Abstract
The 86-kDa immediate-early 2 (IE2) protein of human cytomegalovirus (HCMV) is a promiscuous transactivator essential for viral gene expression. IE2 is covalently modified by SUMO at two lysine residues (K175 and K180) and also interacts noncovalently with SUMO. Although SUMOylation of IE2 has been shown to enhance its transactivation activity, the role of SUMO binding is not clear. Here we showed that SUMO binding by IE2 is necessary for its efficient transactivation function and for viral growth. IE2 bound physically to SUMO-1 through a SUMO-interacting motif (SIM). Mutations in SIM (mSIM) or in both SUMOylation sites and SIM (KR/mSIM), significantly reduced IE2 transactivation effects on viral early promoters. The replication of IE2 SIM mutant viruses (mSIM or KR/mSIM) was severely depressed in normal human fibroblasts. Analysis of viral growth curves revealed that the replication defect of the mSIM virus correlated with low-level accumulation of SUMO-modified IE2 and of viral early and late proteins. Importantly, both the formation of viral transcription domains and the association of IE2 with viral promoters in infected cells were significantly reduced in IE2 SIM mutant virus infection. Furthermore, IE2 was found to interact with the SUMO-modified form of TATA-binding protein (TBP)-associated factor 12 (TAF12), a component of the TFIID complex, in a SIM-dependent manner, and this interaction enhanced the transactivation activity of IE2. Our data demonstrate that the interaction of IE2 with SUMO-modified proteins plays an important role for the progression of the HCMV lytic cycle, and they suggest a novel viral mechanism utilizing the cellular SUMO system.
Human cytomegalovirus (HCMV) is an opportunistic pathogen that causes severe disease complications and pathogenesis in immunocompromised individuals. HCMV infection of newborns often results in cytomegalic inclusion disease. During the lytic cycle of HCMV infection, viral genes are expressed in a regulated cascade pattern with immediate-early (IE), early, and late gene expression. Among the IE proteins, the 86-kDa IE2 (also called IE86 or IE2-p86) has various activities, as a strong transactivator of viral and cellular genes, as a repressor of its own major IE (MIE) promoter, and as a cell cycle modulator (41).
The multiple functions of IE2 are attributed to its ability to interact with numerous cellular proteins. IE2 has been shown to interact with components of the basal transcription factor complex, including TFIIB, TATA-binding protein (TBP), and TBP-associated factors (TAFs) such as TAFII110 and TAFII130 (11, 15, 19, 25, 29, 36, 37, 57). IE2 also interacts with numerous transcription factors such as Ap-1, Sp1, Egr-1, CREB, CBP, SP1-1/Pu.1, Tef-1, and P/CAF (10, 31, 37, 54, 55, 64, 66, 68), as well as with histone modifiers such as HDAC1, HDAC2, HDAC3, G9a, and Suvar(3-9)H1 (43, 45, 50). IE2 also binds to cell cycle modulators such as RB (13, 14, 18, 57), p53 (8, 60, 62), and MDM2 (69).
IE2 is covalently modified by the small ubiquitin-like modifiers (SUMO) SUMO-1, SUMO-2, and SUMO-3 at two lysine residues (K175 and K180), and SUMOylation of IE2 enhances its transactivation capacity for diverse cellular and viral promoters (5, 22). Analysis of the amino acid variations of IE2 in different HCMV strains has consistently demonstrated a correlation between the transactivation activity of IE2 and its degree of SUMOylation (6). However, IE2 SUMOylation has not been shown to be essential for viral growth, since a mutant virus encoding K175/180R mutant IE2, which is defective in SUMOylation, was still viable, although the impact of the absence of IE2 SUMOylation on viral replication was dependent on virus strains (7, 32). IE2 has also been shown to directly bind to Ubc9, a SUMO E2 conjugating enzyme (5, 22), and PIAS1, a SUMO E3 ligase (35).
In addition to covalent SUMO attachment, proteins can also noncovalently interact with SUMO through a region of so-called SUMO-interacting motifs (SIMs). Several studies have identified hydrophobic amino acid residues flanked by negatively charged residues, including h-h-X-S-X-S/T-a-a-a (h, hydrophobic; a, acidic; X, any amino acid) or (I/V)-X-(I/V)-(I/V), as SIM consensus sequences (20, 27, 40, 58). SIMs in certain proteins have been found to mediate protein SUMOylation, in addition to also being involved in mediating interactions with other SUMO-modified proteins (27). IE2 also contains a region that resembles a SIM near the SUMO modification sites (5, 7). However, the role of the IE2 SIM in virus infection is not clear.
In this study, we show that the SIM of IE2 is required for the transactivation function of IE2. Analysis of the IE2 SIM mutant virus provides genetic evidence that noncovalent SUMO binding by IE2 is necessary for efficient viral gene expression and lytic growth by promoting the association of IE2 with viral promoters in viral transcription sites. We also show that IE2 interacts with the SUMO-modified form of TAF12, a component of the TFIID complex, in a SIM-dependent manner and that this interaction enhances the transactivation capacity of IE2.
MATERIALS AND METHODS
Cell culture, production of virus stocks, and transfection.
Human foreskin fibroblasts (HF) were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. The IE2(K175/180R) mutant virus (Towne) and its revertant have been described previously (32). IE1-expressing HF (33) were used to grow the IE2 viruses with mutations in SIM (mSIM viruses) and those with mutations in both SUMOylation sites and SIM (KR/mSIM viruses) to high titers. Viral titers were determined on HF using infectious center assays employing an anti-IE1 antibody (Ab) (23). Electroporation of HF was conducted using a Microporator MP-100 (Digital Bio), as previously described (23).
Plasmids.
The IE2 expression plasmids were generated with the IE2 cDNA derived from the Towne strain of CMV. pSG5-based expression plasmids for wild-type IE2 (pJHA124) and K175/180R IE2 (pYX104) have been described previously (5). Plasmids expressing the IE2 SIM mutants (mSIM and KR/mSIM) were generated on the pJHA124 and pYX104 backgrounds, respectively, by replacing I, V, I, and S residues between positions 200 and 203 with A residues using the Stratagene QuikChange site-directed mutagenesis protocol. Plasmids expressing the hemagglutinin (HA)-tagged or myc-tagged versions of IE2 were also generated on a pSG5 background using Gateway technology (Invitrogen). Plasmids for glutathione-S-transferase (GST)-IE2(135-289) and its SIM mutant version were constructed on a pGEX-3X-derived vector. The plasmid for His-SUMO-1 was generated on a pDEST17 (Invitrogen) background. A pET-17b (Novagen)-based plasmid expressing SUMO-1 has been described previously (28). A reporter plasmid containing the HCMV UL54-luciferase (Pol-Luc) reporter gene has been described previously (5). A reporter plasmid containing the UL112-113-luciferase (UL112-113-Luc) (22) was provided by Thomas Stamminger (University Erlangen-Nurnberg, Erlangen, Germany). pT-E1E2S1, which expresses E1, E2, and an active form of SUMO-1, was used to introduce a synthetic SUMO-1 conjugation pathway into Escherichia coli (63). Plasmids for His- or HA-tagged TAF12 (20 kDa) were provided by Robert G. Roeder (The Rockefeller University, New York, NY) and Thomas Oelgeschlager (Marie Curie Research Institute, Oxted, United Kingdom) (9, 21). Plasmids expressing HA-tagged TAF12, TAF12(K19R), or SUMO-1-TAF12 fusion proteins and plasmids expressing GST-TAF12 or GST-SUMO-1-TAF12 were produced on the pSG5 and pGEX-3X backgrounds, respectively, using Gateway technology. The GST-IE1 construct has been described previously (26).
BAC mutagenesis.
The Towne-bacterial artificial chromosome (T-BAC) clone (38) was used as a template for mutagenesis. A 4.1-kb PvuII-SalI restriction fragment containing the wild-type UL122-UL123 allele from the Towne strain and a 2.4-kb BglII-StuI restriction fragment containing the IE2(K175/180R) allele were cloned into the transfer vector, pGS284, a derivative of the positive suicide selection vector (pCV442) (38), and these resulted in pHR8 and pSAN2, respectively. To mutate the IE2 SIM, I, V, I, and S residues between positions 200 and 203 were replaced by A residues on the pHR8 and pSAN2 backgrounds using the Stratagene QuikChange site-directed mutagenesis protocol, resulting in pYH69 and pYH56, respectively. These plasmids were used as transfer vectors. The subsequent procedures for transfer of DNA fragments containing the SIM mutation into E. coli containing the T-BAC clone, and for selection of the cointegrates and the mutant T-BAC clones, have been described previously (23). The mutant T-BAC clones containing the IE2-mSIM and IE2-KR/mSIM mutations were pYH72 and pYH70, respectively. Their revertant T-BAC clones (pYH76 and pYH75, respectively) were generated by the allelic exchange of the mutants using a transfer vector (pHR8) containing the wild-type fragment. The T-BAC clone containing the IE2(K175/180R) mutant (pBAC2) and its revertant clone (pHR14) have been described previously (32). The SIM mutation and its reversion to wild type in the T-BAC clones were confirmed by direct sequencing and by comparing their restriction enzyme patterns using gel electrophoresis, as described previously (23).
