Abstract
The relatively high-level clonality of methicillin-resistant Staphylococcus aureus (MRSA) and its frequent high-level endemicity in nosocomial settings complicate the development of methods for rapid subtyping of MRSA strains that are capable of identifying person-to-person transmission in hospitals. Phage-derived open reading frame (PDORF) typing is an MRSA typing method targeting mobile genetic elements that was recently described and evaluated using a geographically restricted set of isolates. The objective of this study was to develop a multiplex PCR-reverse line blot (mPCR/RLB) assay for PDORF typing and to test its applicability on a broad range of isolates and in an environment where MRSA is highly endemic. The 16 targets were identified using a 23-primer-pair mPCR/RLB assay with two probes for each target. The method was evaluated using 42 MRSA reference strains, including those representing major international clones, and 35 isolates from episodes of suspected nosocomial transmission. In vivo stability was explored using 81 isolate pairs. Pulsed-field gel electrophoresis (PFGE) and spa typing were performed for comparison. Among the 42 reference strains, there were 33 PFGE subtypes, 30 PDORF types, and 22 spa types. Simpson's index of diversity was 0.987, 0.971, and 0.926 for PFGE subtyping, PDORF typing, and spa typing, respectively. Typing of clinical isolates by PDORF typing and PFGE demonstrated concordant results. mPCR/RLB-based PDORF typing has similar discriminatory power to that of PFGE, can assist in tracking MRSA transmission events in a setting of high-level endemicity, and has the advantage of being a high-throughput technique.
A range of methods for rapid identification and subtyping of methicillin-resistant Staphylococcus aureus (MRSA), which can improve the effectiveness of infection control procedures, have been reported (9, 13, 15, 16). However, their ability to detect person-to-person transmission in settings with high-level endemicity has not been evaluated fully. Phage-derived open reading frame (PDORF) typing is a typing method for MRSA that was recently described from Japan and was reported to have similar discriminatory power and stability to those of pulsed-field gel electrophoresis (PFGE) (16). PDORF typing interrogates the presence or absence of 16 variable elements within the S. aureus genome: 13 from integrated prophages, 1 from a genomic island, and 2 from the SCCmec element. In its original form, PDORF typing involves four 4-plex PCRs, each with product detection by agarose gel electrophoresis (16).
Reverse line blot (RLB) assay is a high-throughput and inexpensive method for product detection from multiplex PCR (mPCR) assays (6). Due to the high sensitivity of RLB probes, “megaplex” PCRs can be utilized, usually allowing amplification of all targets in a single reaction tube. Up to 43 isolates can be interrogated with up to 43 probes on a single reusable membrane. Several membranes can be processed simultaneously, with a total turnaround time of 12 h. The objectives of this study were to develop a high-throughput mPCR-based RLB assay (mPCR/RLB) for PDORF typing and to determine whether PDORF typing would retain its discriminatory power in other geographic settings or in a setting with high-level MRSA endemicity.
MATERIALS AND METHODS
Selection of isolates.
Isolates studied fell into the following four groups: (i) 5 S. aureus isolates for which whole genome sequences are publicly available (MRSA isolates COL, MW2, Mu3, and Mu50 [GenBank accession numbers CP000046, BA000033, AP009324, and BA000017] and methicillin-susceptible S. aureus strain NCTC8325 [GenBank accession number CP000253]), utilized for in silico analysis; (ii) 42 molecularly well-characterized MRSA reference strains which were temporally and/or geographically unrelated and represented major clones circulating in Australia and overseas (2); (iii) 35 MRSA clinical isolates from two Sydney tertiary hospitals, collected between September 2006 and July 2007; and (iv) pairs of isolates obtained from 81 patients at intervals ranging from 34 to 993 days (median, 146 days) for study of in vivo stability. The 35 clinical isolates consisted of 14 isolates from inpatients with nosocomial acquisition, that is, those who had had a documented negative MRSA screen (nose, axilla, and perineum) and subsequently acquired MRSA infection or colonization during the same admission, along with isolates with matching antibiograms from MRSA-colonized patients who shared the same ward at the time of MRSA acquisition and thus were likely sources of transmission. Both hospitals have a high prevalence of MRSA colonization and infection. At one hospital, a recent point prevalence survey indicated that 25% of patients within surgical wards carried MRSA, with a hospital-onset MRSA bacteremia rate of approximately 1.1 per 10,000 occupied bed days for the whole facility.
Primer and probe design.
Primers were modified from the originally published set (16) to produce a melting temperature of approximately 60°C. When necessary, additional inner primers were designed to produce amplicon lengths of <400 bp. In addition to the PDORF targets, primers and probes for nuc, mecA, tst, and pvl were included in the mPCR/RLB assay. Two probes (one sense and one antisense) were designed for each target. Primer modification and probe design were based on bacteriophage and gene sequences referenced in the original paper (16) and published in NCBI GenBank (Table 1 ). Primer and probe specificities were verified using BLASTn searches of published sequences in NCBI GenBank.
