Abstract
Four human coronaviruses (HCoV-229E, HCoV-HKU1, HCoV-NL63, and HCoV-OC43) are associated with a range of respiratory outcomes, including bronchiolitis and pneumonia. Their epidemiologies and clinical characteristics are poorly described and are often reliant on case reports. To address these problems, we conducted a large-scale comprehensive screening for all four coronaviruses by analysis of 11,661 diagnostic respiratory samples collected in Edinburgh, United Kingdom, over 3 years between July 2006 and June 2009 using a novel four-way multiplex real-time reverse transcription-PCR (RT-PCR) assay. Coronaviruses were detected in 0.3 to 0.85% of samples in all age groups. Generally, coronaviruses displayed marked winter seasonality between the months of December and April and were not detected in summer months, which is comparable to the pattern seen with influenza viruses. HCoV-229E was the exception; detection was confined to the winter of 2008 and was sporadic in the following year. There were additional longer-term differences in detection frequencies between seasons, with HCoV-OC43 predominant in the first and third seasons and HCoV-HKU1 dominating in the second (see Results for definitions of seasons). A total of 11 to 41% of coronaviruses detected were in samples testing positive for other respiratory viruses, although clinical presentations of coronavirus monoinfections were comparable to those of viruses which have an established role in respiratory disease, such as respiratory syncytial virus, influenza virus, and parainfluenza viruses. The novel multiplex assay for real-time pan-coronavirus detection enhances respiratory virus diagnosis, overcomes potential diagnostic problems arising through seasonal variation in coronavirus frequency, and provides novel insights into the epidemiology and clinical implications of coronaviruses.
Four human coronaviruses (human coronavirus 229E [HCoV-229E], HCoV-HKU1, HCoV-NL63, and HCoV-OC43) are associated with a range of respiratory symptoms, including high-morbidity outcomes such as pneumonia and bronchiolitis (26, 31, 35). Specifically, HCoV-NL63 has been associated with croup (33) and HCoV-HKU1 with febrile convulsion (18). Coronaviruses are frequently codetected with other respiratory viruses, particularly with human respiratory syncytial virus (HRSV) (17). Whether coronaviruses contribute to disease severity in such coinfections is currently unclear. Other coronaviruses infecting humans include human enteric coronavirus, which is closely related to HCoV-OC43 and is associated with necrotizing enterocolitis and gastroenteritis (10, 27).
Coronaviruses are globally distributed (7, 32, 34, 38), although there are differences in the frequency of detection of the four viruses in different parts of the world at different times (6, 11, 15, 16, 22, 28, 29). Longitudinal studies of coronavirus epidemiology are lacking in the literature and are restricted to descriptions representing a maximum of 1 year for all four respiratory coronaviruses or 2 years for three coronaviruses (9, 17, 18). This inevitably makes direct comparisons of the coronaviruses difficult with respect to their epidemiologies, clinical presentations, and etiological roles in respiratory disease.
To directly address this situation, we conducted a large-scale survey of the incidences and epidemiological and clinical correlations of the four coronaviruses detected among 11,661 respiratory samples collected over a 3-year study period in Edinburgh, United Kingdom. Clinical presentations of study subjects infected with each of the coronaviruses were directly compared, and their roles in pediatric and adult respiratory disease were assessed.
MATERIALS AND METHODS
Sample collection.
Respiratory samples collected at hospital and primary care settings in southeast Scotland are referred to the Royal Infirmary of Edinburgh (RIE) Specialist Virology Centre (SVC) for routine respiratory virus screening. Referral of samples to the RIE SVC is done by patient assessment following established clinical protocols. Samples were labeled with anonymous identifiers and deposited in the SVC sample archive prior to testing. Samples were stored at −80°C. A range of sample types are referred, including bronchial, nasal, nasopharyngeal (the most common category), oral, and tracheal samples.
