Abstract
In vitro gamma interferon release assays (IGRAs) are increasingly used as an alternative to the traditional tuberculin skin test for the diagnosis of latent Mycobacterium tuberculosis infection. Evaluation of the QuantiFERON-TB Gold in-tube assay (QFT-IT) prior to large-scale implementation at the Stanford Hospital and Clinics for a health care worker screening program revealed a critical preanalytical factor affecting the results. We found that incubation delay significantly increased the frequency of indeterminate results. In this study, QFT-IT was performed with samples from healthy volunteers, and replicate tubes were incubated at 37°C either immediately or after a delay at room temperature for 6 and 12 h. No indeterminate results (0/41) were seen when the assay was performed with immediate incubation. Incubation delays of 6 and 12 h yielded indeterminate results at rates of 10% (2/20) (P = 0.10) and 17.1% (7/41) (P = 0.01), respectively. The increased rate of indeterminate results was due to a decrease in the mean values for the mitogen-nil tubes when incubation was delayed for 6 h (P = 0.004) and 12 h (P < 0.001). The rates of concordance of positive or negative results obtained following immediate incubation and following 6- and 12-h delays were 77.8% (14/18) and 79.4% (27/34), respectively. Subsequent implementation of the immediate incubation procedure in our screening program for 14,830 health care workers yielded an indeterminate result rate of 0.36% over a period of 12 months, a significant improvement over the reported rates of 5 to 40% for QFT-IT. We conclude that immediate incubation of QFT-IT tubes is an effective way to minimize indeterminate results. The effect of incubation delay on the accuracy of QFT-IT remains to be determined.
Mycobacterium tuberculosis is among the leading infectious causes of death worldwide (12). Prophylactic treatment of individuals with latent infection is an effective strategy to prevent progression to active disease and further spread (9). After more than a century of using the tuberculin skin test (TST) for diagnosis of latent tuberculosis infection (LTBI), the development of T-cell-based gamma interferon (IFN-γ) release assays (IGRAs) represents a significant advancement in the field of tuberculosis (TB) diagnostics (1, 14). These assays rely on the measurement of increased IFN-γ secretion by effector T cells which have previously been exposed to M. tuberculosis antigens (Ags) from an infection when the cells are stimulated in vitro with purified M. tuberculosis-specific antigens, such as the early secretory antigen 6 (ESAT-6) and culture filtrate protein 10 complex (CFP-10) (2). The advantages of IGRAs over TST include in vitro testing, which eliminates the boosting effect of TST; direct measurement of cell-mediated immune responses; the absence of cross-reactivity with M. bovis BCG vaccination; and the need for only one patient visit (10). The disadvantages of IGRAs include the requirement for laboratory infrastructure and, similar to TST, a lack of distinction between active and latent infection.
A widely used commercial IGRA-based assay, the QuantiFERON-TB Gold assay (QFT-G), was approved for use by the FDA in December 2006. An in-tube version (QFT-IT), approved in October 2007, permits blood to be drawn directly into tubes precoated with antigens and incubated in the same tubes prior to IFN-γ measurement, thus minimizing the preanalytic processing of blood samples. Each QFT-IT consists of three tubes: (i) the TB antigen tube, containing the specific M. tuberculosis antigens, ESAT-6, CFP-10, and TB7.7; (ii) the mitogen control tube, containing nonspecific T-cell-stimulating antigens and serving as a positive control; and (iii) a nil control tube, containing no antigens and serving as a negative control. Each tube is inoculated with 1 ml of whole blood, and after vigorous shaking of the tubes, the tube set is incubated at 37°C for 16 to 24 h. According to the manufacturer's instructions for use, initiation of the incubation can be delayed for up to 16 h. A quantitative enzyme-linked immunosorbent assay (ELISA) for IFN-γ is then performed and the results are interpreted as positive, negative, or indeterminate.
