Abstract
In a previous paper, we reported on a large number of cheetah blood specimens that gave positive signals only for Babesia and/or Theileria genus-specific probes on the reverse line blot (RLB) assay, indicating the presence of a novel species or variant of an existing species. Some of these specimens were investigated further by microscopic, serological, sequencing, and phylogenetic analyses. The near-full-length 18S rRNA genes of 13 samples, as well as the second internal transcribed spacer (ITS2) region, were amplified, cloned, and sequenced. A species-specific RLB probe, designed to target the hypervariable V4 region of the 18S rRNA gene for detection of the novel Babesia sp., was used to screen an additional 137 cheetah blood specimens for the presence of the species. The prevalence of infection was 28.5%. Here we describe the morphology and phylogenetic relationships of the novel species, which we have named Babesia lengau sp. nov.
The cheetah (Acinonyx jubatus, Schreber, 1775) is regarded as a vulnerable species according to the 2008 World Conservation Union (IUCN) Red List of Threatened Species (http://www.iucnredlist.org) and is listed in Appendix I (which includes species that are most threatened) of the Convention of International Trade in Endangered Species (CITES) (9). This is mainly because of loss of habitat in the wild and conflicts with farmers in remaining habitats (23). Between 12,000 and 15,000 cheetahs remain in the wild, mostly in isolated populations in 24 to 26 countries in Africa. Free-ranging cheetahs still inhabit a broad section of Africa, including areas of North Africa, the Sahel, and East and southern Africa. The farmlands of north-central Namibia have the largest free-ranging population, of about 300 animals (16, 24). The Asiatic cheetah (Acinonyx jubatus venaticus), found only in the Kavir Desert region of Iran, is critically endangered, with an estimated population of ca. 50 mature individuals (IUCN).
Both large (>2.5 μm) and small (<1.5 μm) intraerythrocytic piroplasms have been reported from a variety of domestic and wild felid species from several continents (17). Small piroplasms reported from felids include Babesia felis (12), reported for African wild cats (Felis sylvestris ocreata) and caracals (Felis caracal) (38) in Africa; Babesia leo, reported for lions (Panthera leo) in South Africa (38) and Tanzania (32); and Cytauxzoon felis, reported for domestic cats and bobcats (Lynx rufus) (15, 45), mountain lions (Felis concolor), and Florida panthers (Felis concolor coryi) (7, 41) in North America.
Records of Babesia parasites in wild cheetahs are surprisingly rare, with reports of occurrence of piroplasms in three animals in the Serengeti National Park in Tanzania (36) and South Africa (6). Theileria-like piroplasms have also been reported for cheetahs in the Serengeti National Park and Ngorongoro Crater, Tanzania (2). Babesia felis and B. leo have been reported from cheetahs in southern Africa, but not as mixed infections (6). Although the prevalence of infection with these two species in free-ranging cheetahs in Namibia was low (7.5%), a large number (52.9%) of cheetahs were infected with an as yet undescribed Babesia sp. (6). The aim of the current study was to characterize this undescribed Babesia sp. by phylogenetic analysis based on sequences from the 18S rRNA gene and the second internal transcribed spacer region (ITS2).
MATERIALS AND METHODS
Samples.
Thirteen EDTA-blood specimens, previously collected from captive cheetahs (6), were selected for this study. These specimens had been reextracted and screened with a reverse line blot (RLB) hybridization assay, which makes it possible to simultaneously detect and differentiate blood parasites (31). It was found that the amplicons hybridized only with the Babesia and/or Theileria genus-specific probe, which suggested the presence of a novel species or variant of a species (6).
Light microscopy.
Thin blood smears were prepared, and slides were stained using Kyro-Quick stain (Kyron Laboratories, South Africa) and examined for the presence of Babesia parasites. Slides were screened under a Zeiss microscope with a ×100 oil immersion objective. Measurements were made with a Leitz screw micrometer calibrated against a standard stage micrometer. Images were captured with a Nikon Coolpix 4500 digital camera attached to a Zeiss triocular microscope.
IFA.
Eight samples were screened for the presence of antibodies against B. felis by use of an immunofluorescence assay (IFA). Antigen slides were prepared (27) by using blood from a domestic cat with a known B. felis infection. Serum collected from a B. leo-positive lion was used as a control. Twofold dilutions of the test sera and controls were prepared in phosphate-buffered saline. Commercial rabbit anti-cat immunoglobulin G conjugated with fluorescein isothiocyanate (capped; MP Biomedicals, Aurora, OH) was used at a 1:150 dilution. Slides were examined under an Olympus BX41 fluorescence microscope with a ×50 UV objective.
