Abstract
Sphingosine 1-phosphate (S1P)-metabolizing enzymes regulate the level of sphingolipids and have important biological functions. However, the effects of S1P-metabolizing enzymes on host defense against invading viruses remain unknown. In this study, we investigated the role of S1P-metabolizing enzymes in modulating cellular responses to influenza virus infection. Overexpression of S1P lyase (SPL), which induces the degradation of S1P, interfered with the amplification of infectious influenza virus. Accordingly, SPL-overexpressing cells were much more resistant than control cells to the cytopathic effects caused by influenza virus infection. SPL-mediated inhibition of virus-induced cell death was supported by impairment of the upregulation of the proapoptotic protein Bax, a critical factor for influenza virus cytopathogenicity. Importantly, influenza virus infection of SPL-overexpressing cells induced rapid activation of extracellular signal-regulated kinase (ERK) and STAT1 but not of p38 mitogen-activated protein kinase (MAPK), Akt, or c-Jun N-terminal kinase (JNK). Blockade of STAT1 expression or inhibition of Janus kinase (JAK) activity elevated the level of influenza virus replication in the cells, indicating that SPL protects cells from influenza virus via the activation of JAK/STAT signaling. In contrast to that of SPL, the overexpression of S1P-producing sphingosine kinase 1 heightened the cells' susceptibility to influenza virus infection, an effect that was reversed by the inhibition of its kinase activity, representing opposed enzymatic activity. These findings indicate that the modulation of S1P-metabolizing enzymes is crucial for controlling the host defense against infection with influenza virus. Thus, S1P-metabolizing enzymes are novel potential targets for the treatment of diseases caused by influenza virus infection.
Influenza virus continues to threaten humans and remains a major worldwide health concern. Influenza virus causes an average of 36,000 deaths and 200,000 hospitalizations annually in the United States (50), imposing a significant economic burden (33). Further, there is fear of the recurrence of a devastating influenza pandemic similar to the Spanish influenza pandemic in 1918/1919, which killed as estimated 40 to 50 million people worldwide (34). Indeed, on 11 June 2009, the World Health Organization (WHO) declared the spread of the 2009 influenza A (H1N1) virus (initially known as swine flu virus) a global influenza pandemic (14, 45, 51). In addition, outbreaks of avian H5N1 influenza elevated vigilance against the occurrence of an influenza pandemic (4). A substantial number of circulating seasonal influenza viruses, as well as the avian H5N1 influenza virus with pandemic potential, were found to be resistant to antiviral drugs (10). Thus, identifying new therapeutic targets and understanding the mechanisms of host-virus interactions are important biomedical goals.
Sphingolipids are bioactive lipid mediators characterized by the presence of a serine head group with one or two fatty acid tails (7, 44). One of the sphingolipids, sphingosine, and its downstream product sphingosine 1-phosphate (S1P), have emerged as the modulators of multiple cellular processes, such as cell growth, survival, differentiation, and migration, and have therapeutic potential. For instance, a sphingosine analog, FTY720, is a promising biomedical drug candidate that is currently being tested in phase III clinical trials for the treatment of multiple sclerosis (20). S1P, which is generated inside cells, can trigger intracellular signaling or is secreted to act as an exogenous lipid mediator stimulating S1P receptor-mediated signaling (44, 47).
The level of S1P is tightly regulated by the S1P-metabolizing enzymes sphingosine kinase (SK) and S1P lyase (SPL). Its synthesis from sphingosine is catalyzed by SK, while SPL catalyzes the degradation of S1P to phosphoethanolamine and hexadecanal (46). These S1P-metabolizing enzymes were revealed to modulate diverse cellular stresses induced by anticancer drugs (30, 31), DNA damage (39), or serum deprivation (38, 43). Cells overexpressing SK1 displayed increased resistance to anticancer drugs such as cisplatin, carboplatin, and doxorubicin (30), whereas cells overexpressing SPL were more sensitive to drug-mediated cell death (31).
Recently, the sphingosine analog AAL-R was shown to display immunomodulatory activity to alleviate influenza virus-induced immune pathology (27, 28). The phosphorylated analog acted directly on S1P receptors to regulate the expression of inflammatory cytokines, although it did not significantly alter influenza virus propagation (28). However, the role of intracellular S1P-metabolizing enzymes in host defensive mechanisms against influenza virus infection has not been studied.
Here, we now show the contribution of the S1P-metabolizing enzymes SPL and SK1 to cellular responses to influenza virus infection. Overexpression of SPL interfered with influenza virus amplification and virus-induced cell death, with the early activation of STAT1 and extracellular signal-regulated kinase (ERK) molecules. Treatment with inhibitors blocking STAT1 expression or Janus kinase 1 (JAK1) activation increased influenza virus replication preferentially in SPL-overexpressing cells, demonstrating the importance of JAK/STAT signaling for SPL-mediated host defense. The suppression of influenza virus-induced cellular apoptosis by SPL was supported by the diminished expression of both the proapoptotic protein Bax and the cleaved product of poly(ADP-ribose) polymerase (PARP). In contrast, the overexpression of SK1 made cells more permissive to influenza virus infection, which was reversed by the inhibition of its kinase activity. Collectively, our results demonstrate that S1P-metabolizing enzymes regulate influenza virus propagation and represent novel therapeutic targets.
MATERIALS AND METHODS
Virus and cells.
Influenza A/WSN/33 virus (H1N1) was provided by Yoshihiro Kawaoka (University of Wisconsin—Madison) and was used in this study. For the titration of viruses, at various times after infection, WSN virus-infected cells and supernatants containing released viruses were harvested. Viruses that were associated with cells were isolated by one or two cycles of freezing and thawing. Virus titers were determined on Madin-Darby canine kidney (MDCK) cells by a plaque assay (36). Human embryonic kidney (HEK) 293 cells and other established cell lines (SPL and SK1 cells) were maintained as described previously (30, 31).
Western blot analysis.
Specific antibodies against actin, influenza virus nucleoprotein (NP), Bax, Bcl-2, PARP, STAT1, pSTAT1, STAT2, pSTAT2, ERK, pERK, p38, p-p38, Akt, pAkt, Jun N-terminal protein kinase (JNK), pJNK, and FLAG for SPL and SK1 were purchased from Cell Signaling Technology, Abcam, Upstate, or Santa Cruz Biotechnology. Total proteins were extracted by a radioimmunoprecipitation assay (RIPA) buffer supplemented with inhibitors blocking proteases and phosphatases, and levels were then normalized by using a Bradford assay. The protein samples (20 μg each) were run on a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and were transferred to a Protran nitrocellulose (NC) membrane (Whatman). Membrane-bound antibodies were detected by enhanced chemiluminescence (Pierce). All the data presented were obtained by experiments repeated at least twice with independent experimental settings.
RNA interference.