Unambiguous positive and negative results are obviously desired, while indeterminate results, defined as an abnormally high level of IFN-γ in the nil tube (>8 international units [IU] per milliliter) or a low response in the mitogen tube (mitogen and nil tubes, <0.5 IU/ml), pose diagnostic challenges for clinicians and frustration for the individuals undergoing testing (7). The reported frequency of indeterminate results for QFT-IT ranges from 5% to 40% in all participants and 6% in health care workers (4, 8, 11). The manufacturer attributes these indeterminate results to technical error or the testing of patients with immunocompromised or hyperinflammatory states (3). The most common approach to resolving indeterminate results includes repeat testing, which requires a new visit and blood draw, which add to health care costs.
Prior to large-scale implementation of QFT-IT as part of an employee screening program at our academic hospital (Stanford Hospital and Clinics), an in-house evaluation of the assay was performed. Prior studies using enzyme-linked immunospot (ELISpot) and whole-blood IGRAs showed that delays in processing and incubation of blood samples before stimulation with M. tuberculosis antigens result in a lower number of detectable IFN-γ-producing T cells (5, 6, 13). Thus, the focus of this study was to investigate the impact of incubation delay on QFT-IT results.
MATERIALS AND METHODS
Study design.
Stanford Hospital Occupational Health screens all employees at Stanford Hospital and the Lucile Packard Children's Hospital for LTBI prior to employment and annually thereafter. The QFT-G was introduced in December 2006, and QFT-IT was implemented in June 2008. Prior to implementation of QFT-IT, volunteers underwent repeated QFT-IT testing to investigate the effect of incubation delay on the results. This study was approved by the Stanford University Institutional Review Board. The volunteers consisted of 27 health care workers who were recruited to participate on the basis of their known histories of LTBI, determined by prior testing with TST and QFT-G recorded in the Occupational Health database. There were 18 individuals with prior negative TST and negative QFT-G results, 5 with prior positive TST and negative QFT-G results, and 4 with prior positive TST and positive QFT-G results.
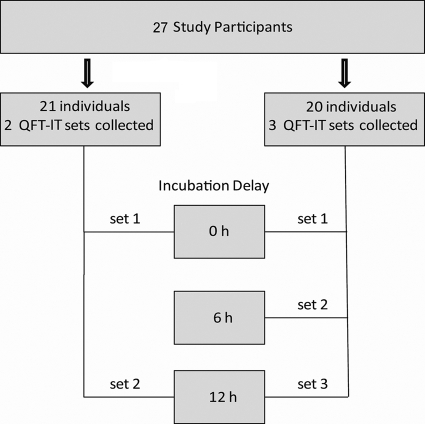
Among the 27 health care workers who volunteered for this study, 21 participated in group 1 and 20 participated in group 2 (Fig. 1). In group 1, two sets of blood samples were drawn in QFT-IT tubes. One set was incubated at 37°C immediately after collection, while incubation of the other set was delayed at room temperature for 12 h. In group 2, three sets of samples were drawn in QFT-IT tubes. One set was incubated immediately, while incubation of the other two sets was delayed for 6 and 12 h, respectively.
FIG. 1.
Schematic overview of the study design. Volunteers underwent duplicate and triplicate QFT-IT testing to investigate the effects of immediate and delayed incubation on indeterminate results.
QuantiFERON assay.
Blood draws were performed by a trained laboratory phlebotomist. One milliliter of blood was drawn directly into the QFT-IT tubes. After the contents of the tubes were vigorously mixed by hand for 5 s, they were either immediately placed in a 37°C incubator or held at room temperature for 6 and/or 12 h before the contents were remixed and the tubes were incubated at 37°C. The tubes were incubated at 37°C for 18 to 23 h. Quantitative ELISA for IFN-γ, measured in IU/ml, was performed on a DSX automated system (Dynex Technologies, Chantilly, VA). Interpretation of the results was done according to the algorithm provided by the manufacturer (3). An indeterminate result was defined as a value for the nil tube of >8 IU/ml and/or a value for the mitogen-nil tube of <0.5 IU/ml and a value for the TB antigen-nil tube of <0.35 IU/ml. A positive result was defined as a value for the TB Ag-nil tube of ≥0.35 IU/ml.
Statistical analysis.
The proportion of indeterminate results was calculated for each group, and Fisher's exact test was used to determine the statistical significance of the difference between the groups. Variations in the mean values for the nil and mitogen-nil tubes for the different incubation delays were analyzed using a paired t test.
