Abstract
Hepatitis C virus (HCV) replication involves many viral and host factors. Here, we employed a lentivirus-based RNA interference (RNAi) screening approach to search for possible cellular factors. By using a kinase-phosphatase RNAi library and an HCV replicon reporter system, we identified a serine-threonine kinase, Polo-like kinase 1 (Plk1), as a potential host factor regulating HCV replication. Knockdown of Plk1 reduced both HCV RNA replication and nonstructural (NS) protein production in both HCV replicon cells and HCV-infected cells while it did not significantly affect host cellular growth or cell cycle. Overexpression of Plk1 in the knockdown cells rescued HCV replication. Interestingly, the ratio between the hyperphosphorylated form (p58) and the basal phosphorylated form (p56) of NS5A was lower in the Plk1 knockdown cells and Plk1 kinase inhibitor-treated cells than in the control groups. Further studies showed that Plk1 could be immunoprecipitated together with NS5A. Both proteins partially colocalized in the perinuclear region. Furthermore, Plk1 could phosphorylate NS5A to both the p58 and p56 forms in an in vitro assay system; the phosphorylation efficiency was comparable to that of the reported casein kinase. Taken together, this study shows that Plk1 is an NS5A phosphokinase and thereby indirectly regulates HCV RNA replication. Because of the differential effects of Plk1 on HCV replication and host cell growth, Plk1 could potentially serve as a target for anti-HCV therapy.
Hepatitis C virus (HCV) is the major causative agent of non-A/non-B hepatitis (26). More than 170 million people, or 3% of the population in the world, are infected with HCV (29). It establishes chronic infection in at least 85% of infected individuals and is associated with liver cirrhosis and hepatocellular carcinoma. Current treatment, which combines polyethylene glycol-interferon (PEG-IFN) and ribavirin, is ineffective in 22% of patients with non-genotype 1 and in 45% of patients with genotype 1 HCV (1, 16, 23, 55). Therefore, identification of new targets for HCV therapy is an important issue, and cellular genes involved in the HCV life cycle may serve as good candidates.
HCV is a positive-strand RNA virus and the only known member of Hepacivirus genus in the family Flaviviridae. Its genome has a length of about 9,600 nucleotides coding for a single polyprotein. The long polyprotein is further processed into at least 10 different products, including four structural proteins (core, E1, E2, and p7) and six nonstructural (NS) proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B). Nonstructural proteins NS3-NS5B are components of the membrane-associated HCV replication complex (8, 13, 36, 45). NS3 is a bifunctional protein containing an N-terminal protease domain and a C-terminal helicase/NTPase domain, and NS4A serves as a cofactor for NS3 protease. NS4B protein is known to induce intracellular membrane changes that probably serve as the site for viral RNA replication (8). NS5A is required for RNA replication, but little is known about its function. NS5B is the RNA-dependent RNA polymerase (reviewed in reference 47).
NS5A is phosphorylated on multiple serine and threonine residues and exists in basal phosphorylated (p56) and hyperphosphorylated (p58) forms (49). Increasing evidence suggests that the regulation of NS5A phosphorylation is important for HCV RNA replication. Adaptive mutations or kinase inhibitors, which reduce NS5A hyperphosphorylation, increased the replication of an HCV replicon in cell culture (HCVcc) systems (2, 4, 38). However, when an adaptive replicon with reduced p58 was further treated with the same kinase inhibitor or introduced with a second adaptive mutation, RNA replication was completely blocked (32, 38). Furthermore, the mutations that reduce NS5A hyperphosphorylation and promote RNA replication in cell culture, paradoxically, prevented productive replication in the chimpanzee model (6). These results imply that the tight control of the p58/p56 ratio is important for HCV replication. The detailed mechanism is still not clear, but a clue was provided by the finding of differential association of NS5A phospho-forms with the host vesicle-associated membrane protein-associated protein A (VAP-A) protein, which is an essential molecule for HCV replicase (9, 12). On the other hand, NS5A phosphorylation was recently found to regulate the production of infectious virus (34, 50). Alanine substitutions in the C-terminal domain III of NS5A impaired NS5A phosphorylation, leading to a decrease in NS5A-core protein interaction, disturbance of subcellular localization of NS5A, and disruption of virion production (3, 34, 50). In summary, phosphorylation on NS5A is not only important for HCV RNA replication but also critical for infectious virus production.
Since the phosphorylation state of NS5A is correlated with HCV RNA replication and virion production, cellular kinases responsible for NS5A phosphorylation may serve as good candidates for drug targets. Several kinases have been shown to target NS5A in vitro, including casein kinase I (CKI), CKII, MEK1, MKK6, MKK7, AKT, and p70S6K (7, 24). Among these proteins, CKI and CKII are better characterized for NS5A phosphorylation. CKIα has been identified as the target of kinase inhibitors which decrease the hyperphosphorylation of NS5A and was further confirmed as a direct kinase of NS5A (41, 42). CKI requires prephosphorylation of residues near the predicted phosphorylation site in NS5A for effective modification, suggesting that other kinases are also involved in this process (42). CKII has been shown to bind to the C-terminal domain of NS5A and phosphorylate NS5A in vitro (24). Inhibition of CKII with chemical compounds or small interfering RNA (siRNA) did not significantly affect HCV RNA replication but severely disrupted virus production (50).
In this study, using lentivirus-based RNA interference (RNAi) screening, we identified a serine/threonine kinase, Polo-like kinase 1 (Plk1), which is involved in HCV replication. Expression of short hairpin RNAs (shRNAs) targeting Plk1 decreased HCV replication and virus production. Moreover, silencing of Plk1 decreased the hyperphosphorylated form of NS5A. In cells treated with a Plk1-specific kinase inhibitor, HCV replication and NS5A hyperphosphorylation were significantly reduced, indicating that Plk1 kinase activity is required for this process. Further studies showed that Plk1 was coimmunoprecipitated and partially colocalized with NS5A, suggesting NS5A as a possible substrate for Plk1. Finally, NS5A is hyperphosphorylated by Plk1 in vitro, supporting the proposition that Plk1 regulates HCV replication through hyperphosphorylation of NS5A.
MATERIALS AND METHODS
Cells, media, and reagents.
Huh-7, Huh-7.5, and HEK293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, nonessential amino acids, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 5% CO2 incubator. Three HCV subgenomic replicons—HCV-EV71I-Luc, HCVrep-His, and HCVrep-HA (where EV is enterovirus and HA is hemagglutinin)—were derived from HCV N strain 1bneo/delS (14). HCV-EV71I-Luc was modified by insertion of an EV71-IRES-driven luciferase gene (where IRES is internal ribosome entry site) between the neo gene and the encephalomyocarditis virus (EMCV)-IRES (Fig. 1 A); HCVrep-His or HCVrep-HA was modified by insertion of a His or an HA tag in the C-terminal region of NS5A, as previously described (35). In vitro transcribed replicon RNAs were electroporated into Huh-7 cells (975μF and 220V) and then selected with G418 at a concentration of 0.5 to 1 mg/ml for 3 weeks. For the HCV-EV71I-Luc replicon, 50 single colonies were picked, expanded, and assayed for luciferase activity. The expanded colony with the highest luciferase activity and HCV protein level was chosen and maintained in G418 (0.5 mg/ml) for subsequent experiments. For HCVrep-His and HCVrep-HA replicons, the G418-resistant cells were pooled together and maintained in 0.5 mg/ml of G418. BI2536, a specific Plk1 kinase inhibitor, was purchased from Axon Medchem.
FIG. 1.
