity, the CAST/EiJ model should prove useful for dissecting genetic differences between the two clades of MPXV.
FIG. 5.
i.n. infection of CAST/EiJ mice with MPXV-USA-C1. Groups of five to eight 5- to 8-week-old female CAST/EiJ mice were infected i.n. with doses of MPXV-USA-C1 from 102 to 106 PFU. Animals were monitored three times per week for weight loss (A) and daily for death (B).
TABLE 2.
Comparison of lethality of Z79 and USA strains of MPXV in CAST/EiJ mice
| Dose | % Survivors |
Maximum wt loss |
||
|---|---|---|---|---|
| Z79 | USA | Z79 | USA | |
| 106 | 0 | 0 | 25 | 25.7 |
| 105 | 0 | 0 | 27.5 | 25.7 |
| 104 | 0 | 50 | 26.4 | 18.1 |
| 103 | 40 | 87.5 | 22.4 | 10.5 |
| 102 | 100 | 100 | 9 | 2.1 |
Virus replication in organs of CAST/EiJ mice infected with MPXV-USA.
Lungs, liver, spleen, and ovaries were removed on the day of death from mice infected i.n. with various doses of MPXV-USA-C1, and the titers were determined (Fig. 6). At the dose of 104 PFU, the virus yield was higher in the lungs (7.1 × 107 PFU) than in the liver (3.9 × 105 PFU) or spleen (1.4 × 106 PFU) as seen with animals similarly infected with MPXV-Z79-CB2 (Fig. 4). Replication in the lungs was independent of inoculum dose, whereas replication in the liver and spleen was increased when the mice were infected with 105 and 106 PFU of MPXV, suggesting enhanced virus dissemination. High titers of virus (greater than 2.9 × 107 PFU) were recovered from the ovaries of all animals, and this was independent of the inoculation dose. Titers of virus in the ovaries of mice infected with MPXV-Z79-CB2 were not determined but would not be expected to differ from what was found with MPXV-USA-C1-infected animals.
FIG. 6.
Virus titer in organs of CAST/EiJ mice infected with MPXV-USA-C1. On the day of death lung, liver, spleen, and ovaries were removed from mice infected i.n. with 104 (n = 4), 105 (n = 6), or 106 (n = 4) PFU of MPXV-USA-C1. Organs were weighed and stored frozen. After thawing and homogenization, titers were determined by plaque assay on BS-C-1 cells.
Utility of the CAST/EiJ-MPXV model for testing vaccines and therapeutics.
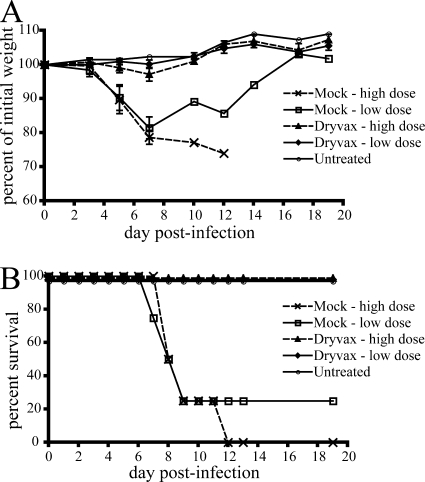
To assess the CAST/EiJ-MPXV model for evaluation of vaccines and therapeutics, we determined the ability of Dryvax, the previously licensed smallpox vaccine consisting of live VACV, and cidofovir, an inhibitor of VACV DNA replication, to protect against a lethal challenge with MPXV. Dryvax (approximately 9 × 105 PFU) was administered by scarification of the shaved back of 6- to 11-week-old mice, and a similar number of animals were mock vaccinated. Lesions developed at the site of Dryvax inoculation but healed within 11 days. Four weeks later, mice (four animals/group) were challenged with either 8 × 103 (10 × LD50) or 8 × 104 PFU (100 × LD50) of MPXV-Z79-CB2. Weight loss and death were monitored for 20 days postchallenge. All of the mock-vaccinated animals in both challenge dose groups lost weight rapidly (Fig. 7 A). The lethalities were 100 and 75% at the high- and low-challenge doses, respectively (Fig. 7B) for these animals. In contrast, full protection was afforded to all of the vaccinated mice at both challenge doses; all animals remained healthy and showed no signs of disease.