RESULTS
Effect of incubation delay on rate of indeterminate results.
The effect of incubation delay on the rate of indeterminate results was determined with blood from healthy volunteers by initiating incubation of the QFT-IT tubes at 37°C either immediately after blood collection or following 6 and/or 12 h of storage at room temperature (Fig. 1). Immediate incubation at 37°C resulted in no indeterminate results (0/41), whereas the rates of indeterminate results were 10% (2/20; P = 0.10) and 17.1% (7/41; P = 0.01) after incubation delays of 6 and 12 h, respectively. The indeterminate result rate for 12 h of delay remained elevated at 18.5% (5/27), when data for duplicate volunteers were eliminated. On the basis of these results, QFT-IT using the immediate incubation procedure was implemented at our institution for screening of health care workers. During the first year, 14,830 QFT-IT tests were performed, yielding 59 indeterminate results for an indeterminate result rate of 0.36%.
Effect of incubation delay on values for nil and mitogen-nil tubes.
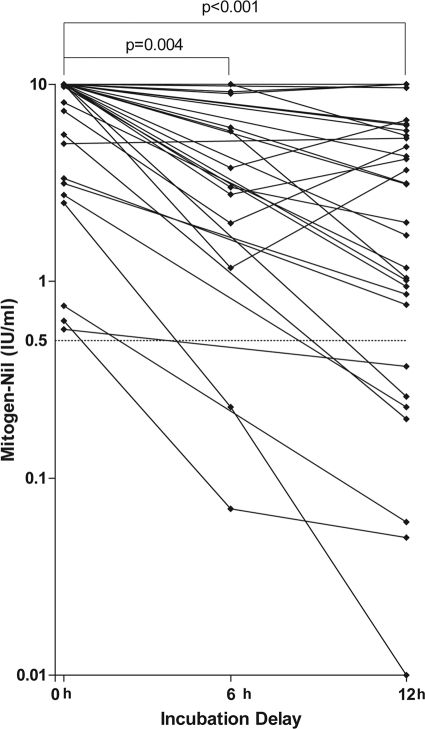
To investigate the cause of the indeterminate results, the values for the nil and mitogen-nil tubes between the immediate and delayed incubations were compared to determine if there was an increase in the value for the nil tube to >8 IU/ml or a decline in the value for the mitogen-nil tube to <0.5 IU/ml. The mean values for the nil tube for the immediate incubation and the 6- and 12-h-delayed incubations were 0.43, 0.63, and 0.14 IU/ml, respectively. Compared to the immediate incubation, the difference in the value for the nil tube was not statistically significant at 6 h (P = 0.9) or 12 h (P = 0.6). In contrast, the mean value for the mitogen-nil tube significantly declined from 8.3 IU/ml for immediate incubation to 6.6 IU/ml for the 6-h delay (P = 0.004) and 5.0 IU/ml for the 12-h delay (P < 0.001) (Fig. 2). The average value for the mitogen-nil tube for immediate incubation is likely underestimated because the maximum value for the mitogen-nil tube is capped at 10 IU/ml. A value for the mitogen-nil tube of less than 0.5 IU/ml was the cause of all indeterminate results with delayed incubation (Fig. 2).
FIG. 2.
Mitogen control results for QFT-IT following immediate and delayed incubations. The cutoff for indeterminate results (mitogen-nil tube value of <0.5 IU/ml) is marked with a dashed line. Values for the mitogen-nil tube greater than 10 IU/ml are shown as 10 IU/ml. The paired t test was used to compare differences in means.
Concordance of results obtained with different incubation delays.
After indeterminate results were excluded, we assessed the concordance of negative and positive results for tests performed with 0-, 6-, and 12-h delays before incubation. The rates of agreement between the results obtained with immediate incubation and those obtained with 6- and 12-h delays were 77.8% (14/18) and 79.4% (27/34), respectively. The agreement between the 6- and 12-h delays was 100% (18/18).
