Abstract
We evaluated a commercially available immunochromatographic dipstick test to detect Trypanosoma cruzi infection in 366 human serum samples with known serological results from Argentina, Ecuador, Mexico, and Venezuela. One hundred forty-nine of 366 (40.7%) and 171/366 (46.7%) samples tested positive by dipstick and serology, respectively. Dipstick sensitivity was calculated to be 84.8% (range between countries, 77.5 to 95%), and specificity was 97.9% (95.9 to 100%).
Chagas disease is caused by Trypanosoma cruzi and is found in wildlife, domestic animals, and humans in rural as well as peri-urban areas of Mexico, Central America, and South America; in the United States, T. cruzi is found in wildlife, but human cases are rare (29). Although transmission of T. cruzi can occur orally, congenitally, or transfusionally, most transmission to mammalian hosts is through the feces of blood-feeding triatomine bugs when T. cruzi trypomastigotes in the feces contaminate the bite wound or enter the host through mucosal surfaces (22). By causing the loss of an estimated 670,000 disability-adjusted life years (i.e., a measure that sums years of potential life lost due to premature mortality and years of productive life lost due to disability), Chagas disease is the most important parasitic disease in the Americas; 8 to 10 million people are currently infected with T. cruzi, with up to 100 million at risk of contracting the disease (32).
There are several methods to diagnose T. cruzi infection (11), but none are ideal when mass screening of samples is required (e.g., epidemiological surveys, blood unit screening). While comparatively easy to use and sensitive, serological tests (i.e., enzyme-linked immunosorbent assay [ELISA], immunofluorescence antibody test [IFAT], indirect hemagglutination test [IHAT], or radioimmunosorbent assay [RIA]) are of varied specificities (i.e., 60 to 100%) (12, 16, 26). Molecular tests, including PCR-based approaches, are very specific but lack sensitivity (i.e., 30 to 95%) and require technological expertise and specialized, expensive laboratory equipment (11, 21, 23). Hemoculture and xenodiagnosis are the current gold standard for T. cruzi parasitological diagnosis (6, 11, 21). Though these techniques are specific, their sensitivity in the chronic phase of infection is quite variable (e.g., 0 to 50% [6]); they also are labor-intensive and time-consuming (e.g., because of the necessity of mass-rearing bugs for xenodiagnosis and examination of them). Thus, a rapid, sensitive, and specific diagnostic test to detect T. cruzi infection would be extremely valuable for mass-screening surveys and intervention campaigns as well as during the onset of outbreaks; results could be read immediately, and control measures could be implemented in situ.
Immunochromatographic dipstick tests have been developed for a range of tropical diseases, including malaria (31), leishmaniasis (7), and schistosomiasis (3); until recently (4, 5, 8, 14, 17, 20, 25, 28, 30), none was available for Chagas disease.
Recently, the World Health Organization announced renewed efforts to eliminate Chagas disease (27). For such efforts to succeed, an easy-to-use, sensitive, and specific diagnostic test will be crucial for both detecting and treating cases early as well as monitoring the implementation of elimination efforts and evaluating their impact (18, 24).
We evaluated the sensitivity and specificity of a commercially available immunochromatographic dipstick test to detect antibodies to T. cruzi infection in human serum samples with known serological results collected in areas of both Chagas disease endemicity and nonendemicity in four different Latin American countries.
Test samples.
Study samples were collected between 2000 and 2007 from the general population, cases suspected of having the disease, or blood donors during a range of epidemiological studies in Argentina, Ecuador, Mexico, and Venezuela (Table 1). Samples had been stored at −20°C and had previously been tested by at least one of the following methods according to standard in-country protocols: ELISA (n = 366), IHAT (n = 166), and IFAT (n = 101) (Table 1). Samples were considered negative and positive as per in-country Chagas disease diagnostic guidelines (Table 1).
TABLE 1.