FIG. 7.
Vaccination of CAST/EiJ mice protects against lethal MPXV infection. Groups of four 6- to 11-week-old female CAST/EiJ mice were vaccinated with Dryvax or mock vaccinated with diluent by scarification on the shaved back. Four weeks later, the animals were challenged i.n. with either 7 × 103 PFU (low dose) or 7 × 104 PFU (high dose) of MPXV-Z79-CB2 and monitored for 20 days for weight loss (A) and death (B).
To test for the ability of cidofovir to protect against MPXV challenge, groups of 11- to 13-week-old mice were infected with 104 PFU of MPXV-Z79-CB2 (14 LD50). At 4 and 24 h postinfection, cidofovir or diluent was administered to each of six mice. None of the cidofovir-treated animals lost weight as a result of MPXV infection. One animal in the cidofovir group died on day 17 postinfection, although unlike all other animals that succumbed to MPXV-induced death, no signs of disease were noted, and the cause of death was not determined. In contrast, animals in the mock-treated group lost an average of 20% of their starting weight (data not shown). Only two of the six animals in this group died. This low death rate could be due to the fact that animals in this experiment were 11 to 13 weeks of age at the time of MPXV infection, in contrast to the younger age (6 to 8 weeks) of animals in the LD50 experiment.
Immune responses in mice vaccinated with Dryvax.
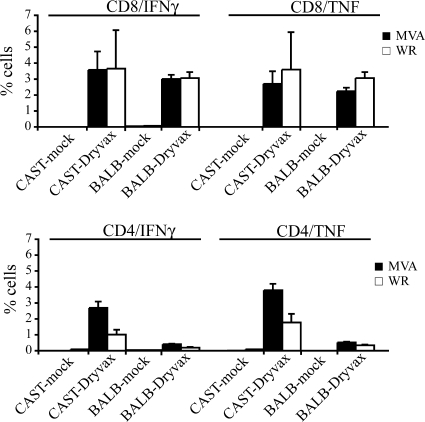
To evaluate the immune competence of CAST/EiJ mice, we measured their Dryvax-induced cell mediated and humoral responses and compared them to those of BALB/c mice. Two weeks after scarification with Dryvax, the CD8 and CD4 responses to VACV and modified VACV Ankara were determined in splenocytes from individual animals. Stimulation with modified VACV Ankara was included because we had previously found it to give superior responses to those of VACV (29), presumably because of lower cell toxicity. As shown in Fig. 8, the CD8/IFN-γ and CD8/TNF VACV-specific responses were similar in CAST/EiJ and BALB/c mice, whereas the CD4 responses were higher in the CAST/EiJ mice than in BALB/c mice. A separate group of CAST/EiJ mice was similarly scarified and bled at 4 weeks postinfection. VACV-specific IgG titers were determined by ELISA with sera from individual animals. The mean titer was 28,500 (±4,800), severalfold higher than that found in Dryvax-infected BALB/c mice (29). Thus, CAST/EiJ mice do not exhibit impaired acquired immunity.
FIG. 8.
CD8 and CD4 T-cell responses in mice vaccinated with Dryvax. Groups of CAST/EiJ and BALB/c mice were vaccinated with Dryvax or mock vaccinated. Two weeks later splenocytes were prepared and stimulated with either MVA or VACV WR. The percentage of VACV-specific IFN-γ- and TNF-expressing CD8 and CD4 cells was determined by flow cytometry.