A total of 11,661 respiratory samples from 7,383 patients referred to the RIE SVC between July 2006 and June 2009 were labeled with anonymous identifiers and archived with approval from the Lothian Regional Ethics Committee (08/S11/02/2). Stored data included age band, recorded clinical information, referral source, month of sample collection, and results of routine virological testing of the sample. As part of routine virological screening, RNA and DNA were extracted using a Qiagen BioRobot MDx system and screened for HRSV, adenovirus (AdV), parainfluenza viruses 1, 2, and 3 (PIV-1 to PIV-3) and influenza A and B viruses by the use of multiplex one-step real-time reverse transcription-PCR (RT-PCR) (14, 30). Extracted nucleic acids were stored at −20°C.
Clinical data interpretation.
Clinical symptoms recorded on sample referral forms were categorized into seven groups—immunocompromised, chronic respiratory condition, lower respiratory tract infection (LRTI), upper respiratory tract infection (URTI), other, none, and no data. Inevitably, some samples were assignable to two or more categories, and so categories were treated in a hierarchical manner, whereby samples were preferentially assigned to categories in the order listed above. The immunocompromised group included patients with carcinoma and transplant patients. Coryza, cough, and sore throat were classified as URTIs. Bronchitis, bronchiolitis, pneumonia, influenza-like illness, and suspected HRSV infection were classified as LRTIs. Samples for which no clinical data were available were excluded from the clinical data analysis. Chronic respiratory conditions included asthma and cystic fibrosis. The “other” group included symptoms which may be associated with but are not restricted to infection with respiratory pathogens, e.g., pyrexia, neurological and gastrointestinal symptoms, and requirement for ventilation. The “none” group included symptoms or data not associated with respiratory tract infection, e.g., rash or urinary tract infection.
Sensitivity of real-time multiplex PCR for coronavirus detection.
RNA transcripts of the four coronaviruses were used as standards to determine assay sensitivity. HCoV-229E, HCoV-NL63, and HCoV-OC43 cDNAs (processed using nucleic acid extraction and reverse transcription as described below) were subjected to single-round real-time PCR as described below. PCR products were cloned into PCR2.1 T/A cloning vector (Invitrogen, Paisley, United Kingdom) according to the manufacturer's instructions for transformation of electrocompetent Escherichia coli. Cultures were plated on agar containing lactose and kanamycin (50 μg/ml) and grown overnight at 37°C. Blue colonies were picked, and successful transformation was confirmed by sequence analysis. Products were purified using a Qiagen (Crawley, United Kingdom) Qiaprep spin miniprep kit and were transcribed in vitro using a Promega (Southampton, United Kingdom) T7 RiboMAX large-scale RNA production system.
HCoV-HKU1-positive cDNA underwent PCR, using a sense primer incorporating a T7 promoter site for DNA amplification. DNA was cleaned using a Qiagen (Crawley, United Kingdom) QIAquick PCR purification kit and transcribed in vitro using a Promega (Southampton, United Kingdom) T7 RiboMAX large-scale RNA production system. Reactions were terminated and RNAs purified using a Qiagen (Crawley, United Kingdom) RNeasy mini-kit.
Coronavirus RNA transcript purity and concentration were established using a nanodrop spectrophotometer (Nanodrop Technologies). The sensitivity of the multiplex assay was established for each coronavirus by testing transcripts of known concentrations serially diluted in Tris-EDTA (TE) buffer. Cycle threshold (CT) values for each set of transcripts were established using multiplex and monoplex assays to detect any loss of sensitivity resulting from multiplexing.
Specificity of real-time multiplex PCR for coronavirus detection.
A panel of respiratory viruses and bacteria commonly found in the respiratory tract was used to determine the specificity of the multiplex PCR. Nucleic acids were extracted from samples positive for influenza A virus, influenza B virus, HRSV, PIV-1 to PIV-4, AdV type 5, human metapneumovirus, rhinoviruses 1b and 16, echovirus 7, mumps virus, measles virus, Bordetella bronchiseptica, Bordetella parapertussis, Bordetella pertussis, Burkholderia cepacia, Chlamydia pneumoniae, Haemophilus influenzae, Klebsiella pneumoniae, Legionella pneumophila, Mycoplasma pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae and tested by multiplex PCR under the conditions described below.