TABLE 1.
Primers and probes used in this study
| Target | Oligonucleotidea | Sequence (5′-3′)b | GenBank accession no. |
|---|---|---|---|
| Tn554 tnpB | N046Fb | 1746-TGATGGAGAGGAGTGGGATA-1765 | X03216 |
| N046AP | 1789-AGTTACGTCTATCCCAAACGTCTT-1766 | X03216 | |
| N046SP | 2096-TGGAAGGACTATTTAGTACCCTTCTTA-2122 | X03216 | |
| N046Rb | 2143-GAACATCATCCCATTCTAGCC-2123 | X03216 | |
| Mu50 SaGIm SAV0803 | SAV0803Fb | 881430-CAATATCCAAACCACGACCC-881411 | BA000017 |
| SAV0803AP | 881376-GATGAAAAGCAAATATACATATATAACTCTACATG-881410 | BA000017 | |
| SAV0803SP | 881245-TCTTTTAATATTTCTTTATGATTGTATTTATTATATGTATATG-881203 | BA000017 | |
| SAV0803Rb | 881183-AAAAATAGCGCCAACAGTCC-881202 | BA000017 | |
| φMu50B SAV0881 | SAV0881Fb | 932269-TGCTTGTTGTCATATCGCC-932287 | BA000017 |
| SAV0881AP | 932316-TGTTTTGGTAACTAGCCACTGTATAGATA-932288 | BA000017 | |
| SAV0881SP | 932418-TCAAATTTCTTTTTGAATAGTAAGTCAGA-932446 | BA000017 | |
| SAV0881Rb | 932468-CCTAGCTTGTATGTCTGCGCTA-932447 | BA000017 | |
| φMu50B SAV0898 | SAV0898Fb | 24676-GAAGATGCAGTTGTAGATCGC-24696 | AF424781 |
| SAV0898AP | 24721-CAACTTCCCAAGCTTCATACAATA-24697 | AF424781 | |
| SAV0898SP | 24781-GGTTTCCACAATAAATTTGAATTAAAA-24807 | AF424781 | |
| SAV0898Rb | 24829-CATCAATACCGTTAGCTTCTGC-24808 | AF424781 | |
| φPV83 ORF 2 | PV83ORF2Fb | 1269-GGCGCTTCTTCTTACAGGAG-1288 | AB044554 |
| PV83ORF2AP | 1323-CATTGTTAGATATTTATATGGTATGTAACCTAAAA-1289 | AB044554 | |
| PV83ORF2Rinner | 1356-GATAATCTTGTTTTTTTCACTAACTAAACCTAT-1324 | AB044554 | |
| PV83ORF2Finner | 1625-TGTTTAATAACAACGGTAAACCAGTATTT-1653 | AB044554 | |
| PV83ORF2SP | 1654-ATAGTTATTAAAGACTTTGAAAACAGAATCATT-1686 | AB044554 | |
| PV83ORF2R2b | 1715-GAATTATAGGTTTTAAGTTCACCCTCTTC-1687 | AB044554 | |
| φMu50B SAV0858 | SAV0858Fb | 6334-ATCTAAATTGCCTGTCGAAGC-6314 | AP001553 |
| SAV0858AP | 6294-TATTTGCGGCTTTAGCGTAA-6313 | AP001553 | |
| SAV0858SP | 6063-CATTTGAGAAAGTCTTTTGTCGATACT-6037 | AP001553 | |
| SAV0858Rb | 6017-CCAAGAACAGGGACATCGAC-6036 | AP001553 | |
| φMu50A SAV1998 | SAV1998Fb | 2122883-CAGTAAACTCACGCCTCCAAG-2122903 | BA000017 |
| SAV1998AP | 2122928-TGCATAGTTAAGCACATTTTTTTGT-2122904 | BA000017 | |
| SAV1998SP | 2123071-CCGAAATGGTTAAAGCACCTAT-2123092 | BA000017 | |
| SAV1998Rb | 2123111-TGCTAAATCATGTGGTGGG-2123093 | BA000017 | |
| SCCmec II kpdC | CN009F | 80974-GGACAACAATGGACAGAACC-80993 | BA000017 |
| CN009AP | 81019-CACTGATACGTCCATGGAAATATTTA-80994 | BA000017 | |
| CN009SP | 81061-GGCGGACCTGCTTCAG-81076 | BA000017 | |
| CN009Rb | 81097-AATTGCCGTAGTTTGAGCC-81079 | BA000017 | |
| φ11 nt 4427-5251 | phi11-4563Fb | 4563-GATATGCAAGATCAGACAATGCC-4585 | AF424781 |
| phi11-4610AP | 4610-CCTCGCTATCAACATGATTTCTAAT-4586 | AF424781 | |
| phi11-4632Rinner | 4632-CTAAATTGGTGCGTCAGTTTGT-4611 | AF424781 | |
| phi11-5026Finner | 5026-CAAACTACTACACGAAGCTAGACTACAAC-5054 | AF424781 | |