DISCUSSION
The wide host range of MPXV among wild rodents (22, 26) contrasted with previous studies that had shown resistance of several inbred laboratory strains of mice (10, 17, 24). However, the classical inbred mouse strains were derived from a very small number of founder animals and are closely related genetically. We considered that mice, like many other rodents, might not be intrinsically resistant to MPXV and therefore conducted an extensive screen of mouse strains. The 38 mice selected for screening were Mouse Phenome Database priority strains (http://www.jax.org/phenome). The screen was carried out by i.n. challenge because that is thought to be an important route for smallpox transmission, although not necessarily for monkeypox (21). Based on preliminary experiments indicating that BALB/c mice are resistant to challenge with 106 PFU of a Congo-derived MPXV strain, we carried out the screen with 2 × 104 PFU in order to identify mice that were at least 100-fold more sensitive. At that challenge dose three mouse strains (CAST/EiJ, MOLF/EiJ, and PERA/EiJ) of the 38 suffered severe weight loss and mortality and 12 others exhibited loss of 3 to 14% of their initial weight but recovered. Interestingly, the three highly susceptible strains are wild-derived subspecies, of Mus musculus castaneus, M. musculus molossinus (a hybrid of M. musculus musculus and M. musculus castaneus [32]), and M. musculus domesticus isolated from Thailand, Japan, and Peru, respectively. Wild-derived strains are genetically pure as they were captured and then bred to homozygosity. The subspecies castaneous and domesticus diverged from a common ancestor more than a million years ago and CAST/EiJ has a higher density of polymorphisms than any other strain (12). Of the 35 resistant mouse strains, four are also wild-derived and the other 31 are classical inbred strains. In retrospect, it is not too surprising that the closely related classical inbred strains exhibited a similar resistance to MPXV. At this point, however, we can only speculate about possible MPXV susceptibility or resistance genes. Available congenic mouse strains derived from CAST/EiJ on a C57BL/6 background may be useful in genetically defining the basis for MPXV sensitivity (3) as may high-density genotyping arrays (31).
Both CAST/EiJ and MOLF/EiJ are among 16 mouse strains in the tier 1 priority list at the Mouse Phenome Database, ensuring their continued characterization. We limited further study to CAST/EiJ mice because of their better availability and lower cost, although further studies with MOLF/EiJ and PERA/EiJ are warranted. The CAST/EiJ mice exhibited a dose response to i.n. administration of MPXV with some weight loss and clinical symptoms at 102 PFU and 100% mortality at 104 PFU. An LD50 of 680 PFU was calculated by using the method of Reed and Muench (20). The mice were even more susceptible when challenged by the i.p. route, with a calculated LD50 of 14 PFU. No skin lesions were observed after either route of inoculation. Footpad inoculation with 104 PFU resulted in a local lesion, leg swelling, and mild weight loss. Analysis of organ titers after i.n. and i.p. inoculation showed that the virus was present in the lungs, liver, and spleen. However, the titers were higher in the lungs after i.n. administration and higher in the liver and spleen after i.p. infection. Further studies with a West African-derived MPXV indicated that a 10-fold-higher dose was required for disease and death, a finding consistent with the lower prevalence of severe disease in humans (14, 18, 19) and in some other animal models (2, 11, 22). At necropsy, after i.n. challenge, the titers in the internal organs were similar to that with the Congo strain. The clear difference in virulence of the West African and Congo strains in CAST/EiJ mice should allow the identification of the responsible MPXV genes.
It is relevant to compare the susceptibility to MPXV of CAST/EiJ mice with that of other animals. Ground squirrels and African dormice are highly susceptible to MPXV infection with 100% lethality at i.n. doses of 100 and 200 PFU, respectively (22, 23). In contrast, 105 PFU of MPXV administered i.p. but not i.n. killed all prairie dogs (30). Cynomolgus monkeys receiving MPXV aerosolized (104 to 105 PFU) (33), intratracheal (106 to 107 PFU) (25), and intravenous (5 × 107 PFU) (4, 5) doses had high mortality. The susceptibility ranking appears to be as follows: ground squirrel > dormouse > CAST/EiJ mice > prairie dog > cynomolgus monkeys. Thus, the susceptibility of the wild-derived CAST/EiJ mice to MPXV is in line with that of many other wild rodent species.