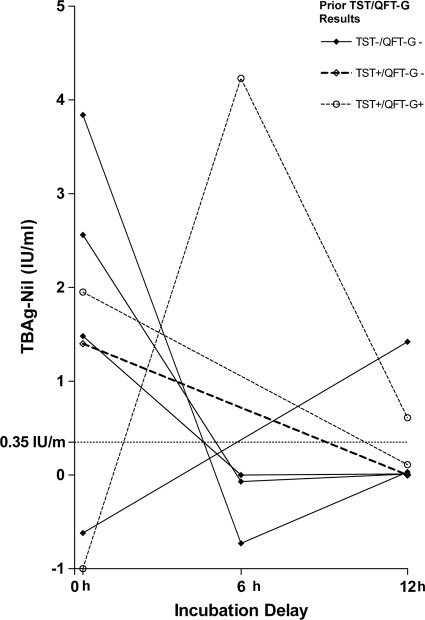
A total of seven volunteers had discordant results when the results for immediate incubation were compared to those for delayed incubation (Fig. 3). Five volunteers went from positive to negative and two went from negative to positive. The risk factors for LTBI in these individuals, based on previous TST and QFT-G results, are shown in Fig. 3. For four volunteers, the results with 6- and 12-h delays were in agreement with previous TST and QFT-G results. For two volunteers, the results for immediate testing were in agreement with previous TST and QFT-G results. For the final volunteer, the result for immediate testing was in agreement with the previous TST result and the result with 12 h of delay was in agreement with the previous QFT-G result.
FIG. 3.
TB-antigen results for volunteers with discordant QFT-IT results following immediate and delayed incubations. The cutoff for negative results (TB Ag-nil tube value, <0.35 IU/ml) is marked with a dashed line. Prior TST and QFTG results are shown. TB Ag-nil tube values less than −1 IU/ml are shown as −1 IU/ml.
DISCUSSION
Indeterminate results from QFT-IT have significant consequences for the individual patient, the health care provider, and the health care system. For the patient, an equivocal result can lead to repeat testing and even a workup for immunologic disease with concomitant uncertainty and inconvenience. In the case of a latent TB diagnosis, repeated equivocal results and/or discrepancies between QFT and TST results create diagnostic challenges for the provider, and there is no consensus on how to resolve them (14), particularly with regard to whether to initiate prophylaxis with isoniazid, a therapy with known toxic side effects. Further, repeated testing, additional diagnostic evaluation, and over- or undertreatment all add to health care costs and reduce efficiency. Therefore, efforts to decrease the rate of indeterminate results have become the focus of quality improvement projects at several institutions. For example, a recent study from the Cleveland Clinic reported a significant reduction in the rate of indeterminate results from 11% to 6.1% after implementation of technical modifications (vortexing) to the manufacturer's processing procedure (11). Despite these efforts, the indeterminate result rates reported in many studies remain relatively high, ranging from 5% to 40% (7), and novel strategies are needed to further reduce the rates.
The consequences of indeterminate results is particularly great because these assays are used primarily as screening tests. Given the potential for QFT-IT to replace TST as a screening test for LTBI, any improvement can have a large impact. In our study, a seemingly minor intervention, immediate incubation of QFT-IT tubes was found to reduce the indeterminate result rate to 0.36% in our screening program of 14,830 employees. On the basis of the reported indeterminate result rate of 5 to 40% prior to this study, this intervention eliminated an excess 742 to 5,932 indeterminate results in 1 year. In a patient population with a higher representation of immunocompromised individuals who are more likely to have indeterminate results, immediate incubation may also yield more interpretable results and aid in diagnosis.
According to the manufacturer, there are several factors that may contribute to an indeterminate result. Both high IFN-γ levels in the negative-control nil tube or a low response in the positive-control mitogen tube can result in an indeterminate result. A high background IFN-γ level may be due to the presence of heterophile antibodies (e.g., human anti-mouse) or spontaneous IFN-γ secretion during an infection or following vaccination (3). A decreased T-cell response in the positive-control mitogen tube may be due to an impaired cellular immune response associated with a decrease in the number or function of T lymphocytes, as seen in HIV infection and cancer (4). In some centers an indeterminate result due to a low mitogen response will trigger an evaluation for a possible immunocompromised state (11). Other causes for indeterminate QFT-IT results include preanalytical errors, such as storage of tubes outside the recommended temperature range, over- or underfilling of tubes with blood, and insufficient mixing. Incomplete washing of the ELISA plate can also result in indeterminate results (3).
