Abstract
Cell-based measurement of prion infectivity is currently restricted to experimental strains of mouse-adapted scrapie. Having isolated cell cultures with susceptibility to prions from diseased elk, we describe a modification of the scrapie cell assay allowing evaluation of prions causing chronic wasting disease, a naturally occurring transmissible spongiform encephalopathy. We compare this cervid prion cell assay to bioassays in transgenic mice, the only other existing method for quantification, and show this assay to be a relatively economical and expedient alternative that will likely facilitate studies of this important prion disease.
Prions consist largely or entirely of PrPSc, a β-sheet-rich conformer of the prion protein (PrP). During disease, PrPSc coerces the normal PrPC protein to adopt the PrPSc conformation. While protease-sensitive forms of PrPSc exist (20), PrPSc is usually partially resistant to limited proteinase K (PK) digestion (4). Bioassays in susceptible animals have, until recently, been the sole means of assessing prion infectivity. The scrapie cell assay (SCA) (12), which relies on detection of protease resistant PrPSc, while a substantial advance, has been limited to the detection of mouse-adapted scrapie prions, and the development of analogous systems for naturally occurring prions is a high priority. Chronic wasting disease (CWD), a burgeoning epidemic of deer, elk, and moose, is of particular importance.
We first generated a cell line susceptible to infection by cervid prions. While rabbit kidney epithelial RK13 cells expressing sheep, mouse, and bank vole PrP supported prion replication from the corresponding species (8, 14, 22), expression of human PrP did not confer susceptibility to human prions (14). We produced RK13 cells expressing elk PrP (RKE cells) and infected them with CWD brain homogenates (5). To analyze cervid PrPSc (CerPrPSc) by Western blotting, detergent extracts containing equal amounts protein were treated with 40 mg/ml PK for 1 h at 50°C and centrifuged for 1 h at 100,000 × g. Alternately, CerPrPSc in cells was analyzed by cell blotting (5). Infection of RKE cells with CWD resulted in detectable CerPrPSc 3 passages after infection; however, the progressive reduction of CerPrPSc upon repeated passage (Fig. 1 A) showed that infection was not sustained.
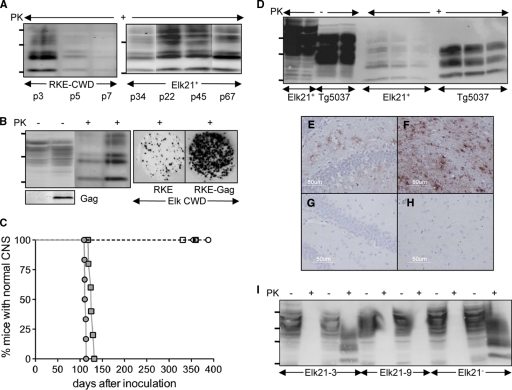
FIG. 1.
Characterization of cell cultures for studying CWD prions. (A) Western blots showing accumulation of CerPrPSc in RKE cells challenged with CWD brain homogenates from elk isolate 012-09442, passaged in Tg5037 mice (RKE-CWD) (left) and Elk21+ cells (right). Passage numbers (p) of cell cultures are indicated. (B) Expression of CerPrPC and HIV-Gag and accumulation of CerPrPSc in RKE and RKE-Gag cells infected with CWD brain homogenates. Cultured cells were also analyzed by cell blotting (right). (C) Bioassay of Elk21+ cells propagating elk CWD 012-09442 prions (filled circles), elk CWD 012-09442 prions in Tg5037 mice (filled squares), uninfected RKE-Gag (open circles), Elk21− 13 passages after DS-500 treatment (open triangles), Elk21− 30 passages after DS-500 treatment (filled triangles), the Elk21-3 clone (open diamonds), and the Elk21-9 clone (open squares). (D) Western blots of CerPrPC (100 and 50 μg total protein loaded in each case) and CerPrPSc (200, 100, and 50 μg total protein loaded in each case) produced in Elk21+ cells and Tg5037 mice inoculated with Elk21+ cell extracts. (E to H) CerPrPSc deposition in the hippocampus (E and G) and thalamus (F and H) of Tg5037 mice inoculated with either Elk21+ (E and F) or uninfected RKE-Gag (G and H) cell extracts. (I) Western blots demonstrating susceptibility of Elk21-3, Elk21-9, and Elk21− to reinfection with elk CWD 012-09442 prions. For each cell line, the first two lanes show extracts from mock (phosphate-buffered saline [PBS])-infected cells, while the second two lanes show extracts from cells exposed to CWD brain homogenates. In all Western blots, samples were either PK treated (+) or untreated (−), and the positions of protein molecular mass markers at 37, 25, and 20 kDa (from top to bottom) are shown.