Antibodies and indirect immunofluorescence assay (IFA).
Mouse monoclonal antibody (MAb) 12E2 against IE2 and anti-HA MAb 3F10 conjugated with horseradish peroxidase (HRP) or labeled with fluorescein were purchased from Chemicon and Roche, respectively. Mouse MAb 810R, which detects epitopes present in both IE1 and IE2, and mouse MAb 6E1, which is specific to IE1, were obtained from Chemicon and Vancouver Biotech, respectively. The anti-peptide rabbit polyclonal Ab (PAb) P3 for IE2 has been described previously (48). The anti-myc mouse MAb 9E10 conjugated with peroxidase was purchased from Roche. Mouse MAb M23 and the rabbit PAb against the UL112-113 proteins have been described previously (46). Mouse MAbs against p52 (UL44) and pp28 (UL99) were obtained from Virusys. A rabbit anti-peptide PAb referred to as PML(C), directed against amino acids 484 to 498 of premyelocytic leukemia protein (PML), has been described previously (2). An anti-HDAC2 rabbit PAb and an anti-β-actin mouse MAb were purchased from Zymed and Sigma, respectively. An anti-His mouse MAb conjugated with HRP and an anti-GST mouse MAb (B-14) were purchased from Santa Cruz. An anti-RNA polymerase II (anti-Pol II) MAb (8WG16) was purchased from Covance.
For the IFA, cells were fixed in ice-cold methanol and rehydrated in cold Tris-buffered saline (TBS) or fixed with 1% paraformaldehyde and permeabilized using 0.2% Triton X-100. All subsequent procedures have been described previously (5). Slides were examined and photographed using a Zeiss Axiophot microscope. For confocal microscopy, a Carl Zeiss Axioplan 2 confocal microscope system with LSM510 software (Carl Zeiss) was used.
Immunoblot analysis.
For immunoblot analysis, DNA-transfected cells were washed with phosphate-buffered saline (PBS), and total-cell extracts were prepared by boiling the cell pellets in sodium dodecyl sulfate (SDS) loading buffer. Equal amounts of the clarified cell extracts were separated on SDS-8% or 12% polyacrylamide gels and were subjected to a standard enhanced chemiluminescence system procedure (Amersham).
In vitro binding assays.
The GST, GST-fusion, SUMO-1, and His-SUMO-1 proteins were generated in E. coli. The myc-IE2 proteins were synthesized in vitro using the TNT Quick-Coupled transcription/translation system (Promega). The standard procedure for the GST pulldown assays has been described previously (45). The SUMO-1-modified and unmodified His-TAF12 or GST-IE1 proteins were produced by the E. coli SUMOylation system using pT-E1E2S1 (63). Coimmunoprecipitation (CoIP) assays using these proteins were performed with an anti-myc antibody as described previously (23).
Luciferase reporter assay.
For luciferase reporter assays, electroporated HF were collected and cell lysates were prepared using three freeze-thaw steps in 100 μl of 0.25 M Tris-HCl (pH 7.9) plus 1 mM dithiothreitol. Subsequent procedures have been described previously (5). A TD-20/20 luminometer (Turner Designs) was used to measure light output (in relative light units) for the 10-s assay.
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were performed using a ChIP assay kit (Upstate Biotechnology Inc.) with minor modifications. In brief, DNA-transfected HF (1.6 × 106) were fixed in 1% formaldehyde for 10 min and were then lysed using the lysis buffer provided with the kit. ChIP assays were performed with an anti-RNA polymerase II Ab, an anti-IE2 Ab (12E2), or control IgG. One-third of the lysates was used for quantitation of the amount of DNA present in different samples prior to immunoprecipitation. For detection of the HCMV MIE, UL54 (Pol), or UL112-113 promoter, DNAs purified from immune complexes were amplified by PCR using the following primers: for the MIE promoter, 5′-TGGGACTTTCCTACTTGG-3′ (sense) and 5′-CCAGGCGATCTGACGGTT-3′ (antisense); for the UL54 promoter, 5′-TTGACACAGAGACTTGTGATA-3′ (sense) and 5′-TCTGCAAAAACTGTTTCTGTG-3′ (antisense for infection experiments) or 5′-TCAGATACGGGTTGAAAA-3′ (antisense for reporter assays); for the UL112-113 promoter, 5′-GCACGCTGTTTTACTTTTGTCGGG-3′ (sense) and 5′-CATCATCTTTCCAGCCCGCCTAGC-3′ (antisense); and for the MIE exon5 region, 5′-ACCATGCAGGTGAACAACAA-3′ (sense) and 5′-CATGAGGAAGGGAGTGGAGA-3′ (antisense).
RESULTS
Interaction of IE2 with SUMO and defining the IE2 SIM.
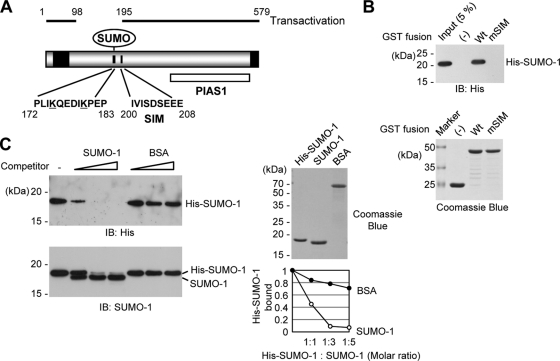
A predicted SIM of HCMV IE2 was found between amino acid residues 200 and 208 (IVISDEEE) near the SUMO modification sites (K175 and K180) (Fig. 1A). To investigate whether the SIM of IE2 indeed mediates noncovalent interaction with SUMO, in vitro binding assays were performed with IE2 and SUMO-1 purified from bacteria. The IE2(135-289) fragment, which contains both the SUMO modification sites and SIM, and its SIM-mutant (mSIM) version with IVIS-to-AAAA mutations between residues 200 and 203, were expressed as GST fusions in E. coli. Untagged and His-tagged SUMO-1 was also produced in E. coli. The results of GST pulldown assays showed that the wild-type IE2 fragment bound to His-SUMO-1 efficiently, whereas the mSIM IE2 did not interact with SUMO at all (Fig. 1B). When a competition assay was performed under the same reaction conditions, the addition of increasing amounts of untagged SUMO-1 efficiently replaced the IE2-bound His-SUMO-1 with untagged SUMO-1 (Fig. 1C). These results demonstrate that the SIM of IE2 is indeed required for SUMO binding.
FIG. 1.
Interaction of IE2 with SUMO through a SIM. (A) SUMO-related domain structures of IE2. Two SUMO conjugation sites (K175 and K180), a SIM (between residues 200 and 208), and the protein inhibitor of the activated STAT1 (PIAS1)-binding region (PIAS1) are indicated. Two transactivation domains identified using the GAL4 swap assays (48) are represented as black boxes. The regions of IE2 required for transactivation (residues 1 to 98 and 195 to 579) are also shown. The numbers are amino acid positions of the protein. (B) In vitro binding of IE2 with SUMO-1 through a SIM. The proteins were purified from bacteria and were used in GST pulldown assays. Five micrograms of GST-IE2(135-289) or its SIM mutant version, IE2(135-289/mSIM), was immobilized on glutathione-Sepharose beads and was incubated with 0.5 μg of His-SUMO-1. The bound proteins were fractionated by SDS-PAGE and were detected by immunoblotting with an anti-His Ab (top), and 17% of the GST or GST fusion protein used in the reaction was also fractionated by SDS-PAGE and stained by Coomassie blue (bottom). Five percent of the His-SUMO-1 used in the binding assays is shown as an input control. (C) Competition assays. The in vitro binding assay for which results are shown in panel B was conducted in the presence of increasing amounts of untagged SUMO-1 or BSA (1:1, 1:3, and 1:5 molar ratio with His-SUMO-1). The bead-bound proteins were detected by immunoblotting (IB) with an anti-His Ab (left, top) or with anti-SUMO-1 Ab (left, bottom). Two micrograms of the SUMO-1 and BSA proteins used is shown by Coomassie blue staining (right, top). The relative amounts of His-SUMO-1 bound to IE2 in the presence of competitors were quantified and are shown in the graph (right, bottom).
In HCMV-infected cells, IE2 is initially localized adjacent to PML-associated nuclear bodies (NBs), where deposition of the input viral genome and viral IE transcription occurs (3, 4, 24). Late after infection, IE2 accumulates at the viral DNA replication sites (3, 4, 24). However, when expressed alone by transfection, IE2 is distributed as a mixed nuclear pattern combining a background of diffuse distribution and a punctate form that is perfectly colocalized with PML-NBs (3). To investigate whether the SIM contributes to this IE2 localization pattern in transfected cells, HF were transfected with wild-type IE2, a K175/190R (KR) mutant defective in SUMOylation, a SIM mutant (mSIM), and a KR/mSIM mutant containing both the KR and mSIM mutations, and the localization patterns were determined by an IFA. The results showed that, consistent with our earlier observation (32), the KR mutant protein was distributed in a pattern similar to that of the wild-type protein, forming foci that colocalize with PML-NBs, whereas both mSIM and KR/mSIM mutants did not form foci at all but were just distributed in a nuclear diffuse form (see Fig. S1 in the supplemental material). Considering that SUMO-modified proteins are highly concentrated in PML-NBs, these results suggest that the IE2 SIM may provide a surface for interactions with other SUMO-modified proteins.