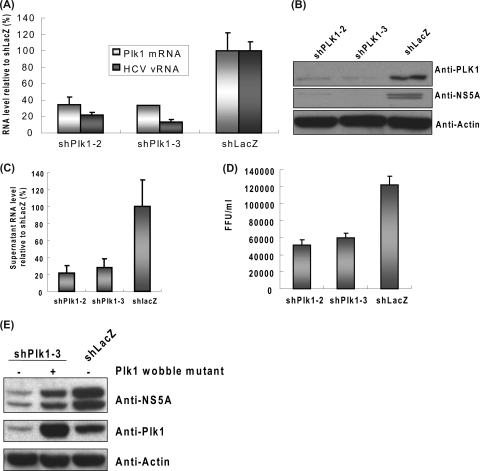
Decrease of HCV replication in Plk1 knocked down HCV replicon cell. (A) Schematic representation of the configuration of HCV-EV71I-Luc replicon construct. (B) Relative luciferase activity (L)/cell viability (M) ratio of Plk1 knockdown cells in the primary screen. The detailed experimental design is described in Materials and Methods. Means of two independent experiments including standard deviations of the means are shown. (C) Cellular RNA of HCVrep-His replicon cells was extracted at posttransduction day 3 and measured with quantitative RT-PCR. RNA levels of target genes were normalized by GAPDH RNA. Means and standard deviations of two independent experiments are shown. (D) Western blot analysis of HCV NS5A and Plk1 proteins in HCVrep-His replicon cells transduced by different shRNAs. Cell lysates were collected at day 4 posttransduction. The proteins were separated by 10% SDS-PAGE and then transferred to a polyvinylidene difluoride membrane, followed by standard Western blotting. Plk1 and HCV intensity levels are quantified by the densitometric scanning of the film using a bioluminescence imaging system (BioSpectrum Imaging System; UVP). The band intensity is normalized with the shLacZ control (set as 100%). (E) MTS assay of lentivirus-infected HCVrep-His replicon cells was performed on each day posttransduction until cell harvest (days 0 to 4) (mean ± standard deviation; n = 3). (F) The cell cycle of knocked down cells. The percentages of G1/S/G2 phases are indicated. shPlk1-2 and shPlk1-3 represent the lentiviruses harboring independent shRNAs targeted to different regions of Plk1 gene. shLacZ is the control virus targeting the LacZ gene.
Screening for cellular factors required for HCV RNA replication by lentiviral shRNAs.
High-titer vesicular stomatitis virus G protein (VSV-G) pseudotyped lentiviruses expressing distinct shRNA were prepared by the National RNAi Core Facility, Academia Sinica, Taiwan. A subset named KP (human kinase and phosphatase genes) was selected for our screening. The KP subset consists of 6,188 unique lentiviral shRNAs, targeting 1,210 human kinase- and phosphatase-related genes. HCV-EV71I-Luc replicon cells were plated (1 × 104 cells per well) 24 h prior to infection in 96-well plates. Then cells were infected with KP subset lentiviruses at a multiplicity of infection (MOI) of 3 in the presence of Polybrene (hexadimethrine bromide; Sigma H9268) at a final concentration of 8 μg/ml. Viruses were inoculated and incubated with the cells for 24 h, and then the medium was replaced with selective medium containing puromycin (3 μg/ml). Luciferase activities (L) and cell viabilities (M) were detected at posttransduction day 5. Luciferase activities were determined by using a Bright-Glo Luciferase Assay System (Promega). Cell viabilities were measured by using an MTS [3,4-(5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt] assay as described below. The knockdown effects on HCV replication were evaluated by the L/M ratio. The relative L/M values were normalized by the L/M ratio of noninfected control cells without puromycin incubation.
Plasmids and viruses.
All the plasmids required for lentivirus production were provided by the National RNAi Core Facility, Academia Sinica, Taiwan. The four pLKO.1-shRNA vectors used for knockdown of Plk1 are the following: TRCN0000006249 (shPlk1-1), TRCN0000006246 (shPlk1-2), TRCN0000006247 (shPlk1-3), and TRCN0000006248 (shPlk1-4). The pLKO.1-shLacZ control plasmid is TRCN0000072237 (shLacZ). The Trans-IT transfection reagent (Mirus Bio) was used for lentiviral production in 293T cells with a packaging construct (pCMV-ΔR8.91), an envelope construct (pMD.G), and different shRNA or rescue constructs, according to the protocol on the RNAi Core website (http://rnai.genmed.sinica.edu.tw/file/protocol/2_LentivirusProductionV4.pdf). The viral titer was determined in Huh-7 cells by using a cell viability assay (relative infectious unit [RIU] method) according to the RNAi Core's instructions (http://rnai.genmed.sinica.edu.tw/file/protocol/4_1_EstimationLentivirusTiterRIUV1.pdf). To generate the plasmid for rescue of Plk1, the full-length cDNA of Plk1 was amplified from Huh-7 total cDNA and inserted into NheI and PmeI sites of pLKO_AS3w.neo lentivector (provided by RNAi Core). Then the wobble mutations (CGA TAC TAC CTA CGG CAA → CGG TAT TAT TTG CGT CAG) that eliminate targeting by shPlk1-3 but do not change the amino acid sequence of the resultant Plk1 protein were introduced by using the jumping PCR method (17). The resultant construct was confirmed by sequencing and named pLKO_AS3w.neo-Plk1-3R. Production of rescue (pLKO_AS3w.neo-Plk1-3R) and control (pLKO_AS3w.neo) lentiviruses was carried out as described above. The pJC1 plasmid, which encodes a chimera genome of HCV J6CF/JFH1, was constructed as previously described (39). The JC1 viruses were produced and titrated in Huh-7.5 cells based on a previously described method (28). pUI-Plk1 was constructed by insertion of Plk1 from pLKO_AS3w.neo-Plk1 into NheI and SmaI sites of pUI vector, which was derived from pCI (Promega) by replacement of the cytomegalovirus (CMV) promoter with a ubiquitin promoter. pCI-HA-Plk1 was cloned by using PCR amplification. The forward primer carries an EcoRI site followed by an in-frame HA tag, and the reverse primer contains an XbaI site. The amplified EcoRI-HA-Plk1-XbaI fragment was cloned into pCI vector. pUI-NS3-5b was constructed from HCV 1bneo/delS replicon cDNA. The cDNA plasmid was digested with PmeI and XbaI, and then the NS3-NS5B fragment was cloned into NheI (Klenow) and XbaI sites of pUI vector. pCI-HA-NS3/4A, pCI-NS5A-HA, and pCI-HA-GST (where GST is glutathione S-transferase) have been described previously (27, 28). pEGFP-N1 was obtained from Clontech. For in vitro kinase assays, full-length and truncated forms of NS5A-coding sequences were amplified by using PCR and HCV 1bneo/delS as a template. The six different cDNA fragments of NS5A are the following: wild type ([WT] nucleotides [nt] 1 to 1350), I (nt 1 to 645), II (nt 646 to 1071), III (nt 1072 to 1350), I+II (nt 1 to 1071), and II+III (nt 646 to 1350). PCR primers were designed to create a 5′ EcoRI site followed by an ATG start codon and 3′ XhoI site for the in-frame fusion with the C-terminal polyhistidine epitopes of the pET21a vector (Novagen). pET-21a-MHVnsp15 was cloned from the genome cDNA of mouse hepatitis virus ([MHV] strain JHM). pET-22b-NS3 was derived from cDNA of HCV JC1 strain. All the clones were confirmed by DNA sequencing.
Abs.