Molecular characterization. (i) Amplification, cloning, and sequencing of the 18S rRNA gene.
DNA was extracted from blood collected in EDTA by use of a QIAamp DNA Mini kit (Qiagen, Southern Cross Biotechnology, South Africa). The near-full-length 18S rRNA gene (∼1,700 bp) was amplified using primers Nbab_1F (35) and TB Rev (25). The PCR mixture consisted of 2× PCR master mix (Fermentas, Inqaba Biotech, South Africa), a 50 pM concentration of each primer (Inqaba Biotech, South Africa), and 2.5 μl (70 to 100 ng) purified DNA to a final volume of 25 μl. Amplification was performed in an automated thermocycler (Perkin Elmer, Foster City, CA). The PCR cycling conditions included an initial denaturation step at 94°C for 2 min, followed by 40 cycles of 94°C for 30 s, 67°C for 45 s, and 72°C for 1 min. Final extension was performed at 72°C for 7 min. The obtained PCR amplicons were purified using a QIAquick PCR purification kit (Qiagen, Southern Cross Biotechnology, South Africa). Amplicons were cloned into the pGEM-T Easy vector system (Promega, Whitehead Scientific, South Africa) according to the manufacturer's instructions. Recombinant plasmids were directly sequenced using an ABI BigDye Terminator cycle sequencing ready reaction kit (PE Applied Biosystems), using 350 ng plasmid DNA and 3.2 pmol of primer (25, 35). Purified products were analyzed on an ABI3130 XL automated DNA sequencer at the Equine Genetics Laboratory, Faculty of Veterinary Science, University of Pretoria.
(ii) Amplification and sequencing of the second internal transcribed spacer region.
The ITS2 genes/regions of 13 samples were amplified using primers ITS-2-F and ITS-2-R (21). PCR was performed by using the KAPALongRange system (KAPA Biosystems, Cape Town, South Africa) according to the manufacturer's instructions. The PCR conditions included an initial denaturation of 94°C for 3 min, followed by 36 cycles of 96°C for 30 s, 52°C for 30 s, and 72°C for 1 min. Final extension was performed at 72°C for 7 min. Amplified products were purified as described above and directly sequenced at Inqaba Biotech, Pretoria, South Africa.
(iii) Sequence analysis.
Sequence data for the near-full-length 18S rRNA gene were assembled and edited to a total length of 1,652 bp by using GAP 4 of the Staden package (version 1.6.0 for Windows) (5, 43, 44). The assembled sequences were aligned with sequences of related genera by use of ClustalX (version 1.81 for Windows). The alignment was manually truncated to the size of the shortest sequence. Similarity matrices were constructed using the two-parameter model of Kimura (19) and the Jukes and Cantor correction model for multiple base changes (18). Phylogenetic trees were constructed using the neighbor-joining (42) and maximum parsimony methods in the Mega 3.0 software package (22). This package was used in combination with the bootstrap method (14) (1,000 replicates/tree for distance methods and 100 replicates/tree for parsimony methods).
Sequence data for the ITS2 region were assembled and edited as described above. Construction of phylogenetic trees was done as described above.
Design of a species-specific probe to detect DNA from the newly described parasite in blood specimens collected from cheetahs.
The obtained 18S rRNA gene sequence data were used to design a species-specific RLB oligonucleotide probe specific for the hypervariable V4 region of the 18S rRNA gene to detect this novel parasite. The probe (5′-CTC CTG ATA GCA TTC-3′) (with a melting temperature of 44°C) was synthesized with an N-terminal N-(trifluoracetamidohexyl-cyanoethyl,N,N-diisopropyl phosphoramidite [TFA])-C6 amino linker at the 5′ end (Operon, Southern Cross Biotechnologies, South Africa) and was prepared for use in the RLB assay (31). The 13 cheetah blood isolates used in this study were screened for the presence of the novel parasite by use of the RLB assay as previously described (6). Subsequently, 137 cheetah samples were tested with the newly developed probe for the novel parasite.
Nucleotide sequence accession numbers.
Sequences for the near-full-length 18S rRNA gene were deposited in GenBank under accession numbers GQ411405, GQ411406, GQ411407, GQ411408, GQ411409, GQ411410, GQ411411, GQ411412, GQ411413, GQ411414, GQ411415, GQ411416, and GQ411417. Sequences for the ITS2 region were deposited in GenBank under accession numbers GQ411418, GQ411419, GQ411420, GQ411421, GQ411422, GQ411423, GQ411424, GQ411425, GQ411426, GQ411427, GQ411428, GQ411429, and GQ411430.