IE2 SIM is required for efficient transactivation function.
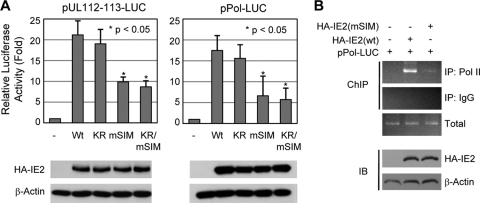
SUMOylation of IE2 enhanced its transactivation capacity for several viral and cellular promoters (5, 22, 35). However, our previous studies using a Towne strain-based recombinant virus encoding the K175/180R (KR) mutant form of IE2, which is defective in SUMOylation, demonstrated that IE2 SUMOylation is not essential for viral replication in permissive HF (32). Notably, two SUMO modification sites, K174 and K180, are located beyond the region that was previously mapped and identified as necessary for the transactivation function of IE2 (1, 48, 49); however, the IE2 SIM lies within this region (Fig. 1A). We examined the role of the IE2 SIM in the IE2 transactivation function. The results of reporter assays performed in HF showed that, consistent with our earlier observation (5), the IE2 KR mutant transactivated the viral UL112-113 and polymerase (UL54) promoters to similar or just marginally reduced levels as wild-type IE2 in a situation where SUMO is not overexpressed (Fig. 2A). However, the SIM mutants of IE2, either mSIM or KR/mSIM, showed significantly lower levels of transactivation activity for these promoters than wild-type IE2 (Fig. 2A). The results of ChIP assays performed in similarly transfected cells showed that cellular RNA polymerase II was less efficiently recruited to the viral polymerase promoter in cells transfected with the mSIM mutant than in cells transfected with wild-type IE2 (Fig. 2B). These results demonstrate that the noncovalent SUMO-binding activity of IE2 through a SIM plays an important role in the transactivation function.
FIG. 2.
Effect of the SIM mutation on the transactivation function of IE2. (A) (Top) HF (2 × 105) were electroporated with 0.5 μg of a plasmid containing a luciferase reporter gene driven by the HCMV UL112-113 promoter (pUL112-113-Luc) or polymerase promoter (pPol-Luc) and 1.5 μg of a plasmid expressing wild-type or mutant (KR, mSIM, or mSIM/KR) IE2. At 48 h after transfection, whole-cell lysates were prepared and assayed for luciferase activity. Luciferase activities are indicated as fold activation over the basal level of a parent vector. The results shown are the mean values with standard errors for three independent experiments. (Bottom) The expression levels of HA-IE2 proteins and β-actin in cell lysates of a representative assay are shown in immunoblots. (B) (Top) ChIP assays. HF in 150-mm-diameter dishes were electroporated with 2 μg of a plasmid containing the Pol-Luc reporter gene and 6 μg of a plasmid expressing wild-type or mSIM IE2. At 48 h after transfection, the ChIP assays (see Materials and Methods) were performed with an anti-RNA polymerase II (anti-Pol II) Ab and IgG (as a control). (Bottom) The expression levels of wild-type or mutant HA-IE2 proteins and β-actin in cell lysates are shown in immunoblots.
Growth defects of recombinant viruses encoding mSIM- or KR/mSIM-IE2s.
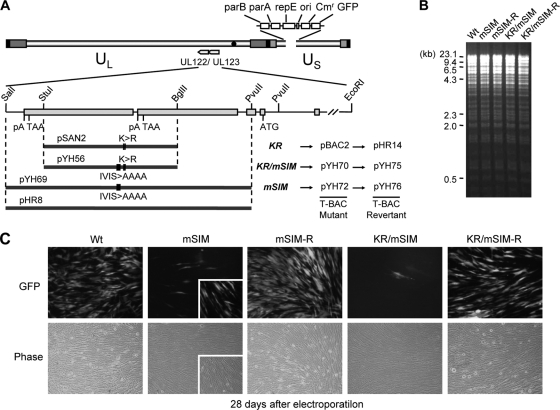
To evaluate the role of the IE2 SIM in viral growth in the context of virus infection, we constructed two T-BAC clones encoding the SIM mutants of IE2 (mSIM and KR/mSIM) and their revertants (Fig. 3A). The introduction of the desired mutation was confirmed by direct sequencing. Comparison of the restriction enzyme-digested patterns of the wild-type, mutant, and revertant T-BAC clones did not show any apparent alteration of their T-BAC DNAs (Fig. 3B). When permissive HF were transfected with the BAC DNAs via electroporation, both mSIM and KR/mSIM viruses grew very slowly compared to wild-type and revertant viruses (Fig. 3C). These results suggest that the SIM mutation causes severely defective growth of the Towne virus in HF.
FIG. 3.
Generation of the IE2 SIM mutant and its revertant HCMV (Towne) BAC clones and their infectivities in transfected HF. (A) Scheme for the generation of the IE2 SIM mutant (mSIM) Towne (T)-BACs and their revertant T-BAC clones. A pGS284 (38) derivative (pYH69), which harbors a 4.1-kb DNA fragment containing the mSIM allele of IE2, was used for homologous recombination with the parental T-BAC clone. This recombination event resulted in the IE2(mSIM) T-BAC clone (pYH72). Similarly, the IE2(KR/mSIM) T-BAC clone (pYH70) was produced using a GS284 derivative (pYH56) containing a 2.4-kb IE2 KR/mSIM allele. A pGS284 derivative (pHR8) containing a 4.1-kb wild-type DNA fragment was used to make their revertant T-BAC clones (pYH76 and pYH75). The IE2 KR mutant T-BAC (pBAC2) and its revertant (pHR14) have been described previously (32). (B) Restriction fragment DNA patterns obtained following EcoRI/BamHI digestion of the wild-type, mutant, and revertant T-BAC DNAs were analyzed by agarose gel electrophoresis. λ-HindIII was used as the molecular weight standard. (C) Infectivities of the transfected T-BAC DNAs. HF were transfected with T-BAC clones (Wt, mSIM [pYH72], mSIM-R [pYH76], KR/mSIM [pYH70], and KR/mSIM-R [pYH75]) via electroporation (see Materials and Methods). The cells were monitored for the spread of GFP signals. GFP images (top) and their phase-contrast images (bottom) were photographed 4 weeks after electroporation. Representative images from at least four independent experiments are shown. The inserts in the images of mSIM virus infection include some GFP-positive cells. Note that the spread of these GFP signals in cells transfected with the mSIM and KR/mSIM T-BAC DNAs was usually incomplete compared to the spread in cells transfected with the wild-type or revertant T-BAC DNAs.
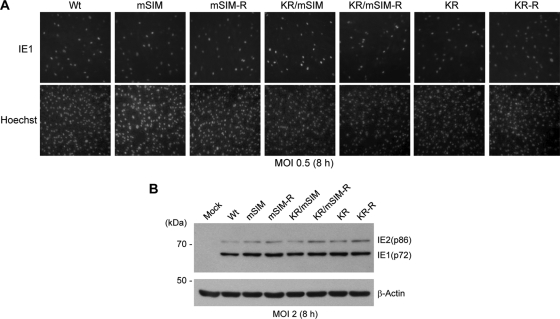
IE2-complementing cells are not currently available, probably because IE2 is highly toxic. However, we obtained high titers of mSIM- and KR/mSIM-IE2 viruses by growing them in HF overexpressing IE1, an HCMV-encoded regulator that inhibits both PML-mediated and type I interferon-mediated antiviral responses (2, 23, 34, 47) and that augments the transactivation activity of IE2 (41). When normal HF were infected with wild-type, SIM mutant (mSIM and KR/mSIM), and KR mutant viruses (32) and their revertant (mSIM-R, KR/mSIM-R, and KR-R) viruses at a multiplicity of infection (MOI) of 0.5 and were stained with an anti-IE1 antibody (Ab) early in the infection (8 h), the numbers of IE1-positive cells were comparable among all viral infections (Fig. 4A). When HF were infected with viruses at an MOI of 2, the expression levels of the 72-kDa IE1 and 86-kDa IE2, measured by immunoblotting 8 h after infection, were similar among these viruses (Fig. 4B). These results demonstrate that the absence of the IE2 SIM does not notably affect the accumulation of unmodified IE1 and IE2 proteins early in the infection.
FIG. 4.
Expression levels of unmodified IE1 and IE2 in recombinant virus-infected HF at early times. (A) HF cultured in chamber slides were infected with the indicated viruses at an MOI of 0.5. At 8 h after infection, cells were fixed in cold methanol and were stained with the IE1-specific Ab 6E1. Hoechst stain was used to visualize the nuclei of all cells in the field. (B) HF in 6-well plates were infected with the indicated viruses at an MOI of 2. Whole-cell lysates were prepared at 8 h after infection, and the expression levels of unmodified IE1 and IE2 were determined by immunoblotting using 810R (for IE1 and IE2) and anti-β-actin mouse MAbs.