The small interfering RNA (siRNA) targeting SPL (si-SPL) was synthesized by Invitrogen Life Technologies. The si-SPL compounds are double-stranded RNAs (dsRNAs) containing proprietary chemical modifications that enhance RNA stability and reduce off-target effects. siRNA used as a control (si-CTR) was purchased from Cell Signaling Technology. Cells were transfected with 10 nM siRNA using Lipofectamine RNAiMAX (Invitrogen Life Technologies) according to the manufacturer's instructions. Then cells were infected with influenza virus at day 3 after transfection. Knockdown of SPL expression was verified by Western blot analysis. The experiment was independently repeated twice with similar results.
Inhibitor assays.
For the JAK inhibitor assay, HEK and SPL cells were either left uninfected or infected with WSN virus at a multiplicity of infection (MOI) of 1. At 2 h postinfection (hpi), cells were treated with either a solvent control (1% dimethyl sulfoxide [DMSO]), JAK inhibitor I (2 or 10 μM), AG490 (a JAK2 inhibitor; 2 μM), or JAK3 inhibitor I (2 μM) (Calbiochem). For the inhibition of STAT1 expression, HEK and SPL cells were pretreated with fludarabine (1 μM) (Sigma) (13) or its solvent control (1% DMSO) for 6 h and were then infected with WSN virus at an MOI of 1. To inhibit SK activity, SK1 cells were preincubated with N,N,-dimethylsphingosine (DMS) (Cayman Chemical) or its solvent (1% DMSO) as a control for 3 h and were then infected with WSN virus. Results were confirmed by repeated experiments.
Sphingolipids.
FTY720, d-erythro-sphingosine, and S1P were purchased from Cayman Chemicals. HEK cells were infected with WSN virus and were simultaneously treated with FTY720 (1 μM), d-erythro-sphingosine (1 μM), or its solvent (1% DMSO). Similarly, virus-infected cells were treated with S1P (1 μM) or its solvent (3 mM NaOH). These experiments were repeated two more times with similar results.
Flow cytometric analysis.
For detection of viral NP and Bax, HEK, SPL, or SK1 cells were either left uninfected or infected with WSN virus at an MOI of 0.1 or 1. At 2 or 3 days postinfection (dpi), cells were incubated with anti-Bax and anti-viral NP antibodies for 1 h and were then stained with phycoerythrin (PE)- and allophycocyanin (APC)-conjugated secondary antibodies (BD) for 1 h as described previously (27). Apoptotic cell death was detected by using an annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BD Pharmingen) according to the manufacturer's instructions. Cells (1 × 105) were washed twice with cold phosphate-buffered saline (PBS) and were then incubated with annexin V-FITC for 15 min at room temperature (RT) in the dark. Data were immediately collected by a CyAn ADP flow cytometer (Beckman Coulter) and were analyzed with FlowJo (Treestar) software. The data shown are representative of three independent experiments.
Immunocytochemistry.
HEK or SPL cells were plated on four-well chamber slides (Nunc) and were infected with WSN at an MOI of 1. At 2 dpi, cells were fixed in 4% paraformaldehyde and were then permeabilized in 0.5% Triton X-100 (Sigma) for 10 min. Cells were blocked in 1% bovine serum albumin (BSA) solution for 2 h and were then incubated with an anti-Bax antibody (BD; clone 6A7) and an antibody against viral NP (Abcam) overnight at 4°C. Cells were stained with Alexa Fluor 488-conjugated anti-mouse IgG and Alexa Fluor 546-conjugated anti-rabbit IgG for 2 h and were then incubated in 4′,6-diamidino-2-phenylindole (DAPI) solution (300 nM; Invitrogen). Images were obtained on a Zeiss LSM 510 META confocal microscope. Representative fields are shown in the figures: images for uninfected controls were selected from 5 different fields, and images for WSN-infected cells were chosen from >10 fields. Results were equivalent in the repeated experiment.
Real-time PCR.
Total cellular RNA was purified by using Tri reagent (Sigma-Aldrich) according to the manufacturer's description and was treated with DNase I to remove contaminated DNAs. Total RNA was reverse transcribed, and the resulting cDNA was analyzed by real-time PCR using gene-specific primer sets. Primers for beta interferon (IFN-β) (5′-CGC CGC ATT GAC CAT CTA-3′ and 5′-GAC ATT AGC CAG GAG GTT CTC A-3′) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (5′-TCA CCA CCA TGG AGA AGG-3′ and 5′-GAT AAG CAG TTG GTG GTG CA-3′) were used. Quantitative real-time PCRs were performed with SYBR green I chemistry using an ABI 7900 HT real-time PCR instrument. The authenticity of the PCR products was verified by melting curve analysis. cDNA quantities were normalized to the GAPDH RNA quantities measured in the same samples. The experiment was independently repeated twice with similar results.
Statistical analysis.
All error bars indicate the standard errors of the means (SEM), and averages were compared using a bidirectional unpaired Student t test.
RESULTS
S1P lyase overexpression inhibits influenza virus propagation.
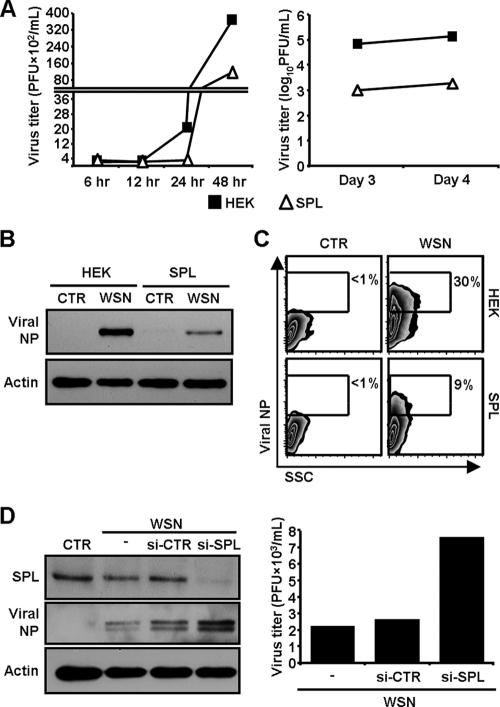
To investigate whether SPL affects influenza virus propagation, HEK 293 cells and SPL-overexpressing HEK cells (SPL cells) (31) were infected with influenza A/WSN/33 (WSN) virus at an MOI of 0.1 and were then monitored for viral amplification. Viral titers were determined by a plaque assay at 6, 12, 24, or 48 hpi (Fig. 1A, left) and 3 or 4 dpi (Fig. 1A, right). The virus produced its progeny on HEK cells with a 10-fold increase in the titer when assessed at 24 hpi, compared to SPL cells at that time point (24 hpi) (Fig. 1A). The increase in the viral titer over time was prominent with HEK cells compared to SPL cells. Indeed, at 3 and 4 dpi, approximately 100-fold fewer viruses were produced from SPL cells than from HEK cells. Accordingly, increased SPL expression strongly impaired the synthesis of influenza virus nucleoprotein (NP) in the cells as evidenced by Western blot analysis (Fig. 1B). Further, the number of NP-expressing (NP+) cells was strongly decreased in SPL cells (NP+ HEK cells, 30%; NP+ SPL cells, 9%) (Fig. 1C) when they were assessed at 3 dpi. The mean fluorescence intensity (MFI) of NP+ SPL cells, which represents the level of NP (MFI, 88), was lower than that of NP+ HEK cells (MFI, 100), which was observed in repeated experiments (12 to 28% decrease in the MFI of NP+ SPL cells compared to that of NP+ HEK cells). These results suggest that SPL inhibits influenza virus replication, leading to the diminished production of virus particles that could infect neighbor cells. To confirm the SPL-mediated inhibition of virus propagation, a small interfering RNA (siRNA) approach was utilized. Specific siRNA targeting SPL effectively downregulated the expression of SPL in SPL cells. Consequently, the expression of viral NP (Fig. 1D, left) and the viral titer (Fig. 1D, right) clearly increased over that observed under nonspecific siRNA (si-CTR) or mock-treated conditions. Therefore, these results demonstrate that overexpression of SPL inhibits the amplification of infectious influenza virus.