Since previous reports demonstrated that retroviral Gag mediated enhanced release of mouse-adapted scrapie from cell cultures (15), RKE cells were further transfected with pcDNA3-gag expressing the HIV-1 GAG precursor protein (9), generating RKE-Gag cells. CerPrPSc levels in infected RKE-Gag were enhanced ∼2-fold (Fig. 1B). Clones of infected RKE-Gag and RKE cells were derived by limited dilution. Of 40 clones isolated in each case, single RKE and RKE-Gag clones produced CerPrPSc. While CerPrPSc was not detected beyond passage 4 of cloned RKE cells (data not shown), Fig. 1A shows that CerPrPSc production in the infected RKE-Gag clone, referred to as Elk21+, was sustained for 67 passages in culture, which equates to ∼223 cell doublings.
Other approaches for producing cells chronically infected with CWD brain homogenates, including infection of N2a cells stably expressing elk PrP, were unsuccessful (Fig. 2), either because N2a cells are resistant to CWD brain homogenates or because CerPrPC-to-CerPrPSc conversion is inhibited by expression of endogenous mouse PrPC (21).
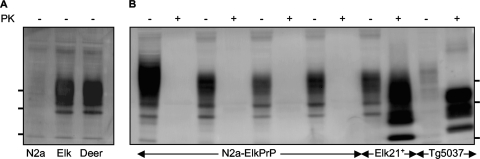
FIG. 2.
Lack of susceptibility of N2a cells expressing elk PrP to CWD. (A) Western blot showing elk and deer PrPC expression in N2a cells. (B) Elk PrPc-expressing N2a cells (N2a-ElkPrP) infected with CWD isolates remain uninfected after four passages. Pairs of lanes show extracts of N2a-ElkPrP cells challenged with brain homogenates from diseased Tg(CerPrP-E226)5037+/− mice infected with elk isolates 012-022012, 012-09442, 99W12389, and 7178-47 (from left to right). Results are also shown for Elk21+ cells and CWD-infected Tg(CerPrP-E226)5037+/− mice. Blots were probed with MAb 9E9, which recognizes only cervid PrP. Samples were either PK treated (+) or untreated (−), and the positions of protein molecular mass markers at 37, 25, and 20 kDa (from top to bottom) are shown.
After 25 passages, Elk21+ cells were bioassayed in Tg(CerPrP-E226)5037+/− mice expressing elk PrP (3), referred to as Tg5037 mice. Mice developed prion disease with a mean incubation time of 112 ± 1 days. Tg5037 mice inoculated with the same prions as those used to produce Elk21+ cells developed disease with a mean incubation time of 126 ± 2 days, while Tg5037 mice challenged with RKE-Gag cells remained asymptomatic (Fig. 1C). Mice were inoculated with infected brain and cell culture preparations containing similar amounts of PrPSc as quantified by Western blot analysis. For bioassays of uninfected cells, mice were inoculated with preparations containing amounts of total protein equivalent to those in infected cell cultures.
Levels of CerPrPC and CerPrPSc and their electrophoretic migration and glycosylation patterns differed between Elk21+ cells and Tg5037 mice (Fig. 1D and 2). CerPrPSc deposition in the brains of Tg5037 mice infected with Elk21+ cells was diffuse and granular (Fig. 1E and F), in accordance with previous reports (3); no disease occurred in Tg5037 mice inoculated with RKE-Gag cells (Fig. 1G and H).
