Abstract
During dengue virus replication, an incomplete cleavage of the envelope glycoprotein prM, generates a mixture of mature (prM-less) and prM-containing, immature extracellular particles. In this study, sequential immunoprecipitation and cryoelectron microscopy revealed a third type of extracellular particles, the partially mature particles, as the major prM-containing particles in a dengue serotype 2 virus. Changes in the proportion of viral particles in the pr-M junction mutants exhibiting altered levels of prM cleavage suggest that the partially mature particles may represent an intermediate subpopulation in the virus maturation pathway. These findings are consistent with a model suggesting the progressive mode of prM cleavage.
Dengue viruses are enveloped, positive-strand RNA viruses in the genus Flavivirus of the family Flaviviridae (19). The viral genome encodes three structural proteins (C, prM/M, and E) and seven nonstructural proteins (19). Two types of genome-containing particles, the immature and mature particles, can be distinguished by the differences in size and surface morphology and the presence and cleavage status of the envelope glycoprotein prM (19, 20). The immature particles are assembled in the endoplasmic reticulum as spherical “spiky” particles of about 60 nm in diameter (36). Each of the spikes is formed by a noncovalent association of three prM-E heterodimers, with the pr portion of prM on the outermost part of the spike providing the main contact (18, 36). During the export, the low-pH environment of the trans-Golgi network induces the rearrangement of prM-E heterodimers into a flattened conformation that allows for an internal cleavage of prM by furin (34). The complete prM cleavage generates the mature particles, which are about 50 nm in diameter and present a smooth surface (17). These infectious particles contain 90 E homodimers arranged in groups of three parallel dimers in the “herringbone” pattern (17). Further complexity of the viral particles was observed in studies of dengue virus and West Nile virus in the form of particles having an intermediate conformation between those of the mature and immature particles (3, 24, 35).
Cleavage of prM is a prerequisite for an acquisition of infectivity, as the pr portion of prM functions as a mechanical barrier to protect the fusion loop in the receptor-binding E glycoprotein from undergoing low pH-mediated fusion (7, 18, 29). Inhibition of the prM cleavage by mutation of the furin cleavage site, treatment of the infected cells with acidotropic amines, or growth of the virus in furin-deficient cells generates noninfectious particles in the extracellular compartment (7, 8, 10, 25, 37). During the replication of dengue virus, cleavage of prM is, however, usually incomplete (1, 4, 9, 11, 16, 21, 25, 27, 32, 33). This reflects an inhibition of cleavage mediated by a highly conserved acidic residue at the P3 cleavage position of the pr-M junction (15). Currently, it is not clear how the prM molecules are collectively cleaved in each viral particle. In the “all-or-none” prM cleavage model, the cleavage is either complete or nil for the 180 prM molecules on a particle. In this model, the extracellular particles represent a mixture of strictly the mature particles and the immature particles. Alternatively, the cleavage may be “progressive,” as each of the prM molecules on immature particles is independently cleaved, generating the third subpopulation of partially mature particles that retain from 1 to 179 molecules of prM in any particle. Evidence in support of the existence of partially mature particles includes the electron microscopic visualization of flavivirus particles that do not fit the structure of intact mature or immature particles (3, 24, 35) and the findings that prM present on infectious particles affects pH sensitivity (8), viral tropism (5), and the neutralization potency of certain antibodies that recognize poorly accessible sites on the E protein (22).