FIG. 1.
Effect of SPL overexpression on influenza virus amplification. (A) HEK cells (filled squares) or SPL cells (open triangles) were infected with WSN virus at an MOI of 0.1. At 6, 12, 24, or 48 hpi (left) and at 3 or 4 dpi (right), viral titers were determined by a plaque assay. (B) HEK cells or SPL cells were either left uninfected (control [CTR]) or infected with WSN virus at an MOI of 1. At 2 dpi, influenza virus NP and actin were detected by Western blot analysis. (C) Cells were either left uninfected (CTR) or infected with WSN at an MOI of 0.1 and were analyzed for the expression of viral NP by flow cytometry at 3 dpi. The percentages of virus NP+ cells are given. SSC, side scatter. (D) SPL cells were either mock transfected (−) or transfected with control siRNA (si-CTR) or siRNA targeting SPL (si-SPL); then the cells were infected with WSN virus at an MOI of 1. Uninfected SPL cells are shown as CTR. At 2 dpi, Western blot analysis was performed to detect SPL, influenza virus NP, and actin (left); viral titers were quantified by a plaque assay (right).
S1P lyase overexpression renders cells resistant to influenza virus-induced cytopathic effect.
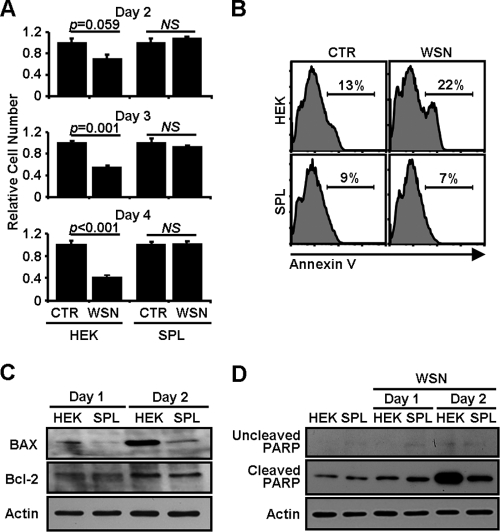
Influenza virus induces apoptosis in the infected cells (21, 48). As expected, the infection of HEK cells with WSN virus caused apparent cytopathic effects (CPEs) when the cells were analyzed at 2 to 4 dpi by using a trypan blue exclusion assay (Fig. 2 A) or by visual inspection with phase-contrast microscopy (data not shown). The viability of HEK cells was strongly diminished by WSN virus infection (day 2, 30% decrease; day 3, 45% decrease; day 4, 59% decrease). However, SPL cells were notably more resistant to influenza virus infection-induced CPEs than HEK cells (Fig. 2A). The inhibition of virus-induced apoptosis by SPL was also observed when the cells were stained with a fluorescence-conjugated annexin V, an early marker of cellular apoptosis, and then analyzed by flow cytometry (Fig. 2B).
FIG. 2.
Reduced CPEs in influenza virus-infected SPL cells. (A) HEK cells or SPL cells were either left uninfected (CTR) or infected with WSN virus at an MOI of 1. At 2, 3, or 4 dpi, cellular viability was monitored by using a trypan blue exclusion assay. The number of uninfected cells was set at 1.0, and the relative numbers of virus-infected groups were compared. Three separate wells per group were used. Values are means ± SEM. P values are included to show statistical significance. NS, no significant difference. (B) HEK cells or SPL cells were either left uninfected or infected with WSN virus at an MOI of 5. Cells were stained with annexin V at 1 dpi and were then analyzed by flow cytometry. Percentages of annexin V+ cells are shown. (C and D) HEK cells or SPL cells were infected with WSN virus at an MOI of 1. Cell lysates were used for Western blot analysis to detect Bax, Bcl-2, and actin (C) or uncleaved PARP, cleaved PARP, and actin (D) at 1 or 2 dpi.
The finding of SPL-mediated reduction of CPEs upon influenza virus infection prompted us to define the underlying molecular mechanism. Recently, the activation of the proapoptotic protein Bax was reported to be critical for efficient induction of apoptosis caused by influenza virus through caspase activation (29). Therefore, we evaluated the change in the Bax level in influenza virus-infected cells following the overexpression of SPL. After influenza virus infection, Bax was upregulated in HEK cells over time (Fig. 2C). Overexpression of SPL strongly inhibited the virus-induced elevation of Bax expression. However, the expression of an antiapoptotic protein. Bcl-2, did not change (Fig. 2C). We further evaluated the activation of the apoptotic pathway downstream of Bax. The cleaved 84-kDa form of PARP is known to be generated by activated caspase-3 (49) and to be increased by influenza virus infection (23, 32). In support of Bax regulation, the level of cleaved PARP markedly increased in HEK cells at 2 dpi, but not in SPL cells (Fig. 2D). Thus, SPL mediated profound suppression of Bax expression and PARP cleavage, reflecting the increased viability of SPL cells upon influenza virus infection.
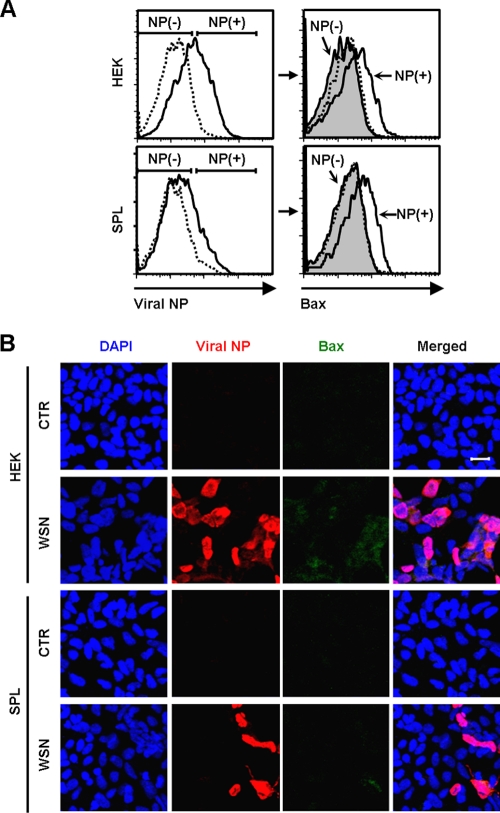
To determine whether apoptosis is restricted to influenza virus-infected cells, we conducted flow cytometric analysis. As previously demonstrated, HEK cells were more sensitive to the virus infection and displayed higher NP expression (Fig. 3 A, left panels) and Bax expression (data not shown) than SPL cells. In both HEK cells and SPL cells, some NP+ cells were coexpressing Bax, whereas the majority of NP− cells were not (Fig. 3A, right panels). However, not all virus-infected NP+ cells were coexpressing Bax. This is presumably because at the early stage of influenza virus replication in the nucleus, the cells might not be undergoing apoptosis, and virus-induced apoptosis is associated with the nuclear export of the ribonucleoprotein complex of influenza virus (52). The finding was further confirmed by immunocytochemistry (Fig. 3B). In agreement with the result of flow cytometry, NP+ or Bax+ cells were readily detectable in influenza virus-infected HEK cells compared to infected SPL cells; most of the Bax+ cells were expressing viral NP simultaneously.