RESULTS
Taxonomic review.
Family: Felidae
Parasite: Babesia lengau sp. nov.
Type host: Acinonyx jubatus (Schreber)
Type locality: de Wildt Cheetah Centre, North West Province, South Africa
Vector: currently unknown but assumed to be a species of ixodid tick
Etymology: named for the Setswana name for the cheetah
Description.
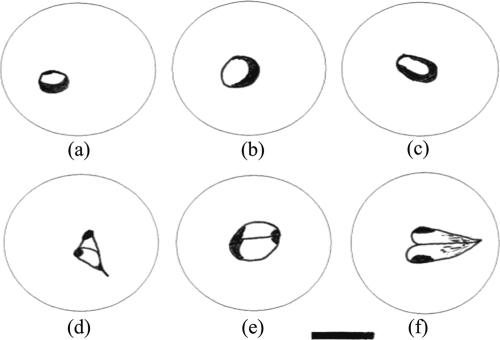
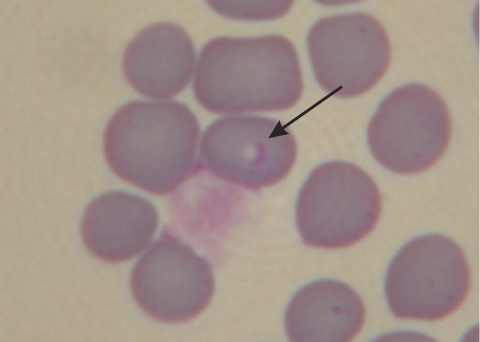
The organism is a typical small Babesia species whose developing trophozoites usually occupy a central to subcentral position within the host cell erythrocyte (Fig. 1 a to c and 2). The cytoplasm is pale to hyaline, with a purple-staining nucleus around the periphery; the small forms are round to slightly ovoid, averaging 1.3 μm (standard deviation [SD], 0.2 μm), with a range of 0.8 to 1.8 μm (n = 40). As the parasite grows, the nuclear chromatin begins to separate and a fine dividing line can be seen (Fig. 1d); the thickened nucleus at one end and chromatin at the other become clearer just prior to division (Fig. 1e). The parasite finally divides by binary fission into two merozoites (Fig. 1f). Measurements of dividing trophozoites are 1.1 by 1.9 μm (SD, 0.2 μm), with a range of 0.6 to 1.3 by 1.6 to 2.3 μm (n = 12). Figure 2 illustrates a microscopic view of the described parasite.
FIG. 1.
Babesia lengau sp. nov. from Acinonyx jubatus. (a to c) Small ring-form trophozoites. (d) Parasite with early division of chromatin. (e) Parasite almost ready to divide. (f) Parasite dividing by binary fission into two trophozoites. Bar = 2 μm.
FIG. 2.
Microscopic view of Babesia lengau sp. nov. Slides were screened under a Zeiss microscope with a ×100 oil immersion objective. Measurements were made with a Leitz screw micrometer calibrated against a standard stage micrometer. Images were captured with a Nikon Coolpix 4500 digital camera attached to a Zeiss triocular microscope.
Hapantotype.
G 465380 from A. jubatus (2813) coll. Caldwell, 24 July 2003, de Wildt Cheetah Centre, North West Province, South Africa, deposited in the Queensland Museum, Brisbane, Australia.
Parahapantotypes.
G 465381 from A. jubatus (1985) coll. Koeppel, 21 May 2003, de Wildt Cheetah Centre, North West Province, South Africa; G 465382 from A. jubatus (2817) coll. Caldwell, 24 July 2003, de Wildt Cheetah Centre, North West Province, South Africa; and G 465383 from A. jubatus (2811) coll. Caldwell, 24 July 2003, de Wildt Cheetah Centre, North West Province, South Africa. All are deposited in the Queensland Museum, Brisbane, Australia.
Serology.
Sera of 8 of the 13 cheetah samples were tested for the presence of antibodies against B. felis. All samples tested negative. Complete sets of a blood smear, serum, and EDTA-blood were obtained only for 8 animals. An IFA for the presence of B. leo antibodies was not performed.
Molecular sequencing.