Characteristics of samples and serological assays useda
| Country | No. of samples that were |
Total | ELISA description |
IFAT description | IHAT description | Antigen used | Definition (per in-country guidelines) of: |
Reference(s) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | From patients with other infections | I | II | Positive sample | Negative sample | Discordant samples | ||||||
| Argentina | 40 | 51 | 10 | 101 | In-house protocol (cutoff, 0.2 + SD) | In-house protocol (cutoff, 1:32) | In-house protocol (cutoff, 1:32) | T. cruzi epimastigotes, Tulahuén strain | A sample was considered seropositive if at least two serological tests were positive following testing by ELISA, IHAT, and IFAT | Readings were negative following testing by three different tests (ELISA, IFAT, and IHA) | Readings were discordant following testing by three different tests (ELISA, IFAT, and IHAT) | 9, 19 | |
| Ecuador | 51 | 8 | 41 | 100 | Chagas III (BiosChile Ingeniería Genética S.A., Santiago, Chile) (cutoff, [CPos + CNeg]·0.35) | Chagatek ELISA (bioMérieux, Buenos Aires, Argentina) (cutoff, CNeg·0.100) | ND | ND | Commercial kit antigen | Readings were positive following testing by two different ELISAs | Readings were negative following testing by two different ELISAs | Readings were discordant following testing by two different ELISAs, or one reading was close to the test's cutoff (±10%); the third reading by either test was considered definitive | 1 |
| Mexico | 40 | 50 | 10 | 100 | Kit Chagas III (BiosChile Ingenieria Genetica S.A., Santiago, Chile) (cutoff, [CPos + CNeg]·0.35) | ND | ND | Commercial kit antigen | Readings were positive following testing by two different ELISAs | Reading was negative following testing by one ELISA | Readings were discordant following testing by two different ELISAs, or one reading was close to the test's cutoff; the third reading by either test was considered definitive | N. Pavia-Ruzb | |
| Venezuela | 40 | 25 | 65 | In-house protocol (cutoff, 0.2 + 2 SD) | ND | In-house protocol (cutoff, 1:4) | T. cruzi epimastigotes, PM strain locally isolated from a patient in 1985 | Readings were positive by serological testing of two patient samples taken 1 month apart | Reading was negative following testing by one ELISA | Readings were discordant following testing by two different ELISAs, or one reading was close to the test's cutoff; the third reading by either test was considered definitive | 10, 13 | ||
Positive and negative samples included samples that had previously tested positive and negative by serological methods, as per in-country Chagas disease diagnostic guidelines. Abbreviations: CPos and CNeg, optical density value of positive and negative controls, respectively; ND, not done.
Personal communication.
Dipstick.
The dipstick test (Trypanosoma Detect MRA rapid test; Inbios, Seattle, WA) was carried out according to the manufacturer's instructions, where 20 μl of serum followed by 3 to 4 drops of buffer (150 to 200 μl) were added to the dipstick's sample pad. After 10 min, a red control line and, if positive, a second line appeared on the test field of the dipstick cassette. The test is based on a proprietary gold mix containing multiepitope recombinant antigen derived from different T. cruzi antigens. All dipsticks originated from the same lot and were distributed to the various study locations in January 2007; all dipsticks were used on study samples between May and October 2007, within the specified date of expiration of the dipstick.
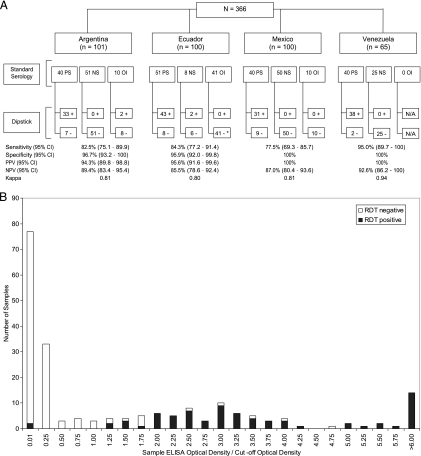
Figure 1 A summarizes the dipstick and serology results of samples evaluated. One hundred forty-nine of 366 (40.7%) and 171/366 (46.7%) samples tested positive by the dipstick test and serology, respectively. A total of 30 (8.2%) samples had discordant results when tested by conventional serological tests and with dipsticks (Fig. 1A). Samples that were negative by standard serology but positive by the dipstick method included 2 of the 47 (4.3%) negative-control samples and 2 of the 61 (3.3%) samples from individuals with other infections (i.e., HIV, syphilis, hepatitis B virus [HBV], hepatitis C virus [HCV], brucellosis, and tuberculosis). The remaining 26 samples yielding discordant results were from individuals living in areas of T. cruzi endemicity and who had tested positive by conventional serology but negative with the dipstick. For samples that were tested by ELISA and for which exact optical density values were available, an association between ELISA optical density and dipstick positivity was observed (Fig. 1B).