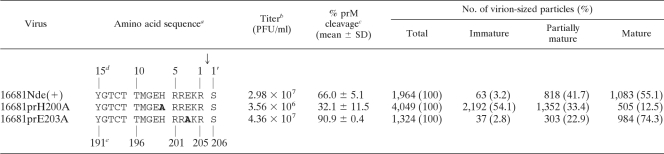
In our previous study, a comparison of prM and M, the particle-bound product of prM cleavage, revealed that approximately 30 to 40% of prM remained in the extracellular particles of a dengue serotype 2 virus, strain 16681Nde(+), following its replication in mosquito cells (15) (Table 1). If the remaining prM molecules were restricted to the immature particles in this virus, it should be possible to separate the prM-containing minor subpopulation from the rest of the prM-less mature particles by immunoprecipitation with anti-prM antibody. When unbound particles are subsequently reacted with anti-E antibody, a comparison of the E protein content in both precipitates would yield the proportion of prM-containing particles in the total precipitable particles. To assess the distribution of the prM molecules on viral particles, C6/36 mosquito cells (14) were infected with 16681Nde(+) at a multiplicity of infection of 0.2 and the progeny viral particles were metabolically labeled with [35S]methionine and [35S]cysteine (Express 35S protein labeling mix; Perkin Elmer, Boston, MA). Under the conditions employed, the majority of particles were in the form of virion-sized particles while subviral particles represented the minor subpopulation (15). At 19 h after labeling, the culture medium was incubated with protein G Sepharose beads overnight to reduce the content of proteins capable of binding nonspecifically with the beads. Viral particles were then allowed to bind protein G Sepharose beads that had been precoated with an anti-prM monoclonal antibody, prM-6.1, which reacts with the pr portion of the prM protein (15). Following the separation of the beads by centrifugation, unbound viral particles were subsequently pulled down with beads that were coated with either prM-6.1, with 3H5, a serotype 2-specific anti-E antibody that recognizes a domain III epitope (12), with 4G2, a group-specific anti-E antibody that recognizes an epitope located in or nearby the fusion loop (31), or with MOPC-21, an irrelevant plasmacytoma protein. Bound particles were dissociated from beads and the viral proteins separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and then visualized and quantitated with a phosphorimager (Typhoon 6410; GE Healthcare Bio-Sciences, Piscataway, NJ) (Fig. 1).
TABLE 1.
Sequences of the dengue virus pr-M junction and some characteristics of the parent virus and the pr-M junction mutants
Amino acid letters in bold represent mutated residues. The arrow indicates the pr-M cleavage site.
Infectious virus titer was determined by employing a plaque assay on BHK cells.
The extent of prM cleavage was determined by metabolic labeling, partial purification by isopycnic centrifugation, and SDS-polyacrylamide gel electrophoresis, as described in reference 15.
Numbers refer to the positions of the amino acids relative to the pr-M cleavage site in the proximal direction (without apostrophe) and distal direction (with apostrophe).
Numbers refer to the positions of the amino acids in the polyprotein according to reference 2.
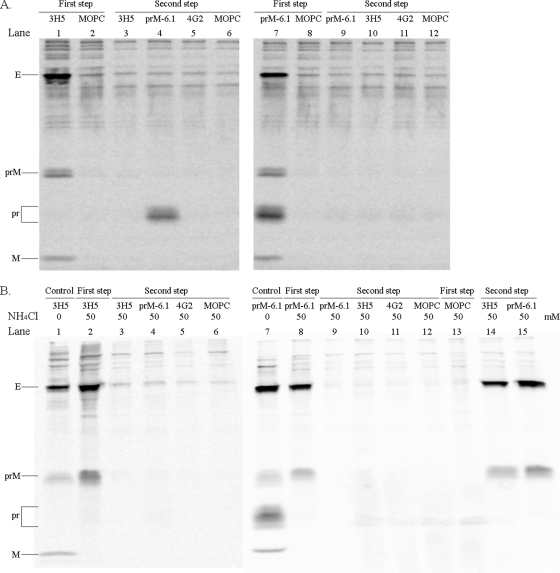
FIG. 1.