Several studies have shown that laboratory bred rodents, which are immunologically immature or have impaired innate or adaptive immune responses, exhibit enhanced susceptibility to MPXV. Although adult rabbits, guinea pigs, and hamsters were reported to be resistant to MPXV infection, 10-day-old rabbits and 8- to 12-day-old white mice (strain not indicated) developed generalized infections with high lethality after oral or i.n. administration of 106 to 107 PFU of MPXV (15). Enhanced sensitivity to MPXV occurs with normally resistant mice that are deficient in STAT1, a member of the signal transducers and activators of transcription factors family that upregulates genes due to a signal from IFN-α or IFN-β stimulation (24) or have severe combined immunodeficiency (17). We could find no indication in the literature that CAST/EiJ, MOLF/EiJ, or PERA/EiJ mice are highly susceptible to other viruses or exhibit a functional immunodeficiency. CAST/EiJ mice that survived MPXV infection had relatively high levels of antibody. Furthermore, CAST/EiJ mice mounted humoral and T-cell responses comparable to that of BALB/c mice when immunized with VACV and were protected against subsequent MPXV challenge. CAST/EiJ mice contain a susceptibility gene for Bacillus anthracis lethal toxin (16) and resistance genes for flaviviruses (8) and mouse gammaretroviruses (28), although it is unlikely that these have any bearing on their susceptibility to MPXV. Listings of numerous phenotypic characteristics of CAST/EiJ mice are available at the Mouse Phenome Database (http://www.jax.org/phenome), and we plan to investigate their relevance to MPXV susceptibility, as well as the ability of these mice to make innate immune responses after MPXV inoculation and survival after infection with other viruses.
In conclusion, our identification of inbred strains of mice that are highly susceptible to MPXV provides a platform for studying pathogenicity and testing candidate vaccines and therapeutics. Inbred mouse strains have important advantages over other MPXV animal models. The mice are commercially available and well characterized genetically and phenotypically. Moreover, numerous immunological and other reagents available for the mouse are difficult or impossible to obtain for other animals.
Acknowledgments
We thank Inger Damon for MPXV seed stocks, Mark Buller for dormouse breeders, and Robin Kastenmayer for maintenance of our dormouse breeding colony. We also thank Catherine Cotter for assistance with plaque purification of MPXV clones and Jack Bennink for monoclonal antibody 2.4G2.
This study was supported by the Intramural Research Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Published ahead of print on 2 June 2010.
REFERENCES
- 1.Breman, J. G., R. Kalisa, M. V. Steniowski, E. Zanotto, A. I. Gromyko, and I. Arita. 1980. Human monkeypox, 1970-79. Bull. World Health Organ. 58:165-182. [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, N., G. Li, M. K. Liszewski, J. P. Atkinson, P. B. Jahrling, Z. Feng, J. Schriewer, C. Buck, C. Wang, E. J. Lefkowitz, J. J. Esposito, T. Harms, I. K. Damon, R. L. Roper, C. Upton, and R. M. Buller. 2005. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 340:46-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis, R. C., A. Jin, M. Rosales, S. Yu, X. Xia, K. Ranola, E. E. Schadt, and A. J. Lusis. 2007. A genome-wide set of congenic mouse strains derived from CAST/Ei on a C57BL/6 background. Genomics 90:306-313. [DOI] [PubMed] [Google Scholar]