The 18S rRNA genes of the 13 cheetah isolates were amplified, cloned, and sequenced. Resulting sequences were identical, indicating the presence of only one parasite species. A BLAST search performed with the 18S rRNA gene sequence (∼1,652 bp) revealed no identical sequences in the public database, and it was therefore designated Babesia lengau sp. nov. The most closely related sequences (∼96% identity) were from Babesia conradae (GenBank accession no. AF158702 and AF231350), isolated from a Californian dog, and Babesia duncani WA1 (GenBank accession no. AY 231350), isolated from humans in Washington State (8).
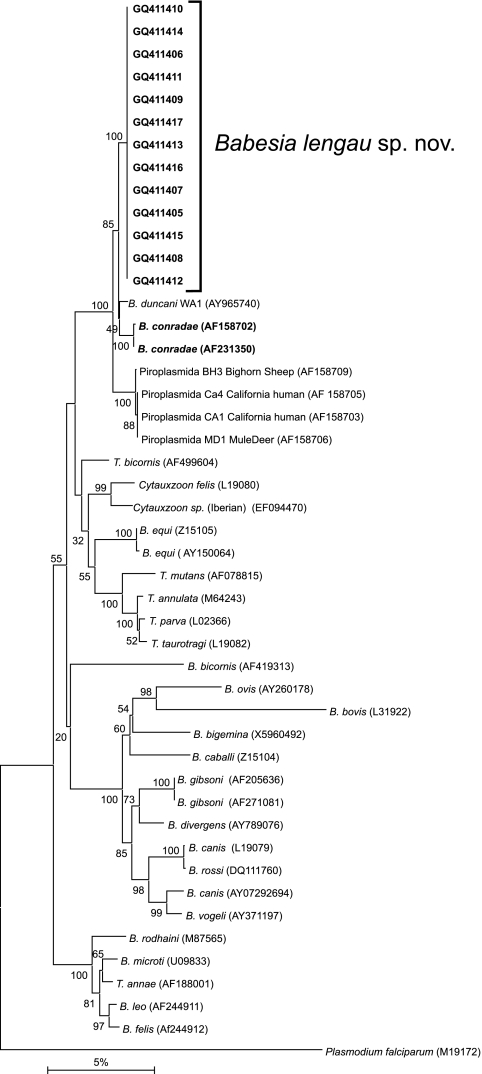
The phylogenetic relationships between Babesia lengau sp. nov. and other Babesia and Theileria species were analyzed using both neighbor-joining and maximum parsimony phylogenetic analyses. No significant changes in the topologies of the trees were found by either of these methods. A representative tree, obtained by the neighbor-joining method with the Kimura two-parameter distance (19) calculation, is shown in Fig. 3. Babesia lengau sp. nov. formed a monophyletic group with B. conradae (20, 21) and with piroplasms previously isolated from humans and wildlife in the western United States. This clade was distinct from the Babesia spp. sensu stricto (Babesia bigemina, Babesia canis, Babesia divergens, and Babesia gibsoni), Theileria spp., and Babesia microti and related species (Fig. 3).
FIG. 3.
Results of neighbor-joining analysis of the 18S rRNA gene, showing the phylogenetic relationship of Babesia lengau sp. nov. with other known Babesia and Theileria species. Branch lengths are proportional to the estimated genetic distances between the species. The scale bar represents the percent nucleotide difference. Plasmodium falciparum (M19172) was used as the outgroup.
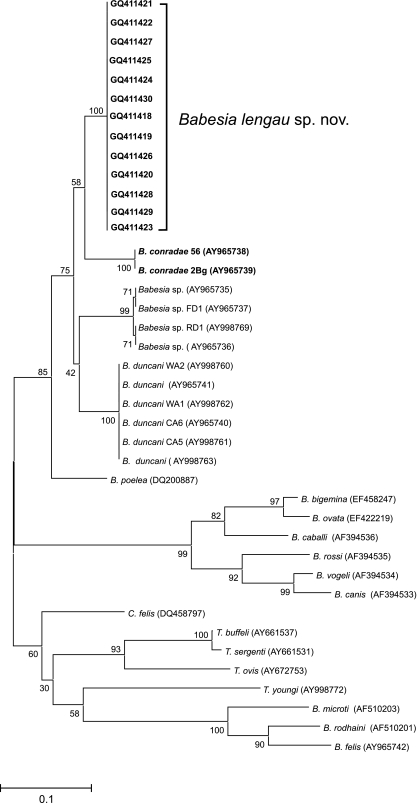
The lengths of the ITS2 gene sequences ranged from 428 to 1,438 bp. Phylogenetic analysis of a 420-bp region of the ITS2 gene (Fig. 4) was in concordance with that obtained with the 18S rRNA gene. Babesia lengau sp. nov. grouped with B. conradae, B. duncani, and the rest of the “western clade” of piroplasms, which is made up of piroplasm isolates from wildlife and humans from California and Washington. As with the 18S rRNA gene sequence analysis, the Babesia spp. sensu stricto, the B. microti group of Babesia spp., and the Theileria spp. were grouped separately.