Sequential immunoprecipitation of extracellular viral particles. (A, left panel) The radiolabeled particles in a pretitrated volume (200 μl) of the culture fluid of virus-infected C6/36 cells were reacted with protein G Sepharose beads overnight and then with 3H5 (lane 1)- or MOPC-21 (lane 2)-coated beads in 1 ml at 4°C for 36 h. After a wash, particles were dissociated from beads with 2.5% 2-mercaptoethanol-1.5% SDS-40 mM Tris-HCl (pH 6.8)-0.02% bromophenol blue at 37°C for 30 min. In the coating step, 15 μg (3H5) or 10 μg (other antibodies) of protein G Sepharose column-purified antibodies and, in the majority of experiments, a fixed amount (5 μl) of an ascites, which was devoid of anti-dengue virus activity, were incubated with 30 μl of 50% (vol/vol) protein G Sepharose in 1 ml of 50 mM Tris-HCl (pH 7.4)-4 mM EDTA (pH 8.0)-300 mM NaCl overnight. Unbound particles from the 3H5 reaction were separated by spinning them in a microcentrifuge at 14,000 rpm for 5 min, and then precipitated with a set of beads that had been coated with 3H5 (lane 3), prM-6.1 (lane 4), 4G2 (lane 5), or MOPC-21 (lane 6). The eluted proteins were separated by electrophoresis in 0.1% SDS-15% polyacrylamide gel and the radioactivity signals captured with a phosphorimager. (A, right panel) The radiolabeled viral particles were reacted with protein G Sepharose and then with prM-6.1 (lane 7)- or MOPC-21 (lane 8)-coated beads overnight prior to elution. Subsequent precipitation of the unbound particles was performed with beads that had been coated with prM-6.1 (lane 9), 3H5 (lane 10), 4G2 (lane 11), or MOPC-21 (lane 12). (B) Viral particles derived from the 50 mM NH4Cl-treated, infected C6/36 culture were employed in the sequential immunoprecipitation with 3H5 (lanes 2 to 6) or prM-6.1 (lanes 8 to 12) as described for panel A. Lanes 1 and 7 represent the reaction of particles from NH4Cl-untreated cultures with 3H5 and prM-6.1, respectively, to ensure the correct use of intended antibodies. Lanes 13 to 15 represent an initial precipitation of particles from NH4Cl-treated cultures with MOPC-21 (lane 13) and subsequent reactions of unbound materials with 3H5 (lane 14) or prM-6.1 (lane 15). The antibody specificities were determined previously (15). The viral proteins are indicated. Note that C is not observed, as it is inefficiently labeled with the isotopes used.
As a control, radiolabeled particles were reacted initially with 3H5 or MOPC-21. In the first-step reaction, 3H5 precipitated a much higher proportion of radiolabeled particles than MOPC-21 (mean E proportions of 91.5% versus 8.5%; standard error [SE] = 2.3; n = 3) (Fig. 1A, lanes 1 and 2). A lack of pr peptide in the 3H5 first-step eluate (Fig. 1A, lane 1) and the presence of this soluble protein in the prM-6.1 second-step eluate (Fig. 1A, lane 4) were consistent with a specific binding of E-containing viral particles with 3H5. Unbound particles from the 3H5 reaction were next precipitated with 3H5, prM-6.1, 4G2, or MOPC-21, and the E protein signal in the first-step precipitate was compared with those in the 4 second-step precipitates. Only a small proportion of E-containing particles remained to be pulled down subsequently with these antibodies (Fig. 1A, lanes 3 to 6, and Table 2). These results indicated that the majority of viral particles were removed by 3H5 in the first step and the remainders were brought down in the second step mainly through a nonspecific association with the beads. Under the similar conditions, an unexpectedly high proportion of particles were precipitated with prM-6.1, as the proportions of E in the prM-6.1 first-step precipitate were in the same range as those in the 3H5 immunoprecipitation reactions (Fig. 1A, right panel, and Table 2). When immature particles derived from NH4Cl-treated, 16681Nde(+)-infected C6/36 cells were employed in the sequential immunoprecipitation (Fig. 1B), slightly increased proportions of these particles were pulled down in the first step with prM-6.1, but not 3H5, compared with the level for the particles from NH4Cl-untreated cells (Table 2). When MOPC-21 was employed in the initial immunoprecipitation and the subsequent step was performed with 3H5 or prM-6.1, the results were compatible with a minimal nonspecific loss of particles during the first immunoprecipitation reaction (Fig. 1B, lanes 13 to 15). Our inability to specifically precipitate viral particles with 3H5 or 4G2 following the prM-6.1 first-step immunoprecipitation (Fig. 1A, lanes 10 and 11) indicated that the proportion of prM-containing particles in 16681Nde(+) was far greater than the 30 to 40% level predicted by the “all-or-none” prM cleavage model. These results, therefore, suggested that there exist a number of dengue virus particles in which complete cleavage of prM had not occurred.