The mouse monoclonal antibodies (MAb) specific for HCV genotype 1b NS5A was purchased from Biodesign. The mouse MAb for JC1 NS5A detection was obtained from Austral Biologicals. Mouse MAb against Plk1 (clone 3F8) was a gift from Sheng-Chung Lee, Institute of Molecular Medicine, National Taiwan University. Mouse MAb against actin was obtained from Millipore. Rat MAb against the HA epitope was purchased from Roche Diagnostics. Rabbit polyclonal anti-HA antibody for immunofluorescence staining was obtained from Santa Cruz. Alexa Fluor 488-conjugated anti-mouse and Alexa Fluor 647-conjugated anti-rabbit secondary antibodies (Abs) were purchased from Invitrogen Molecular Probes.
Quantitative detection of HCV and Plk1 RNA by quantitative reverse transcription-PCR (qRT-PCR).
For intracellular RNA detection, total RNA was extracted using TRIZol (Invitrogen). RNA in the culture supernatant was extracted by using a QIAamp Viral RNA Mini Kit (Qiagen) according to the manufacturer's protocol. Extracted RNA concentration was determined by a spectrophotometer. cDNA from 150 ng of total RNA or 11 μl of supernatant RNA was synthesized in a 20-μl total volume by using a SuperScript III First-Strand Synthesis System (Invitrogen). The primers for reverse transcription are oligo(dT)20 and an HCV-specific RT primer (5′-CACTCGCAAGCACCCTATCA-3′). For real-time PCR, we followed the standard TaqMan method with the Universal Probe Library System and LightCycler (Roche Diagnostics). The primers are the following: for HCV, 5′-CATGGCGTTAGTATGAGTGTCG-3′ (sense) and 5′-GGTTCCGCAGACCACTATG-3′ (antisense) with Universal Probe 75 (Roche); for Plk1, 5′-CACAGTGTCAATGCCTCCA-3′ (sense) and 5′-TTGCTGACCCAGAAGATGG-3′ (antisense) with Universal Probe 30 (Roche). As an internal control, we amplified human GAPDH (glyceraldehyde 3-phosphate dehydrogenase) with the following primer set: 5′-AGCCACATCGCTCAGACAC-3′ (sense) and 5′-GCCCAATACGACCAAATCC-3′ (antisense) with Universal Probe 60 (Roche).
MTS assay.
A CellTiter 96 AQueous One Solution Cell Proliferation Assay Kit (Promega) was used to evaluate cell viability. Cells (0.6 × 104 to 1 × 104) were seeded onto a 96-well microtiter plate. After 24 h of incubation, cells were inoculated with lentiviruses (MOI of 3) or incubated with various concentrations of BI2536 (Axon Medchem). The MTS assays were performed on the day indicated in the figure legends using the following protocol: cell medium was replaced with phenol red-free DMEM (Invitrogen) containing 10% CellTiter 96 AQueous One Solution Reagent. The plates were incubated for an additional 1 h at 37°C. Light absorbance was measured at 490 nm with a 96-well plate reader. Each experiment was performed in three replicate wells.
Flow cytometry and cell-cycle analysis.
For cell cycle analysis, knocked down or BI2536-treated cells were stained with 20 μg/ml propidium iodide in the presence of 0.1% sodium citrate and 0.1% Triton X-100 for 2 h. The data were acquired on a FACScan flow cytometer (BD Biosciences). Subsequent analysis was performed using FlowJo software (TreeStar, San Carlos, CA).
Coimmunoprecipitations.
To test whether endogenous Plk1 could be coimmunoprecipitated with HCV replicon NS5A, Huh-7 and HCVrep-HA cells were subcultured into a 10-cm dish 2 days before protein collection. For overexpression and immunoprecipitation, various expression plasmids were transfected into HEK293T cells with Lipofectamine 2000 transfection reagent (Invitrogen). Cells were collected 48 h after transfection. The preparation of total lysates and immunoprecipitation for HA-tagged proteins were performed according to instructions provided with the Profound Mammalian HA-Tag IP/CO-IP kit (Pierce). The immunoprecipitated proteins were run on a 7.5% SDS-polyacrylamide gel, and immunoblot analysis was performed using antibodies described above.
Immunofluorescence staining.
Huh-7 and HCVrep-HA cells were cultured on glass chamber slides (Lab-Tek II). Cells were washed twice with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde-PBS for 20 min, and then permeabilized with 0.2% Triton X-100-PBS. Samples were blocked with 0.25% bovine serum albumin (BSA)-PBS for 1 h. Primary Abs were diluted in blocking buffer and incubated with cells for 1 h. After three washes in PBS, Alexa Fluor 488- and 647-conjugated secondary Abs along with 4′,6′-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) were diluted in blocking buffer and added to cells for 1 h of incubation. After staining, slides were washed three times with PBS and mounted with ProLong Antifade (Invitrogen Molecular Probes). All the procedures were carried out at room temperature. Photographs were taken with a confocal microscope (Zeiss LSM 710 confocal laser scanning microscope). Image analysis and colocalization coefficient were performed using the standard system operating software provided with the microscope.
In vitro kinase assay.
Overexpression of different fragments of HCV 1b neo/delS strain NS5A protein and two control proteins, MHV nsp15 and HCV NS3, was performed in BL21(DE3) strain of Escherichia coli. The procedure of expression and purification was performed as previously described (18). Plk1 recombinant proteins were purchased from Cell Signaling Technology. CKIδ and CKII were obtained from New England Biolabs. Protein kinase R (PKR) proteins were purchased from Abcam. All reactions were performed in a final volume of 10 μl with 0.1 μg of substrate, 50 μM cold ATP, 10 μCi of [γ-32P]ATP, and 1 μl of reaction buffer; buffers consisted of the following: 10× buffer for Plk1 was composed of 50 mM (morpholinepropanesulfonic acid) MOPS (pH 7.2), 25 mM β-glycerophosphate, 10 mM EGTA, 4 mM EDTA, 50 mM MgCl2, and 0. 5 mM dithiothreitol (DTT); CKIδ (10×) and CKII (10×) reaction buffers were provided by New England Biolabs; kinase buffer (10×) for PKR was purchased from Cell Signaling. Plk1 (100 ng), 10 units of CKI, 20 units of CKII, or 100 ng of PKR, respectively, was applied to the reaction mixture for the time indicated in the figure legends at 30°C. The reactions were stopped by the addition of the same volume of 2× Lane Marker Non-Reducing Sample Buffer (Thermo Scientific), followed by boiling for 5 min. Proteins were separated by either 7.5% (for fragments WT, I+II, and II+III) or 12% (for fragments I, II, and III) SDS-PAGE. After separation, the SDS-PAGE gels were stained with Coomassie blue to determine the position of unphosphorylated bands. After destaining, the SDS-PAGE gels were vacuum dried, followed by film-based autoradiography for the detection of radiolabeled proteins.
RESULTS
Silencing of Plk1 decreases the HCV replicon replication.