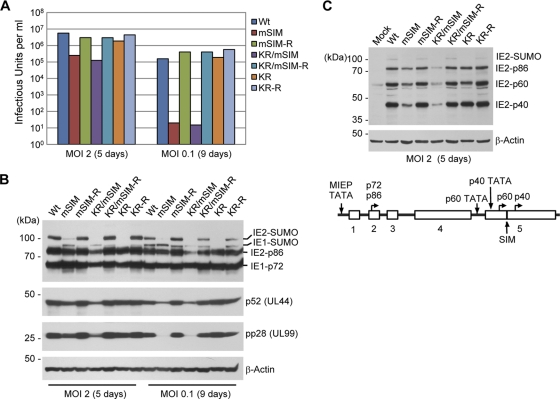
We next compared the titers of wild-type and mutant (mSIM, KR, and KR/mSIM) viruses and their revertant viruses produced from infected HF. When the total amounts of virus produced (intracellular and extracellular combined) were measured at 5 days after infection at an MOI of 2 or at 9 days after infection at an MOI of 0.1, the titers of mSIM and KR/mSIM viruses were 20-fold lower at an MOI of 2 and 10,000-fold lower at an MOI of 0.1 than those of wild-type and revertant viruses (Fig. 5A). Consistent with our earlier observation (32), the titers of the KR mutant virus were similar to those of wild-type virus at both MOIs (Fig. 5A). In immunoblot assays with cell extracts prepared at the same time points, the mSIM virus showed reduced accumulation of SUMO-modified IE2, p52 (an early protein), and pp28 (a late protein) at both MOIs (Fig. 5B). For the KR/mSIM virus, as expected, no SUMO-modified IE2 was detected, and the levels of the IE2, p52, and pp28 proteins were significantly reduced (Fig. 5B). Importantly, in cells infected at a low MOI, both mSIM and KR mutant viruses expressed comparable amounts of unmodified IE2 and little or no SUMO-modified IE2 (Fig. 5B); however, the titer of the IE2 SIM mutant was 10,000-fold less than that of the KR mutant IE2 virus (Fig. 5A), suggesting that abrogation of SIM has a more profound effect on viral growth than absence of SUMOylation in this virus. Two late proteins, p60 and p40, are expressed within exon 5. It has been found that the SIM mutation in the viral genome does not disturb the p60 and p40 initiator methionine residues (located at positions 170 and 220, respectively) or their upstream TATAA sequences (53). We found that generation of the SIM mutant did not abrogate the expression of the smaller IE2 forms (Fig. 5C). The reduced titers of mSIM and KR/mSIM viruses also correlated well with the slow spread of green fluorescent protein (GFP) signals to neighboring cells (see Fig. S2 in the supplemental material).
FIG. 5.
Comparison of the progeny virus titers of wild-type, SIM mutant, SUMOylation-defective mutant, and revertant viruses, and accumulation of viral proteins in infected cells. (A) HF in 12-well plates were infected with wild-type, mSIM, KR/mSIM, or KR viruses and their revertant viruses at an MOI of 2 for 5 days or at an MOI of 0.1 for 9 days. The progeny virus titers are shown as infectious units in cell culture supernatants, which were determined by infectious center assays. The results shown are the averages of data from two independent assays. (B and C) Accumulation of viral IE, early, and late proteins in infected cells. HF in 6-well plates were infected as described for panel A, and whole-cell lysates were prepared and were subjected to SDS-8% PAGE, followed by immunoblotting with 810R (for IE1 and IE2), anti-p52, anti-pp28, and anti-β-actin MAbs (B) and with an anti-peptide rabbit PAb (P3) for IE2, which detects all of the p86, p60, and p40 IE2 proteins (48) (C, top). The positions of the IE2 SIM and the TATA sequences and start codons for p60 and p40 are shown (C, bottom).
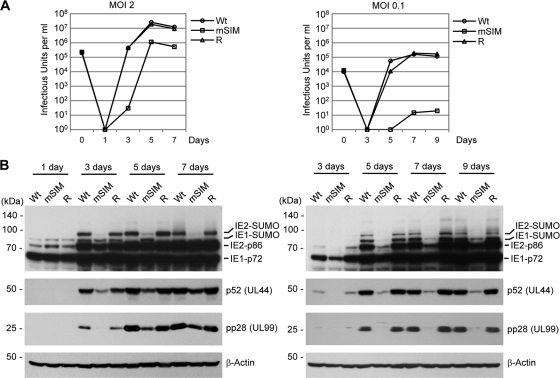
We further analyzed the growth curves of mSIM virus at high and low MOIs in HF. The results showed that at an MOI of 2, the growth of mSIM virus was delayed 1 or 2 days and the highest titer was 20-fold less than the titers of wild-type and revertant viruses (Fig. 6A, left). At an MOI of 0.1, the growth of mutant virus was more profoundly delayed, and the maximum titer was 10,000-fold lower than the titers of wild-type and revertant viruses (Fig. 6A, right). The reduced growth of mSIM virus at an MOI of 2 correlated with the reduced accumulation of SUMO-modified IE2, p52, and pp28 (Fig. 6B, left). At an MOI of 0.1, the accumulation of the IE1, IE2, p52, and pp28 proteins was more severely reduced in mSIM virus-infected cells (Fig. 6B, right). Overall, these data demonstrate that the growth defect of mSIM virus is attributed to less-efficient accumulation of SUMO-modified IE2 and early and late proteins. This observation is consistent with the reduced transactivation activity of IE2 in the SIM mutant (Fig. 2).
FIG. 6.
Growth curves of wild-type, SIM mutant, and revertant viruses and the expression levels of viral proteins. (A) HF in 12-well plates were infected with the wild-type (Wt), SIM mutant (mSIM), and revertant viruses at an MOI of 2 or 0.1. The time course results represent the total numbers of cell-free viruses produced in cell culture supernatants at the indicated sampling times, which were measured as described in the legend to Fig. 5A. The results are averages of data from two independent assays. (B) HF in 6-well plates were infected with recombinant viruses at an MOI of 2 or 0.1 as for panel A. Accumulation of viral proteins at the indicated sampling times was determined by immunoblotting as described in the legend to Fig. 5B (left, MOI of 2; right, MOI of 0.1).
Reduced formation of viral transcription domains in mSIM virus infection.
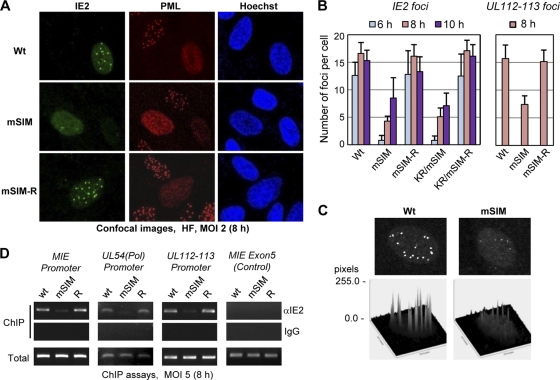
We investigated whether the formation of IE2-containing viral transcription domains (4, 24, 59) at early times is affected in mSIM virus-infected cells. HF were infected with viruses at an MOI of 2, double-labeled for IE2 and PML 8 h after infection, and analyzed using a confocal microscope. At this time point, wild-type IE2 was localized in viral transcription domains (i.e., as nuclear foci) on the nuclear diffuse background; however, the formation of viral transcription domains was markedly lower in mSIM virus-infected cells than in wild-type and revertant virus-infected cells (Fig. 7A). In quantitative assays performed at 6, 8, and 10 h after infection, the numbers of IE2 foci produced in mSIM or KR/mSIM virus-infected cells were about 10-fold lower at 6 h than those in cells infected with wild-type or revertant viruses, although they reached about 50% of IE2 foci produced in wild-type and revertant viruses by 10 h after infection (Fig. 7B, left graph). The intensity of IE2 foci was also weaker in mSIM virus-infected cells than in wild-type and revertant virus-infected cells (Fig. 7C). The inefficient targeting of IE2 to viral transcription domains may be attributed to its aberrant localization property, as seen in transfected cells (see Fig. S1 in the supplemental material). However, when we examined the localization patterns of UL112-113 and HDAC2, which accumulate at viral transcription sites (4, 45), both proteins also accumulated less efficiently at viral transcription domains in mSIM virus-infected cells (Fig. 7B, right graph; see also Fig. S3 in the supplemental material). These results suggest that the IE2 SIM is required for the efficient targeting of IE2 and other IE2-associated proteins, such as UL112-113 and HDAC2, to viral transcription sites. Consequently, the development of normal-sized viral replication compartments in the infected-cell nuclei was also delayed in mSIM virus-infected cells (see Fig. S4 in the supplemental material).
FIG. 7.