Elk21+ cells were treated with the antiprion compound dextran sulfate 500 (DS-500) (7, 11, 13). CerPrPSc was undetectable after 5 weeks and did not reemerge when cells were returned to medium lacking drug (Fig. 1I). Tg5037 mice remained asymptomatic for >355 days following inoculation (Fig. 1C). Cells cured of PrPSc by DS-500, referred to as Elk21− cells, retained the ability to sustain production of CerPrPSc when rechallenged with elk CWD brain homogenates (Fig. 1I). The process of cloning of Elk21+ cells after 58 passages in culture also resulted in elimination of PrPSc in a subset of subclones. While Western and cell blotting detected CerPrPSc in 3 subclones, 11 subclones did not produce CerPrPSc. Of the 11 “negative” subclones rechallenged with CWD brain homogenates, 10 produced CerPrPSc (for example, clone Elk21-3 [Fig. 1I]), while clone Elk21-9 was resistant to CWD (Fig. 1I). The CWD-free statuses of clones Elk21-3 and Elk21-9 were confirmed in Tg5037 mice (Fig. 1C).
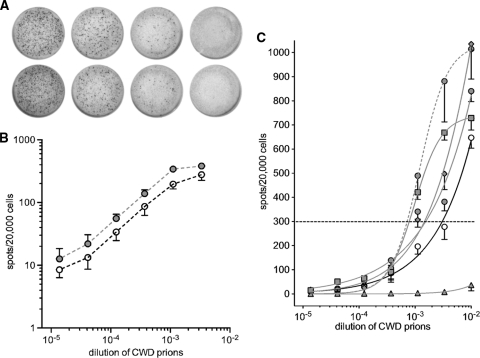
We adapted the SCA (12, 16, 17) to visualize infected Elk21− or Elk21-3 cells. We refer to this as the cervid prion cell assay (CPCA) (see the supplemental material). Briefly, susceptible Elk21− cells in 96-well plates were exposed to serial dilutions of CWD brain homogenates ranging from 10−2 to 10−5 in a volume of 100 μl. Cell cultures were split once at 1:4 and twice at 1:7, which effectively diluted out CerPrPSc in the inoculum. Inclusion of RK13 cells stably transfected with empty vector (RKV cells) showed that positive spots detected after three splits were the result of newly generated CerPrPSc. After the final passage, 20,000 cells were filtered onto Multiscreen IP 96-well, 0.45-μm filter plates (enzyme-linked immunospot [ELISPOT] assay plates; Millipore, Billerica, MA) or AcroWell 96-well, 0.45-μm BioTrace filter plates (Pall, East Hills, NY). Cells were subjected to PK digestion and denaturation with guanidinium thiocyanate. CerPrPSc-producing cells were detected by an enzyme-linked immunosorbent assay (ELISA) using anti-PrP monoclonal antibody (MAb) 6H4, followed by alkaline phosphatase (AP)-conjugated secondary anti-mouse IgG, and developed with NBT/BCIP. Images were scanned with CTL ELISPOT equipment, and spot numbers were determined using ImmunoSpot3 software (Cellular Technology, Ltd., Shaker Heights, OH). Figure 3 A depicts magnifications of ELISPOT filters of infected Elk21− cells.
FIG. 3.
Quantification of elk CWD prion infectivity by the transgenic mouse bioassay and the cervid prion cell assay. (A) Representative wells of an ELISPOT plate showing spots given by duplicate Elk21+ cells exposed to 3-fold serial dilutions of pooled elk CWD brain homogenates, between 10−3 and 10−4.4. (B) Double-logarithmic plot of spot number versus brain homogenate dilution showing the linear response of the CPCA. Elk21− cells infected with dilutions of pooled elk CWD brain homogenates (open circles) and pooled elk CWD brain homogenates passaged in Tg5037 mice (filled circles). In each case, the mean is derived from 6 independent experiments performed in triplicate, with error bars indicating the standard errors of the means (SEM). (C) Responsiveness of Elk21− and Elk21-3 cells to various CWD brain homogenates. The cells were infected with serial 1:3 dilutions of homogenates of CWD-infected brains and subjected to the CPCA. In each case, the dilution required to yield 300 positive cells per 20,000 cells after the third split was calculated. Solid black line, CPCA of CWD brain homogenates from diseased elk brain, using Elk21− cells; solid gray lines, CPCA of CWD brain homogenates from diseased Tg5037 mice, using Elk21− cells; dashed gray line, CPCA using Elk21-3 cells. Filled triangles, D10 CWD isolate; open circles, pooled elk CWD brain homogenate; filled circles, brain homogenate of Tg5037 mice infected with pooled elk CWD; filled diamonds, 012-09442 CWD isolate; filled squares, 01-0306 CWD isolate.