To identify cellular factors involved in HCV replication, we performed an RNAi high-throughput screening. A tricistronic HCV replicon, HCV-EV71I-Luc, harboring a subgenomic HCV (genotype 1b) and an EV71-IRES-driven luciferase gene, was introduced into Huh-7 cells to establish a stable cell line (Fig. 1A). Then a KP (human kinase and phosphatase genes) subset of VSV-G pseudotyped lentiviruses expressing distinct shRNA was employed for screening. By measuring the luciferase activity (L) and cell viability (M), the knockdown performance on HCV replication in transduced cells was evaluated by the L/M ratio. Two independent primary screens were performed to ascertain that candidate genes were reproducible. Candidates were selected, in which each gene hit was shown by at least two unique shRNAs, resulting in more than a 50% enhancing or inhibitory effect on HCV replication. Several genes known to be involved in the HCV life cycle, including TYK2, PAK1, and RHO (5, 15, 20, 40), were among the 40 candidate genes identified. The candidates were further confirmed by quantitative RT-PCR to detect the RNA of target genes and the HCV replicon. The candidates were ruled out if their gene knockdown efficiencies did not match the changes in HCV replication. Finally, four genes were selected for further investigation. Polo-like kinase 1 (Plk1), a serine/threonine kinase, is one of these candidates. Plk1 was hit by two unique shRNAs showing around a 50% reduction in the L/M ratio (Fig. 1B). The reduction of HCV replication in shPlk1-transduced cells was confirmed by determination of viral RNA and NS5A protein levels (Fig. 1C and D). Compared to the shLacZ control, both quantitative RT-PCR and Western blot results showed that all four different clones of shPlk1 decreased HCV replication, and the knockdown efficiency correlated well with the phenotype. Among these four clones, clone 2 and clone 3 showed particularly high knockdown efficiencies and HCV inhibition, consistent with the result of the primary screening. Furthermore, knockdown of Plk1 did not significantly affect the cell survival rate or cell cycle (Fig. 1E and F); so, the decrease of HCV replication was not the result of an effect on host cell viability or cell cycle progression. The positive correlation between Plk1 and HCV level indicates a role of Plk1 in HCV replication.
Knockdown of PLK1 decreases HCV replication and virion release in the HCVcc system.
Because the replicon RNA contains several heterologous sequences, we further determined the importance of Plk1 in the HCV life cycle using an HCV infection system. Huh-7.5 cells were transduced with shPlk1 or shLacZ lentiviruses. After puromycin selection, cells were reseeded and infected with HCV JC1 strain. On the third day postinfection, cells were collected for assays. Analysis of intracellular HCV RNA and NS5A protein revealed that shPlk1 significantly inhibited HCV replication in the infectious virus system (Fig. 2 A and B). The supernatant HCV RNA and infectious viral titers were also decreased proportionally in Plk1 knockdown cells, indicating that virion production was also affected (Fig. 2C and D). Furthermore, overexpression of a Plk1 wobble mutant reversed the decrease in HCV replication caused by shPlk1 (Fig. 2E). These results together indicate that Plk1 is involved in HCV replication. Moreover, because the replicon and JC1 virus belong to the 1b and 2a genotypes, respectively, these results also suggest that the involvement of Plk1 in HCV replication is not limited to a certain genotype.
FIG. 2.
Depletion of Plk1 decreased HCV replication and virion production in infectious HCVcc system. (A to D) shPlk1-2 and shPlk1-3 were transduced into Huh-7.5 cells, and the transduced cells were selected with puromycin for 2 days. Cells were then subcultured and infected with JC1 virus (MOI of 1). Three days after infection, cells were collected and analyzed with quantitative RT-PCR (A) (normalized to GAPDH RNA; mean ± standard deviation; n = 3) or Western blotting (B). Supernatant was collected for RNA extraction for quantitative PCR (C) and viral titer determination (D). The viral RNA was normalized by the input volume of supernatant (mean ± standard deviation; n = 3). In panel D the serially diluted supernatant from the JC1-infected shPlk1/shLacZ knocked down cells was applied to Huh-7.5 cells in 96-well plates. The number of focus forming units (FFU) was calculated at day 3 postinfection by staining of core protein (mean ± standard deviation; n = 3). (E) The lentiviruses carrying the rescue (pLKO_AS3w.neo-Plk1-3R)- or control (pLKO_AS3w.neo)-expressing vector were transduced in Huh-7.5 cells to establish stable cell lines and then infected with shPlk1-3 or shLacZ viruses as indicated. The subsequent HCV infection was as described above.
Hyperphosphorylation of NS5A is decreased in Plk1-depleted or Plk1 kinase inhibitor-treated cells.
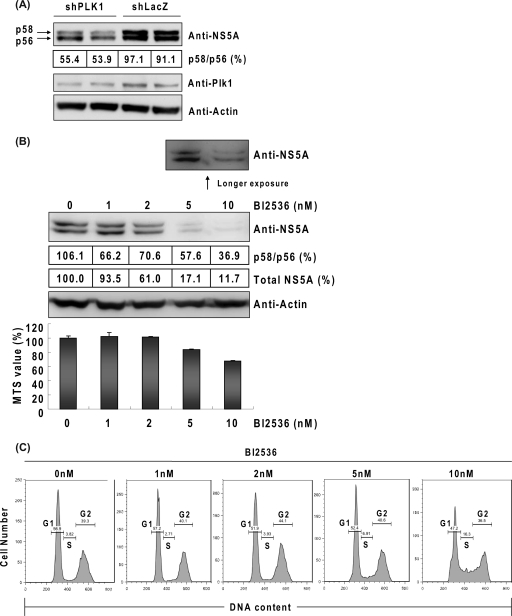
NS5A is phosphorylated on multiple serine residues and, to a much lesser extent, on threonine residues (19, 22, 44). The importance of tight control of NS5A phosphorylation for HCV replication has become evident (2, 4, 6, 9, 32, 38). In the results shown in Fig. 2E, we found that the ratio between the hyperphosphorylated form (p58) and the basal phosphorylated form (p56) of NS5A was slightly decreased in the Plk1-depleted cells and recovered when Plk1 was overexpressed. This result was confirmed in replicon cells, as shown in Fig. 3 A. These findings raised a possibility that Plk1 may regulate HCV replication by phosphorylating NS5A. Since Plk1 is a serine/threonine kinase, we next investigated whether the kinase activity of Plk1 is required for this regulation. BI2536, a Plk1-specific kinase inhibitor (46), was applied to Huh-7.5 cells and followed by HCV JC1 virus infection. On the third day postinfection, NS5A was detected by using immunoblotting. At the same time point, MTS assays and cell cytometry studies were performed to assess cell viability and cell cycle progression. The immunoblotting results showed that both the ratio of p58/p56 and the total amount of NS5A protein were decreased by BI2536 in a dose-dependent manner (Fig. 3B, upper panel). Treatment with 2 nM BI2536 reduced the p58/p56 ratio by 30% and the total amount of NS5A protein by 39% without a decrease in cell viability or retardation of the cell cycle (Fig. 3B and C). At higher concentrations, BI2536 caused a reduction of cell viability (16.5% at 5 nM and 32.3% at 10 nM) and S-phase arrest in a dose-dependent manner; nevertheless, the total amount of NS5A protein was more dramatically diminished (83% at 5 nM and 90% at 10 nM) than the degree of cell death or the extent of cell cycle arrest (Fig. 3B and C). These results suggested that the reduction of NS5A by the Plk1 inhibitor was not entirely the result of cell death or cell cycle blockage. Therefore, we conclude that the kinase activity of Plk1 is positively correlated with NS5A hyperphosphorylation and HCV replication. These results together suggest that Plk1 is a potential kinase of NS5A and that it regulates HCV replication through hyperphosphorylation of NS5A.
FIG. 3.