Reduced formation of the viral transcription domains in IE2 SIM mutant virus infection. (A) HF in chamber slides were infected with wild-type, IE2 SIM mutant (mSIM), or revertant (mSIM-R) viruses at an MOI of 2. At 8 h after infection, the cells were fixed in cold methanol, followed by a confocal double-label IFA using anti-IE2 and anti-PML Abs. DNA in cell nuclei was stained with Hoechst dye. (B) Quantitation of the viral transcription domains. HF were infected as for panel A. Cells were fixed at 6, 8, and 10 h after infection and were stained with an anti-IE2 Ab or were fixed at 8 h and stained with an anti-UL112-113 Ab as for panel A. The IE2 or UL112-113 foci were counted in at least 20 infected (i.e., IE2- or UL112-113-positive) cells, and the results are shown in the graphs, as the mean values for the number of IE2 or UL112-113 foci per cell with standard errors. (C) Comparison of the intensities of IE2 signals in foci between wild-type and mSIM virus-infected cells. Surface plot analysis of an infected cell nucleus at 10 h after infection was performed with the ImageJ program (NIH). (D) ChIP assessment of the amounts of IE2 loaded on viral promoters (MIE, UL54, and UL112-113) in infected cells. HF were infected with wild-type, mSIM, or revertant viruses at an MOI of 5 for 8 h. ChIP assays were conducted with an anti-IE2 mouse MAb or nonspecific mouse immunoglobulin G (IgG). The amounts of coprecipitated DNA fragments were determined by PCR and agarose gel electrophoresis. The MIE exon 5 region was tested as a negative control.
We next investigated whether the association of IE2 with the HCMV major IE (MIE), UL54, and UL112-113 promoters is affected in mSIM virus infection. Chromatin immunoprecipitation (ChIP) assays were performed on HF infected with wild-type, mSIM, or revertant viruses 8 h after infection. The results showed that the level of association of IE2 with these promoters was significantly lower in mSIM virus-infected cells than in wild-type or revertant virus-infected cells (Fig. 7D). IE2 did not associate with the MIE exon 5 region in a control experiment (Fig. 7D). This result suggests that the reduced formation of viral transcription domains in mSIM virus may result from the reduced association of IE2 with viral promoters. The minimal region of IE2 for DNA-binding activity is residues 346 to 542 (12). Since the IE2 SIM lies beyond this region, it is unlikely that the mSIM mutation affected the direct DNA binding activity of IE2. Indeed, the mSIM mutant still autoregulated its own promoter in reporter assays (data not shown).
IE2 interacts with SUMO-modified TAF12 through a SIM, and this interaction contributes to the IE2-mediated transactivation function.
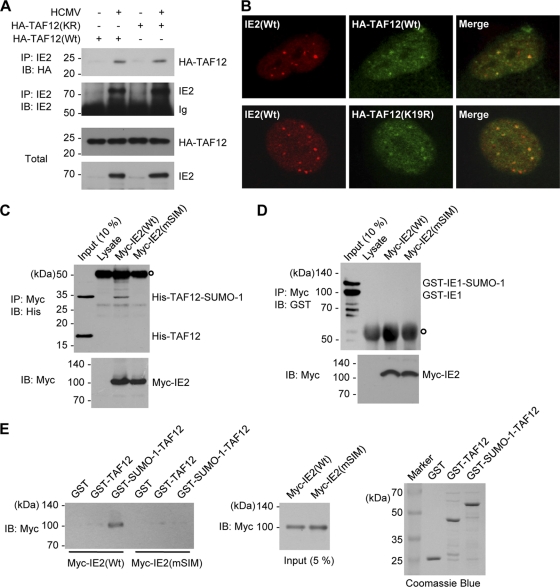
Among general transcription factors, recombinant TBP and TBP-associated factors (TAFs), such as TAF5 and TAF12, have recently been shown to be modified by SUMO (9, 51). TBP has been shown to interact physically with unmodified IE2 (11, 19, 25, 57). In this study, we investigated the possible association of IE2 with SUMO-modified TAF12. When HF were first transfected with hemagglutinin (HA)-tagged wild-type and SUMOylation-defective K19R mutant TAF12 and were then infected with HCMV, IE2 was found to form a complex with both wild-type and K19R mutant TAF12 proteins (Fig. 8A). In similarly transfected and infected cells, both wild-type and mutant TAF12 proteins were shown to be colocalized with IE2 in viral transcription domains, demonstrating that the recruitment of TAF12 to viral transcription sites is independent of its SUMOylation (Fig. 8B).
FIG. 8.
Interaction of IE2 with the SUMO-modified form of TAF12. (A) CoIP assays for IE2 and TAF12 in infected cells. HF in 100-mm-diameter dishes were first transfected with wild-type or K19R mutant TAF2 via electroporation and then infected with HCMV at an MOI of 5. At 8 h, whole-cell lysates were prepared, and CoIP assays were performed with an anti-IE2 Ab. (B) Colocalization of IE2 and TAF12 in infected cells. HF cultured in chamber slides were transfected and infected as described for panel A. Cells were fixed in cold methanol, and confocal double-label IFA was performed with anti-IE2 and anti-HA Abs. (C) In vitro interaction of IE2 with SUMO-modified TAF2 in a SIM-dependent manner. His-TAF12 and SUMO-1-modified His-TAF12 proteins, which were produced in E. coli cotransformed with plasmids expressing the SUMO conjugating enzymes (pT-E1E2-S1) (see Materials and Methods) and His-TAF12, were incubated with the control rabbit reticulocyte lysates or in vitro-translated myc-IE2(Wt) or myc-IE2(mSIM) proteins. After immunoprecipitation with an anti-myc Ab, the coprecipitated proteins were immunoblotted with an anti-His Ab (top). Ten percent (each) of the His-TAF12 proteins and myc-IE2 proteins is shown as an input control. The open circle at the top left indicates immunoglobulins. (D) An assay similar to that for which results are shown in panel C was conducted using GST-IE1 and SUMO-modified GST-IE1, which were produced in E. coli. (E) In vitro GST pulldown assays. GST, GST-TAF12, and GST-SUMO-1-TAF12 fusion proteins produced in bacteria were incubated with in vitro-translated myc-IE2(Wt) or myc-IE2(mSIM) proteins. The bound IE2 proteins were identified by immunoblotting with an anti-Myc Ab (left panel). The amounts of myc-IE2 and GST or GST fusion proteins used are shown as input controls (center and right panels).
We next investigated whether IE2 interacts physically with intact or SUMO-modified TAF12. Unmodified and SUMO-1-modified forms of His-TAF12 were produced in E. coli and used for in vitro binding assays with IE2 translated in vitro. The results showed that SUMO-modified TAF12, but not unmodified TAF12, interacts with IE2 in an IE2 SIM-dependent manner (Fig. 8C). IE2 did not interact with SUMO-modified IE1 under similar experimental conditions (Fig. 8D). This suggests that either the interaction between IE2 and SUMO-modified TAF12 is context dependent or the SUMO moiety conjugated to lysine 450 of IE1 is masked from being recognized by IE2. We also used the SUMO-1-TAF12 fusion protein as a surrogate for the SUMO-modified form of TAF12. In GST pulldown assays using bacterially produced GST, GST-TAF12, and GST-SUMO-1-TAF12 proteins, in vitro-translated IE2 interacted only with GST-SUMO-1-TAF12, not with GST or GST-TAF12, and mSIM IE2 did not interact with GST-SUMO-1-TAF12 (Fig. 8E). Collectively, these in vitro binding assay results demonstrate that IE2 interacts with SUMO-modified TAF12 through a SIM.
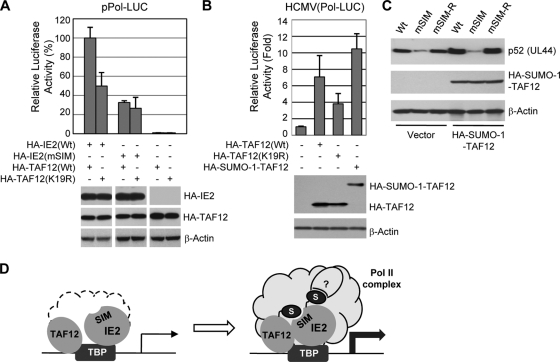
To evaluate the role of the interaction between IE2 and SUMO-modified TAF12 in the IE2 transactivation function, we performed reporter assays with HF using a viral DNA polymerase (UL54)-luciferase reporter construct. The results showed that the IE2-mediated transactivation capacity for the polymerase promoter under TAF12-overexpressing conditions was 50% less when a SUMOylation-defective K19R mutant was used instead of wild-type TAF12; however, the transactivation activity mediated by mSIM IE2 was similar in wild-type and K19R mutant TAF12-expressing cells (Fig. 9A). We also measured the effect of the interaction between IE2 and SUMO-modified TAF12 on the activation of the polymerase promoter in virus-infected cells. When HF were first transfected with HA-tagged wild-type, K19R mutant, and SUMO-1-TAF12 fusion proteins and were then infected with a recombinant virus containing the polymerase-luciferase reporter gene, IE2 activated the viral polymerase promoter more efficiently in cells transfected with wild-type TAF12 than in cells transfected with the K19R mutant, and the transactivation activity of IE2 was higher in cells expressing the SUMO-1-TAF12 fusion protein than in cells expressing wild-type TAF12 (Fig. 9B).
FIG. 9.