| phi11-5055SP | 5055-GAAAAGTAAATAAACAGTGGGTGCTTTA-5082 | AF424781 | |
| phi11-5103Rb | 5103-CTCTTGCCCATGTGTTCTGAG-5083 | AF424781 | |
| φSLT ORF 257 | SLTorf257Fb | 26802-GTGTTATCGCTATGAGTGGTGAC-26824 | EF462198 |
| SLTorf257AP | 26855-TTAAAAAACTATTTTTGTGCATAAAAATAGT-26825 | EF462198 | |
| SLTorf257SP | 27093-TCTCTAAAGAGCAATATAAGCGTTTC-27118 | EF462198 | |
| SLTorf257Rb | 27142-CTTTAAATCTTCTGGGACGTTCTC-27119 | EF462198 | |
| φMu50B SAV0850 | SAV0850F2b | 12192-ACCACAAGTTGACGTATGGC-12211 | AF410775 |
| SAV0850AP | 12236-TGAACTTCGATTGGTCTAAAATGTT-12212 | AF410775 | |
| SAV0850SP | 12419-GGAACTCACTATGGCGAGTATTCTATTA-12446 | AF410775 | |
| SAV0850Rb | 12467-AAACTCTCAACGGCTCAAATG-12447 | AF410775 | |
| φSLT ORF 175 | SLTorf175Fb | 15837-AAATGCTAGAATGCCCGAAC-15856 | EF462198 |
| SLTorf175AP | 15878-CCTGCATCCGTCTTATGATTTC-15857 | EF462198 | |
| SLTorf175SP | 15967-GGCTATGTCGGGCTGTTAACTA-15988 | EF462198 | |
| SLTorf175Rb | 16014-CGTTTTACTACTTACACCACTACGG-15990 | EF462198 | |
| φN315 SA1801 | SA1801Fb | 5235-CAATCAGCGGTCGAGAACT-5253 | EF462197 |
| SA1801AP | 5279-GAGTCTTAACCTCTAATGCTTGATGA-5254 | EF462197 | |
| SA1801Rinner | 5305-CATTCTTTCAAACCATTTTTTGTATG-5280 | EF462197 | |
| SA1801Finner | 5687-CGCAGATTGTTTGAGTGGTTA-5707 | EF462197 | |
| SA1801SP | 5708-CGTCAAAACGGATTCCTTATTAAA-5731 | EF462197 | |
| SA1801Rb | 5751-TTATAATCCACACCCTTGCG-5732 | EF462197 | |
| φMu50B SAV0913 | SAV0913Fb | 958598-TTATTCATAACGACGCAGGAAG-958619 | BA000017 |
| SAV0913AP | 958636-CTGCTGTTGCCCCTTTG-958620 | BA000017 | |
| SAV0913SP | 958868-GCGCTAGATTGTTGAAAAAATG-958889 | BA000017 | |
| SAV0913Rb | 958910-TTTCTGTTTGCTGGTAATCCC-958890 | BA000017 | |
| φMu50A SAV1974 | SAV1974Fb | 2110912-GCCACAAGAAAAGGCAGTG-2110894 | BA000017 |
| SAV1974AP | 2110869-TGCTTACAGCTACATCTGTTTTGAT-2110893 | BA000017 | |
| SAV1974SP | 2110719-GATATGAGTAACTTTGGTCGGAGTC-2110695 | BA000017 | |
| SAV1974Rb | 2110673-ATACTTTCCATCTATCCCAGCAG-2110694 | BA000017 | |
| φSLT ORF 182 | SLTorf182Fb | 1080359-AAATGATAGGAAAGCGACACG-1080379 | CP000736 |
| SLTorf182AP | 1080413-TTGATATAACCTTTAATGTCTCTTACTAAATTGT-1080380 | CP000736 | |
| SLTorf182SP | 1080493-CAACCTTGTTACCTACTAACCAAAAA-1080518 | CP000736 | |
| SLTorf182R2b | 1080540-GTTTGCTACTATGTCGCAACCT-1080519 | CP000736 | |
| mecA | mecAP4b | 1190-TCCAGATTACAACTTCACCAGG-1211 | Y00688 |
| mecAAP | 1236-TGCTGTTAATATTTTTTGAGTTGAAC-1211 | Y00688 | |
| mecASP | 1309-GATAAATCTTGGGGTGGTTACAAC-1332 | Y00688 | |
| mecAP7b | 1357-CATTTACCACTTCATATCTTGTAACG-1332 | Y00688 |
Oligonucleotides whose names end in “b” are 5′-biotinylated primers. Oligonucleotides whose names end in “P” are 5′-amine-labeled probes. Inner primers were unlabeled. mecA primers were derived from the work of Oliveira et al. (11). Primers and probes for nuc, tst, and pvl were unchanged from those previously published (2).