Depletion of Plk1 or impairment of Plk1 kinase activity inhibits NS5A hyperphosphorylation. (A) Western blot detection of hyperphosphorylated form (p58) and basal phosphorylated form (p56) of NS5A in Plk1-depleted cells. Huh-7 cells bearing an HCVrep-His replicon were inoculated with shPlk1-3 or shLacZ lentivirus. After 4 days, cells were lysed in the presence of phosphatase inhibitor (Thermo) and protease inhibitor (Thermo), and then standard immunoblotting was performed. After application with the ECL substrate, the band intensities of p58 and p56 were directly visualized and quantified by a bioluminescence imaging system (BioSpectrum Imaging System; UVP). (B) BI2536 at different concentrations was applied to Huh-7.5 cells and followed by HCV JC1 virus infection at an MOI of 1. Cells were collected at day 3 postinfection, and immunoblotting was performed. Quantification of the p58/p56 ratio and total NS5A protein was performed as described above. MTS assay of BI2536-treated cells was also performed on the third day postinfection (mean ± standard deviation; n = 3). (C) Cell cycle analysis of BI2536-treated cells. The percentages of G1/S/G2 are indicated.
Direct interaction and colocalization between Plk1 and NS5A.
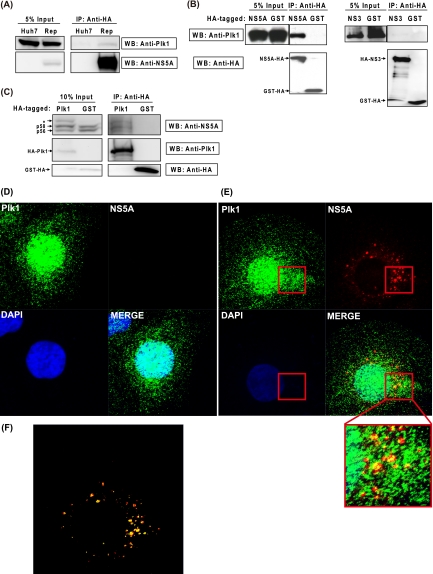
To study whether NS5A is a potential substrate of Plk1, we next examined a possible protein-protein interaction between Plk1 and NS5A. Huh-7 and HCVrep-HA cells were collected for immunoprecipitation of HA-tagged NS5A. As shown in Fig. 4 A, the endogenous Plk1 was coimmunoprecipitated with NS5A in the replicon cells. Although the band was weak, it was consistently detected in three independent experiments. To demonstrate more convincingly the Plk1-NS5A interaction, we cotransfected 293T cells with Plk1 and NS5A-HA or GST-HA-expressing vectors and used them for coimmunoprecipitation experiments. For this purpose, we used 293T rather than Huh-7 cells because a higher transfection efficiency (>80%) could be achieved in 293T cells. In the case of NS5A-HA, the anti-HA antibody coprecipitated both NS5A and Plk1. In the case of GST-HA, in contrast, the anti-HA antibody precipitated GST only and not Plk1 (Fig. 4B, left panel). Under the same conditions, Plk1 was not coprecipitated with NS3 (Fig. 4B, right panel), revealing the specificity of the interaction between NS5A and Plk1. Furthermore, a reciprocal experiment was also performed. When the HA-Plk1 and NS3-NS5B polyprotein were cooverexpressed in 293T cells and Plk1 was precipitated with anti-HA, NS5A was also coimmunoprecipitated (Fig. 4C). In this case, p58 was detected together with p56 since the presence of NS3, NS4A, and NS4B in the polyprotein facilitated hyperphosphorylation (25, 37, 49). Interestingly, compared to the GST control, overexpression of Plk1 increased the p58/p56 ratio, consistent with our hypothesis. Compared to the GST control, one more band was detected in Plk1-overexpressing cells by NS5A antibody (Fig. 4C, asterisk). It is possible that this band represents the NS5A protein with a greater degree of phosphorylation, but this possibility requires more experiments to confirm.
FIG. 4.
Plk1 associates and partially colocalizes with NS5A. (A) Coimmunoprecipitation of replicon NS5A and endogenous Plk1. Lysates from Huh-7 and HCVrep-HA replicon cells were collected and immunoprecipitated with anti-HA agarose, and then the eluate was subjected to Western blotting with anti-Plk1 and anti-NS5A antibodies. (B) 293T cells were cotransfected with pUI-Plk1 and NS5A-HA-, HA-NS3/4A-, or GST-HA-expressing vectors. At 48 h posttransfection, immunoprecipitation and Western blotting were performed as above. Anti-HA was used for NS proteins and GST detection. (C) Western blot analysis of HA-Plk1 immunoprecipitates. 293T cells were cotransfected with pCI-HA-Plk1/pCI-GST-HA and pUI-NS3-5B. At 48 h posttransfection, immunoprecipitation and Western blotting were performed similar to the experiments described in panels A and B. IP, immunoprecipitation; WB, Western blotting. (D and E) Immunohistochemical staining of NS5A-HA and Plk1 in Huh-7 cells (D) and HCVrep-HA replicon (E). Cells were fixed and stained at 2 days after subculture. Green, anti-Plk1; red, anti-HA (NS5A); blue, DAPI (nucleus). (F) The image analysis of colocalization coefficient using the Zen 2009 program provided with the LSM710 confocal microscope (Zeiss). Red, NS5A signals not colocalizing with Plk1; yellow, NS5A signals colocalizing with Plk1.
Previous studies have shown that Plk1 is expressed at low levels in G1/S phase but at higher levels in S and G2 phase and is localized to both the cytoplasm and the nucleus (48). In an attempt to examine the subcellular distribution of NS5A and Plk1, we performed indirect immunofluorescence staining in a nonsynchronized cell population. In both Huh-7 and HCVrep-HA cells, endogenous Plk1 was found in the nucleus and the cytoplasm, consistent with the previous observations (Fig. 4D and E). Furthermore, the NS5A protein was surrounded and partially colocalized with Plk1 in the perinuclear region (Fig. 4E). Overlay analysis indicated that 54.1% of NS5A signal colocalized with Plk1, and most of the large NS5A spots, representing the replication complexes, partially colocalized with Plk1 (Fig. 4F). The association and colocalization of these two proteins suggest a potential interaction between Plk1 and NS5A and imply that NS5A is a potential kinase substrate of Plk1.
Hyperphosphorylation of NS5A occurs upon incubation with Plk1 in vitro.
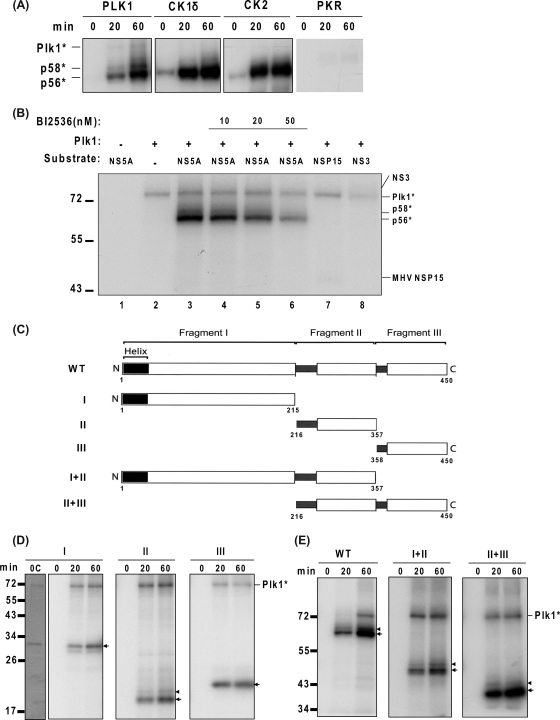
To test if Plk1 is a direct kinase of NS5A, an in vitro kinase assay was carried out. The recombinant genotype 1b NS5A purified from E. coli was incubated with several protein kinases, including Plk1, casein kinase I delta (CKIδ), casein kinase II (CKII), and protein kinase R (PKR). Casein kinase I alpha and delta were the only known kinases capable of NS5A hyperphosphorylation in vitro (42). Here, we used CKIδ because its activity has been commercially verified. CKII has been reported to phosphorylate NS5A in vitro to the basal phosphorylated form but not the hyperphosphorylated form (24); PKR has been reported not to phosphorylate NS5A in vitro (11). Our results are consistent with the previous studies. Furthermore, incubation of NS5A with Plk1 recombinant protein phosphorylated NS5A to not only the basal phosphorylated form (p56) but also the hyperphosphorylated form (p58) (Fig. 5 A). Plk1 also autophosphorylated itself. The efficiency of Plk1 in phosphorylating NS5A appeared to be comparable to that of CKI (Fig. 5A). To confirm the specificity of the Plk1 kinase, we included several controls (Fig. 5B). Neither the p58 nor p56 band was detected in the reaction mixture containing NS5A or Plk1 only (lanes 1 and 2). When Plk1 inhibitor BI2536 was added, both hyperphosphorylation and basal phosphorylation were decreased in a dose-dependent manner (lanes 4 to 6). Furthermore, mouse hepatitis virus (MHV) nsp15 and HCV NS3 were included as negative controls. Plk1 did not phosphorylate either of these two proteins (lanes 7 and 8). These results showed that the Plk1 is a specific kinase which can phosphorylate NS5A to both p56 and p58.