Role of the interaction between IE2 and SUMO-modified TAF12 in the transactivation function of IE2. (A) HF (2 × 105) were transfected by electroporation with a plasmid containing the Pol (UL54)-luciferase reporter gene and plasmids expressing wild-type or mutant HA-IE2 or HA-TAF12 as indicated. At 24 h after transfection, whole-cell lysates were prepared, and luciferase assays were performed. Luciferase activities are expressed as percentages of the level in cells transfected with both the wild-type IE2 and the wild-type TAF12 protein. The results shown are the mean values for three independent experiments with standard errors. The expression levels of HA-IE2 and HA-TAF12 proteins and β-actin in cell lysates are shown in immunoblots. (B) HF were transfected with plasmids encoding wild-type TAF12, TAF12(K19R), or the HA-SUMO-1-TAF12 fusion protein and were then infected with a recombinant virus containing a Pol-luciferase reporter gene (Pol-LUC) at an MOI of 1. At 24 h after infection, whole-cell lysates were prepared, and luciferase activities were assayed as for panel A. The expression levels of the HA-TAF12 and HA-SUMO-1-TAF12 proteins and β-actin in cell lysates are shown in immunoblots. (C) HF were transfected with an empty vector or a plasmid encoding HA-SUMO-1-TAF12 and were then infected with wild-type, mSIM mutant, or revertant (mSIM-R) virus at an MOI of 1. At 48 h after infection, cell lysates were prepared and analyzed by immunoblotting. (D) Hypothetical model showing the role of the covalent and noncovalent SUMO attachment of IE2 in transactivation activity. Both IE2 and TAF12 can be associated with TBP in the viral transcription domain. The binding of IE2 with SUMO-modified TAF12 through a SIM may contribute to the enhanced stabilization of the transcription initiation complex containing RNA Pol II. It is also likely that SUMO-modified IE2 may interact with other SIM-containing transcription factors, which may also promote the formation of the transcription initiation complex.
To investigate the role of the interaction between IE2 and SUMO-modified TAF12 in virus-infected cells, control HF and cells expressing the SUMO-TAF12 fusion protein were infected with wild-type, mSIM, and mSIM-R viruses, and the expression level of p52 (UL44) was examined. The results showed that the p52 levels in wild-type and mSIM-R virus infections were higher in cells expressing the SUMO-1-TAF12 fusion protein than in control cells, but the p52 level in mSIM virus infection was not increased in SUMO-1-TAF12-expressing cells (Fig. 9C). These results suggest that the interaction of IE2 with SUMO-modified TAF12 contributes to the transactivation function of IE2 in virus-infected cells. The possible role of noncovalent interaction of IE2 with SUMO-modified TAF12 and its covalent SUMO modification in enhancing the formation of the IE2-containing transcription initiation complex is illustrated in Fig. 9D.
DISCUSSION
In this study, we provide evidence that the noncovalent SUMO-binding activity of IE2 plays an important role in the transactivation function of IE2 and in the progression of HCMV lytic growth. Roles for noncovalent SUMO binding in the enhancement of protein SUMOylation have been reported in studies using several SIM-containing SUMO substrates, such as USP25, Sp100, BLM, and HIPK2 (30, 39, 70). A SIM in proteins is thought to be recognized by a SUMO covalently linked to active-site cysteine (C93) (70) or to lysine (K14) (30) residues of Ubc9, a SUMO E2 enzyme, leading to enhanced transfer of SUMO from Ubc9 to target lysine residues of substrate proteins. IE2 belongs to a group of SUMO substrates in which SUMOylation is enhanced by noncovalent SUMO binding through a SIM (7, 28). In our study, the SUMO-modified IE2 consistently accumulated less efficiently in IE2 SIM mutant virus-infected cells (Fig. 5 and 6).
Although SUMOylation of IE2 enhances its transactivation activity, and the IE2 SIM is necessary for efficient SUMOylation of IE2, it is unlikely that the reduced transactivation activity of the mSIM IE2 can be attributed solely to the reduction in IE2 SUMOylation. In our reporter assays performed under conditions without SUMO overexpression, the IE2 KR mutant, which is defective in SUMOylation, had a level of transactivation activity similar to that of wild-type IE2; however, under the same conditions, the IE2 SIM mutant showed significantly lower transactivation activity than wild-type IE. Therefore, noncovalent SUMO binding by IE2 is also able to promote the IE2 transactivation function in a manner independent of the degree of IE2 SUMOylation. In this regard, it is notable that the minimum IE2 region required for transactivation functioning, which was determined by reporter assays, includes the SIM but not the SUMO conjugation site (Fig. 1A).
Our analysis of the growth characteristics of recombinant viruses clearly demonstrates that the SIM mutant viruses (both mSIM and mSIM/KR viruses) have severely defective growth at both high and low MOIs, indicating the important role of the IE2 SIM in the promotion of viral growth. In addition to reduced transactivation activity in reporter assays, the less efficient formation of viral transcription domains and the reduced association of the mSIM IE2 with viral promoters in mutant virus-infected cells (Fig. 7) may account for defective growth by the mSIM IE2 viruses. Since the mSIM IE2, unlike the wild-type protein, did not target PML-NBs in transfected cells (see Fig. S1 in the supplemental material), the IE2 SIM is thought to provide a surface for interaction with other cellular proteins. The role of SIMs in building a protein-protein interaction network has been well demonstrated with PML (56).We consistently observed that some cellular proteins, which were isolated as IE2-interacting partners in yeast two-hybrid screening, interacted with IE2 in a SIM-dependent manner (data not shown). More importantly, we showed in this study that IE2 interacts with SUMO-modified TAF12 and that this interaction promotes the IE2-mediated transactivation function on the viral polymerase promoter. SUMOylation of TAF12 does not appear to be necessary for the recruitment of TAF12 to viral transcription domains, since SUMOylation-defective TAF12(K19R) was still associated with IE2 in CoIP assays and IFAs. However, the direct interaction of IE2 with the SUMO-modified form of TAF12 through a SIM is thought to enhance the formation of a stable transcription initiation complex (Fig. 9C). This interaction may also explain how the IE2 SIM promotes the transactivation function in our reporter assays. This scenario is consistent with an earlier observation that transactivation by IE2 occurs after the recruitment of TBP (29). However, given that many transcription factors and the subunits of RNA polymerase I, II, and III (44, 65) are SUMO substrates, it is possible that the IE2 SIM is also recognized by other SUMO-modified transcription factors. For many cellular transcription factors, SUMOylation often promotes the recruitment of corepressors and subsequently inhibits target gene expression (17). Many corepressors are also modified by SUMO, and their SUMOylation enhances repression activity (16). Therefore, it may also be possible that IE2 interacts with SUMO-modified transcription repressors and interferes with the formation of the SUMO-associated repressor complexes.
The 72-kDa IE1 protein is also covalently modified by SUMO (61, 67). Interestingly, we found that the accumulation levels of the SUMO-modified form of IE1 in infected cells were generally higher at a low MOI than at a high MOI, and in cells infected with a SIM mutant virus than in those infected with wild-type virus (Fig. 5B). This suggests that the level of IE1 SUMOylation may be regulated by different MOIs and by the SUMO-binding ability of IE2. The role of IE1 SUMOylation is not clear. It has been suggested that IE1 SUMOylation promotes viral growth (42) and affects the intracellular localization of IE1 (52). However, it has also been reported that the absence of IE1 SUMOylation did not affect the abilities of IE1 to augment the IE2-mediated transactivation function in reporter assays (34) and to complement the defective growth of IE1-deleted mutant virus in IE1-expressing cells (61). We recently found that SUMOylation of IE1 interferes with the binding of IE1 to STAT2, which is thought to be a viral mechanism to inhibit type I interferon responses (23), suggesting that, unlike IE2, IE1 SUMOylation may be a mechanism of cellular defense against HCMV infection. In this regard, increased IE1 SUMOylation at a low MOI and in SIM mutant viruses also suggests a possible role for the IE2 SIM in limiting IE1 SUMOylation. Whether HCMV has a strategy to downregulate IE1 SUMOylation is an intriguing question that should be addressed.
Supplementary Material
Acknowledgments
We thank Robert G. Roeder, Thomas Oelgeschlager, Thomas Stamminger, and Gary S. Hayward for reagents.
This work was supported by the Ubiquitome Research Program (2009-0065395) and the Mid-Career Research Program (2009-0078805) through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology.