Modifications of the primers originally published by Suzuki et al. (16) are indicated by underlines. Numbers flanking the sequences indicate the starting and ending nucleotide positions in the indicated GenBank sequence.
In silico analysis.
Results of the mPCR/RLB assay were predicted by performing in silico PCR and probe binding, using FastPCR software (PrimerDigital), against five published S. aureus genomes: Mu3, Mu50, MW2, COL, and NCTC8325. First, in silico mPCR was performed to predict PCR products for each genome. These products were then used to predict probe binding. The predicted results were compared to the in vitro findings as part of assay validation.
mPCR and reverse line blot assay.
DNA was extracted by suspending 1 or 2 colonies in 400 μl molecular-grade H2O and heating them to 100°C for 10 min. This solution was frozen at −20°C and then thawed and centrifuged at 16,100 × g for 5 min before the supernatant was used as a PCR template. A 23-plex PCR and product detection by RLB assay were performed as described previously (6). The positive control consisted of isolates which, in combination, produced positive results for each probe (Mu3, COL, SJOG30, and 14176-5170). The specificity of positive controls was confirmed by in silico analysis (Mu3 and COL) or by single PCR and sequencing (SJOG30 and 14176-5170). The negative control consisted of PCR master mix without a DNA template.
Single PCR and sequencing.
The specificity of probe signals was confirmed where necessary by using single-primer-pair PCR, with sequencing of the product in the forward and reverse directions on an Applied Biosystems 3730xl DNA analyzer.
Interpretation of results.
Probe signals were considered strong if they were equal to or of greater intensity than the positive-control result for the corresponding probe. Probe signals were considered weak if they were present but of clearly lower intensity than that of the positive-control result. Probes for which no signal was detected were considered negative. Isolates belonging to the same PDORF type shared a unique pattern for the 16 genes. Since each gene was represented by two probes, for the purposes of PDORF type assignment, a gene was considered present if at least one strong probe signal or two weak probe signals were found and absent if only one weak probe signal or no probe signal was found (i.e., a solitary weak signal for one probe with a negative signal for the second probe was considered nonspecific).
PFGE and spa typing.
PFGE was performed according to the Harmony protocol (10), and fingerprint patterns were examined by Bionumerics v3.00 software (Applied Maths, Sint-Martens-Latem, Belgium). Optimization was set at 0.6%, with a band tolerance of 2% and a change toward the end of the fingerprint of 0.5%. Cluster analysis of PFGE patterns was performed using the Dice coefficient and the unweighted-pair group method using average linkages (UPGMA). PFGE subtypes were defined by patterns which were indistinguishable (100% similarity). spa sequencing and type assignment were performed as previously published (2).
Statistics.
Simpson's index of diversity and 95% confidence intervals (95% CI) were calculated using standard methods (3, 4).
RESULTS
In silico analysis.
Computational predictions confirmed in vitro experiments for isolates Mu3, Mu50, MW2, COL, and NCTC8325 (Fig. 1). It was noted that weak signal strength was associated with amplicons of 341 bp or more (Tn554 tnpB, 398 bp; φSLT ORF 257, 341 bp). No signal was found for the antisense probe for φMu50B SAV0858 in isolates Mu3 and Mu50, despite in silico analysis predicting only a single base-pair mismatch with the PCR product. However, this mismatch reduced the predicted melting temperature of the probe-PCR product binding from 52.1°C to 42.8°C, which was the likely explanation for the lack of signal on the RLB. This was confirmed by single PCR and sequencing of φMu50B SAV0858 in Mu3 and Mu50 in addition to isolate 14176-5170 (for which both probes gave a strong signal and no mismatch was found using the antisense probe).
FIG. 1.
Comparison of in vitro and in silico analyses of five isolates. For each isolate, the in vitro analysis is shown on the left and the in silico analysis is shown on the right. For the in silico analysis, black squares indicate a 100% match, while gray squares indicate a one- or two-base-pair mismatch between the predicted amplicon and the probe. Sense probes are shown above the antisense probes for each target gene.
For isolates Mu3 and Mu50, weak, nonspecific probe signals were noted for the antisense probe for φSLT ORF 182. Single PCR using primers against φSLT ORF 182 did not yield any PCR product for these isolates.
Analysis of reference collection.