TABLE 2.
Sequential immunoprecipitation of radiolabeled viral particles with anti-E and anti-prM antibodies
| Condition | Mean % proportion of E in the first-step precipitate (SE) for indicated first- and second-step antibodiesa |
|||||||
|---|---|---|---|---|---|---|---|---|
| 3H5 |
prM-6.1 |
|||||||
| 3H5 | prM-6.1 | 4G2 | MOPC-21 | prM-6.1 | 3H5 | 4G2 | MOPC-21 | |
| Without NH4Cl (n = 5) | 93.0 (1.1) | 91.8 (2.0) | 92.0 (2.0) | 93.9 (1.3) | 91.9 (0.7) | 90.3 (1.1) | 91.0 (0.5) | 92.2 (0.8) |
| With NH4Cl (n = 2) | 94.9 (2.7) | 96.6 (1.1) | 95.6 (3.2) | 96.1 (2.6) | 96.7 (0.5) | 97.1 (0.0) | 96.7 (0.8) | 97.6 (0.6) |
| Pb | 0.46 | 0.22 | 0.39 | 0.45 | 0.01 | 0.01 | <0.01 | 0.01 |
The first-step antibodies are indicated above the corresponding second-step antibodies. The proportion of E in the first-step precipitate was determined from the following equation: 100 × (mean E signal in the first step precipitate)/(mean E signal in the first-step precipitate + mean E signal in the second-step precipitate).
P values were determined by using the 2-sided t test. A P value of <0.05 indicates statistical significance.
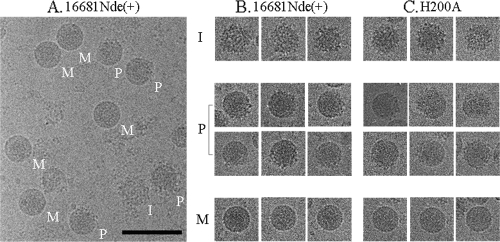
The proportion of particles with prM was next assessed with cryoelectron microscopy (cryo-EM). Extracellular particles of 16681Nde(+) amplified in C6/36 cells in the absence of an acidotropic agent were concentrated and then purified by rate-zonal centrifugation in a 10- to 35-g% potassium tartrate step gradient. The vitrified water-embedded viral particles were examined on a Philips CM300 field emission gun transmission electron microscope (Eindhoven, Netherlands) as previously reported (17). Inspection of virion-sized particles in 39 photomicrographs revealed that mature particles exhibiting smooth surface constituted slightly more than half of the large particles (55.1%) (Fig. 2 and Table 1). The remainder consisted of the immature particles with a spiky surface (36) and another distinct group of particles that displayed patches of smooth and spiky surfaces on the same particle, “the partially mature particles” (Fig. 2). For 16681Nde(+), the immature particles represented a minor (3.2%) subpopulation of the extracellular particles while the partially mature particles were more abundant (41.7%) (Table 1). It should be noted that cryo-EM might underestimate the proportion of the partially mature particles, as only one hemisphere in each particle could be examined in the micrographs. With an overall prM cleavage of 60 to 70% for 16681Nde(+), there might be a tendency toward a misidentification of partially mature particles for the mature ones. Despite this limitation, these data indicated that the partially mature particles constituted a significant fraction of the extracellular particles in this dengue virus.
FIG. 2.