FIG. 1.
(A) Comparative diagnosis of T. cruzi in 366 human blood samples. Samples were evaluated by different serological tests and considered positive or negative as per in-country Chagas disease diagnostic guidelines. The number of samples that were tested in each country is specified, as is whether they were serologically positive or negative, whether they were samples from patients with other infections (and also serologically negative), and how they tested when the dipsticks were used. Sensitivities, specificities, positive and negative predictive values, kappa values, and 95% confidence intervals are also given. Shown below the standard-serology results are results for samples that had serologically concordant and discordant results and how they tested with the dipstick. One sample from Ecuador from a case with another infection (indicated with *) had discordant ELISA results, testing negative and testing borderline positive; it retested negative. Abbreviations: PS, samples testing positive as per country guidelines; NS, samples testing negative as per country guidelines; OI, samples from individuals with other infections (i.e., brucellosis, HBV, HCV, HIV, syphilis, tuberculosis); Dipstick, Trypanosoma cruzi Detect dipstick; N/A, not available; 95% CI, 95% confidence interval; PPV, positive predictive value; NPV, negative predictive value. (B) Relationship between ELISA optical density values and rapid diagnostic test positivity. Samples represented are samples for which exact optical density values were available, i.e., samples from Ecuador and Mexico (n = 200).
Based on the total number of samples testing positive or negative by serology, the sensitivity and specificity of the rapid diagnostic test was calculated to be 84.8% (range between countries, 77.5 to 95.0%) and 97.9% (95.9 to 100%); negative and positive predictive values were 88.0% (85.0 to 92.6%) and 97.3% (94.3 to 100%), respectively (Fig. 1A). The kappa value, which is an index comparing the observed agreement between tests against the agreement which might have been expected by chance, was calculated to be 0.83 (range between countries, 0.80 to 0.94).
To our knowledge, this is the first multicenter study to evaluate the Inbios Trypanosoma Detect test to detect T. cruzi infection in blood samples from people in the general population living in areas of T. cruzi endemicity or nonendemicity, cases suspected of having the disease, or blood donors. Our study indicates that the tested dipstick test has high specificity (97.9%) and moderate to high sensitivity (84.8%). Agreement between the results of standard serological tests and the tested dipstick was good. This complements the results from other studies testing the same dipstick (5, 14, 30) or dipsticks from other manufacturers (i.e., Chagas Stat-Pak, Chembio Diagnostics Inc.; SD Bioline Chagas Ab rapid test (Standard Diagnostics); and OnSite Chagas Ab rapid test cassette, CTK Biotech) (4, 8, 14, 20, 25, 28).
Four samples that were negative by standard serological tests were positive with the dipstick, and we are currently assessing whether these samples are true or false positives; clearly, the tested dipstick yielded false negatives (n = 26). As similar studies evaluating dipsticks for malaria (31) and leishmaniasis (7) diagnosis have shown, variability in dipstick performance will depend on factors such as the type of diagnostic antigen and conjugate used. Commercially available dipstick tests can be highly variable in terms of sensitivity and specificity (7). Rates of false positivity for malaria dipsticks can be as high as 28%, which may, for example, be due to cross-reactivity to rheumatoid factor (15). For samples from individuals suspected of T. cruzi infections, false-positive results may be caused by other protozoan organisms, including Leishmania spp. (2).