- 4.Earl, P. L., J. L. Americo, L. S. Wyatt, L. A. Eller, D. C. Montefiori, R. Byrum, M. Piatak, J. D. Lifson, R. R. Amara, H. L. Robinson, J. W. Huggins, and B. Moss. 2007. Recombinant modified vaccinia virus Ankara provides durable protection against disease caused by an immunodeficiency virus as well as long-term immunity to an orthopoxvirus in a non-human primate. Virology 366:84-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Earl, P. L., J. L. Americo, L. S. Wyatt, O. Espenshade, J. Bassler, K. Gong, S. Lin, E. Peters, L. Rhodes, Jr., Y. E. Spano, P. M. Silvera, and B. Moss. 2008. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc. Natl. Acad. Sci. U. S. A. 105:10889-10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earl, P. L., N. Cooper, L. S. Wyatt, B. Moss, and M. W. Carroll. 1998. Preparation of cell cultures and vaccinia virus stocks, p. 16.16.1-16.16.3. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Greene Publishing Associates/Wiley-Interscience, New York, NY. [Google Scholar]
- 7.Earl, P. L., C. Cotter, B. Moss, T. VanCott, J. Currier, L. A. Eller, F. McCutchan, D. L. Birx, N. L. Michael, M. A. Marovich, M. Robb, and J. H. Cox. 2009. Design and evaluation of multi-gene, multi-clade HIV-1 MVA vaccines. Vaccine 27:5885-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson, W., S. Dvora, J. Gallo, A. Orth, and S. Boissinot. 2008. Long-term balancing selection at the West Nile virus resistance gene, Oas1b, maintains transspecific polymorphisms in the house mouse. Mol. Biol. Evol. 25:1609-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guarner, J., B. J. Johnson, C. D. Paddock, W. J. Shieh, C. S. Goldsmith, M. G. Reynolds, I. K. Damon, R. L. Regnery, and S. R. Zaki. 2004. Monkeypox transmission and pathogenesis in prairie dogs. Emerg. Infect. Dis. 10:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutson, C. L., J. A. Abel, D. S. Carroll, V. A. Olson, Z. H. Braden, C. M. Hughes, M. Dillon, C. Hopkins, K. L. Karem, I. K. Damon, and J. E. Osorio. 2009. Comparison of West African and Congo Basin monkeypox viruses in BALB/c and C57BL/6 mice. PLoS One 5:e8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutson, C. L., V. A. Olson, D. S. Carroll, J. A. Abel, C. M. Hughes, Z. H. Braden, S. Weiss, J. Self, J. E. Osorio, P. N. Hudson, M. Dillon, K. L. Karem, I. K. Damon, and R. L. Regnery. 2009. A prairie dog animal model of systemic orthopoxvirus disease using West African and Congo Basin strains of monkeypox virus. J. Gen. Virol. 90:323-333. [DOI] [PubMed] [Google Scholar]
- 12.Ideraabdullah, F. Y., E. de la Casa-Esperon, T. A. Bell, D. A. Detwiler, T. Magnuson, C. Sapienza, and F. P. de Villena. 2004. Genetic and haplotype diversity among wild-derived mouse inbred strains. Genome Res. 14:1880-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langohr, I. M., G. W. Stevenson, H. L. Thacker, and R. L. Regnery. 2004. Extensive lesions of monkeypox in a prairie dog (Cynomys sp). Vet. Pathol. 41:702-707. [DOI] [PubMed] [Google Scholar]
- 14.Likos, A. M., S. A. Sammons, V. A. Olson, A. M. Frace, Y. Li, M. Olsen-Rasmussen, W. Davidson, R. Galloway, M. L. Khristova, M. G. Reynolds, H. Zhao, D. S. Carroll, A. Curns, P. Formenty, J. J. Esposito, R. L. Regnery, and I. K. Damon. 2005. A tale of two clades: monkeypox viruses. J. Gen. Virol. 86:2661-2672. [DOI] [PubMed] [Google Scholar]
- 15.Marennikova, S. S., and E. M. Seluhina. 1976. Susceptibility of some rodent species to monkeypox virus, and course of the infection. Bull. World Health Organ. 53:13-20. [PMC free article] [PubMed] [Google Scholar]
- 16.Moayeri, M., N. W. Martinez, J. Wiggins, H. A. Young, and S. H. Leppla. 2004. Mouse susceptibility to anthrax lethal toxin is influenced by genetic factors in addition to those controlling macrophage sensitivity. Infect. Immun. 72:4439-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osorio, J. E., K. P. Iams, C. U. Meteyer, and T. E. Rocke. 2009. Comparison of monkeypox viruses pathogenesis in mice by in vivo imaging. PLoS One 4:e6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker, S., A. Nuara, R. M. Buller, and D. A. Schultz. 2007. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2:17-34. [DOI] [PubMed] [Google Scholar]