FIG. 5.
NS5A is hyperphosphorylated in vitro by Plk1. (A) In vitro kinase assay. His-tagged NS5A proteins were expressed in E. coli and purified with Ni-affinity gel and dialyzed. These purified NS5A proteins were then incubated with the kinases as indicated in the presence of [γ-32P]ATP. Reaction time ranged from 0 min to 60 min. (B) The specificity of Plk1 kinase. BI2536 treatment and negative-control proteins, MHV nsp15 and HCV NS3, were indicated. The reaction mixture was incubated for 60 min. (C) Schematic presentation of the NS5A derivatives used in this study. The diagram of the full-length NS5A structure is modified from Tellinghuisen et al. (51). The N-terminal and C-terminal amino acids of each fragment are labeled below. (D and E) Incubation of His-tagged truncated NS5A proteins with Plk1 in vitro. The basal phosphorylated forms corresponding to the size of unphosphorylated fragments are indicated with arrows; the hyperphosphorylated forms are denoted by arrowheads. Reaction time ranged from 0 min to 60 min as indicated. 0C, Coomassie blue staining of the proteins at 0 min of the reaction. Plk1*, autophosphorylated Plk1. Molecular sizes are indicated on the left (in kDa).
Next, we dissected the NS5A protein to identify the region responsible for Plk1-dependent hyperphosphorylation in vitro. NS5A was separated into three fragments (I, II, and III) (Fig. 5C), as suggested by Tellinghuisen et al. (51). As shown in Fig. 5D, each of the fragments I, II, and III yielded p56, but only fragment II showed a weak p58. Although there are two bands detected in Fig. 5D, fragment I, the upper band was determined to be the basal phosphorylated band by Coomassie blue staining of the unphosphorylated protein (Fig. 5D, panel I, lane 0C). These results suggest that basal phosphorylation occurs at multiple sites but that hyperphosphorylation occurs only in fragment II. The poor efficiency of hyperphosphorylation (p58) in vitro may suggest that the hyperphosphorylation requires larger protein regions of NS5A. This possibility was confirmed by the finding that both fragments I+II and II+III yielded significant amounts of both p56 and p58 (Fig. 5E). Since fragment II contains Ser-2197, Ser-2201, and Ser-2204 residues known to be important for NS5A hyperphosphorylation and HCV replication, our results suggest that Plk1 may phosphorylate these serines in the domain II region and thereby regulate HCV replication. In summary, our results combined showed that Plk1 regulates HCV replication through direct hyperphosphorylation of NS5A protein.
DISCUSSION
Phosphorylation of NS5A is conserved in Flavivirus and generally thought to play an important role in the Flavivirus life cycle (43). Phosphorylation on the C-terminal region of NS5A by CKII was found to regulate HCV virus production (50), whereas hyperphosphorylation of the central region of NS5A has been shown to correlate with virus replication/infectivity (2, 4, 6, 9, 32, 38). CKI is known as a direct kinase of NS5A, capable of hyperphosphorylation of the latter (41, 42). Typically, the CKI recognition sites have to be primed by a previous phosphorylation at the N terminus of the CKI recognition site (10, 33); thus, other kinase(s) are likely involved in the phosphorylation of NS5A. Here, we identified a new NS5A kinase, Plk1, by using RNAi screening. Knockdown of Plk1 or treatment with a Plk1-specific kinase inhibitor decreased both NS5A hyperphosphorylation and HCV replication in vivo, and Plk1 can induce both the basal phosphorylation and hyperphosphorylation forms of NS5A in vitro. Our results indicate that Plk1 regulates HCV replication through hyperphosphorylation of NS5A.
The consensus sequence for the Plk1 binding motif is one or two negatively charged/uncharged amino acid residues preceding the Ser or Thr, followed by a hydrophobic/uncharged residue (21, 31, 52-54). Interestingly, Ser-2197, Ser-2201, and Ser-2204, which are putative sites of NS5A hyperphosphorylation (49), conform to this motif. Our results of the in vitro kinase assay are also consistent with this prediction. Hyperphosphorylation of NS5A specifically occurred on fragment II, which contains these three serine residues (Fig. 5D and E). Furthermore, efficient phosphorylation of an NS5A peptide by CKI requires prephosphorylation of Ser-2201 (42), raising a possibility that Plk1 and CKI may act sequentially to regulate NS5A phosphorylation.
Tight control of the p58/p56 ratio has been proposed to regulate HCV replication. Previous studies showed that adaptive mutations which decrease NS5A hyperphosphorylation augmented HCV replicon replication, whereas introducing a second mutation or treating the adaptive replicon with the kinase inhibitor completely blocked the replication (4, 32, 38). It has also been shown that there is an inverse relationship between NS5A phosphorylation and human VAP-A (hVAP-A) interaction (9), thus providing a possible explanation for the requirement for a delicate balance between basal and hyperphosphorylation. It is worth noting that the replicon used in our experiments was previously modified by deleting the Ser-2202 residue to improve its replication efficiency (14). Therefore, impairment of HCV adaptive replicon replication by knockdown of Plk1 is consistent with these findings. The p58/p56 ratio may have hit the balance in the adaptive replicon; inhibition of Plk1 by shRNA or inhibitor disrupts this balance and thus affects HCV replication. Reduction of p58 by knockdown of Plk1 may retain NS5A in the replication complex via association with VAP-A, thereby blocking other important functions of NS5A, such as translation and regulation of cellular genes. We have also tested other replicons with various mutations in the hyperphosphorylation sites in NS5A. Although their replication efficiency was much lower than that of the adaptive replicon with a deletion of Ser-2202, Plk1 knockdown universally decreased the replicon replication (data not shown).
Plk1 is an important regulator of cell cycle progression. However, in our experiments, knockdown of Plk1 did not retard the cell growth rate. One of the possible reasons is that the requirement of Plk1 is cell type specific. It was reported that knockdown of Plk1 in human telomerase reverse transcriptase (hTERT-RPE1) cells slowed down the cell cycle, whereas the same procedure in MCF10A cells resulted in normal proliferation rates with no obvious cell cycle arrest (30). Therefore, Plk1 may be dispensable in cell cycle function of some cell lines including Huh-7 cells. Another possibility is that the knockdown efficiency of shPlk1 is only 70% in our experiments. The remaining 30% of Plk1 may be enough for the cell cycle but not for NS5A phosphorylation. Our experiment using a Plk1 inhibitor also supports the interpretation that, compared to cell cycle regulation, NS5A phosphorylation and HCV replication are more sensitive to Plk1 depletion.