FIG. 3.
Coexpression of Bax and viral NP in influenza virus-infected HEK or SPL cells. (A) HEK cells or SPL cells were either left uninfected (dotted lines) or infected (solid lines) with WSN virus at an MOI of 1. (Left) At 2 dpi, the expression of viral NP was assessed by flow cytometry. Virus-treated cells (solid lines) were separated into NP-expressing (NP+) and nonexpressing (NP−) cells. (Right) The Bax expression of the NP+ cells (solid lines) and the NP− cells (shaded areas) was compared with that of uninfected cells (dotted lines). (B) Cells were either left uninfected (CTR) or infected with WSN at an MOI of 1. They were fixed, permeabilized, and stained with DAPI, to detect nuclei, and with antibodies against viral NP and Bax at 2 dpi. Representative confocal images are shown (original magnification, ×200). Bar, 20 μm.
S1P lyase overexpression induces the activation of STAT1 and ERK1/2 upon influenza virus infection.
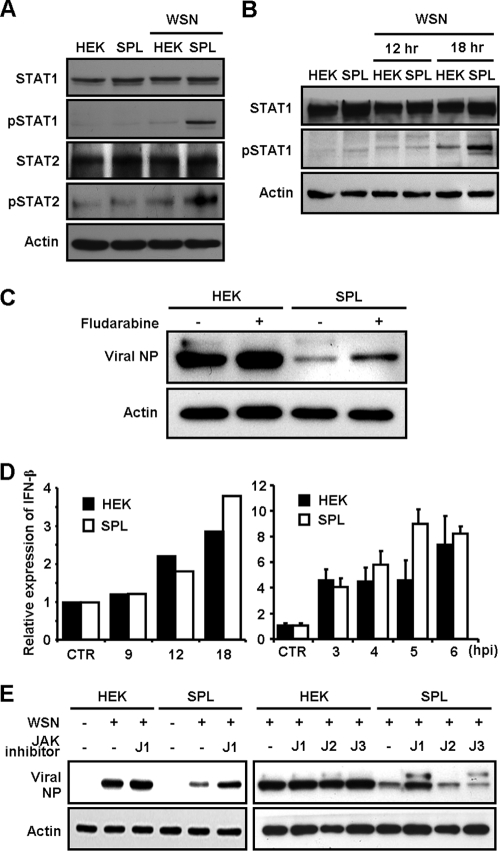
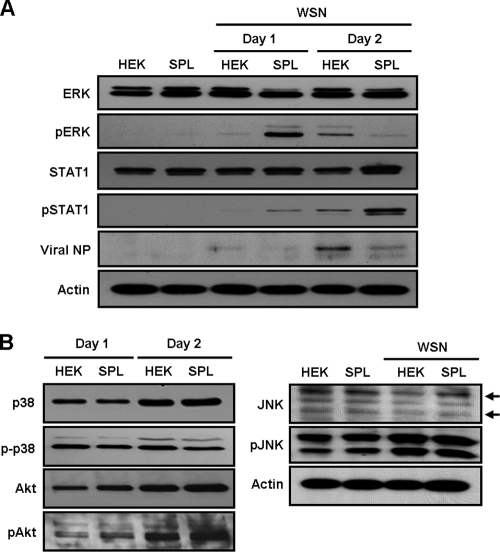
Since STAT1/2 molecules are transcriptional activators essential for the type I interferon (IFN) signaling (1, 8, 41) that induces the antiviral state (16, 18, 22, 40), we examined their activation in HEK cells and SPL cells upon WSN virus infection. Interestingly, overexpression of SPL strongly increased the expression of phosphorylated STAT1 (pSTAT1) and slightly upregulated pSTAT2 at 1 dpi (Fig. 4 A). Further analysis revealed that the elevation of pSTAT1 occurred on SPL cells between 12 and 18 hpi (Fig. 4B). When the expression of STAT1 was blocked by its inhibitor fludarabine, viral NP (Fig. 4C) and Bax (data not shown) were upregulated preferentially in SPL cells. Therefore, STAT1 is an important cellular component for SPL's inhibition of influenza virus replication and viral cytopathogenicity. However, STAT1/2 activation does not appear to be due to the increased type I IFN synthesis in SPL cells, because the amounts of type I IFN at the level of mRNA hardly changed over time compared to that in HEK cells (Fig. 4D). Since STAT1/2 are phosphorylated by nonreceptor tyrosine kinases of Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2) in type I IFN signaling (1, 8, 41), we performed inhibitor studies blocking their kinase activities to further determine the involvement of JAK/STAT signaling in SPL's modulation of virus replication. HEK cells or SPL cells were either left uninfected or infected with influenza virus and were then treated with a JAK1 inhibitor (JAK inhibitor I) that blocks primarily JAK1 activity and secondarily JAK2, JAK3, and TYK2 activities. The inhibition of these kinase activities strongly elevated the expression level of viral NP in WSN virus-infected SPL cells, whereas a slight increase in NP expression was detected in JAK1 inhibitor-treated HEK cells (left panel of Fig. 4E). In contrast, inhibitors specifically blocking the activation of JAK2 or JAK3 did not increase viral NP expression in SPL cells (right panel of Fig. 4E). These results indicate that the activation of the STAT signaling pathway by JAK1/TYK2 is critical for the suppression of influenza virus replication observed in SPL cells.
FIG. 4.
Analysis for the activation of JAK/STAT following influenza virus infection of SPL cells. HEK cells or SPL cells were either left uninfected or infected with WSN virus at an MOI of 1. (A) At 1 dpi, cell lysates were used for Western blot analysis to detect STAT1, pSTAT1, STAT2, pSTAT2, and actin. (B) At 0, 12, or 18 hpi, whole proteins were extracted and then used for Western blot analysis to detect STAT1, pSTAT1, and actin. (C) HEK or SPL cells were pretreated with fludarabine (1 μM), blocking STAT1 expression, and at 2 dpi, the expression of viral NP and actin was analyzed. (D) HEK cells (filled bars) and SPL cells (open bars) were either left uninfected (CTR) or infected with WSN virus at an MOI of 1 (left) or 10 (right). Reverse transcription followed by quantitative real-time PCR was performed to detect the relative mRNA level of IFN-β. Three reactions per sample were carried out for the right panel. Values are means ± SEM. (E) HEK or SPL cells were treated with inhibitors of JAK1 (J1 [10 μM for the left panel or 2 μM for the right panel]), JAK2 (J2 [2 μM]), or JAK3 (J3 [2 μM]) at 2 hpi. After 2 days, the levels of viral NP and actin were assessed by Western blot analysis.