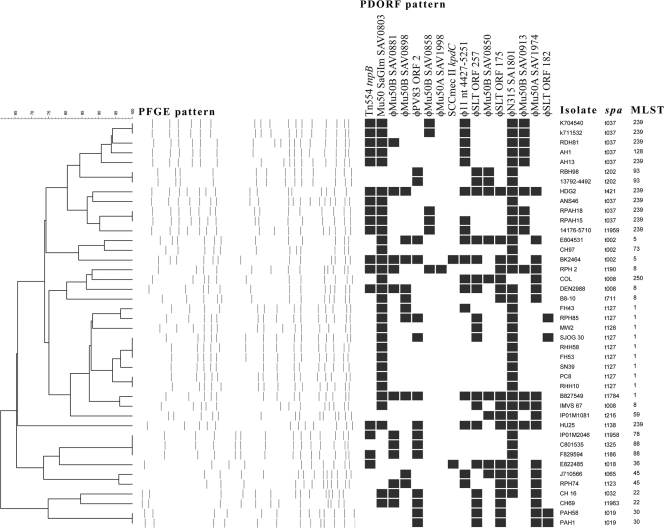
Analysis of the 42 reference strains revealed 33 PFGE subtypes (D = 0.9872; 95% CI, 0.9765 to 0.9980) and 30 PDORF types (D = 0.9710; 95% CI, 0.9447 to 0.9973) (Fig. 2). There were two PFGE subtypes that were comprised of three isolates each and five subtypes that were comprised of two isolates each. The remaining 26 PFGE subtypes contained only one isolate each. The largest group of PDORF types contained six isolates. Four of the PFGE subtypes with multiple members corresponded exactly with groups of PDORF types. For two of the PFGE subtypes, the isolates differed by a single PDORF target, while for one PFGE subtype, one isolate contained three PDORF targets that were absent in the other two isolates (Fig. 2). spa sequence typing produced 22 spa types (D = 0.9257; 95% CI, 0.8791 to 0.9722), and there were 15 types obtained by multilocus sequence typing (MLST) (D = 0.8815; 95% CI, 0.8225 to 0.9405).
FIG. 2.
PFGE, PDORF typing, spa typing, and MLST results for 42 reference strains. Clustering is based on the PFGE pattern. A black rectangle indicates detection of the relevant PDORF target by mPCR/RLB assay.
Analysis of clinical isolates.
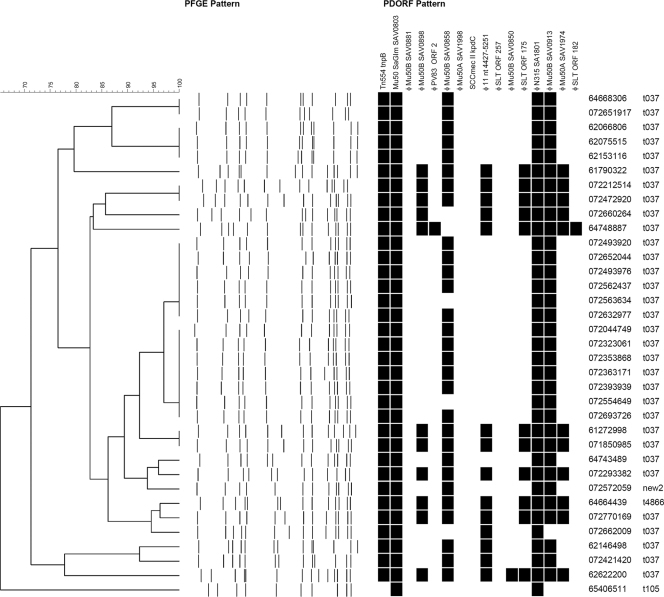
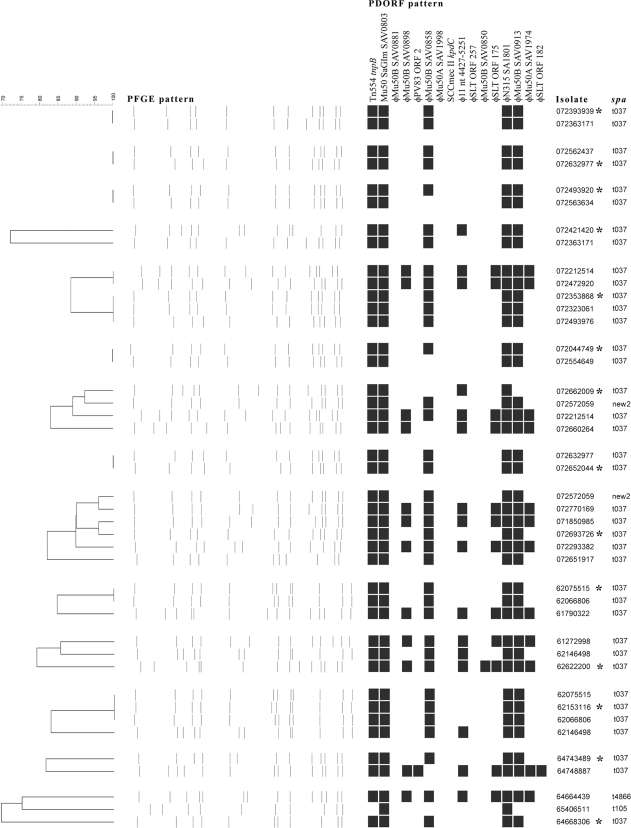
Analysis of the epidemiologically restricted set of 35 clinical isolates demonstrated 19 PFGE subtypes, 8 PDORF types, and 4 spa types (Fig. 3). All of these isolates were multiresistant, with 32 carrying spa type t037, which is typical of the MLST239-SCCmec III MRSA clone that is currently endemic in many Australian hospitals. Figure 4 shows data for the same isolates, with 14 isolates representing cases of nosocomial acquisition grouped with isolates from MRSA carriers who had contact with the patients around the time of acquisition. In 8 of these acquisition events, an MRSA carrier with an identical PFGE subtype was identified. Of these, 6 also had identical PDORF types; for the remaining 2, the PDORF type differed only by the absence of one marker. There was only one instance when isolates from a newly colonized patient and his contact had identical PDORF types but different PFGE types (minimum similarity, 82.1%). One novel spa type was identified, with the repeat sequence 15-12-16-02-25-17-12-16-02-25-17-24.