Footnotes
Published ahead of print on 2 June 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Ahn, J. H., C. J. Chiou, and G. S. Hayward. 1998. Evaluation and mapping of the DNA binding and oligomerization domains of the IE2 regulatory protein of human cytomegalovirus using yeast one and two hybrid interaction assays. Gene 210:25-36. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, J. H., and G. S. Hayward. 2000. Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection. Virology 274:39-55. [DOI] [PubMed] [Google Scholar]
- 3.Ahn, J. H., and G. S. Hayward. 1997. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J. Virol. 71:4599-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn, J. H., W. J. Jang, and G. S. Hayward. 1999. The human cytomegalovirus IE2 and UL112-113 proteins accumulate in viral DNA replication compartments that initiate from the periphery of promyelocytic leukemia protein-associated nuclear bodies (PODs or ND10). J. Virol. 73:10458-10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn, J. H., Y. Xu, W. J. Jang, M. J. Matunis, and G. S. Hayward. 2001. Evaluation of interactions of human cytomegalovirus immediate-early IE2 regulatory protein with small ubiquitin-like modifiers and their conjugation enzyme Ubc9. J. Virol. 75:3859-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrasa, M. I., N. Harel, Y. Yu, and J. C. Alwine. 2003. Strain variations in single amino acids of the 86-kilodalton human cytomegalovirus major immediate-early protein (IE2) affect its functional and biochemical properties: implications of dynamic protein conformation. J. Virol. 77:4760-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berndt, A., H. Hofmann-Winkler, N. Tavalai, G. Hahn, and T. Stamminger. 2009. Importance of covalent and noncovalent SUMO interactions with the major human cytomegalovirus transactivator IE2p86 for viral infection. J. Virol. 83:12881-12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonin, L. R., and J. K. McDougall. 1997. Human cytomegalovirus IE2 86-kilodalton protein binds p53 but does not abrogate G1 checkpoint function. J. Virol. 71:5861-5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer-Guittaut, M., K. Birsoy, C. Potel, G. Elliott, E. Jaffray, J. M. Desterro, R. T. Hay, and T. Oelgeschlager. 2005. SUMO-1 modification of human transcription factor (TF) IID complex subunits: inhibition of TFIID promoter-binding activity through SUMO-1 modification of hsTAF5. J. Biol. Chem. 280:9937-9945. [DOI] [PubMed] [Google Scholar]
- 10.Bryant, L. A., P. Mixon, M. Davidson, A. J. Bannister, T. Kouzarides, and J. H. Sinclair. 2000. The human cytomegalovirus 86-kilodalton major immediate-early protein interacts physically and functionally with histone acetyltransferase P/CAF. J. Virol. 74:7230-7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caswell, R., C. Hagemeier, C. J. Chiou, G. Hayward, T. Kouzarides, and J. Sinclair. 1993. The human cytomegalovirus 86K immediate early (IE) 2 protein requires the basic region of the TATA-box binding protein (TBP) for binding, and interacts with TBP and transcription factor TFIIB via regions of IE2 required for transcriptional regulation. J. Gen. Virol. 74(Pt. 12):2691-2698. [DOI] [PubMed] [Google Scholar]
- 12.Chiou, C. J., J. Zong, I. Waheed, and G. S. Hayward. 1993. Identification and mapping of dimerization and DNA-binding domains in the C terminus of the IE2 regulatory protein of human cytomegalovirus. J. Virol. 67:6201-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi, K. S., S. J. Kim, and S. Kim. 1995. The retinoblastoma gene product negatively regulates transcriptional activation mediated by the human cytomegalovirus IE2 protein. Virology 208:450-456. [DOI] [PubMed] [Google Scholar]
- 14.Fortunato, E. A., M. H. Sommer, K. Yoder, and D. H. Spector. 1997. Identification of domains within the human cytomegalovirus major immediate-early 86-kilodalton protein and the retinoblastoma protein required for physical and functional interaction with each other. J. Virol. 71:8176-8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furnari, B. A., E. Poma, T. F. Kowalik, S. M. Huong, and E. S. Huang. 1993. Human cytomegalovirus immediate-early gene 2 protein interacts with itself and with several novel cellular proteins. J. Virol. 67:4981-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Dominguez, M., and J. C. Reyes. 2009. SUMO association with repressor complexes, emerging routes for transcriptional control. Biochim. Biophys. Acta 1789:451-459. [DOI] [PubMed] [Google Scholar]
- 17.Gill, G. 2005. Something about SUMO inhibits transcription. Curr. Opin. Genet. Dev. 15:536-541. [DOI] [PubMed] [Google Scholar]
- 18.Hagemeier, C., R. Caswell, G. Hayhurst, J. Sinclair, and T. Kouzarides. 1994. Functional interaction between the HCMV IE2 transactivator and the retinoblastoma protein. EMBO J. 13:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagemeier, C., S. Walker, R. Caswell, T. Kouzarides, and J. Sinclair. 1992. The human cytomegalovirus 80-kilodalton but not the 72-kilodalton immediate-early protein transactivates heterologous promoters in a TATA box-dependent mechanism and interacts directly with TFIID. J. Virol. 66:4452-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannich, J. T., A. Lewis, M. B. Kroetz, S. J. Li, H. Heide, A. Emili, and M. Hochstrasser. 2005. Defining the SUMO-modified proteome by multiple approaches in Saccharomyces cerevisiae. J. Biol. Chem. 280:4102-4110. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann, A., C. M. Chiang, T. Oelgeschlager, X. Xie, S. K. Burley, Y. Nakatani, and R. G. Roeder. 1996. A histone octamer-like structure within TFIID. Nature 380:356-359. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann, H., S. Floss, and T. Stamminger. 2000. Covalent modification of the transactivator protein IE2-p86 of human cytomegalovirus by conjugation to the ubiquitin-homologous proteins SUMO-1 and hSMT3b. J. Virol. 74:2510-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huh, Y. H., Y. E. Kim, E. T. Kim, J. J. Park, M. J. Song, H. Zhu, G. S. Hayward, and J. H. Ahn. 2008. Binding STAT2 by the acidic domain of human cytomegalovirus IE1 promotes viral growth and is negatively regulated by SUMO. J. Virol. 82:10444-10454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishov, A. M., R. M. Stenberg, and G. G. Maul. 1997. Human cytomegalovirus immediate early interaction with host nuclear structures: definition of an immediate transcript environment. J. Cell Biol. 138:5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jupp, R., S. Hoffmann, R. M. Stenberg, J. A. Nelson, and P. Ghazal. 1993. Human cytomegalovirus IE86 protein interacts with promoter-bound TATA-binding protein via a specific region distinct from the autorepression domain. J. Virol. 67:7539-7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang, H., E. T. Kim, H. R. Lee, J. J. Park, Y. Y. Go, C. Y. Choi, and J. H. Ahn. 2006. Inhibition of SUMO-independent PML oligomerization by the human cytomegalovirus IE1 protein. J. Gen. Virol. 87:2181-2190. [DOI] [PubMed] [Google Scholar]
- 27.Kerscher, O. 2007. SUMO junction—what's your function? New insights through SUMO-interacting motifs. EMBO Rep. 8:550-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, E. T., K. K. Kim, M. J. Matunis, and J. H. Ahn. 2009. Enhanced SUMOylation of proteins containing a SUMO-interacting motif by SUMO-Ubc9 fusion. Biochem. Biophys. Res. Commun. 388:41-45. [DOI] [PubMed] [Google Scholar]
- 29.Kim, J. M., Y. Hong, K. T. Jeang, and S. Kim. 2000. Transactivation activity of the human cytomegalovirus IE2 protein occurs at steps subsequent to TATA box-binding protein recruitment. J. Gen. Virol. 81:37-46. [DOI] [PubMed] [Google Scholar]
- 30.Knipscheer, P., A. Flotho, H. Klug, J. V. Olsen, W. J. van Dijk, A. Fish, E. S. Johnson, M. Mann, T. K. Sixma, and A. Pichler. 2008. Ubc9 sumoylation regulates SUMO target discrimination. Mol. Cell 31:371-382. [DOI] [PubMed] [Google Scholar]
- 31.Lang, D., S. Gebert, H. Arlt, and T. Stamminger. 1995. Functional interaction between the human cytomegalovirus 86-kilodalton IE2 protein and the cellular transcription factor CREB. J. Virol. 69:6030-6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, H. R., and J. H. Ahn. 2004. Sumoylation of the major immediate-early IE2 protein of human cytomegalovirus Towne strain is not required for virus growth in cultured human fibroblasts. J. Gen. Virol. 85:2149-2154. [DOI] [PubMed] [Google Scholar]
- 33.Lee, H. R., Y. H. Huh, Y. E. Kim, K. Lee, S. Kim, and J. H. Ahn. 2007. N-terminal determinants of human cytomegalovirus IE1 protein in nuclear targeting and disrupting PML-associated subnuclear structures. Biochem. Biophys. Res. Commun. 356:499-504. [DOI] [PubMed] [Google Scholar]
- 34.Lee, H. R., D. J. Kim, J. M. Lee, C. Y. Choi, B. Y. Ahn, G. S. Hayward, and J. H. Ahn. 2004. Ability of the human cytomegalovirus IE1 protein to modulate sumoylation of PML correlates with its functional activities in transcriptional regulation and infectivity in cultured fibroblast cells. J. Virol. 78:6527-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, J. M., H. J. Kang, H. R. Lee, C. Y. Choi, W. J. Jang, and J. H. Ahn. 2003. PIAS1 enhances SUMO-1 modification and the transactivation activity of the major immediate-early IE2 protein of human cytomegalovirus. FEBS Lett. 555:322-328. [DOI] [PubMed] [Google Scholar]
- 36.Lukac, D. M., N. Y. Harel, N. Tanese, and J. C. Alwine. 1997. TAF-like functions of human cytomegalovirus immediate-early proteins. J. Virol. 71:7227-7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukac, D. M., J. R. Manuppello, and J. C. Alwine. 1994. Transcriptional activation by the human cytomegalovirus immediate-early proteins: requirements for simple promoter structures and interactions with multiple components of the transcription complex. J. Virol. 68:5184-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchini, A., H. Liu, and H. Zhu. 2001. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 75:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meulmeester, E., M. Kunze, H. H. Hsiao, H. Urlaub, and F. Melchior. 2008. Mechanism and consequences for paralog-specific sumoylation of ubiquitin-specific protease 25. Mol. Cell 30:610-619. [DOI] [PubMed] [Google Scholar]
- 40.Minty, A., X. Dumont, M. Kaghad, and D. Caput. 2000. Covalent modification of p73α by SUMO-1. Two-hybrid screening with p73 identifies novel SUMO-1-interacting proteins and a SUMO-1 interaction motif. J. Biol. Chem. 275:36316-36323. [DOI] [PubMed] [Google Scholar]
- 41.Mocarski, E. S., T. Shenk, and R. F. Pass. 2007. Cytomegaloviruses, p. 2701-2772. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA.