To determine the dose-response relationship of Elk21− cells to CWD brain homogenates, we used a pooled elk CWD inoculum titrated in two different transgenic mouse lines (3, 6). In the case of Tg5037 mice, we estimated the titer to be 107.0 intracerebral (i.c.) 50% infective doses (ID50)/g of brain, and the titer in Tg(CerPrP)1536+/− mice expressing deer PrP was estimated at 107.2 i.c. ID50/g (Table 1) (19).
TABLE 1.
CWD infectivity assays
| Dilution | Tg(CerPrP-M132)1536+/− |
Tg(CerPrP-E226)5037+/− |
No. of CPCA units (mean ± SD)a |
|||
|---|---|---|---|---|---|---|
| Incubation time (days) (mean ± SD) | No. of sick mice/total no. of mice | Incubation time (days) (mean ± SD) | No. of sick mice/total no. of mice | Elk pool 1 | Elk pool 2 | |
| 10−2 | 293 ± 31 | 6/6 | 126 ± 9 | 8/8 | 647 ± 184 | 840 ± 189 |
| 10−3 | 233 ± 23 | 6/6 | 128 ± 4 | 7/7 | 196 ± 134 | 341 ± 135 |
| 10−4 | 288 ± 31 | 7/7 | 147 ± 12 | 6/6 | 34 ± 39 | 56 ± 42 |
| 10−5 | 402 ± 3 | 5/5 | 263 ± 79 | 3/6 | 9 ± 9 | 13 ± 25 |
| 10−6 | 436 ± 3 | 2/6 | 248 ± 51 | 3/6 | ND | ND |
| 10−7 | >533 | 0/6 | >537 | 0/6 | ND | ND |
| 10−8 | >543 | 0/6 | >539 | 0/6 | ND | ND |
| 10−9 | >546 | 0/7 | >538 | 0/7 | ND | ND |
| 10−10 | >554 | 0/6 | >553 | 0/6 | ND | ND |
| 10−11 | >566 | 0/8 | >562 | 0/5 | ND | ND |
Elk pool 1, elk CWD pool inoculum; elk pool 2, elk CWD pool inoculum passaged in Tg(CerPrP-E226)5037+/− mice; ND, not determined.
Proportions of infected Elk21− cells were assessed following exposure to serial 10−2-to-10−5 dilutions of titrated CWD (Fig. 3 and Table 1). Double logarithmic plots from 6 independent experiments, each performed in triplicate, showed a linear response between dilutions of ∼10−3 and ∼10−4.4 (Fig. 3B). An increased dose-response relationship was recorded for brain homogenates of diseased Tg5037 mice, reflecting increased CWD titers. Figure 3C shows plots of CerPrPSc-positive cells as a function of log dilution of CWD prion inocula. The number of CerPrPSc-positive cells reflects the prion titer (12, 16). We determined that 100 μl of a 10−2.5 dilution of the elk CWD pool yielded 300 spots, the point used to determine the response index in the SCA (16), which corresponds to 106.0 CPCA units/g. The CPCA was also performed on the elk CWD pool passaged in Tg5037 mice, as well as 2 other elk inocula passaged in Tg5037 mice, producing CPCA titers of 106.3, 106.3, and 106.6 units/g of brain, respectively, again reflecting higher CWD prion titers (Fig. 3C). We also determined the response of the Elk21-3 clone to the elk CWD pool passaged in Tg5037 mice; in this case, the CPCA titer was 106.6.
In summary, we generated CWD-susceptible cells by ex vivo transgenesis in RK13 cells. While CerPrPSc purification as described for other CWD cell culture systems (18) was not a prerequisite for sustained cellular infection, we show that expression of retroviral Gag facilitated prion susceptibility. As described previously (5, 12), selection of susceptible clones was critical. While the mechanism of Gag action and identification of cellular infection factors will be of considerable interest, Gag expression and/or cloning may be required to facilitate isolation of cell lines with susceptibilities to human (14) and other prions. Identification of susceptible clones by “curing” of infected counterparts also greatly facilitated our approach. CWD-susceptible transgenic mice not only provided a convenient and controlled source of CWD brain homogenates for infections, and a benchmark for quantifying CWD prion infectivity (Table 1), but also allowed us to ascertain the CWD infection statuses of cell cultures (Fig. 1C).