FIG. 3.
PFGE, PDORF typing, and spa typing results for 35 clinical isolates. Clustering is based on the PFGE pattern. A black rectangle indicates detection of the relevant PDORF target by mPCR/RLB assay.
FIG. 4.
Results of typing of clinical isolates involved in suspected nosocomial transmission events. Isolates marked with asterisks are from patients who had negative MRSA screens after admission. Each of these is grouped with isolates with the same antibiogram from patients in the same ward at the time of acquisition. Some isolates appear more than once in different groups.
In vivo stability and reproducibility.
For 42 of the 81 stability isolate pairs, the typing results were identical by PFGE and PDORF typing, while for 9 pairs the typing results were different by both methods (Table 2). For 22 pairs, PDORF typing results were indistinguishable but PFGE results differed. For 6 of these 22 pairs, the PFGE similarity was >95%, equivalent to a single band difference; for another 14, the PFGE similarity was 80 to 95%. Eight isolate pairs had indistinguishable PFGE patterns but different PDORF patterns. Three of these differed by two PDORF targets, and the remaining five differed by one PDORF target. The median time between collections for pairs that had identical patterns for both PFGE and PDORF typing was 140.5 days; for those with different PDORF patterns, it was 243 days, while it was 197 days for those that had different PFGE patterns (Mann-Whitney test; P = 0.99).
TABLE 2.
Results of in vivo stability study of pairs of isolates from 81 patients
| PDORF result | No. of isolate pairs with PFGE result (median time [days] between collections) |
Total no. of isolate pairs (median time [days] between collections) | |
|---|---|---|---|
| Identical | Different | ||
| Identical | 42 (140.5) | 22 (107.5) | 64 (135) |
| Different | 8 (100) | 9 (355) | 17 (243) |
| Total | 50 (138) | 31 (197) | 81 (146) |
Forty-two isolates had DNA extracted on two separate occasions and were tested by PDORF typing, using two separate PCRs with product detection on two identical RLB membranes prepared on different days. There was 100% concordance between the results produced on the two occasions.
DISCUSSION
Despite a long turnaround time, low throughput level, and labor-intensive analysis procedure, PFGE remains the gold standard for typing of S. aureus outbreaks due to its high discriminatory power and its proven reflection of epidemiologic relationships. With the aim of overcoming the limitations of PFGE, newer, PCR-based typing methods have been developed, such as spa sequence typing (9), multilocus variable-number-tandem-repeat analysis (MLVA) (14), and repetitive sequence-based PCR (rep-PCR) (13). However, no method has yet been accepted as superior to PFGE, since the newer methods either are less discriminatory, require analysis of a gel-based fingerprint, lack reproducibility, or are too expensive.
Binary typing involves interrogating the bacterial genome for the presence or absence of a set of markers. It has theoretical advantages over other typing methods because of the ability to express results as positive or negative for each marker, which facilitates harmonization of test protocols and creation of typing databases for interrun and interlaboratory comparisons of results. Furthermore, no specialized equipment is needed, with the minimum requirements being a PCR cycler and detection of amplicons by agarose gel electrophoresis.
We use the mPCR/RLB format for binary typing because it offers a number of advantages over other methods. The probe hybridization with chemiluminescence detection which is utilized in the mPCR/RLB assay is highly sensitive, permitting somewhat inefficient PCR amplification conditions; this allows amplification of a large number of targets in a single megaplex PCR. At the same time, probe-based detection, particularly with two probes per product, is highly specific. The membrane and its attached probes can be reused more than 20 times (after a simple washing step), up to 43 isolates may be tested on a single membrane, and multiple membranes can be processed simultaneously by one technician. This makes mPCR/RLB assay a cost-effective (approximately $2 [U.S. currency] per sample) and high-throughput option. Furthermore, the flexibility of the method allows for inclusion of additional targets of interest, such as pvl or tst (6).