Real-time multiplex PCR for coronavirus detection.
Nucleic acids were pooled in groups of 10. Pools were screened for HCoV-229E, HCoV-HKU1, HCoV-NL63, and HCoV-OC43 by one-step multiplex RT-PCR using a Qiagen (Crawley, United Kingdom) one-step RT-PCR kit for amplification of the membrane glycoprotein (HCoV-229E and HCoV-OC43) or the nucleocapsid phosphoprotein (HCoV-HKU1 and HCoV-NL63). Each 20-μl reaction mixture contained 5 μl of 5× reaction buffer, 1 μl of deoxynucleoside triphosphate (dNTP) (10 mM), 2 μl of MgCl2 (25 mM), 0.3 μl of each of the inner primers for HCoV-HKU1, HCoV-NL63, and HCoV-OC43 (10 mM) (Table 1 ), 0.4 μl of each inner primer for HCoV-229E, 0.25 μl of each fluorescent probe for each coronavirus (Table 1) (50 mM), and 1 μl of enzyme mix. Cycling conditions were 50°C for 30 min, 95°C for 15 min, and then 50 cycles of 95°C for 30 s, 55°C for 40 s, and 72°C for 30 s. Amplified nucleic acid was detected using a Bioluminescence ABI machine.
TABLE 1.
Species-specific PCR reagents and conditions for coronavirus amplification
| CoV | Probea | Outer sense primerb | Outer antisense primerb | Inner sense primera,b | Inner antisense primera,b | Annealing temp (°C)b | No. of PCR cyclesb |
|---|---|---|---|---|---|---|---|
| 229E | FAM | AAT GCA ATC ACT GTC ACA ACC GTG | AAC CCA GCC TGT GCT ATT TTG TG | CAT ACT ATC AAC CCA TTC AAC AAG | CAC GGC AAC TGT CAT GTA TT | 50 | 35 |
| HKU1 | Cy5 | ACT TCT AYT CCC TCC GAT GTT TC | TTA TGC CTR ATT TCC TTG GC | TCC TAC TAY TCA AGA AGC TAT CC | AAT GAA CGA TTA TTG GGT CCA C | 52 | 33 |
| NL63 | TxR | CCT CCT CCT TCA TTT TAC ATG CC | ACA GAG AGC TCT GGA GGC AA | GTT CTG ATA AGG CAC CAT ATA GG | TTT AGG AGG CAA ATC AAC ACG | 52 | 30 |
| OC43 | YAK | TAT GTT AGG CCG ATA ATT GAG GAC | CCT GAT GGT TGC TGA GAR GT | CAT ACY CTG ACG GTC ACA ATA ATA | ACC TTA GCA ACA GTC ATA TAA GC | 52 | 37 |
Used in real-time PCR. FAM, 6-carboxyfluorescein; YAK, Yakima Yellow.
Used in nested PCR.
Conventional nested PCR for coronavirus detection.
Component nucleic acids from coronavirus-positive pools were screened using reverse transcription and nested PCR for their respective coronaviruses. This method was shown to be as sensitive as the real-time PCR (data not shown) and was undertaken (instead of repeated real-time PCRs) to reduce diversion of resources, a strategy that was of particular relevance during the influenza pandemic, and also served to reduce the cost of the reagents required to screen so many samples. Reverse transcription was conducted using a Promega (Southampton, United Kingdom) A3500 reverse transcription system. Each 20-μl reaction mixture included 4 μl of MgCl2 (25 mM), 2 μl of 2× reaction buffer, 2 μl of dNTPs (10 mM), 1 μl of random primers (10 mM), 0.5 μl of RNAsin, and 15 units of reverse transcriptase. Reactions were conducted according to the manufacturer's instructions, with an extended elongation step of 55 min. Each 20-μl PCR mixture contained 4 μl of MgCl2 (25 mM), 0.2 μl of dNTP (3 mM), 1 μl of each species-specific sense and antisense primer (10 mM) (Table 1), and 0.4 units of Taq polymerase. The first-round reaction mixture contained 2 μl cDNA, and the second-round mixture contained 1 μl of the first-round product. Cycling conditions for first- and second-round PCRs were 94°C for 21 s, the virus-specific annealing temperature (Table 2) for 18 s, and 72°C for 90 s; cycle numbers were also virus specific (Table 2) and were followed by a hold at 72°C for 5 min. PCR products were detected by agarose gel electrophoresis.