There are many potential advantages of using dipsticks over other diagnostic methods. First, when using dipsticks, a large number of samples can be processed quickly and with minimum effort. Second, compared to the technological expertise needed for serology, molecular methods, or xenodiagnosis, the expertise (i.e., training of personnel) necessary to perform the dipstick tests is minimal, as is the requirement for specialized laboratory equipment. Another advantage of dipstick tests is that patients can see the results for themselves, which will contribute to a better working relationship between local communities and people carrying out the testing (e.g., during surveys). Third, from an epidemiological point of view, a dipstick test allows intervention strategies to be implemented in situ, such as for serologic surveillance, vaccine or clinical trials, and rapid initiation of treatment of infected individuals during outbreaks of acute Chagas disease. Compared to what is required following currently used serological tests, the need for follow-up visits to surveyed individuals is reduced and hence operational costs are reduced. Fourth, in settings normally considered nonsupportive of T. cruzi and Chagas disease (22, 29), dipstick tests could easily be included in laboratory testing algorithms. These could apply to people potentially exposed to T. cruzi when traveling (e.g., tourists, military personnel) as well as blood and organ donors from countries where Chagas disease is endemic.
Immunochromatographic dipstick tests are comparatively expensive (the tested dipstick has a retail price of $1.40), but considering the above, a sensitive and specific dipstick test such as the one tested here could prove very cost-effective relative to currently available diagnostic tests, especially when used in mass-screening surveys, investigations of acute outbreaks of Chagas disease, and even tests in blood donor units.
A more comprehensive multicenter study is now planned to evaluate dipsticks using a standardized protocol on batches of sera from known negative and positive controls and a random selection of samples from areas of endemicity and nonendemicity, as well as to assess the relationship between dipstick positivity and standard serology and the heat stability and ease of use of dipsticks.
Acknowledgments
We thank Otilia Rodríguez Carvajal from the Instituto Mexicano del Seguro Social in Mérida, Mexico, as well as Ana María de Rissio from the Instituto Nacional de Parasitología Mario Fatala Chabén in Buenos Aires, Argentina, for testing and providing the serum samples used in this study.
R.E.G. is supported by awards from the NIH/NSF Ecology of Infectious Diseases program, which is funded by the Fogarty International Center and the National Institute of Environmental Health Sciences (grant R01 TW05836 to Uriel Kitron and R.E.G.); the Agencia Nacional de Promoción Científica y Técnica (Argentina); and the University of Buenos Aires.
M.V.C. and R.E.G. are members of CONICET's Researcher's Career.
The opinions expressed are those of the authors and may not reflect the position of their employing organization or of their work's sources of funding.
Footnotes
Published ahead of print on 9 June 2010.
REFERENCES
- 1.Abad-Franch, F., and M. H. Aguilar. 2007. Control de la enfermedad de Chagas en Ecuador, p. 1-164. OPS/OMS Ministeriode Salud Pública del Ecuador, Quito, Ecuador.
- 2.Amato Neto, V., V. S. Amato, F. F. Tuon, E. Gakiya, C. R. de Marchi, R. M. de Souza, and C. R. Furucho. 2009. False-positive results of a rapid K39-based strip test and Chagas disease. Int. J. Infect. Dis. 13:182-185. [DOI] [PubMed] [Google Scholar]
- 3.Bosompem, K. M., I. Ayi, W. K. Anyan, T. Arishima, F. K. Nkrumah, and S. Kojima. 1997. A monoclonal antibody-based dipstick assay for diagnosis of urinary schistosomiasis. Trans. R. Soc. Trop. Med. Hyg. 91:554-556. [DOI] [PubMed] [Google Scholar]