FIG. 4.
Results of phylogenetic analysis of the ITS2 genes of Babesia lengau sp. nov. and other piroplasm species.
Based on the 18S rRNA gene sequences obtained, a Babesia lengau sp. nov.-specific RLB oligonucleotide probe was designed to target the hypervariable V4 region of the gene. The probe was shown to bind to its target sequence during RLB analysis and did not cross-hybridize with any other amplified Theileria or Babesia spp. It was subsequently used to screen 137 cheetah samples, of which 39 (28.5%) were infected with Babesia lengau sp. nov.
DISCUSSION
Phenotypic and genotypic data on a Babesia parasite present in cheetahs support the differentiation and naming of this piroplasm as a distinct species.
Babesia lengau sp. nov. is a parasite like many other species of small babesias, with no particular distinguishing morphological features. Type slides were selected from nine blood smears from individual cheetahs to provide the morphometric data for the description, but all infections were low-level infections, with parasitemias of <1.0%. Moreover, since all infections were chronic, dividing forms were scarce, but the parasite does appear to divide by binary fission only, as no evidence of cruciform division was observed. No parasites were observed in which the separation of dividing forms had progressed beyond that depicted in Fig. 1f. Dividing parasites do not appear to occupy any position other than a central position within the host cell. The round forms of Babesia lengau sp. nov. are similar in appearance to those of B. felis. For comparison, two slides containing B. felis from a domestic cat were screened and the parasites measured, and these were 1.3 μm in diameter (SD, 0.2 μm), with a range of 0.9 to 1.9 μm (n = 60), very similar to those of B. lengau sp. nov. No dividing forms were seen for B. felis.
Molecular tools have been used successfully in resolving the phylogenies of Babesia parasites (11, 20, 38). The 18S rRNA gene was used in this study because it has been used successfully to resolve phylogenies of Babesia parasites (10, 20, 38). Both the 18S rRNA and ITS2 gene sequence analyses were distinct from those of other felid babesias, such as B. felis and B. leo, and other blood parasites, such as Cytauxzoon felis. The only other genotypically related piroplasm is a canine parasite, B. conradae, indicating that Babesia lengau sp. nov. forms part of the previously described “western clade” of piroplasms, which includes B. conradae, B. duncani, and piroplasms isolated from both wildlife and humans from the western United States. Babesia lengau sp. nov. clustered separately from the Babesia spp. sensu stricto, the B. microti clade, and the Theileria and Cytauxzoon species. Babesia conradae was described in 2006 from a dog in California. This parasite is associated with hemolytic anemia in dogs in the United States. It was suggested that coyotes may serve as a reservoir for B. conradae (20, 21). It was also mentioned that B. conradae had not been detected outside California, although studies were limited. It has also been shown that B. conradae is closely related to Babesia spp. in humans in the United States (8, 20, 21). The piroplasm that is most closely related to B. conradae is B. duncani, isolated from a human in the western United States (8, 40). The results obtained from 18S rRNA gene sequences correspond with the ITS2 results of Kjemtrup and Conrad (20).
Cheetahs are suspected to be particularly vulnerable to infectious diseases. It has been argued that this is due mainly to the fact that cheetahs lack heterogeneity at major histocompatibility complex (MHC) loci that encode peptide-mediating immune responsiveness to pathogens (33, 34). It has been shown, however, that a physiologic response to the environment is likely of equal or perhaps greater importance than genetic influences in determining health in cheetahs (30). Captive cheetahs also have a high prevalence of atypical diseases, such as veno-occlusive disease and glomerulosclerosis, that rarely occur in other species (4, 28). Also, Helicobacter and feline infectious peritonitis (FIP) virus, which are common infectious agents, cause persistent and severe inflammatory disease in cheetahs (13, 28). These unusual diseases also occur in captive South African cheetahs, whose environment and diet more closely mimic those of free-ranging cheetahs (29).