- 19.Reed, K. D., J. W. Melski, M. B. Graham, R. L. Regnery, M. J. Sotir, M. V. Wegner, J. J. Kazmierczak, E. J. Stratman, Y. Li, J. A. Fairley, G. R. Swain, V. A. Olson, E. K. Sargent, S. C. Kehl, M. A. Frace, R. Kline, S. L. Foldy, J. P. Davis, and I. K. Damon. 2004. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 350:342-350. [DOI] [PubMed] [Google Scholar]
- 20.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 21.Reynolds, M. G., K. L. Yorita, M. J. Kuehnert, W. B. Davidson, G. D. Huhn, R. C. Holman, and I. K. Damon. 2006. Clinical manifestations of human monkeypox influenced by route of infection. J. Infect. Dis. 194:773-780. [DOI] [PubMed] [Google Scholar]
- 22.Sbrana, E., S. Y. Xiao, P. C. Newman, and R. B. Tesh. 2007. Comparative pathology of North American and central African strains of monkeypox virus in a ground squirrel model of the disease. Am. J. Trop. Med. Hyg. 76:155-164. [PubMed] [Google Scholar]
- 23.Schultz, D. A., J. E. Sagartz, D. L. Huso, and R. M. Buller. 2009. Experimental infection of an African dormouse (Graphiurus kelleni) with monkeypox virus. Virology 383:86-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stabenow, J., R. M. Buller, J. Schriewer, C. West, J. E. Sagartz, and S. Parker. 2010. A lethal mouse model for evaluating prophylactics and therapeutics against monkeypox virus. J. Virol. 84:3909-3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stittelaar, K. J., J. Neyts, L. Naesens, G. van Amerongen, R. F. van Lavieren, A. Holy, E. De Clercq, H. G. Niesters, E. Fries, C. Maas, P. G. Mulder, B. A. van der Zeijst, and A. D. Osterhaus. 2006. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature 439:745-748. [DOI] [PubMed] [Google Scholar]
- 26.Tesh, R. B., D. M. Watts, E. Sbrana, M. Siirin, V. L. Popov, and S. Y. Xiao. 2004. Experimental infection of ground squirrels (Spermophilus tridecemlineatus) with monkeypox virus. Emerg. Infect. Dis. 10:1563-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Magnus, P., E. Andersen, and K.-A. Petersen. 1959. A Pox-like disease in cynomolgous monkeys. Acta Pathol. Microbiol. Scand. 46:156-176. [Google Scholar]
- 28.Wu, T., Y. Yan, and C. A. Kozak. 2005. Rmcf2, a xenotropic provirus in the Asian mouse species Mus castaneus, blocks infection by polytropic mouse gammaretroviruses. J. Virol. 79:9677-9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyatt, L. S., P. L. Earl, L. A. Eller, and B. Moss. 2004. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. U. S. A. 101:4590-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao, S. Y., E. Sbrana, D. M. Watts, M. Siirin, A. P. da Rosa, and R. B. Tesh. 2005. Experimental infection of prairie dogs with monkeypox virus. Emerg. Infect. Dis. 11:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang, H., Y. Ding, L. N. Hutchins, J. Szatkiewicz, T. A. Bell, B. J. Paigen, J. H. Graber, F. P. de Villena, and G. A. Churchill. 2009. A customized and versatile high-density genotyping array for the mouse. Nat. Methods 6:663-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yonekawa, H., O. Gotoh, Y. Tagashira, Y. Matsushima, L. I. Shi, W. S. Cho, N. Miyashita, and K. Moriwaki. 1986. A hybrid origin of Japanese mice “Mus musculus molossinus.” Curr. Top. Microbiol. Immunol. 127:62-67. [PubMed] [Google Scholar]
- 33.Zaucha, G. M., P. B. Jahrling, T. W. Geisbert, J. R. Swearengen, and L. Hensley. 2001. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis). Lab. Invest. 81:1581-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]