Previously, the increased sensitivity of SPL cells to cisplatin was reported to be dependent on p38 mitogen-activated protein kinase (MAPK) and partly on c-Jun N-terminal kinase (JNK) signaling but not to involve activation of ERK (31). Additionally, these cellular pathways could affect the STAT signaling cascade (3, 9, 17, 24, 37). For instance, ERK2 was reported to interact with STAT1 and type I IFN receptor (9). Thus, we further determined the activation of the diverse signaling molecules ERK, p38 MAPK, Akt, and JNK following infection of SPL cells with influenza virus. Interestingly, the virus strongly induced the activation of ERK1/2 (p44/p42 MAPK), especially ERK2 (p42), as well as STAT1 in SPL cells at 1 dpi compared to ERK2 expression in HEK cells (Fig. 5 A). Then, in contrast to that of pSTAT1, the level of activated pERK quickly diminished in SPL cells at 2 dpi, when pERK was upregulated in HEK cells (Fig. 5A). However, the phosphorylation of p38 MAPK (p-p38), Akt (p-Akt), or JNK (p-JNK) was not significantly affected by influenza virus infection of SPL cells compared to their expression in virus-infected HEK cells (Fig. 5B). Therefore, the early activation of STAT1 and ERK is a specific event occurring in SPL cells following influenza virus infection.
FIG. 5.
Preferential activation of ERK, but not of p38, Akt, or JNK, in SPL cells. HEK cells or SPL cells were either left uninfected or infected with WSN virus at an MOI of 1. (A) At 1 or 2 dpi, whole proteins were used for Western blot analysis to record the expression of ERK, pERK, STAT1, pSTAT1, viral NP, and actin. (B) Cell lysates were obtained at 1 or 2 dpi and were analyzed by Western blotting to determine the levels of p38, p-p38, Akt, pAkt, JNK, pJNK, and actin.
Sphingosine kinase 1 increases the susceptibility of cells to influenza virus infection.
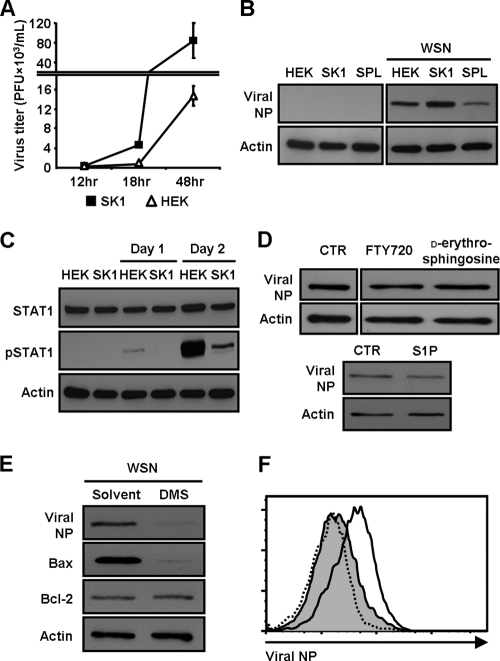
SK1 produces S1P via its kinase function, whereas S1P lyase induces the degradation of S1P (46). Thus, we examined whether overexpression of SK1 affects influenza virus propagation differently from SPL cells. In contrast to SPL cells, SK1-overexpressing cells (SK1 cells) (30) were more susceptible to viral infection than control HEK cells (Fig. 6 A). The WSN virus-infected SK1 cells produced more progeny virus than HEK cells at 18 or 48 hpi (Fig. 6A) and at 3 dpi (data not shown), and phenotypic death of the former cells was prominent by visual inspection with phase-contrast microscopy (data not shown). The level of viral protein expression was greater in SK1 cells than in HEK cells or SPL cells, supporting the increased viral replication in SK1 cells (Fig. 6B). Further, pSTAT1 expression was inhibited in infected SK1 cells compared to HEK cells (Fig. 6C). These findings indicate that overexpression of SK1 heightens cells' susceptibility to influenza virus infection, which correlated with the suppression of STAT1 activation. These outcomes reflect the opposing enzymatic activities. However, direct treatment of cells with the synthetic sphingosine analog FTY720, d-erythro-sphingosine, or S1P failed to enhance the synthesis of influenza virus NP (Fig. 6D). These results suggest that the increase in influenza virus replication is not mediated by S1P receptor signaling but is regulated by SK1, presumably via intracellular S1P signaling or the altered balance among sphingolipids.
FIG. 6.
Enhanced susceptibility of SK1 cells to influenza virus infection. (A) HEK cells (open triangles) or SK1 cells (filled squares) were infected with WSN virus at an MOI of 0.1. At 12, 18, or 48 hpi, virus titers were determined by a plaque assay. Three separate samples of virus-infected cells per group were used at each time point. Values are means ± SEM. (B) Western blot analysis was conducted at 2 dpi to detect viral NP and actin. (C) Cell lysates were used for Western blot analysis to detect STAT1, pSTAT1, and actin. (D) HEK cells were infected with WSN virus at an MOI of 1 and were treated with either a solvent control (CTR), FTY720 (1 μM), d-erythro-sphingosine (1 μM), or S1P (1 μM). At 2 dpi, total proteins were extracted for the detection of viral NP and actin by Western blot analysis. (E) SK1 cells were pretreated with DMS (0.1 μM) or its solvent control for 3 h and were then either left uninfected or infected with WSN virus at an MOI of 1. At 3 dpi, the expression of viral NP, Bax, Bcl-2, and actin was analyzed by Western blotting. (F) SK1 cells were pretreated with DMS (0.5 μM) or its solvent control and were then either left uninfected or infected with WSN virus at an MOI of 1. At 2 dpi, the expression of viral NP was detected by flow cytometry. Dotted line, non-virus-infected cells; solid line, virus-infected cells; shaded area, DMS-treated, virus-infected cells.
Thus, we investigated whether the inhibition of SK's enzymatic action reverses the increased susceptibility of SK1 cells to influenza virus infection. To this end, we performed an inhibitor assay using N,N,-dimethylsphingosine (DMS) (11, 53), a known inhibitor of SK activity. The data show that DMS dramatically decreased the expression of influenza virus NP and Bax, but did not alter the expression of Bcl-2, at 3 dpi (Fig. 6E). The inhibitory effect of DMS on virus replication, i.e., the decrease in the expression of NP, was confirmed by flow cytometric analysis (Fig. 6F) and immunocytochemistry (data not shown). These results further support our conclusion that SK1 makes cells more permissive to influenza virus.