The CPCA takes ∼24 days; the bioassay using Tg5037 mice requires a dilution series out to 10−8 and a minimum of 400 days (Table 1). On the basis of these considerations, the costs of assaying a CWD sample by endpoint titration are ∼$15,500, compared with ∼$135 per sample (triplicate determination including controls) for the CPCA. Thus, the bioassay is, conservatively, >100-fold more expensive and takes >16-fold longer than the CPCA. While endpoint titration with Tg mice produced elk CWD pool titers of ∼107, the titers for the CPCA ranged from ∼106 to 106.5. The 106.6-CPC-unit value in Elk21-3 cells (Fig. 3C) suggests that the sensitivity of this clone is higher and that isolation of clones with improved responses will be possible.
Finally, while D10 deer prions induced disease in Tg5037 mice (3), they failed to elicit a CPCA response (Fig. 3C). We have performed infectivity assays of D10 in Tg mice on several previous occasions (1-3, 6, 10). The inoculum also contains high levels of PK-resistant CerPrPSc (6). We previously reported that the mean incubation time for a 10−2 dilution of D10 brain in Tg1536 mice was 225 ± 1 days and that the mean incubation time for an equivalent dilution of the deer D92 isolate was 268 ± 15 days. We determined the endpoint titer of D92 in Tg1536 mice to be 6 log i.c. ID50/g. The source of D10 prions for infection of Elk21− cells was Tg5037 mice, which developed disease with a mean incubation time of 201 ± 1 days (3). These characteristics suggest that the lack of a CPCA response to D10 is not due to low prion titers but rather due to differences in the strain properties of these prions. Our findings also suggest the possibility of distinguishing cervid prion strains by adapting the CPCA to a cell panel assay format (16).
Supplementary Material
Acknowledgments
These studies were supported by grants 2RO1 NS040334-04 (from the National Institute of Neurological Disorders and Stroke) and 1P01AI077774-01 (from the National Institute of Allergy and Infectious Diseases). V. Khaychuck was supported by the T32 DA022738 Training Program in Therapeutic Strategies for Neurodegeneration.
We thank Tanya Seward for technical support. We thank our colleagues at Prionics AG, Schlieren, Switzerland, for MAb 6H4.
Footnotes
Published ahead of print on 2 June 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Angers, R. C., S. R. Browning, T. S. Seward, C. J. Sigurdson, M. W. Miller, E. A. Hoover, and G. C. Telling. 2006. Prions in skeletal muscles of deer with chronic wasting disease. Science 311:1117. [DOI] [PubMed] [Google Scholar]
- 2.Angers, R. C., H. E. Kang, D. Napier, S. Browning, T. Seward, C. Mathiason, A. Balachandran, D. McKenzie, J. Castilla, C. Soto, J. Jewell, C. Graham, E. A. Hoover, and G. C. Telling. 2010. Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science 328:1154-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angers, R. C., T. S. Seward, D. Napier, M. Green, E. A. Hoover, T. Spraker, K. O'Rourke, A. Balachandran, and G. C. Telling. 2009. Prions in antler velvet of CWD-infected Elk. Emerg. Infect. Dis. 15:696-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolton, D. C., M. P. McKinley, and S. B. Prusiner. 1982. Identification of a protein that purifies with the scrapie prion. Science 218:1309-1311. [DOI] [PubMed] [Google Scholar]
- 5.Bosque, P. J., and S. B. Prusiner. 2000. Cultured cell sublines highly susceptible to prion infection. J. Virol. 74:4377-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning, S. R., G. L. Mason, T. Seward, M. Green, G. A. Eliason, C. Mathiason, M. W. Miller, E. S. Williams, E. Hoover, and G. C. Telling. 2004. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J. Virol. 78:13345-13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caughey, B., and G. J. Raymond. 1993. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J. Virol. 67:643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courageot, M. P., N. Daude, R. Nonno, S. Paquet, M. A. Di Bari, A. Le Dur, J. Chapuis, A. F. Hill, U. Agrimi, H. Laude, and D. Vilette. 2008. A cell line infectible by prion strains from different species. J. Gen. Virol. 89:341-347. [DOI] [PubMed] [Google Scholar]