To date, no evidence has been seen to suggest that Babesia lengau sp. nov. causes any clinical signs in the host. The low-level parasitemias observed with chronic infections suggest that patent levels may exist for long periods, which would be required in order for ticks to become infected, given the normal low-density rates of cheetahs in the wild. It may well be that infection is acquired by the cubs and then persists at a chronic level for a long time, possibly with relapses when stress or other factors prevail. Clinical babesiosis due to Babesia bicornis, in association with stressful conditions, has been well documented for black rhinoceroses (Diceros bicornis) (26, 31). There is also circumstantial evidence that the death of the famous lioness “Elsa,” attributed to babesiosis, was stress related (1, 3, 37).
The geographic distribution of B. lengau sp. nov. in cheetahs is currently unknown, but the parasite is assumed to have a wide, if sporadic, occurrence throughout the host's range in Africa. Whether the parasite also occurs in cheetahs outside Africa is speculative, and the small numbers of animals surviving in these areas may well exclude exposure to infection, but this remains to be investigated. The development of a new B. lengau sp. nov.-specific RLB probe makes it possible, for the first time, to determine the prevalence of this parasite in the cheetah populations in Africa and to discern the parasite's geographic distribution.
The mode of transmission of felid babesias is unknown (17, 39). In a phylogenetic study in which felid babesias were compared to other related Babesia, Theileria, and Cytauxzoon species, B. felis was grouped with Babesia microti, suggesting that these species have the same mode of transstadial transmission by tick vectors (38). Babesia lengau sp. nov. grouped phylogenetically with B. conradae, and preliminary tick transmission studies suggest that B. conradae may be transmitted by Rhipicephalus sanguineus ticks (46). The tick vector for Babesia lengau sp. nov. is still unknown.
In conclusion, both phenotypic and phylogenetic analyses support recognition of the new piroplasm Babesia lengau sp. nov. present in cheetahs as a distinct species.
Acknowledgments
We thank Milana Troskie for assistance with light microscopy analysis, Karen Ebersohn and Elke Mashianoke for their assistance in the laboratory, and Gerty Pretorius for providing the samples.
This report emanates from project 36-5-613, approved by the Research Committee of the Faculty of Veterinary Science and the Animal Use and Care Committee of the University of Pretoria. This research was funded by a South African National Research Foundation grant (NRF grant GUN 2069496) to B.L.P.
Footnotes
Published ahead of print on 2 June 2010.
REFERENCES
- 1.Adamson, J. 1962. Living free. Collins & Harvill, London, United Kingdom.
- 2.Averbeck, G., K. E. Bjork, C. Parker, and L. Herbst. 1990. Prevalence of haematozoans in lions (Panthera leo) and cheetah (Acinonyx jubatus) in Serengeti National Park and Ngorongoro Crater, Tanzania. J. Wildl. Dis. 26:392-394. [DOI] [PubMed] [Google Scholar]
- 3.Barnett, S. F., and D. W. Brocklesby. 1968. Some piroplasms of wild animals. Symp. Zool. Soc. Lond. 24:159-175. [Google Scholar]
- 4.Bolton, L. A., and L. Munson. 1999. Glomerulosclerosis in captive cheetahs (Acinonyx jubatus). Vet. Pathol. 36:14-22. [DOI] [PubMed] [Google Scholar]
- 5.Bonfield, J. K., K. F. Smith, and R. Staden. 1995. A new DNA sequence assembly program. Nucleic Acids Res. 23:4992-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosman, A.-M., E. H. Venter, and B. L. Penzhorn. 2007. Occurrence of Babesia felis and Babesia leo in various wild felids and domestic cats in South Africa, based on reverse line blot analysis. Vet. Parasitol. 144:33-38. [DOI] [PubMed] [Google Scholar]
- 7.Butt, M. T., D. Bowman, M. C. Barr, and M. E. Roelke. 1991. Iatrogenic transmission of Cytauxzoon felis from a Florida panther (Felix concolor coryi) to a domestic cat. J. Wildl. Dis. 27:342-347. [DOI] [PubMed] [Google Scholar]