- 4.Brutus, L., D. Schneider, J. Postigo, M. Romero, J. Santalla, and J. P. Chippaux. 2008. Congenital Chagas disease: diagnostic and clinical aspects in an area without vectorial transmission, Bermejo, Bolivia. Acta Trop. 106:195-199. [DOI] [PubMed] [Google Scholar]
- 5.Cardinal, M. V., R. Reithinger, and R. E. Gürtler. 2006. Use of an immunochromatographic dipstick test for rapid detection of Trypanosoma cruzi in sera from animal reservoir hosts. J. Clin. Microbiol. 44:3005-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerisola, J. A., R. W. Rohwedder, and C. E. Del Prado. 1971. Rendimiento del xenodiagnóstico en la infección chagásica crónica humana, utilizando ninfas de diferentes especias de triatominos. Bol. Chile Parasit. 26:57-58. [PubMed] [Google Scholar]
- 7.Chappuis, F., S. Rijal, A. Soto, J. Menten, and M. Boelaert. 2006. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ 333:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chippaux, J. P., J. R. Postigo, J. A. Santalla, D. Schneider, and L. Brutus. 2008. Epidemiological evaluation of Chagas disease in a rural area of southern Bolivia. Trans. R. Soc. Trop. Med. Hyg. 102:578-584. [DOI] [PubMed] [Google Scholar]
- 9.De Rissio, A. M., K. Scollo, and R. Cardoni. 2009. La transmisión madre-hijo del Trypanosoma cruzi en la Argentina. Medicina (Buenos Aires) 69:529-535. [PubMed] [Google Scholar]
- 10.Diaz-Bello, Z., R. Zavala-Jaspe, M. Diaz-Villalobos, L. Mauriello, A. Maekelt, and B. Alarcón de Noya. 2008. Diagnóstico confirmatorio de anticuerpos anti-Trypanosoma cruzi en donantes referidos por banco de sangre en Venezuela. Invest. Clin. 49:141-150. [PubMed] [Google Scholar]
- 11.Dubner, S., E. Schapachnik, A. R. Riera, and E. E. Valero. 2008. Chagas disease: state-of-the-art of diagnosis and management. Cardiol. J. 15:493-504. [PubMed] [Google Scholar]
- 12.Ferreira, A. W., Z. R. Belem, E. A. Lemos, S. G. Reed, and A. Campos-Neto. 2001. Enzyme-linked immunosorbent assay for serological diagnosis of Chagas disease employing a Trypanosoma cruzi recombinant antigen that consists of four different peptides. J. Clin. Microbiol. 39:4390-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gobierno Bolivariano de Venezuela Ministerio del Poder Popular para la Salud. 2007. Vigilancia de enfermedad de Chagas: guía para el diagnóstico, manejo y tratamiento de enfermedad de Chagas en fase aguda a nivel de los establecimientos de salud, primera edición, p. 1-32. Gobierno Bolivariano de Venezuela Ministerio del Poder Popular para la Salud, Caracas, Venezuela.
- 14.Ji, M. J., J. S. Noh, B. K. Cho, Y. S. Cho, S. J. Kim, and B. S. Yoon. 2009. Evaluation of SD BIOLINE Chagas Ab rapid kit. Korean J. Lab. Med. 29:48-52. [DOI] [PubMed] [Google Scholar]
- 15.Laferi, H., K. Kandel, and H. Pichler. 1997. False positive dipstick test for malaria. N. Engl. J. Med. 337:1635-1636. [DOI] [PubMed] [Google Scholar]
- 16.Lauricella, M. A., M. B. Castañera, R. E. Gürtler, and E. L. Segura. 1998. Immunodiagnosis of Trypanosoma cruzi (Chagas disease) infection in naturally infected dogs. Mem. Inst. Oswaldo Cruz 93:501-507. [DOI] [PubMed] [Google Scholar]
- 17.Luquetti, A. O., C. Ponce, E. Ponce, J. Esfandiari, A. Schijman, S. Revollo, N. Añez, B. Zingales, R. Ramgel-Aldao, A. Gonzalez, M. J. Levin, E. S. Umezawa, and J. Franco da Silveira. 2003. Chagas disease diagnosis: a multicentric evaluation of Chagas Stat-Pak, a rapid immunochromatographic assay with recombinant proteins of Trypanosoma cruzi. Diagn. Microbiol. Infect. Dis. 6:265-271. [DOI] [PubMed] [Google Scholar]
- 18.Médecins sans Frontières. 2008. Campaign for Access to Essential Medicines. International meeting: new diagnostic tests are urgently needed to treat patients with Chagas disease. Rev. Soc. Bras. Med. Trop. 41:315-319. [DOI] [PubMed] [Google Scholar]
- 19.Ministerio de Salud, Presidencia de la Nación. 2008. Normas para el diagnóstico y tratamiento del Chagas, p. 1-28. Ministerio de Salud, Buenos Aires, Argentina.