Cryo-EM visualization of extracellular virus particles. C6/36 cells were infected with dengue viruses at a multiplicity of infection of 0.05. After 5 to 7 days, the culture medium was collected and clarified by centrifugation at 6,000 rpm in a J2-21 rotor (Beckman) at 4°C for 30 min. Viral particles were precipitated with 8 g% polyethylene glycol (PEG) in 12 mM Tris (pH 8.0)-120 mM NaCl-1 mM EDTA buffer at 4°C for overnight. The precipitates were collected by spinning at 9,500 rpm for 50 min in the J2-21 rotor, resuspended with 12 mM Tris (pH 8.0)-120 mM NaCl-1 mM EDTA buffer, and then centrifuged in a 24 g% sucrose cushion (32,000 rpm for 1.5 h at 4°C, using a Beckman SW41 rotor). Particles were further purified in a 10- to 35-g% potassium tartrate gradient (32,000 rpm for 2 h at 4°C in a Beckman SW41 rotor). The visible viral band was collected, concentrated by using a centrifugal filter device, applied onto the carbon-coated cryoelectron grids, and vitrified in liquid ethane. The grids were examined with a transmission electron microscope. Images were recorded at a magnification of 45,000 under low-dose conditions (14 to 17 e−/Å2). The micrographs taken from each virus preparation were digitized at a 7-μm step size to yield a sampling of 2.96 Å/pixel. The morphology of virus particles was visualized using the RobEM program (http://cryoem.ucsd.edu/programDocs/runRobem.txt). (A) The cryo-EM micrograph of the parent virus, strain 16681Nde(+). M, I, and P indicate the mature, immature, and partially mature particles, respectively. (B and C) Cropped cryo-EM images showing single particles of 16681Nde(+) and prH200A, respectively. The bar represents 100 nm.
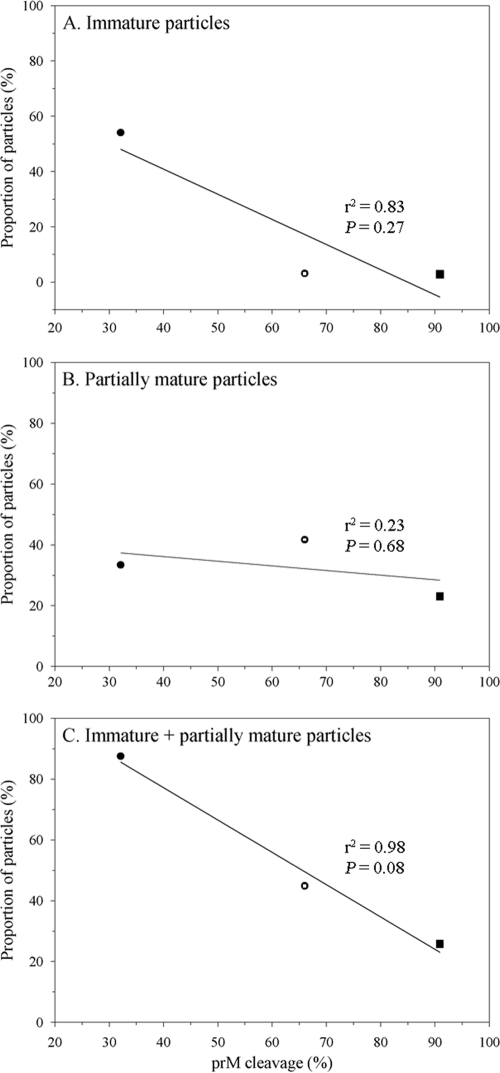
Among flaviviruses, dengue virus and the cell fusing agent virus are unique in the conservation of a cleavage-suppressive acidic residue at the P3 position of the pr-M junction (28). As an incomplete prM cleavage underlies the generation of the three types of extracellular dengue particles, it is intriguing to determine whether the modification of prM cleavage efficiency could affect the proportion of the particle subpopulations. In a previous study, a number of pr-M junction mutant viruses bearing a substitution at the cleavage positions P3 and P5-6 exhibited altered levels of prM cleavage (15). A P3 mutant with an enhanced prM cleavage (prE203A) and another P6 mutant with a reduced prM cleavage (prH200A) were selected for the cryo-EM study (Table 1). The mutant designations refer to the position of the amino acid residue in the viral polyprotein (2). When the P3 mutant was compared with the parent virus, the mature particles were found to be more common in this mutant, and there was a corresponding decrease of the partially mature particles (Table 1), indicating a shift of the partially mature particles toward the mature subpopulation as a result of enhanced prM cleavage. In the P6 mutant, the reciprocal changes in the proportions of immature particles and mature particles were observed while the relative abundance of partially mature particles was minimally affected (Table 1). The reduction of prM cleavage in the P6 mutant likely caused concurrent changes of the partially mature particles: some immature particles were not cleaved into the partially mature particles while certain partially mature particles failed to progress to the mature ones. When the proportion of prM-containing particles was plotted against the extent of prM cleavage (Fig. 3), changes in the proportion of the combined immature and partially mature particles, but not of each type of particles alone, exhibited a linear relationship with the level of prM cleavage.