In this study we showed a significant reduction in the rate of indeterminate QFT-IT results when an immediate incubation protocol was followed. The cause of indeterminate results in our study is not likely to be due to patient-specific factors, such as a hyperinflammatory or immunocompromised state suggested by the manufacturer, since the same blood sample from each volunteer was tested with different incubation delays and yielded interpretable results with immediate incubation but indeterminate results with a delay in incubation. A consistent indeterminate response, regardless of the incubation delay, would be expected, if a true impairment of the cellular immune response was present. Specimen processing is also not likely to contribute, since all samples were handled by the same trained laboratory staff, who followed similar procedures. Instead, all excess of indeterminate results seen with an incubation delay in our study resulted from a decrease in the value for the mitogen-nil tube compared with the values for immediate incubation, indicating that indeterminate results are caused by the delay in incubation itself. The decrease in the value for the mitogen-nil tube may be due to the impaired function of T cells when blood samples are stored at room temperature prior to 37°C incubation.
Although our results clearly show that incubation delay increases the number of indeterminate results, the small study size prevented a full evaluation of the effect of incubation delay on the accuracy of the QFT-IT results. Among 41 volunteers tested, 7 had discordant results based on the time lapse in incubation initiation. Notably, five test results changed from positive to negative when incubation was immediate instead of delayed (Fig. 3). One possibility is that the response to M. tuberculosis antigens seen with immediate incubation in these individuals is real and that the subsequent loss of response with delayed incubation is false negative due to the loss of viability or function of immune cells. This would be consistent with the results of studies using ELISpot and whole-blood assays, in which a delay of 1 to 4 h in processing and incubation of blood samples resulted in a significantly lower number of spot-forming cells (5, 6, 13). However, in three of these individuals, the result seen with delayed incubation was consistent with prior TST and QFT-G results. These observations raise the question of how immediate versus delayed incubation affects the accuracy of the test. Given the potential consequences of misdiagnosis, these findings underscore the need for future controlled studies with a larger cohort of volunteers with clearly delineated LTBI status to adequately address the effect of incubation delay on the reproducibility and accuracy of QFT-IT.
Altogether, our findings suggest that immediate incubation may minimize the number of indeterminate QFT-IT results. At our institution, immediate incubation of tubes has been accomplished by placing incubators at all collection sites. This strategy may not be feasible or cost-effective in all institutions where blood is drawn offsite and transported to the laboratory. In centers where immediate incubation is not feasible, it may be useful to create a standardized practice in terms of incubation delay and temperature while in transport. This is particularly important for interpretation of results for individuals undergoing repeat testing, such as health care workers undergoing routine screening.
The main strength of this study was the identification of immediate incubation as a critical step in QFT-IT, followed by the prospective assessment of this practice with a large cohort of health care workers. Despite the small number of volunteers in our initial evaluation, the indeterminate result rate (0.36%) seen for our health care worker screening program compared to that seen at comparable institutions (6.1%) (11) suggests that immediate incubation may be an important factor. This study utilized healthy volunteers without documented immunosuppressive conditions. Therefore, the results from this study can be applied only to healthy individuals and may not apply to other patient populations, such as immunocompromised hosts. Although we assessed the effect of incubation delay at 6 and 12 h, it is possible that shorter incubation times can reduce the rate of indeterminate results without the need for immediate incubation. It is also possible that altering the conditions of storage (for example, temperature) prior to initiation of 37°C incubation may minimize the negative effects of incubation delay and the risk of indeterminate results. We did not evaluate the effect of our training programs directed at avoiding technical errors by phlebotomists on assay performance. It is possible that this training also contributed in part to the low number of indeterminate results seen at our center.
In summary, we show that immediate incubation is a relatively simple but effective strategy for reducing indeterminate results. Further studies are necessary to determine the effect of incubation delay and other preanalytical factors on the accuracy and reproducibility of the QFT-IT assay.