- 20.Ponce, C., E. Ponce, E. Vinelli, A. Montoya, V. de Aguilar, A. Gonzalez, B. Zingales, R. Rangel-Aldao, M. J. Levin, J. Esfandiari, E. S. Umezawa, A. O. Luquetti, and J. F. da Silveira. 2005. Validation of a rapid and reliable test for diagnosis of Chagas disease by detection of Trypanosoma cruzi-specific antibodies in blood of donors and patients in Central America. J. Clin. Microbiol. 43:5065-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portela-Lindoso, A. A., and M. A. Shikanai-Yasuda. 2003. Chronic Chagas disease: from xenodiagnosis and hemoculture to polymerase chain reaction. Cad. Saude Publica 37:107-115. [DOI] [PubMed] [Google Scholar]
- 22.Prata, A. 2001. Clinical and epidemiological aspects of Chagas disease. Lancet Infect. Dis. 1:92-100. [DOI] [PubMed] [Google Scholar]
- 23.Ramírez, J. D., F. Guhl, E. S. Umezawa, C. A. Morillo, F. Rosas, J. A. Marin-Neto, and S. Restrepo. 2009. Evaluation of adult chronic Chagas heart disease diagnosis by molecular and serological methods. J. Clin. Microbiol. 47:3945-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reithinger, R., R. L. Tarleton, J. A. Urbina, U. Kitron, and R. E. Gürtler. 2009. Eliminating Chagas disease: challenges and a roadmap. BMJ 338:1044-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roddy, P., J. Goiri, L. Flevaud, P. P. Palma, S. Morote, N. Lima, L. Villa, F. Torrico, and P. Albajar-Viñas. 2008. Field evaluation of a rapid immunochromatographic assay for detection of Trypanosoma cruzi infection by use of whole blood. J. Clin. Microbiol. 46:2022-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segura, E. L., E. N. Cura, S. A. Estani, J. Andrade, J. C. Lansetti, A. M. de Rissio, A. Campanini, S. B. Blanco, R. E. Gürtler, and M. Alvarez. 2000. Long-term effects of a nationwide control program on the seropositivity for Trypanosoma cruzi infection in young men from Argentina. Am. J. Trop. Med. Hyg. 62:353-362. [DOI] [PubMed] [Google Scholar]
- 27.Senior, K. 2007. Chagas disease: moving towards global elimination. Lancet Infect. Dis. 7:572. [DOI] [PubMed] [Google Scholar]
- 28.Sosa-Estani, S., M. R. Gamboa-León, J. Del Cid-Lemus, F. Althabe, J. Alger, O. Almendares, M. L. Cafferata, J. P. Chippaux, E. Dumonteil, L. Gibbons, N. Padilla-Raygoza, D. Schneider, J. M. Belizán, and Pierre Buekens Working Group. 2008. Use of a rapid test on umbilical cord blood to screen for Trypanosoma cruzi infection in pregnant women in Argentina, Bolivia, Honduras, and Mexico. Am. J. Trop. Med. Hyg. 79:755-759. [PubMed] [Google Scholar]
- 29.Tarleton, R. L., R. Reithinger, J. A. Urbina, U. Kitron, and R. E. Gürtler. 2007. The challenges of Chagas disease—grim outlook or glimmer of hope. PLoS Med. 4:e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verani, J. R., A. Seitz, R. H. Gilman, C. LaFuente, G. Galdos-Cardenas, V. Kawai, E. de LaFuente, L. Ferrufino, N. M. Bowman, V. Pinedo-Cancino, M. Z. Levy, F. Steurer, C. W. Todd, L. V. Kirchhoff, L. Cabrera, M. Verastegui, and C. Bern. 2009. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am. J. Trop. Med. Hyg. 80:410-415. [PubMed] [Google Scholar]
- 31.Wongsrichanalai, C., M. J. Barcus, S. Muth, A. Sutamihardja, and W. H. Wernsdorfer. 2007. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). Am. J. Trop. Med. Hyg. 77(Suppl. 6):119-127. [PubMed] [Google Scholar]
- 32.World Health Organization. 2004. World health report: changing history. World Health Organization, Geneva, Switzerland.