- 8.Conrad, P. A., A. M. Kjemtrup, R. A. Carreno, J. Thomford, K. Wainwright, M. Eberhard, R. Quick, S. R. Telford, and B. L. Herwaldt. 2006. Description of Babesia duncani n. sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int. J. Parasitol. 36:779-789. [DOI] [PubMed] [Google Scholar]
- 9.Convention on International Trade in Endangered Species of Wild Fauna and Flora. 2007. Appendices I, II, and III. Convention on International Trade in Endangered Species of Wild Fauna and Flora, Geneva, Switzerland. http://www.cites.org/eng/app/e-appendices.pdf. [DOI] [PubMed]
- 10.Craido-Fernelio, A. J., A. Martinez-Marcos, A. Buling-Saraña, and J. C. Barba-Carretero. 2003. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe. II. Phylogenetic analysis and evolutionary history. Vet. Parasitol. 114:173-194. [DOI] [PubMed] [Google Scholar]
- 11.Craido-Fernelio, A., J. Martinez, A. Buling, J. C. Barba, S. Merino, R. Jefferies, and P. J. Irwin. 2006. New data on epizootiology and genetics of piroplasms based on sequences of small ribosomal subunit and cytochrome b genes. Vet. Parasitol. 142:238-247. [DOI] [PubMed] [Google Scholar]
- 12.Davis, L. J. 1929. On a piroplasm of the Sudanese wild cat (Felis ocreata). Trans. R. Soc. Trop. Med. Hyg. 22:523-534. [Google Scholar]
- 13.Eaton, K. A., M. J. Radin, L. W. Kramer, R. F. Wack, R. Sherding, S. Krakowka, J. G. Fox, and D. R. Morgan. 1993. Epizootic gastritis associated with gastric spiral bacilli in cheetahs (Acinonyx jubatus). Vet. Pathol. 30:55-63. [DOI] [PubMed] [Google Scholar]
- 14.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 15.Glenn, B. L., A. A. Kocan, and E. F. Blouin. 1983. Cytauxzoonosis in bobcats. J. Am. Vet. Med. Assoc. 183:1155-1158. [PubMed] [Google Scholar]
- 16.Hanson, L., and P. Stander. 2004. Namibian large carnivore atlas, vol. 1. Predator Conservation Trust, Ministry of Environment and Tourism, Division of Speciality Support Services, Windhoek, Namibia.
- 17.Jacobson, L. S., T. Schoeman, and R. G. Lobetti. 2000. A survey of feline babesiosis in South Africa. J. S. Afr. Vet. Assoc. 71:222-228. [DOI] [PubMed] [Google Scholar]
- 18.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism. Academic Press, New York, NY.
- 19.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions though comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 20.Kjemtrup, A. M., and P. A. Conrad. 2006. A review of the small canine piroplasm from California: Babesia conradae in the literature. Vet. Parasitol. 138:112-117. [DOI] [PubMed] [Google Scholar]
- 21.Kjemtrup, A. M., K. Wainwright, M. Miller, B. L. Penzhorn, and R. A. Carreno. 2006. Babesia conradae, sp. nov., a small canine Babesia identified in California. Vet. Parasitol. 138:103-111. [DOI] [PubMed] [Google Scholar]
- 22.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 23.Marker, L. 2002. Aspects of the Namibian cheetah: biology, ecology, and conservation strategies. Ph.D. dissertation. University of Oxford, Oxford, United Kingdom.
- 24.Marker, L. L., A. J. Pearks Wilkerson, R. J. Sarno, J. Martenson, C. Breitenmoser-Würsten, S. J. O'Brien, and W. E. Johnson. 2007. Molecular genetic insights on cheetah (Acinonyx jubatus) ecology and conservation in Namibia. J. Hered. 99:2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matjila, P. T., A. L. Leisewitz, M. C. Oosthuizen, F. Jongejan, and B. L. Penzhorn. 2008. Detection of Theileria species in dogs in South Africa. Vet. Parasitol. 157:34-40. [DOI] [PubMed] [Google Scholar]
- 26.McCullogh, B., and P. L. Achard. 1960. Mortalities associated with capture, translocation, trade and exhibition of black rhinoceroses. Int. Zoo Yearb. 9:184-195. [Google Scholar]
- 27.Morzaria, S. P., D. W. Brocklesby, and D. F. Harradine. 1977. Evaluation of the indirect fluorescent antibody test for Babesia major and Theileria mutans in Britain. Vet. Rec. 100:484-487. [DOI] [PubMed] [Google Scholar]