FIG. 3.
Relationship between the proportion of prM-containing extracellular particles and the extent of prM cleavage. The immature and partially mature particles present in the purified preparations of 16681Nde(+) (open circle), prH200A (closed circle), and prE203A (closed square) were categorized by cryo-EM based on their surface morphology, and the proportion of each particle subpopulation was plotted against the level of prM cleavage of each virus. The levels of prM cleavage of 16681Nde(+) and the pr-M junction mutants were determined in a previous study (15). Pearson's correlation and linear regression analysis were employed in the evaluation of the relationship between the level of prM cleavage and the proportion of particle subpopulations by using the STATA 8.2 software (StataCorp, TX).
The results of sequential immunoprecipitation and cryo-EM visualization of extracellular particles in this study are compatible with the existence of partially mature dengue virus particles and the progressive mode of prM cleavage. The dynamic changes in the particle subpopulations in the pr-M junction mutants suggest that the partially mature particles represent an intermediate subpopulation in the virus maturation pathway. However, it is not yet possible to exclude the possibility that the partially mature particles represent a “dead-end” subpopulation unrelated to the generation of mature particles. Whether the partially mature particles are infectious or capable of gaining infectivity upon further exposure to furin remains to be elucidated. The partially mature particles are likely to be heterogeneous with regard to the quantity of remaining prM, and a number of particles may be close to becoming the mature particles. During entry, such particles may be converted to an infectious form by furin in the endosome (30) after a threshold amount of prM is cleaved and the pr peptide is released. These particles may underlie the observations that certain anti-prM antibodies are capable of enhancing dengue virus infection of cells bearing the Fc receptors for IgG (6, 13, 25, 26). It is also possible that the partially mature particles participate in the antibody-dependent neutralization of dengue virus infection (23).
Acknowledgments
We thank Amornrat O'Brien for helpful discussions and Matthawee Lausumpao for technical assistance.
This investigation was supported by the Thailand Tropical Diseases Research Program (02-2-DEN-03-003) and the Medical Biotechnology Network, National Center for Genetic Engineering and Biotechnology, Thailand. J.J. is supported by the Thailand Research Fund through the Royal Golden Jubilee Ph.D. program (PHD/0225/2546). This work was also supported by a Public Health Service Program Project Grant (AI45976) from the National Institute of Allergy and Infectious Diseases to R.J.K.
Footnotes
Published ahead of print on 2 June 2010.
REFERENCES
- 1.Anderson, R., S. Wang, C. Osiowy, and A. C. Issekutz. 1997. Activation of endothelial cells via antibody-enhanced dengue virus infection of peripheral blood monocytes. J. Virol. 71:4226-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blok, J., S. M. McWilliam, H. C. Butler, A. J. Gibbs, G. Weiller, B. L. Herring, A. C. Hemsley, J. G. Aaskov, S. Yoksan, and N. Bhamarapravati. 1992. Comparison of a dengue-2 virus and its candidate vaccine derivative: sequence relationships with the flaviviruses and other viruses. Virology 187:573-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherrier, M. V., B. Kaufmann, G. E. Nybakken, S.-M. Lok, J. T. Warren, B. R. Chen, C. A. Nelson, V. A. Kostyuchenko, H. A. Holdaway, P. R. Chipman, R. J. Kuhn, M. S. Diamond, M. G. Rossmann, and D. H. Fremont. 2009. Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. EMBO J. 28:3269-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crabtree, M. B., R. C. Sang, V. Stollar, L. M. Dunster, and B. R. Miller. 2003. Genetic and phenotypic characterization of the newly described insect flavivirus, Kamiti River virus. Arch. Virol. 148:1095-1118. [DOI] [PubMed] [Google Scholar]