- 9.Gottwein, E., and H. G. Krausslich. 2005. Analysis of human immunodeficiency virus type 1 Gag ubiquitination. J. Virol. 79:9134-9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green, K. M., S. R. Browning, T. S. Seward, J. E. Jewell, D. L. Ross, M. A. Green, E. S. Williams, E. A. Hoover, and G. C. Telling. 2008. The elk PRNP codon 132 polymorphism controls cervid and scrapie prion propagation. J. Gen. Virol. 89:598-608. [DOI] [PubMed] [Google Scholar]
- 11.Kimberlin, R. H., and C. A. Walker. 1986. Suppression of scrapie infection in mice by heteropolyanion 23, dextran sulfate, and some other polyanions. Antimicrob. Agents Chemother. 30:409-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klohn, P. C., L. Stoltze, E. Flechsig, M. Enari, and C. Weissmann. 2003. A quantitative, highly sensitive cell-based infectivity assay for mouse scrapie prions. Proc. Natl. Acad. Sci. U. S. A. 100:11666-11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladogana, A., P. Casaccia, L. Ingrosso, M. Cibati, M. Salvatore, Y.-G. Xi, C. Masullo, and M. Pocchiari. 1992. Sulphate polyanions prolong the incubation period of scrapie-infected hamsters. J. Gen. Virol. 73:661-665. [DOI] [PubMed] [Google Scholar]
- 14.Lawson, V. A., L. J. Vella, J. D. Stewart, R. A. Sharples, H. Klemm, D. M. Machalek, C. L. Masters, R. Cappai, S. J. Collins, and A. F. Hill. 2008. Mouse-adapted sporadic human Creutzfeldt-Jakob disease prions propagate in cell culture. Int. J. Biochem. Cell Biol. 40:2793-2801. [DOI] [PubMed] [Google Scholar]
- 15.Leblanc, P., S. Alais, I. Porto-Carreiro, S. Lehmann, J. Grassi, G. Raposo, and J. L. Darlix. 2006. Retrovirus infection strongly enhances scrapie infectivity release in cell culture. EMBO J. 25:2674-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahal, S. P., C. A. Baker, C. A. Demczyk, E. W. Smith, C. Julius, and C. Weissmann. 2007. Prion strain discrimination in cell culture: the cell panel assay. Proc. Natl. Acad. Sci. U. S. A. 104:20908-20913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahal, S. P., C. A. Demczyk, E. W. Smith, Jr., P. C. Klohn, and C. Weissmann. 2008. Assaying prions in cell culture: the standard scrapie cell assay (SSCA) and the scrapie cell assay in end point format (SCEPA). Methods Mol. Biol. 459:49-68. [DOI] [PubMed] [Google Scholar]
- 18.Raymond, G. J., E. A. Olsen, K. S. Lee, L. D. Raymond, P. K. Bryant III, G. S. Baron, W. S. Caughey, D. A. Kocisko, L. E. McHolland, C. Favara, J. P. Langeveld, F. G. van Zijderveld, R. T. Mayer, M. W. Miller, E. S. Williams, and B. Caughey. 2006. Inhibition of protease-resistant prion protein formation in a transformed deer cell line infected with chronic wasting disease. J. Virol. 80:596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed, J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 27:493-497. [Google Scholar]
- 20.Safar, J., H. Wille, V. Itri, D. Groth, H. Serban, M. Torchia, F. E. Cohen, and S. B. Prusiner. 1998. Eight prion strains have PrPSc molecules with different conformations. Nat. Med. 4:1157-1165. [DOI] [PubMed] [Google Scholar]
- 21.Telling, G. C., M. Scott, J. Mastrianni, R. Gabizon, M. Torchia, F. E. Cohen, S. J. DeArmond, and S. B. Prusiner. 1995. Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell 83:79-90. [DOI] [PubMed] [Google Scholar]
- 22.Vilette, D., O. Andreoletti, F. Archer, M. F. Madelaine, J. L. Vilotte, S. Lehmann, and H. Laude. 2001. Ex vivo propagation of infectious sheep scrapie agent in heterologous epithelial cells expressing ovine prion protein. Proc. Natl. Acad. Sci. U. S. A. 98:4055-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.