Acknowledgments
We thank Howard Robin for helpful discussions and Nancy Watz for performing the validation study.
Footnotes
Published ahead of print on 2 June 2010.
REFERENCES
- 1.Agger, E. M., I. Brock, L. M. Okkels, S. M. Arend, C. S. Aagaard, K. N. Weldingh, and P. Andersen. 2003. Human T-cell responses to the RD1-encoded protein TB27.4 (Rv3878) from Mycobacterium tuberculosis. Immunology 110:507-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthet, F. X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 144:3195-3203. [DOI] [PubMed] [Google Scholar]
- 3.Cellestis. November 2009, posting date. Quantiferon-TB Gold In-Tube package insert. http://www.cellestis.com.
- 4.Cummings, K. J., T. S. Smith, E. S. Shogren, R. Khakoo, S. Nanda, L. Bunner, A. Smithmyer, D. Soccorsi, M. L. Kashon, G. H. Mazurek, L. N. Friedman, and D. N. Weissman. 2009. Prospective comparison of tuberculin skin test and QuantiFERON-TB Gold In-Tube assay for the detection of latent tuberculosis infection among healthcare workers in a low-incidence setting. Infect. Control Hosp. Epidemiol. 30:1123-1126. [DOI] [PubMed] [Google Scholar]
- 5.Doherty, T. M., A. Demissie, D. Menzies, P. Andersen, G. Rook, and A. Zumla. 2005. Effect of sample handling on analysis of cytokine responses to Mycobacterium tuberculosis in clinical samples using ELISA, ELISPOT and quantitative PCR. J. Immunol. Methods 298:129-141. [DOI] [PubMed] [Google Scholar]
- 6.Hanekom, W. A., J. Hughes, M. Mavinkurve, M. Mendillo, M. Watkins, H. Gamieldien, S. J. Gelderbloem, M. Sidibana, N. Mansoor, V. Davids, R. A. Murray, A. Hawkridge, P. A. J. Haslett, S. Ress, G. D. Hussey, and G. Kaplan. 2004. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J. Immunol. Methods 291:185-195. [DOI] [PubMed] [Google Scholar]
- 7.Kobashi, Y., T. Sugiu, K. Mouri, Y. Obase, N. Miyashita, and M. Oka. 2009. Indeterminate results of QuantiFERON TB-2G test performed in routine clinical practice. Eur. Respir. J. 33:812-815. [DOI] [PubMed] [Google Scholar]
- 8.Lalvani, A., and M. Pareek. 2010. Interferon gamma release assays: principles and practice. Enferm. Infecc. Microbiol. Clin. 28:245-252. [DOI] [PubMed] [Google Scholar]
- 9.Menzies, D., A. Fanning, L. Yuan, and M. Fitzgerald. 1995. Tuberculosis among health care workers. N. Engl. J. Med. 332:92-98. [DOI] [PubMed] [Google Scholar]
- 10.Menzies, D., M. Pai, and G. Comstock. 2007. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann. Intern. Med. 146:340-354. [DOI] [PubMed] [Google Scholar]
- 11.Miranda, C. M. D., B. P. Yen-Lieberman, P. D. O. Terpeluk, J. W. M. D. Tomford, and S. M. D. Gordon. 2009. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect. Control Hosp. Epidemiol. 30:296-298. [DOI] [PubMed] [Google Scholar]
- 12.Raviglione, M. C. 2003. The TB epidemic from 1992 to 2002. Tuberculosis 83:4-14. [DOI] [PubMed] [Google Scholar]
- 13.Smith, S. G., S. A. Joosten, V. Verscheure, A. A. Pathan, H. McShane, T. H. M. Ottenhoff, H. M. Dockrell, and F. Mascart. 2009. Identification of major factors influencing ELISpot-based monitoring of cellular responses to antigens from Mycobacterium tuberculosis. PLoS One 4:e7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swindells, J. E., S. H. Aliyu, D. A. Enoch, and I. Abubakar. 2009. Role of interferon-gamma release assays in healthcare workers. J. Hosp. Infect. 73:101-108. [DOI] [PubMed] [Google Scholar]