- 5.Davis, C. W., H. Y. Nguyen, S. L. Hanna, M. D. Sanchez, R. W. Doms, and T. C. Pierson. 2006. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 80:1290-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dejnirattisai, W., A. Jumnainsong, N. Onsirisakul, P. Fitton, S. Vasanawathana, W. Limpitikul, C. Puttikhunt, C. Edwards, T. Duangchinda, S. Supasa, K. Chawansuntati, P. Malasit, J. Mongkolsapaya, and G. Screaton. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science 328:745-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elshuber, S., S. L. Allison, F. X. Heinz, and C. W. Mandl. 2003. Cleavage of protein prM is necessary for infection of BHK-21 cells by tick-borne encephalitis virus. J. Gen. Virol. 84:183-191. [DOI] [PubMed] [Google Scholar]
- 8.Guirakhoo, F., R. A. Bolin, and J. T. Roehrig. 1992. The Murray Valley encephalitis virus prM protein confers acid resistance to virus particles and alters the expression of epitopes within the R2 domain of E glycoprotein. Virology 191:921-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He, R.-T., B. L. Innis, A. Nisalak, W. Usawattanakul, S. Wang, S. Kalayanarooj, and R. Anderson. 1995. Antibodies that block virus attachment to Vero cells are a major component of the human neutralizing antibody response against dengue virus type 2. J. Med. Virol. 45:451-461. [DOI] [PubMed] [Google Scholar]
- 10.Heinz, F. X., K. Stiasny, G. Puschner-Auer, H. Holzmann, S. L. Allison, C. W. Mandl, and C. Kunz. 1994. Structural changes and functional control of the tick-borne encephalitis virus glycoprotein E by the heterodimeric association with protein prM. Virology 198:109-117. [DOI] [PubMed] [Google Scholar]
- 11.Henchal, E. A., J. M. McCown, D. S. Burke, M. C. Seguin, and W. E. Brandt. 1985. Epitope analysis of antigenic determinants on the surface of dengue-2 virions using monoclonal antibodies. Am. J. Trop. Med. Hyg. 34:162-169. [DOI] [PubMed] [Google Scholar]
- 12.Henchal, E. A., M. K. Gentry, J. M. McCown, and W. E. Brandt. 1982. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am. J. Trop. Med. Hyg. 31:830-836. [DOI] [PubMed] [Google Scholar]
- 13.Huang, K. J., Y. C. Yang, Y. S. Lin, J. H. Huang, H.-S. Liu, T. M. Yeh, S. H. Chen, C. C. Liu, and H. Y. Lei. 2006. The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J. Immunol. 176:2825-2832. [DOI] [PubMed] [Google Scholar]
- 14.Igarashi, A. 1978. Isolation of Singh's Aedes albopictus cell clone sensitive to dengue and chikungunya viruses. J. Gen. Virol. 40:531-544. [DOI] [PubMed] [Google Scholar]
- 15.Junjhon, J., M. Lausumpao, S. Supasa, S. Noisakran, A. Songjaeng, P. Saraithong, K. Chaichoun, U. Utaipat, P. Keelapang, A. Kanjanahaluethai, C. Puttikhunt, W. Kasinrerk, P. Malasit, and N. Sittisombut. 2008. Differential modulation of prM cleavage, extracellular particle distribution, and virus infectivity by conserved residues at nonfurin consensus positions of the dengue virus pr-M junction. J. Virol. 82:10776-10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keelapang, P., R. Sriburi, S. Supasa, N. Punyadee, A. Songjaeng, A. Jairungsri, C. Puttikunt, W. Kasinrerk, P. Malasit, and N. Sittisombut. 2004. Alterations of pr-M cleavage and virus export in pr-M junction chimeric dengue viruses. J. Virol. 78:2367-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn, R. J., W. Zhang, M. G. Rossmann, S. V. Pletnev, J. Corver, E. Lenches, C. T. Jones, S. Mukhopadhyay, P. R. Chipman, E. G. Strauss, T. S. Baker, and J. H. Strauss. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, L., S.-M. Lok, I.-M. Yu, Y. Zhang, R. J. Kuhn, J. Chen, and M. G. Rossmann. 2008. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science 319:1830-1834. [DOI] [PubMed] [Google Scholar]
- 19.Lindenbach, B. D., H.-J. Thiel, and C. M. Rice. 2007. Flaviviridae: the viruses and their replication, p. 1101-1152. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 5th ed. Lippincott-Raven, Philadelphia, PA.