- 28.Munson, L. 1993. Diseases of captive cheetahs (Acinonyx jubatus): results of the Cheetah Research Council pathology survey 1989-1992. Zoo Biol. 12:105-124. [Google Scholar]
- 29.Munson, L., J. W. Nesbit, D. G. A. Meltzer, L. P. Colly, L. Bolton, and N. P. J. Kriek. 1999. Diseases of captive cheetahs (Acinonyx jubatus jubatus) in South Africa: a 20-year retrospective survey. J. Zoo Wildl. Med. 30:342-347. [PubMed] [Google Scholar]
- 30.Munson, L., K. A. Terio, R. Kock, T. Mlengeya, M. E. Roelke, E. Dubovi, B. Summers, A. R. E. Sinclair, and C. Packer. 2008. Climate extremes promote fatal co-infections during canine distemper epidemics in African lions. PLoS One 3:e2545. doi: 10.1371/journal.pone.0002545.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nijhoff, A., B. L. Penzhorn, G. Lynen, J. O. Mollel, C. Bekker, and F. Jongejan. 2003. Babesia bicornis sp. n. and Theileria bicornis sp. n.: tick-borne parasites associated with mortality in the black rhinoceros (Diceros bicornis). J. Clin. Microbiol. 41:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nijhof, A., V. Pillay, J. Steyl, L. Prozeskey, W. H. Stoltsz, J. A. Lawrence, B. L. Penzhorn, and F. Jongejan. 2005. Molecular characterization of Theileria species associated with mortality in four species of African antelopes. J. Clin. Microbiol. 43:5907-5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien, S. J., and D. E. Wildt. 1983. The cheetah is depauperate in genetic variation. Science 221:459-462. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien, S. J., M. E. Roelke, R. L. Marker, A. Newman, C. A. Winkler, D. Meltzer, L. Colly, and J. F. Evermann. 1985. Genetic basis for species vulnerability in cheetah. Science 227:1428-1434. [DOI] [PubMed] [Google Scholar]
- 35.Oosthuizen, M. C., E. Zweygardt, N. E. Collins, M. Troskie, and B. L. Penzhorn. 2008. Identification of a novel Babesia sp. from sable antelope (Hippotragus niger Harris, 1838). Vet. Parasitol. 163:39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peirce, M. A., M. K. Laurenson, and S. C. Gascoyne. 1995. Hepatozoonosis in cheetahs and wild dogs in the Serengeti ecosystem. Afr. J. Ecol. 33:273-275. [Google Scholar]
- 37.Penzhorn, B. L. 2006. Babesiosis of wild carnivores and ungulates. Vet. Parasitol. 138:11-21. [DOI] [PubMed] [Google Scholar]
- 38.Penzhorn, B. L., A. M. Kjemtrup, L. M. López-Rebollar, and P. A. Conrad. 2001. Babesia leo n. sp. from lions in the Kruger National Park, South Africa, and its relation to other small piroplasms. J. Parasitol. 87:681-685. [DOI] [PubMed] [Google Scholar]
- 39.Penzhorn, B. L., T. Schoeman, and L. S. Jacobson. 2004. Feline babesiosis in South Africa: a review. Ann. N. Y. Acad. Sci. 1026:183-186. [DOI] [PubMed] [Google Scholar]
- 40.Quicke, R. E., B. L. Herwaldt, J. W. Thomford, M. E. Garnett, M. L. Eberhard, M. Wilson, D. H. Spach, J. W. Dickerson, S. R. Telford III, and K. R. Steingart. 1993. Babesiosis in Washington State: a new species? Ann. Intern. Med. 119:284-290. [DOI] [PubMed] [Google Scholar]
- 41.Rotstein, D. S., S. K. Taylor, J. W. Harvey, and J. Bean. 1999. Hematologic effects of cytauxzoonosis in Florida panthers and Texas cougars in Florida. J. Wildl. Dis. 35:613-617. [DOI] [PubMed] [Google Scholar]
- 42.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 43.Staden, R. 1996. The Staden sequence analysis package. Mol. Biotechnol. 5:233-241. [DOI] [PubMed] [Google Scholar]
- 44.Staden, R., K. F. Beal, and J. K. Bonfield. 2000. The Staden package, 1998. Methods Mol. Biol. 132:115-130. [DOI] [PubMed] [Google Scholar]
- 45.Wagner, J. E. 1976. A fatal cytauxzoonosis-like disease in cats. J. Am. Vet. Med. Assoc. 168:585-588. [PubMed] [Google Scholar]
- 46.Yamane, I., I. A. Gardner, S. R. Telford, T. Elward, J. A. Hair, and P. A. Conrad. 1993. Vector competence of Rhipicephalus sanguineus and Dermacentor variabilis for American isolates of Babesia gibsoni. Exp. Appl. Acarol. 17:913-919. [Google Scholar]