Comparative genomic techniques have demonstrated a substantial array of genetic loci that are variably present among even otherwise closely related strains of S. aureus, raising the prospect of a highly discriminatory binary typing system based on such targets (7). We previously developed mPCR/RLB binary typing methods for detection of antibiotic resistance genes (12), exotoxin genes (2), and SCCmec elements (1) in MRSA. Another approach has been to identify discriminatory sequences by randomly amplified DNA analysis and then to clone sequences, label them, and interrogate their presence in genomic DNAs of test isolates by Southern blot hybridization (18, 19). This technique has also been adapted for reverse hybridization (17). Huygens et al. combined five binary targets (pvl, cna, sdrE, pUB110, and pT181) and single nucleotide polymorphism (SNP)-based MLST for an MRSA typing system with improved discriminatory power compared with that of the SNP-based method alone (5).
Mobile genetic elements such as integrated prophages, transposons, and genomic islands have also attracted attention as potentially highly discriminatory typing targets (8). Such markers were used in the typing system developed by Suzuki et al. (16) that has been adapted in this study. In the original study, 60 ORFs were identified by comparative analysis of staphylococcal phage genomes, and locally circulating strains were surveyed for the presence or absence of these markers. A subset of the most discriminatory set of 16 markers was chosen for use in the typing system, which consisted of four 4-plex PCRs and product detection by electrophoresis on four agarose gels. This phage-derived ORF typing system had a similar discriminatory power to that of PFGE (16).
Our findings confirmed that PDORF typing had a similar discriminatory power to that of PFGE for a large collection of epidemiologically unrelated isolates (D = 0.9710 versus 0.9872). In testing the clinical isolates, two of the targets were absent in all clinical isolates tested (φMu50 SAV1998 and SCCmec II kpdC), and three others contributed little to discriminatory power (Mu50 SaGIm SAV0803, N315 SA1801, and φMu50B SAV0850). We did not survey locally circulating Australian strains for the relative frequencies of all 60 ORFs explored in the original study, but if this were done, there would be the potential to identify other binary targets that may further improve the discriminatory power of PDORF typing in our setting.
Mobile genetic elements can be relatively unstable due to the high molecular clock speeds of those components of the genome. While this is exactly what confers the high level of discriminatory power, it could also potentially result in misclassification of the epidemiologic relationships between isolates. It is important that the stability of new typing systems based on mobile elements be assessed comprehensively to ensure that the loci remain unchanged, at least over time frames which are relevant to the proposed application of the method. Studies of in vivo stability seem more relevant for this purpose than in vitro studies, since the acquisition of exogenous DNA and evolutionary changes due to ecological pressures are unlikely to occur simply with serial passage in the laboratory. For the purposes of monitoring hospital epidemiology of MRSA over a short-term period (several months; this time frame is relevant for investigation of nosocomial outbreaks), we have shown that PDORF typing is at least as stable as PFGE, a similar finding to that of Suzuki et al. (16). The binary format of this system lends itself to establishing databases of PDORF typing results, which can be useful for easy comparison of isolates from a suspected outbreak. However, the utility of PDORF typing for studying long-term epidemiology and evolution of MRSA and its relationship with other methods traditionally used for this purpose (such as MLST) need further study.
While PDORF typing separated 35 clinical isolates into fewer groups than PFGE subtyping did (8 versus 19), examination of subgroups of these isolates in an attempt to identify nosocomial transmission events suggested that the ability of PDORF typing to identify related isolates is similar to that of PFGE. Based on these data and the mobile nature of the loci utilized, we suggest that identical PDORF patterns can be used to define epidemiologically related isolates among isolates from an outbreak of MRSA. A difference at a single locus would suggest that the isolates are possibly related in this setting.
Conclusions.
PDORF typing of MRSA has a similar discriminatory power to that of PFGE, but with better stability, can identify nosocomial transmission events in settings of high-level endemicity, and is highly reproducible. mPCR/RLB-based PDORF typing improves throughput compared with that of the original gel-based method. Its main advantages over PFGE include a short turnaround time, high throughput level, low cost, and binary result format. While the method as described utilizes stable targets and is useful for identifying nosocomial transmission events, surveying locally circulating strains for a wider range of phage-derived targets may identify additional markers which could be included in the system for further improvements in discriminatory power.
Acknowledgments
Matthew V. N. O'Sullivan is the recipient of a National Health and Medical Research Council postgraduate student scholarship.
Footnotes
Published ahead of print on 2 June 2010.