- 20.Mukhopadhyay, S., R. J. Kuhn, and M. G. Rossmann. 2005. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 3:13-22. [DOI] [PubMed] [Google Scholar]
- 21.Murray, J. M., J. G. Aaskov, and P. J. Wright. 1993. Processing of the dengue virus type 2 proteins prM and C-prM. J. Gen. Virol. 74:175-182. [DOI] [PubMed] [Google Scholar]
- 22.Nelson, S., C. A. Jost, Q. Xu, J. Ess, J. E. Martin, T. Oliphant, S. S. Whitehead, A. P. Durbin, B. S. Graham, M. S. Diamond, and T. C. Pierson. 2008. Maturation of West Nile virus modulates sensitivity to antibody-mediated neutralization. PLoS Pathog. 4:e1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierson, T. C., D. H. Fremont, R. J. Kuhn, and M. S. Diamond. 2008. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe 4:229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pokidysheva, E., Y. Zhang, A. J. Battisti, C. M. Bator-Kelly, P. R. Chipman, C. Xiao, G. G. Gregorio, W. A. Hendrickson, R. J. Kuhn, and M. G. Rossmann. 2006. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell 124:485-493. [DOI] [PubMed] [Google Scholar]
- 25.Randolph, V. B., G. Winkler, and V. Stollar. 1990. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology 174:450-458. [DOI] [PubMed] [Google Scholar]
- 26.Rodenhuis-Zybert, I. A., H. M. van der Schaar, J. M. da Silva Voorham, H. van der Ende-Metselaar, H.-Y. Lei, J. Wilschut, and J. M. Smit. 2010. Immature dengue virus: a veiled pathogen? PLoS Pathog. 6:e1000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roehrig, J. T., R. A. Bolin, and R. G. Kelly. 1998. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology 246:317-328. [DOI] [PubMed] [Google Scholar]
- 28.Sittisombut, N., P. Keelapang, and P. Malasit. 2006. Functional role of prM glycoprotein in dengue virus replication, p. 169-189. In M. Kalitzky and P. Borowski (ed.), Molecular biology of the flavivirus. Horizon Bioscience, Norfolk, United Kingdom.
- 29.Stadler, K., S. L. Allison, J. Schalich, and F. X. Heinz. 1997. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 71:8475-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas, G. 2002. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 3:753-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trainor, N. B., W. D. Crill, J. A. Roberson, and G.-J. J. Chang. 2007. Mutation analysis of the fusion domain region of St. Louis encephalitis virus envelope protein. Virology 360:398-406. [DOI] [PubMed] [Google Scholar]
- 32.van der Schaar, H. M., M. J. Rust, B.-L. Waarts, H. van der Ende-Metselaar, R. J. Kuhn, J. Wilschut, X. Zhuang, and J. M. Smit. 2007. Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J. Virol. 81:12019-12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, S., R. He, and R. Anderson. 1999. prM- and cell-binding domains of the dengue virus E protein. J. Virol. 73:2547-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu, I.-M., W. Zhang, H. A. Holdaway, L. Li, V. A. Kostyuchenko, P. R. Chipman, R. J. Kuhn, M. G. Rossmann, and J. Chen. 2008. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 319:1834-1837. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, Y., B. Kaufmann, P. R. Chipman, R. J. Kuhn, and M. G. Rossmann. 2007. Structure of immature West Nile virus. J. Virol. 81:6141-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, Y., J. Corver, P. R. Chipman, W. Zhang, S. V. Pletnev, D. Sedlak, T. S. Baker, J. H. Strauss, R. J. Kuhn, and M. G. Rossmann. 2003. Structures of immature flavivirus particles. EMBO J. 22:2604-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zybert, I. A., H. van der Ende-Metselaar, J. Wilschut, and J. M. Smit. 2008. Functional importance of dengue virus maturation: infectious properties of immature virions. J. Gen. Virol. 89:3047-3051. [DOI] [PubMed] [Google Scholar]