Finally, the selective Plk1 inhibitor BI2536 is a potential drug for cancer therapy, and some clinical trials are currently ongoing. Our results showed that BI2536 can diminish HCV replication in the HCVcc system in cell culture, revealing the potential application of BI2536 or other new Plk1 inhibitors to HCV treatment. Further investigation of BI2536 treatment in a xenograft mouse model will be helpful to examine the potential.
Acknowledgments
This study was supported by grants NSC 95-3112-B-001-019, NSC 96-3112-B-001-003, and NSC 97-3112-B-001-001 from the National Science Council, Taiwan.
We thank Yi-Ling Lin, Lih-Hwa Hwang, and Yu-Sun Chang for helpful discussions and suggestions. Plk1 antibody was a gift from Sheng-Chung Lee, Institute of Molecular Medicine, National Taiwan University. The shRNA constructs were obtained from the National RNAi Core Facility, Academia Sinica, Taiwan.
Footnotes
Published ahead of print on 9 June 2010.
REFERENCES
- 1.Abonyi, M. E., and P. L. Lakatos. 2005. Ribavirin in the treatment of hepatitis C. Anticancer Res. 25:1315-1320. [PubMed] [Google Scholar]
- 2.Appel, N., T. Pietschmann, and R. Bartenschlager. 2005. Mutational analysis of hepatitis C virus nonstructural protein 5A: potential role of differential phosphorylation in RNA replication and identification of a genetically flexible domain. J. Virol. 79:3187-3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appel, N., M. Zayas, S. Miller, J. Krijnse-Locker, T. Schaller, P. Friebe, S. Kallis, U. Engel, and R. Bartenschlager. 2008. Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly. PLoS Pathog. 4:e1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. [DOI] [PubMed] [Google Scholar]
- 5.Brazzoli, M., A. Bianchi, S. Filippini, A. Weiner, Q. Zhu, M. Pizza, and S. Crotta. 2008. CD81 is a central regulator of cellular events required for hepatitis C virus infection of human hepatocytes. J. Virol. 82:8316-8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukh, J., T. Pietschmann, V. Lohmann, N. Krieger, K. Faulk, R. E. Engle, S. Govindarajan, M. Shapiro, M. St. Claire, and R. Bartenschlager. 2002. Mutations that permit efficient replication of hepatitis C virus RNA in Huh-7 cells prevent productive replication in chimpanzees. Proc. Natl. Acad. Sci. U. S. A. 99:14416-14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coito, C., D. L. Diamond, P. Neddermann, M. J. Korth, and M. G. Katze. 2004. High-throughput screening of the yeast kinome: identification of human serine/threonine protein kinases that phosphorylate the hepatitis C virus NS5A protein. J. Virol. 78:3502-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egger, D., B. Wolk, R. Gosert, L. Bianchi, H. E. Blum, D. Moradpour, and K. Bienz. 2002. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J. Virol. 76:5974-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans, M. J., C. M. Rice, and S. P. Goff. 2004. Phosphorylation of hepatitis C virus nonstructural protein 5A modulates its protein interactions and viral RNA replication. Proc. Natl. Acad. Sci. U. S. A. 101:13038-13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flotow, H., P. R. Graves, A. Q. Wang, C. J. Fiol, R. W. Roeske, and P. J. Roach. 1990. Phosphate groups as substrate determinants for casein kinase I action. J. Biol. Chem. 265:14264-14269. [PubMed] [Google Scholar]
- 11.Gale, M. J., Jr., M. J. Korth, N. M. Tang, S. L. Tan, D. A. Hopkins, T. E. Dever, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 230:217-227. [DOI] [PubMed] [Google Scholar]
- 12.Gao, L., H. Aizaki, J. W. He, and M. M. Lai. 2004. Interactions between viral nonstructural proteins and host protein hVAP-33 mediate the formation of hepatitis C virus RNA replication complex on lipid raft. J. Virol. 78:3480-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gosert, R., D. Egger, V. Lohmann, R. Bartenschlager, H. E. Blum, K. Bienz, and D. Moradpour. 2003. Identification of the hepatitis C virus RNA replication complex in Huh-7 cells harboring subgenomic replicons. J. Virol. 77:5487-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo, J. T., V. V. Bichko, and C. Seeger. 2001. Effect of alpha interferon on the hepatitis C virus replicon. J. Virol. 75:8516-8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazari, S., L. Taylor, S. Haque, R. F. Garry, S. Florman, R. Luftig, F. Regenstein, and S. Dash. 2007. Reduced expression of Jak-1 and Tyk-2 proteins leads to interferon resistance in hepatitis C virus replicon. Virol. J. 4:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heathcote, J., and J. Main. 2005. Treatment of hepatitis C. J. Viral Hepat. 12:223-235. [DOI] [PubMed] [Google Scholar]
- 17.Higuchi, R., B. Krummel, and R. K. Saiki. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 16:7351-7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, L., E. V. Sineva, M. R. Hargittai, S. D. Sharma, M. Suthar, K. D. Raney, and C. E. Cameron. 2004. Purification and characterization of hepatitis C virus non-structural protein 5A expressed in Escherichia coli. Protein Expr. Purif. 37:144-153. [DOI] [PubMed] [Google Scholar]
- 19.Ide, Y., A. Tanimoto, Y. Sasaguri, and R. Padmanabhan. 1997. Hepatitis C virus NS5A protein is phosphorylated in vitro by a stably bound protein kinase from HeLa cells and by cAMP-dependent protein kinase A-alpha catalytic subunit. Gene 201:151-158. [DOI] [PubMed] [Google Scholar]
- 20.Ishida, H., K. Li, M. Yi, and S. M. Lemon. 2007. p21-activated kinase 1 is activated through the mammalian target of rapamycin/p70 S6 kinase pathway and regulates the replication of hepatitis C virus in human hepatoma cells. J. Biol. Chem. 282:11836-11848. [DOI] [PubMed] [Google Scholar]
- 21.Jackman, M., C. Lindon, E. A. Nigg, and J. Pines. 2003. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat. Cell Biol. 5:143-148. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko, T., Y. Tanji, S. Satoh, M. Hijikata, S. Asabe, K. Kimura, and K. Shimotohno. 1994. Production of two phosphoproteins from the NS5A region of the hepatitis C viral genome. Biochem. Biophys. Res. Commun. 205:320-326. [DOI] [PubMed] [Google Scholar]
- 23.Kim, A. I., and S. Saab. 2005. Treatment of hepatitis C. Am. J. Med. 118:808-815. [DOI] [PubMed] [Google Scholar]
- 24.Kim, J., D. Lee, and J. Choe. 1999. Hepatitis C virus NS5A protein is phosphorylated by casein kinase II. Biochem. Biophys. Res. Commun. 257:777-781. [DOI] [PubMed] [Google Scholar]
- 25.Koch, J. O., and R. Bartenschlager. 1999. Modulation of hepatitis C virus NS5A hyperphosphorylation by nonstructural proteins NS3, NS4A, and NS4B. J. Virol. 73:7138-7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo, G., Q. L. Choo, H. J. Alter, G. L. Gitnick, A. G. Redeker, R. H. Purcell, T. Miyamura, J. L. Dienstag, M. J. Alter, C. E. Stevens, et al. 1989. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 244:362-364. [DOI] [PubMed] [Google Scholar]
- 27.Lai, C. K., K. S. Jeng, K. Machida, Y. S. Cheng, and M. M. Lai. 2008. Hepatitis C virus NS3/4A protein interacts with ATM, impairs DNA repair and enhances sensitivity to ionizing radiation. Virology 370:295-309. [DOI] [PubMed] [Google Scholar]