DISCUSSION
In this study, we found that S1P-metabolizing enzymes regulate cellular responses to influenza virus infection. Importantly, overexpression of SPL impaired the synthesis of the virus protein NP and infectious virus production, whereas SK1 increased virus replication. SPL-mediated inhibition of viral propagation and cytopathogenicity correlated with the activation of STAT1 and ERK and with the decreased levels of the proapoptotic protein Bax and cleaved PARP.
S1P-metabolizing enzymes were known to modulate sensitivity to cellular stresses. For instance, SPL renders cells more sensitive to stresses caused by anticancer drugs or serum deprivation (31, 43), whereas SK1-overexpressing cells become more resistant to cell death induced by these stimuli (30, 38). The result was supported by the increased expression of the antiapoptotic protein Bcl-2 in SPL-deficient cells (5). However, our study revealed that SPL cells were more resistant to influenza virus infection and virus-induced apoptosis, and SK1 cells became more sensitive to the infection and its aftermath. These outcomes are probably due to disparate underlying molecular mechanisms that are influenced by influenza virus, given that virus infection creates a new cellular environment via dynamic interaction between viral components and the cellular machinery. It is likely that SPL suppresses influenza virus replication, leading to the inhibition of virus-induced Bax expression, although we cannot exclude the possibility of SPL-mediated direct inhibition of Bax upregulation upon influenza virus infection. Interestingly, influenza virus infection of SPL cells induced early activation of STAT1/2 signaling, which is known as a powerful host defense mechanism against influenza virus infection (16, 18, 22, 40). Moreover, when STAT1 expression was inhibited or JAK1/TYK2 activity, i.e., phosphorylation of STAT1/2 molecules (1, 8, 41), was blocked by a specific inhibitor, the production of influenza virus NP increased preferentially in SPL cells (Fig. 4C and E). Therefore, these data strongly suggest that early activation of JAK/STAT signaling in SPL-overexpressing cells results in a potent anti-virus status of the cells. Based on this result, we hypothesized that SPL increases the synthesis of type I IFN for the activation of the JAK/STAT signaling cascade. However, the level of type I IFN scarcely changed (Fig. 4D). Alternatively, the overexpression of SPL may induce an intracellular signaling pathway to trigger STAT signaling against influenza virus infection. ERK2 was reported to associate with STAT1 as well as with the α subunit of the type I IFN receptor when the cells were exposed to type I IFN, and thus to modify the JAK/STAT signaling cascade (9). In our results, the activation level of ERK in SPL cells strongly increased upon influenza virus infection, compared to that in control HEK cells at 1 dpi (Fig. 5A). Therefore, it is likely that the activation of ERK leads to the phosphorylation of STAT1. However, the level of pERK dramatically decreased at 2 dpi compared to its expression in HEK cells infected with influenza virus. ERK was also shown to be activated by influenza virus infection and has a critical role in enhancing virus propagation (42), especially at the late stage of virus replication. Conceivably, early short-term activation of ERK is important for STAT1 activation in SPL cells, while the inhibition of ERK at the late stage reflects decreased virus replication in the cells. Therefore, the increased resistance to influenza virus infection that is mediated by SPL could be due to enhanced ERK function, leading directly or indirectly, via JAK activation, to STAT activation, although the cause-effect relationship requires further investigation. Thus, future studies should include the definition of detailed mechanisms for the link between SPL activation and JAK/STAT signaling and the identification of specific IFN-stimulated genes that are responsible for the SPL-mediated inhibition of virus propagation.
Besides ERK, other MAPK family members, p38 MAPK (35) and JNK (25), as well as Akt in the phosphatidylinositol-3-kinase (PI3K) pathway (12, 19), have been known to be important factors in regulating influenza virus propagation. Previous study has shown that overexpression of SPL activated p38 MAPK, in particular, in response to treatment with anticancer drugs such as cisplatin (31). However, the activation of these molecules was not significantly affected by SPL overexpression in cells upon influenza virus infection. These results indicate that the SPL-mediated antiviral cellular mechanism does not employ mechanisms involving the p38 MAPK, Akt, or JNK signaling pathways.
Overexpression of SK1 heightened the cells' susceptibility to influenza virus infection. This altered cellular response was reversed by treatment with an SK inhibitor, DMS, suggesting that SK could be a potential therapeutic target for the treatment of diseases caused by influenza virus infection. However, direct treatment of cells with S1P or the sphingosine analog FTY720 did not significantly affect influenza virus replication (Fig. 6D). Several possibilities could explain these results. First, extracellular S1P might have a different function in modulating cellular responses to influenza virus infection from intracellular S1P. S1P that is generated intracellularly by SK is not only secreted to act as an exogenous lipid mediator via specific S1P receptors (44) but can also remain inside the cell to stimulate cellular signaling components (47). Second, SPL and SK initially change the level of S1P/sphingosine, which subsequently affects the amounts of other sphingolipids. In other words, regulation of S1P-metabolizing enzymes can alter the levels of various types of sphingolipids, including ceramide, sphingosine, and S1P, and their intermediate and terminal metabolites (2). Thus, it is conceivable that the balance between S1P-metabolizing enzymes is more critical than the amount of S1P for cellular defense mechanisms against influenza virus infection (6). Lastly, it is possible that S1P-metabolyzing enzymes have other, as yet unidentified functions in regulating cellular responses to influenza virus infection. However, there has been no evidence that SPL and SK function in any way beyond catalyzing their respective reactions of breaking down or synthesizing S1P (15, 26, 46). Further, inhibition of SK enzymatic activity by DMS results in the inhibition of influenza virus propagation (Fig. 6E and F). Overall, this strongly supports the contention that S1P-metabolizing enzymes regulate the replication of influenza virus via their primary catalytic activity, not by some other, undefined role of the enzymes, although the latter possibility cannot be completely ruled out. Further analysis involving measurement of the levels of sphingolipids, especially sphingosine/S1P, in enzyme-overexpressing cells or enzyme-deficient cells following virus infection would be informative for uncovering mechanisms in detail.
Taken together, our findings indicate that the modulation of S1P-metabolizing enzymes is pivotal for the regulation of host cellular defense mechanisms against influenza virus infection. Manipulation of these enzymes, i.e., specific activation of SPL or inhibition of SK1, could block the propagation of influenza virus and virus-induced pathogenicity. In conjunction with the efforts to discover small molecules specifically targeting S1P-metabolizing enzymes (15), our studies could facilitate the development of novel mechanism-based therapeutics for viral diseases.
Acknowledgments
This work was supported by NIH/NIAID grant AI088363 (to B.H.), a startup grant from the University of Missouri (MU) (to B.H.), a Fellow/Mentor Research grant from the Department of Surgery (to Y.-J.S. and B.H.), and the MU Research Board (to B.H.). S.A. was supported by NIH grant GM53929.
We thank Yoshihiro Kawaoka (University of Wisconsin—Madison) for graciously supplying the influenza WSN virus. Also, we thank the Cell & Immunology Core and Molecular Cytology Core facilities at MU.