TABLE 2.
Detection of respiratory viruses over 3 years from 11,661 samples
| Virusa | No. of samples with indicated virus(es) detectedb |
Detection rate (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AdV | Flu A | Flu B | PIV-1 | PIV-2 | PIV-3 | HRSV | HCoV-229E | HCoV-HKU1 | HCoV-NL63 | HCoV-OC43 | ||
| AdV | 783 | 16 | 3 | 3 | 2 | 49 | 119 | 3 | 3 | 7 | 17 | 6.7 |
| Flu A | 336 | 1 | 0 | 0 | 1 | 9 | 0 | 0 | 0 | 4 | 2.9 | |
| Flu B | 131 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1.1 | ||
| PIV-1 | 75 | 0 | 1 | 3 | 0 | 2 | 0 | 0 | 0.64 | |||
| PIV-2 | 39 | 0 | 6 | 0 | 0 | 0 | 0 | 0.33 | ||||
| PIV-3 | 380 | 11 | 0 | 2 | 2 | 1 | 3.3 | |||||
| HRSV | 1,300 | 1 | 17 | 10 | 26 | 11.1 | ||||||
| HCoV-229E | 35 | 0 | 0 | 0 | 0.30 | |||||||
| HCoV-HKU1 | 61 | 0 | 0 | 0.52 | ||||||||
| HCoV-NL63 | 75 | 2 | 0.64 | |||||||||
| HCoV-OC43 | 111 | 0.85 | ||||||||||
| 1 virus | 565 | 306 | 125 | 66 | 32 | 318 | 1,109 | 31 | 38 | 57 | 67 | 23.2 |
| 2 viruses | 203 | 29 | 6 | 9 | 6 | 59 | 180 | 4 | 22 | 14 | 38 | 4.89 |
| 3 viruses | 15 | 1 | 0 | 0 | 1 | 3 | 11 | 0 | 1 | 4 | 6 | 0.36 |
Flu A, influenza A virus; Flu B, influenza B virus.
Boldface indicates total number of virus detections.
Coronavirus CT values.
Respiratory RNA was tested for coronaviruses by pooling RNAs in groups of 10 and screening using four-way multiplex RT-PCR. Identification of coronavirus-positive components of coronavirus-positive RNA pools was done using reverse transcription, nested PCR, and gel electrophoresis. Coronavirus-positive RNA identified by the nested PCR was then retested using the real-time RT-PCR to establish CT values for individual samples.
Statistical analyses.
Statistical analyses were conducted (unless otherwise stated) using a two-tailed Fisher's exact test with a threshold for significance of P < 0.05, using population parameters defined by the sample set.
RESULTS
Sensitivity and specificity of four-way multiplex RT-PCR for coronavirus detection.
Serial dilutions of coronavirus RNA transcripts were tested using the multiplex PCR. No differences in CT values were found when transcripts were tested using single primers and probes compared to using multiplexed primers and probes (results not shown). The fluorescent signal observed at various dilutions for each virus type corresponded to calculated minimal amounts of detectable RNA copy numbers of 66 for HCoV-229E, 9 for HCoV-HKU1, 69 for HCoV-NL63, and 18 for HCoV-OC43 in 10 μl of RNA. No nonspecific reactions were observed. As RNAs were pooled in groups of 10, 1 μl of each RNA was tested in the initial screening process. Consequently, the minimum theoretical concentration of viral RNA required for detection was likely a 10-fold increase compared to the calculated values.
Detection of coronaviruses in Edinburgh and assay sensitivity.