- 42.Nevels, M., W. Brune, and T. Shenk. 2004. SUMOylation of the human cytomegalovirus 72-kilodalton IE1 protein facilitates expression of the 86-kilodalton IE2 protein and promotes viral replication. J. Virol. 78:7803-7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nevels, M., C. Paulus, and T. Shenk. 2004. Human cytomegalovirus immediate-early 1 protein facilitates viral replication by antagonizing histone deacetylation. Proc. Natl. Acad. Sci. U. S. A. 101:17234-17239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panse, V. G., U. Hardeland, T. Werner, B. Kuster, and E. Hurt. 2004. A proteome-wide approach identifies sumoylated substrate proteins in yeast. J. Biol. Chem. 279:41346-41351. [DOI] [PubMed] [Google Scholar]
- 45.Park, J. J., Y. E. Kim, H. T. Pham, E. T. Kim, Y. H. Chung, and J. H. Ahn. 2007. Functional interaction of the human cytomegalovirus IE2 protein with histone deacetylase 2 in infected human fibroblasts. J. Gen. Virol. 88:3214-3223. [DOI] [PubMed] [Google Scholar]
- 46.Park, M. Y., Y. E. Kim, M. R. Seo, J. R. Lee, C. H. Lee, and J. H. Ahn. 2006. Interactions among four proteins encoded by the human cytomegalovirus UL112-113 region regulate their intranuclear targeting and the recruitment of UL44 to prereplication foci. J. Virol. 80:2718-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paulus, C., S. Krauss, and M. Nevels. 2006. A human cytomegalovirus antagonist of type I IFN-dependent signal transducer and activator of transcription signaling. Proc. Natl. Acad. Sci. U. S. A. 103:3840-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pizzorno, M. C., M. A. Mullen, Y. N. Chang, and G. S. Hayward. 1991. The functionally active IE2 immediate-early regulatory protein of human cytomegalovirus is an 80-kilodalton polypeptide that contains two distinct activator domains and a duplicated nuclear localization signal. J. Virol. 65:3839-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pizzorno, M. C., P. O'Hare, L. Sha, R. L. LaFemina, and G. S. Hayward. 1988. trans-activation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J. Virol. 62:1167-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reeves, M., and J. Sinclair. 2008. Aspects of human cytomegalovirus latency and reactivation. Curr. Top. Microbiol. Immunol. 325:297-313. [DOI] [PubMed] [Google Scholar]
- 51.Rizos, H., S. Woodruff, and R. F. Kefford. 2005. p14ARF interacts with the SUMO-conjugating enzyme Ubc9 and promotes the sumoylation of its binding partners. Cell Cycle 4:597-603. [DOI] [PubMed] [Google Scholar]
- 52.Sadanari, H., R. Yamada, K. Ohnishi, K. Matsubara, and J. Tanaka. 2005. SUMO-1 modification of the major immediate-early (IE) 1 and 2 proteins of human cytomegalovirus is regulated by different mechanisms and modulates the intracellular localization of the IE1, but not IE2, protein. Arch. Virol. 150:1763-1782. [DOI] [PubMed] [Google Scholar]
- 53.Sanders, R. L., C. J. Del Rosario, E. A. White, and D. H. Spector. 2008. Internal deletions of IE2 86 and loss of both of the late IE2 60 and IE2 40 proteins encoded by human cytomegalovirus affect the levels of the UL84 protein but not the amount of UL84 mRNA or the loading and distribution of the mRNA on polysomes. J. Virol. 82:11383-11397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz, R., B. Helmich, and D. H. Spector. 1996. CREB and CREB-binding proteins play an important role in the IE2 86-kilodalton protein-mediated transactivation of the human cytomegalovirus 2.2-kilobase RNA promoter. J. Virol. 70:6955-6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scully, A. L., M. H. Sommer, R. Schwartz, and D. H. Spector. 1995. The human cytomegalovirus IE2 86-kilodalton protein interacts with an early gene promoter via site-specific DNA binding and protein-protein associations. J. Virol. 69:6533-6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen, T. H., H. K. Lin, P. P. Scaglioni, T. M. Yung, and P. P. Pandolfi. 2006. The mechanisms of PML-nuclear body formation. Mol. Cell 24:331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sommer, M. H., A. L. Scully, and D. H. Spector. 1994. Transactivation by the human cytomegalovirus IE2 86-kilodalton protein requires a domain that binds to both the TATA box-binding protein and the retinoblastoma protein. J. Virol. 68:6223-6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song, J., L. K. Durrin, T. A. Wilkinson, T. G. Krontiris, and Y. Chen. 2004. Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc. Natl. Acad. Sci. U. S. A. 101:14373-14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sourvinos, G., N. Tavalai, A. Berndt, D. A. Spandidos, and T. Stamminger. 2007. Recruitment of human cytomegalovirus immediate-early 2 protein onto parental viral genomes in association with ND10 in live-infected cells. J. Virol. 81:10123-10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Speir, E., R. Modali, E. S. Huang, M. B. Leon, F. Shawl, T. Finkel, and S. E. Epstein. 1994. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 265:391-394. [DOI] [PubMed] [Google Scholar]
- 61.Spengler, M. L., K. Kurapatwinski, A. R. Black, and J. Azizkhan-Clifford. 2002. SUMO-1 modification of human cytomegalovirus IE1/IE72. J. Virol. 76:2990-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai, H. L., G. H. Kou, S. C. Chen, C. W. Wu, and Y. S. Lin. 1996. Human cytomegalovirus immediate-early protein IE2 tethers a transcriptional repression domain to p53. J. Biol. Chem. 271:3534-3540. [PubMed] [Google Scholar]
- 63.Uchimura, Y., M. Nakamura, K. Sugasawa, M. Nakao, and H. Saitoh. 2004. Overproduction of eukaryotic SUMO-1- and SUMO-2-conjugated proteins in Escherichia coli. Anal. Biochem. 331:204-206. [DOI] [PubMed] [Google Scholar]
- 64.Wara-aswapati, N., Z. Yang, W. R. Waterman, Y. Koyama, S. Tetradis, B. K. Choy, A. C. Webb, and P. E. Auron. 1999. Cytomegalovirus IE2 protein stimulates interleukin 1β gene transcription via tethering to Spi-1/PU. 1. Mol. Cell. Biol. 19:6803-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wohlschlegel, J. A., E. S. Johnson, S. I. Reed, and J. R. Yates III. 2004. Global analysis of protein sumoylation in Saccharomyces cerevisiae. J. Biol. Chem. 279:45662-45668. [DOI] [PubMed] [Google Scholar]
- 66.Wu, J., J. O'Neill, and M. S. Barbosa. 1998. Transcription factor Sp1 mediates cell-specific trans-activation of the human cytomegalovirus DNA polymerase gene promoter by immediate-early protein IE86 in glioblastoma U373MG cells. J. Virol. 72:236-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu, Y., J. H. Ahn, M. Cheng, C. M. apRhys, C. J. Chiou, J. Zong, M. J. Matunis, and G. S. Hayward. 2001. Proteasome-independent disruption of PML oncogenic domains (PODs), but not covalent modification by SUMO-1, is required for human cytomegalovirus immediate-early protein IE1 to inhibit PML-mediated transcriptional repression. J. Virol. 75:10683-10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoo, Y. D., C. J. Chiou, K. S. Choi, Y. Yi, S. Michelson, S. Kim, G. S. Hayward, and S. J. Kim. 1996. The IE2 regulatory protein of human cytomegalovirus induces expression of the human transforming growth factor β1 gene through an Egr-1 binding site. J. Virol. 70:7062-7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang, Z., D. L. Evers, J. F. McCarville, J. C. Dantonel, S. M. Huong, and E. S. Huang. 2006. Evidence that the human cytomegalovirus IE2-86 protein binds mdm2 and facilitates mdm2 degradation. J. Virol. 80:3833-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu, J., S. Zhu, C. M. Guzzo, N. A. Ellis, K. S. Sung, C. Y. Choi, and M. J. Matunis. 2008. Small ubiquitin-related modifier (SUMO) binding determines substrate recognition and paralog-selective SUMO modification. J. Biol. Chem. 283:29405-29415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.