Footnotes
Published ahead of print on 2 June 2010.
REFERENCES
- 1.Aaronson, D. S., and C. M. Horvath. 2002. A road map for those who don't know JAK-STAT. Science 296:1653-1655. [DOI] [PubMed] [Google Scholar]
- 2.Chalfant, C. E., and S. Spiegel. 2005. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J. Cell Sci. 118:4605-4612. [DOI] [PubMed] [Google Scholar]
- 3.Chung, J., E. Uchida, T. C. Grammer, and J. Blenis. 1997. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol. Cell. Biol. 17:6508-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claas, E. C., A. D. Osterhaus, R. van Beek, J. C. De Jong, G. F. Rimmelzwaan, D. A. Senne, S. Krauss, K. F. Shortridge, and R. G. Webster. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351:472-477. [DOI] [PubMed] [Google Scholar]
- 5.Colié, S., P. P. Van Veldhoven, B. Kedjouar, C. Bedia, V. Albinet, S. C. Sorli, V. Garcia, M. Djavaheri-Mergny, C. Bauvy, P. Codogno, T. Levade, and N. Andrieu-Abadie. 2009. Disruption of sphingosine 1-phosphate lyase confers resistance to chemotherapy and promotes oncogenesis through Bcl-2/Bcl-xL upregulation. Cancer Res. 69:9346-9353. [DOI] [PubMed] [Google Scholar]
- 6.Cuvillier, O., G. Pirianov, B. Kleuser, P. G. Vanek, O. A. Coso, S. Gutkind, and S. Spiegel. 1996. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381:800-803. [DOI] [PubMed] [Google Scholar]
- 7.Cyster, J. G. 2005. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23:127-159. [DOI] [PubMed] [Google Scholar]
- 8.Darnell, J. E., Jr., I. M. Kerr, and G. R. Stark. 1994. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415-1421. [DOI] [PubMed] [Google Scholar]
- 9.David, M., E. Petricoin III, C. Benjamin, R. Pine, M. J. Weber, and A. C. Larner. 1995. Requirement for MAP kinase (ERK2) activity in interferon alpha- and interferon beta-stimulated gene expression through STAT proteins. Science 269:1721-1723. [DOI] [PubMed] [Google Scholar]
- 10.Dharan, N. J., L. V. Gubareva, J. J. Meyer, M. Okomo-Adhiambo, R. C. McClinton, S. A. Marshall, K. St George, S. Epperson, L. Brammer, A. I. Klimov, J. S. Bresee, and A. M. Fry. 2009. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA 301:1034-1041. [DOI] [PubMed] [Google Scholar]
- 11.Edsall, L. C., J. R. Van Brocklyn, O. Cuvillier, B. Kleuser, and S. Spiegel. 1998. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry 37:12892-12898. [DOI] [PubMed] [Google Scholar]
- 12.Ehrhardt, C., H. Marjuki, T. Wolff, B. Nurnberg, O. Planz, S. Pleschka, and S. Ludwig. 2006. Bivalent role of the phosphatidylinositol-3-kinase (PI3K) during influenza virus infection and host cell defence. Cell. Microbiol. 8:1336-1348. [DOI] [PubMed] [Google Scholar]
- 13.Frank, D. A., S. Mahajan, and J. Ritz. 1999. Fludarabine-induced immunosuppression is associated with inhibition of STAT1 signaling. Nat. Med. 5:444-447. [DOI] [PubMed] [Google Scholar]
- 14.Fraser, C., C. A. Donnelly, S. Cauchemez, W. P. Hanage, M. D. Van Kerkhove, T. D. Hollingsworth, J. Griffin, R. F. Baggaley, H. E. Jenkins, E. J. Lyons, T. Jombart, W. R. Hinsley, N. C. Grassly, F. Balloux, A. C. Ghani, N. M. Ferguson, A. Rambaut, O. G. Pybus, H. Lopez-Gatell, C. M. Alpuche-Aranda, I. B. Chapela, E. P. Zavala, D. M. Guevara, F. Checchi, E. Garcia, S. Hugonnet, and C. Roth. 2009. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324:1557-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fyrst, H., and J. D. Saba. 2008. Sphingosine-1-phosphate lyase in development and disease: sphingolipid metabolism takes flight. Biochim. Biophys. Acta 1781:448-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Sastre, A., R. K. Durbin, H. Zheng, P. Palese, R. Gertner, D. E. Levy, and J. E. Durbin. 1998. The role of interferon in influenza virus tissue tropism. J. Virol. 72:8550-8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goh, K. C., S. J. Haque, and B. R. Williams. 1999. p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J. 18:5601-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandvaux, N., B. R. tenOever, M. J. Servant, and J. Hiscott. 2002. The interferon antiviral response: from viral invasion to evasion. Curr. Opin. Infect. Dis. 15:259-267. [DOI] [PubMed] [Google Scholar]
- 19.Hale, B. G., and R. E. Randall. 2007. PI3K signalling during influenza A virus infections. Biochem. Soc. Trans. 35:186-187. [DOI] [PubMed] [Google Scholar]
- 20.Horga, A., and X. Montalban. 2008. FTY720 (fingolimod) for relapsing multiple sclerosis. Expert Rev. Neurother. 8:699-714. [DOI] [PubMed] [Google Scholar]
- 21.Kash, J. C., T. M. Tumpey, S. C. Proll, V. Carter, O. Perwitasari, M. J. Thomas, C. F. Basler, P. Palese, J. K. Taubenberger, A. Garcia-Sastre, D. E. Swayne, and M. G. Katze. 2006. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature 443:578-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 23.Lin, C., R. E. Holland, Jr., J. C. Donofrio, M. H. McCoy, L. R. Tudor, and T. M. Chambers. 2002. Caspase activation in equine influenza virus induced apoptotic cell death. Vet. Microbiol. 84:357-365. [DOI] [PubMed] [Google Scholar]
- 24.Liu, A. M., R. K. Lo, C. S. Wong, C. Morris, H. Wise, and Y. H. Wong. 2006. Activation of STAT3 by G alpha(s) distinctively requires protein kinase A, JNK, and phosphatidylinositol 3-kinase. J. Biol. Chem. 281:35812-35825. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig, S., C. Ehrhardt, E. R. Neumeier, M. Kracht, U. R. Rapp, and S. Pleschka. 2001. Influenza virus-induced AP-1-dependent gene expression requires activation of the JNK signaling pathway. J. Biol. Chem. 276:10990-10998. [PubMed] [Google Scholar]
- 26.Maceyka, M., H. Sankala, N. C. Hait, H. Le Stunff, H. Liu, R. Toman, C. Collier, M. Zhang, L. S. Satin, A. H. Merrill, Jr., S. Milstien, and S. Spiegel. 2005. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 280:37118-37129. [DOI] [PubMed] [Google Scholar]