All coronavirus-positive pools detected by real-time PCR were found to include at least one coronavirus-positive component by nested PCR. Some pools comprised samples positive for different coronaviruses, some contained more than one sample positive for the same coronavirus, and two samples were found to be positive for both HCoV-OC43 and HCoV-NL63. Of the 11,661 samples, 267 (2.30%) were positive for at least one coronavirus (Table 2); of all virus detections, 8.15% were of coronaviruses.
Coronavirus-positive samples detected by the nested PCR were rescreened using real-time RT-PCR to establish CT values; of these, 86.2% (232/269) tested positive for coronavirus by the real-time RT-PCR. In some cases, a sample that tested as negative on rescreening represented the only coronavirus-positive sample in its pool; therefore, these results were not attributed to false positives in the nested PCR screening.
Patient parameters.
Coronaviruses were detected in all age groups, most frequently (4.86%) in the 7- to 12-month age category (Fig. 1). Significantly more males (64.6%; P = 0.0332) were infected with HCoV-OC43 than females. More males than females were infected with HCoV-NL63, but this result did not reach significance (62.7%; P = 0.132). HCoV-HKU1 and HCoV-229E infected approximately equal numbers of males and females (the proportions of those infected who were male were 50.8% and 51.4%, respectively).
FIG. 1.
Percentages of detection frequencies of the four coronaviruses HCoV-229E, HCoV-HKU1, HCoV-NL63, and HCoV-OC43 by age group. The total number of samples within each age band is indicated at head of each column.
Circulation trends.
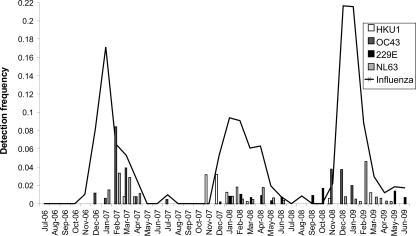
Coronaviruses displayed the marked seasonality typical of other respiratory viruses, with high detection frequencies in winter months but few or no detections in the summer (Fig. 2). The exception was HCoV-229E, which was detectable primarily in the winter of the second year of the study and sporadically through the third year of study.
FIG. 2.
Coronavirus and influenza virus (A and B combined) detection frequencies by month over the 3 years of the study.
HCoV-OC43 displayed biennial peaks in detection frequency; it was present in 2.96% of samples collected in the first season (December 2006 to April 2007), in only January of the second season (November 2007 to March 2008) (0.83%), and in 3.17% of samples collected in the third season (November 2008 to January 2009) (P < 0.0001 for the first and third seasons compared to the second).
For HCoV-HKU1, in contrast to the pattern described for HCoV-OC43, the peak detection frequency occurred in the second season (1.92% of samples collected in November 2007 to March 2008). Conversely, when HCoV-OC43 was circulating at the highest frequency, detection frequencies of HCoV-HKU1 did not exceed 1% (P < 0.0001 for the first and third seasons compared to the second).
HCoV-NL63 was detected later than other coronaviruses in all three seasons (in December or January), and detection frequencies peaked in February to April. Detection frequencies between seasons were more consistent than for the other coronaviruses, although a significantly higher HCoV-NL63 detection frequency was observed in the first year of study than in the second and third years (P < 0.001 and P = 0.0200, respectively).
Clinical associations of coronavirus infections.