REFERENCES
- 1.Cai, L., F. Kong, Q. Wang, H. Wang, M. Xiao, V. Sintchenko, and G. L. Gilbert. 2009. A new multiplex PCR-based reverse line-blot hybridization (mPCR/RLB) assay for rapid staphylococcal cassette chromosome mec (SCCmec) typing. J. Med. Microbiol. 58:1045-1057. [DOI] [PubMed] [Google Scholar]
- 2.Cai, Y., F. Kong, Q. Wang, Z. Tong, V. Sintchenko, X. Zeng, and G. L. Gilbert. 2007. Comparison of single and multilocus sequence typing and toxin gene profiling for characterisation of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 45:3302-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundmann, H., S. Hori, and G. Tanner. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 39:4190-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huygens, F., J. Inman-Bamber, G. R. Nimmo, W. Munckhof, J. Schooneveldt, B. Harrison, J. A. McMahon, and P. M. Giffard. 2006. Staphylococcus aureus genotyping using novel real-time PCR formats. J. Clin. Microbiol. 44:3712-3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong, F., and G. L. Gilbert. 2006. Multiplex PCR-based reverse line blot hybridization assay (mPCR/RLB)—a practical epidemiological and diagnostic tool. Nat. Protoc. 1:2668-2680. [DOI] [PubMed] [Google Scholar]
- 7.Lindsay, J. A., and M. T. G. Holden. 2004. Staphylococcus aureus: superbug, super genome? Trends Microbiol. 12:378-385. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay, J. A., C. E. Moore, N. P. Day, S. J. Peacock, A. A. Witney, R. A. Stabler, S. E. Husain, P. D. Butcher, and J. Hinds. 2006. Microarrays reveal that each of the ten dominant lineages of Staphylococcus aureus has a unique combination of surface-associated and regulatory genes. J. Bacteriol. 188:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellmann, A., A. W. Friedrich, N. Rosenkotter, J. Rothganger, H. Karch, R. Reintjes, and D. Harmsen. 2006. Automated DNA sequence-based early warning system for the detection of methicillin-resistant Staphylococcus aureus outbreaks. PLoS Med. 3:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murchan, S., M. E. Kaufmann, A. Deplano, R. de Ryck, M. Struelens, C. E. Zinn, V. Fussing, S. Salmenlinna, J. Vuopio-Varkila, N. El Solh, C. Cuny, W. Witte, P. T. Tassios, N. Legakis, W. van Leeuwen, A. van Belkum, A. Vindel, I. Laconcha, J. Garaizar, S. Haeggman, B. Olsson-Liljequist, U. Ransjo, G. Coombes, and B. Cookson. 2003. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J. Clin. Microbiol. 41:1574-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira, D. C., and H. de Lencastre. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2155-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Sullivan, M. V., Y. Cai, F. Kong, X. Zeng, and G. L. Gilbert. 2006. Influence of disk separation distance on accuracy of the disk approximation test for detection of inducible clindamycin resistance in Staphylococcus spp. J. Clin. Microbiol. 44:4072-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross, T. L., W. G. Merz, M. Farkosh, and K. C. Carroll. 2005. Comparison of an automated repetitive sequence-based PCR microbial typing system to pulsed-field gel electrophoresis for analysis of outbreaks of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43:5642-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabat, A., J. Krzyszton-Russjan, W. Strzalka, R. Filipek, K. Kosowska, W. Hryniewicz, J. Travis, and J. Potempa. 2003. New method for typing Staphylococcus aureus strains: multiple-locus variable-number tandem repeat analysis of polymorphism and genetic relationships of clinical isolates. J. Clin. Microbiol. 41:1801-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabat, A., N. Malachowa, J. Miedzobrodzki, and W. Hryniewicz. 2006. Comparison of PCR-based methods for typing Staphylococcus aureus isolates. J. Clin. Microbiol. 44:3804-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki, M., Y. Tawada, M. Kato, H. Hori, N. Mamiya, Y. Hayashi, M. Nakano, R. Fukushima, A. Katai, T. Tanaka, M. Hata, M. Matsumoto, M. Takahashi, and K. Sakae. 2006. Development of a rapid strain differentiation method for methicillin-resistant Staphylococcus aureus isolated in Japan by detecting phage-derived open-reading frames. J. Appl. Microbiol. 101:938-947. [DOI] [PubMed] [Google Scholar]
- 17.van Leeuwen, W., C. Libregts, M. Schalk, J. Veuskens, H. Verbrugh, and A. van Belkum. 2001. Binary typing of Staphylococcus aureus strains through reversed hybridization using digoxigenin-universal linkage system-labeled bacterial genomic DNA. J. Clin. Microbiol. 39:328-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Leeuwen, W., M. Sijmons, J. Sluijs, H. Verbrugh, and A. van Belkum. 1996. On the nature and use of randomly amplified DNA from Staphylococcus aureus. J. Clin. Microbiol. 34:2770-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Leeuwen, W., H. Verbrugh, J. van der Velden, N. van Leeuwen, M. Heck, and A. van Belkum. 1999. Validation of binary typing for Staphylococcus aureus strains. J. Clin. Microbiol. 37:664-674. [DOI] [PMC free article] [PubMed] [Google Scholar]