- 27.Marsolais, D., B. Hahm, K. H. Edelmann, K. B. Walsh, M. Guerrero, Y. Hatta, Y. Kawaoka, E. Roberts, M. B. Oldstone, and H. Rosen. 2008. Local not systemic modulation of dendritic cell S1P receptors in lung blunts virus-specific immune responses to influenza. Mol. Pharmacol. 74:896-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsolais, D., B. Hahm, K. B. Walsh, K. H. Edelmann, D. McGavern, Y. Hatta, Y. Kawaoka, H. Rosen, and M. B. Oldstone. 2009. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc. Natl. Acad. Sci. U. S. A. 106:1560-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean, J. E., E. Datan, D. Matassov, and Z. F. Zakeri. 2009. Lack of Bax prevents influenza A virus-induced apoptosis and causes diminished viral replication. J. Virol. 83:8233-8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min, J., A. Mesika, M. Sivaguru, P. P. Van Veldhoven, H. Alexander, A. H. Futerman, and S. Alexander. 2007. (Dihydro)ceramide synthase 1 regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Mol. Cancer Res. 5:801-812. [DOI] [PubMed] [Google Scholar]
- 31.Min, J., P. P. Van Veldhoven, L. Zhang, M. H. Hanigan, H. Alexander, and S. Alexander. 2005. Sphingosine-1-phosphate lyase regulates sensitivity of human cells to select chemotherapy drugs in a p38-dependent manner. Mol. Cancer Res. 3:287-296. [DOI] [PubMed] [Google Scholar]
- 32.Mok, C. K., D. C. Lee, C. Y. Cheung, M. Peiris, and A. S. Lau. 2007. Differential onset of apoptosis in influenza A virus H5N1- and H1N1-infected human blood macrophages. J. Gen. Virol. 88:1275-1280. [DOI] [PubMed] [Google Scholar]
- 33.Molinari, N. A., I. R. Ortega-Sanchez, M. L. Messonnier, W. W. Thompson, P. M. Wortley, E. Weintraub, and C. B. Bridges. 2007. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 25:5086-5096. [DOI] [PubMed] [Google Scholar]
- 34.Morens, D. M., and A. S. Fauci. 2007. The 1918 influenza pandemic: insights for the 21st century. J. Infect. Dis. 195:1018-1028. [DOI] [PubMed] [Google Scholar]
- 35.Nencioni, L., G. De Chiara, R. Sgarbanti, D. Amatore, K. Aquilano, M. E. Marcocci, A. Serafino, M. Torcia, F. Cozzolino, M. R. Ciriolo, E. Garaci, and A. T. Palamara. 2009. Bcl-2 expression and p38MAPK activity in cells infected with influenza A virus: impact on virally induced apoptosis and viral replication. J. Biol. Chem. 284:16004-16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann, G., T. Watanabe, H. Ito, S. Watanabe, H. Goto, P. Gao, M. Hughes, D. R. Perez, R. Donis, E. Hoffmann, G. Hobom, and Y. Kawaoka. 1999. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl. Acad. Sci. U. S. A. 96:9345-9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen, H., C. V. Ramana, J. Bayes, and G. R. Stark. 2001. Roles of phosphatidylinositol 3-kinase in interferon-gamma-dependent phosphorylation of STAT1 on serine 727 and activation of gene expression. J. Biol. Chem. 276:33361-33368. [DOI] [PubMed] [Google Scholar]
- 38.Olivera, A., T. Kohama, L. Edsall, V. Nava, O. Cuvillier, S. Poulton, and S. Spiegel. 1999. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J. Cell Biol. 147:545-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oskouian, B., P. Sooriyakumaran, A. D. Borowsky, A. Crans, L. Dillard-Telm, Y. Y. Tam, P. Bandhuvula, and J. D. Saba. 2006. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc. Natl. Acad. Sci. U. S. A. 103:17384-17389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osterlund, P., J. Pirhonen, N. Ikonen, E. Ronkko, M. Strengell, S. M. Makela, M. Broman, O. J. Hamming, R. Hartmann, T. Ziegler, and I. Julkunen. 2010. Pandemic H1N1 2009 influenza A virus induces weak cytokine responses in human macrophages and dendritic cells and is highly sensitive to the antiviral actions of interferons. J. Virol. 84:1414-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Platanias, L. C. 2005. Mechanisms of type-I and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5:375-386. [DOI] [PubMed] [Google Scholar]
- 42.Pleschka, S., T. Wolff, C. Ehrhardt, G. Hobom, O. Planz, U. R. Rapp, and S. Ludwig. 2001. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 3:301-305. [DOI] [PubMed] [Google Scholar]
- 43.Reiss, U., B. Oskouian, J. Zhou, V. Gupta, P. Sooriyakumaran, S. Kelly, E. Wang, A. H. Merrill, Jr., and J. D. Saba. 2004. Sphingosine-phosphate lyase enhances stress-induced ceramide generation and apoptosis. J. Biol. Chem. 279:1281-1290. [DOI] [PubMed] [Google Scholar]
- 44.Rosen, H., and E. J. Goetzl. 2005. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 5:560-570. [DOI] [PubMed] [Google Scholar]
- 45.Rossman, J. S., and R. A. Lamb. 2010. Swine-origin influenza virus and the 2009 pandemic. Am. J. Respir. Crit. Care Med. 181:295-296. [DOI] [PubMed] [Google Scholar]
- 46.Spiegel, S., and R. Kolesnick. 2002. Sphingosine 1-phosphate as a therapeutic agent. Leukemia 16:1596-1602. [DOI] [PubMed] [Google Scholar]
- 47.Taha, T. A., Y. A. Hannun, and L. M. Obeid. 2006. Sphingosine kinase: biochemical and cellular regulation and role in disease. J. Biochem. Mol. Biol. 39:113-131. [DOI] [PubMed] [Google Scholar]
- 48.Takizawa, T., S. Matsukawa, Y. Higuchi, S. Nakamura, Y. Nakanishi, and R. Fukuda. 1993. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J. Gen. Virol. 74(Pt. 11):2347-2355. [DOI] [PubMed] [Google Scholar]
- 49.Tewari, M., L. T. Quan, K. O'Rourke, S. Desnoyers, Z. Zeng, D. R. Beidler, G. G. Poirier, G. S. Salvesen, and V. M. Dixit. 1995. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 81:801-809. [DOI] [PubMed] [Google Scholar]
- 50.Thompson, W. W., D. K. Shay, E. Weintraub, L. Brammer, C. B. Bridges, N. J. Cox, and K. Fukuda. 2004. Influenza-associated hospitalizations in the United States. JAMA 292:1333-1340. [DOI] [PubMed] [Google Scholar]
- 51.Wang, T. T., and P. Palese. 2009. Unraveling the mystery of swine influenza virus. Cell 137:983-985. [DOI] [PubMed] [Google Scholar]
- 52.Wurzer, W. J., O. Planz, C. Ehrhardt, M. Giner, T. Silberzahn, S. Pleschka, and S. Ludwig. 2003. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 22:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yatomi, Y., F. Ruan, T. Megidish, T. Toyokuni, S. Hakomori, and Y. Igarashi. 1996. N,N-Dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry 35:626-633. [DOI] [PubMed] [Google Scholar]