- 28.Lai, C. K., K. S. Jeng, K. Machida, and M. M. Lai. 2008. Association of hepatitis C virus replication complexes with microtubules and actin filaments is dependent on the interaction of NS3 and NS5A. J. Virol. 82:8838-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauer, G. M., and B. D. Walker. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41-52. [DOI] [PubMed] [Google Scholar]
- 30.Lei, M., and R. L. Erikson. 2008. Plk1 depletion in nontransformed diploid cells activates the DNA-damage checkpoint. Oncogene 27:3935-3943. [DOI] [PubMed] [Google Scholar]
- 31.Lin, H. R., N. S. Ting, J. Qin, and W. H. Lee. 2003. M phase-specific phosphorylation of BRCA2 by Polo-like kinase 1 correlates with the dissociation of the BRCA2-P/CAF complex. J. Biol. Chem. 278:35979-35987. [DOI] [PubMed] [Google Scholar]
- 32.Lohmann, V., S. Hoffmann, U. Herian, F. Penin, and R. Bartenschlager. 2003. Viral and cellular determinants of hepatitis C virus RNA replication in cell culture. J. Virol. 77:3007-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marin, O., V. H. Bustos, L. Cesaro, F. Meggio, M. A. Pagano, M. Antonelli, C. C. Allende, L. A. Pinna, and J. E. Allende. 2003. A noncanonical sequence phosphorylated by casein kinase 1 in beta-catenin may play a role in casein kinase 1 targeting of important signaling proteins. Proc. Natl. Acad. Sci. U. S. A. 100:10193-10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masaki, T., R. Suzuki, K. Murakami, H. Aizaki, K. Ishii, A. Murayama, T. Date, Y. Matsuura, T. Miyamura, T. Wakita, and T. Suzuki. 2008. Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles. J. Virol. 82:7964-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moradpour, D., M. J. Evans, R. Gosert, Z. Yuan, H. E. Blum, S. P. Goff, B. D. Lindenbach, and C. M. Rice. 2004. Insertion of green fluorescent protein into nonstructural protein 5A allows direct visualization of functional hepatitis C virus replication complexes. J. Virol. 78:7400-7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moradpour, D., R. Gosert, D. Egger, F. Penin, H. E. Blum, and K. Bienz. 2003. Membrane association of hepatitis C virus nonstructural proteins and identification of the membrane alteration that harbors the viral replication complex. Antiviral Res. 60:103-109. [DOI] [PubMed] [Google Scholar]
- 37.Neddermann, P., A. Clementi, and R. De Francesco. 1999. Hyperphosphorylation of the hepatitis C virus NS5A protein requires an active NS3 protease, NS4A, NS4B, and NS5A encoded on the same polyprotein. J. Virol. 73:9984-9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neddermann, P., M. Quintavalle, C. Di Pietro, A. Clementi, M. Cerretani, S. Altamura, L. Bartholomew, and R. De Francesco. 2004. Reduction of hepatitis C virus NS5A hyperphosphorylation by selective inhibition of cellular kinases activates viral RNA replication in cell culture. J. Virol. 78:13306-13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pietschmann, T., A. Kaul, G. Koutsoudakis, A. Shavinskaya, S. Kallis, E. Steinmann, K. Abid, F. Negro, M. Dreux, F. L. Cosset, and R. Bartenschlager. 2006. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc. Natl. Acad. Sci. U. S. A. 103:7408-7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plumlee, C. R., C. A. Lazaro, N. Fausto, and S. J. Polyak. 2005. Effect of ethanol on innate antiviral pathways and HCV replication in human liver cells. Virol. J. 2:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quintavalle, M., S. Sambucini, C. Di Pietro, R. De Francesco, and P. Neddermann. 2006. The alpha isoform of protein kinase CKI is responsible for hepatitis C virus NS5A hyperphosphorylation. J. Virol. 80:11305-11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quintavalle, M., S. Sambucini, V. Summa, L. Orsatti, F. Talamo, R. De Francesco, and P. Neddermann. 2007. Hepatitis C virus NS5A is a direct substrate of casein kinase I-alpha, a cellular kinase identified by inhibitor affinity chromatography using specific NS5A hyperphosphorylation inhibitors. J. Biol. Chem. 282:5536-5544. [DOI] [PubMed] [Google Scholar]
- 43.Reed, K. E., A. E. Gorbalenya, and C. M. Rice. 1998. The NS5A/NS5 proteins of viruses from three genera of the family Flaviviridae are phosphorylated by associated serine/threonine kinases. J. Virol. 72:6199-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed, K. E., J. Xu, and C. M. Rice. 1997. Phosphorylation of the hepatitis C virus NS5A protein in vitro and in vivo: properties of the NS5A-associated kinase. J. Virol. 71:7187-7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi, S. T., K. J. Lee, H. Aizaki, S. B. Hwang, and M. M. Lai. 2003. Hepatitis C virus RNA replication occurs on a detergent-resistant membrane that cofractionates with caveolin-2. J. Virol. 77:4160-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steegmaier, M., M. Hoffmann, A. Baum, P. Lenart, M. Petronczki, M. Krssak, U. Gurtler, P. Garin-Chesa, S. Lieb, J. Quant, M. Grauert, G. R. Adolf, N. Kraut, J. M. Peters, and W. J. Rettig. 2007. BI 2536, a potent and selective inhibitor of Polo-like kinase 1, inhibits tumor growth in vivo. Curr. Biol. 17:316-322. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki, T., K. Ishii, H. Aizaki, and T. Wakita. 2007. Hepatitis C viral life cycle. Adv. Drug Deliv. Rev. 59:1200-1212. [DOI] [PubMed] [Google Scholar]
- 48.Taniguchi, E., F. Toyoshima-Morimoto, and E. Nishida. 2002. Nuclear translocation of Plk1 mediated by its bipartite nuclear localization signal. J. Biol. Chem. 277:48884-48888. [DOI] [PubMed] [Google Scholar]
- 49.Tanji, Y., T. Kaneko, S. Satoh, and K. Shimotohno. 1995. Phosphorylation of hepatitis C virus-encoded nonstructural protein NS5A. J. Virol. 69:3980-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tellinghuisen, T. L., K. L. Foss, and J. Treadaway. 2008. Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog. 4:e1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tellinghuisen, T. L., J. Marcotrigiano, and C. M. Rice. 2005. Structure of the zinc-binding domain of an essential component of the hepatitis C virus replicase. Nature 435:374-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toyoshima-Morimoto, F., E. Taniguchi, and E. Nishida. 2002. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 3:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toyoshima-Morimoto, F., E. Taniguchi, N. Shinya, A. Iwamatsu, and E. Nishida. 2001. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature 410:215-220. [DOI] [PubMed] [Google Scholar]
- 54.Yarm, F. R. 2002. Plk phosphorylation regulates the microtubule-stabilizing protein TCTP. Mol. Cell. Biol. 22:6209-6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaman, A., M. B. Fennerty, and E. B. Keeffe. 2003. Systematic review: peginterferon vs. standard interferon in the treatment of chronic hepatitis C. Aliment. Pharmacol. Ther. 18:661-670. [DOI] [PubMed] [Google Scholar]