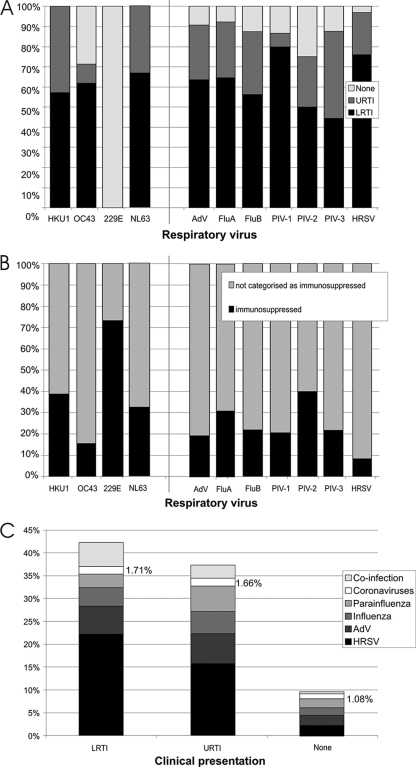
Clinical data were available for 6,068/11,661 (52.0%) of samples included in the study. Although high rates of coinfection with other respiratory viruses were detected (11 to 41% of coronavirus-positive samples tested positive for at least one other respiratory virus), single infections associated with respiratory outcomes were observed for three coronaviruses (HCoV-HKU1, HCoV-NL63, and HCoV-OC43). HCoV-OC43, HCoV-NL63, and HCoV-HKU1 had clinical profiles similar to those of respiratory viruses currently included in routine diagnostic screening (Fig. 3 A), with HCoV-OC43 associated with LRTI in over 40% of single infections for which clinical data were available—a higher proportion than that seen with influenza A virus. HCoV-229E was not associated with any cases of LRTI or URTI in otherwise healthy individuals and was found in over 70% of cases representing samples from immunocompromised patients (Fig. 3B).
FIG. 3.
(A) Comparison of the clinical presentations of study subjects infected with coronaviruses to the clinical presentations of those infected with respiratory viruses included in diagnostic screening (adenovirus [AdV], influenza A virus, influenza B virus, PIV-1, PIV-2, PIV-3, and HRSV). Only single infections are included in the analysis. The y axis indicates proportions of subjects with symptoms or diagnoses of LRTI or URTI or symptoms not associated with respiratory infection (“None”). (B) Proportions of samples testing singly positive for each of the respiratory viruses from patients with immunosuppression. (C) Proportions of samples from patients with LRTI or URTI or with no respiratory symptoms (“None”) testing singly positive for each respiratory virus.
Coronaviruses were not associated only with respiratory outcomes. Febrile convulsions were not reported for any patients testing singly positive for coronavirus, although one patient testing singly positive for HCoV-HKU1 had meningitis and one patient testing singly positive for HCoV-OC43 had seizures. Of 35 samples testing singly positive for HCoV-OC43, two were associated with vomiting, though one of these was taken from an immunocompromised patient.
Croup has previously been associated with HCoV-NL63 infection (19, 33, 37) but was not reported in association with any of the 41 samples with clinical data testing singly positive for HCoV-NL63; 3 samples were associated with cough and 1 was associated with wheezing.
For children under 2 years of age with LRTI, coronaviruses represented the only virus detected in 20 of 916 (2.18%) samples, compared with a rate of 5 of 336 (1.49%) samples collected from patients over the age of 45 with LRTI. The difference was not significant (P = 0.5).
For all four coronaviruses combined, the detection frequency in samples from patients with respiratory symptoms (URTI and LRTI) exceeded the proportion seen with samples taken from patients whose symptoms were categorized as “none” (i.e., no respiratory symptoms; Fig. 3C), providing epidemiologic evidence for the role of these three viruses in the etiology of respiratory disease.
No differences were found between the CT values of coronavirus-positive samples associated with differing respiratory outcomes (data not shown).
Coinfections.
High rates of coinfection with other respiratory viruses were observed for all coronaviruses (Table 2). Similar rates of LRTI and URTI were observed in single coronavirus infections (HcoV-HKU1 and HCoV-OC43) compared to cases in which a coronavirus was detected as part of a mixed infection. LRTI and URTI were observed at a higher frequency in single compared to mixed infections with HCoV-NL63 (Table 3).
TABLE 3.
Detection frequencies of coronavirus cases of LRTI and URTI
| Clinical outcome | Detection frequency (%) |
||||||
|---|---|---|---|---|---|---|---|
| HCoV-229E (all) | HCoV-HKU1 |
HCoV-NL63 |
HCoV-OC43 |
||||
| Single | Mixed | Single | Mixed | Single | Mixed | ||
| LRTI | 0 | 4 | 5 | 12 | 3 | 13 | 16 |
| URTI | 0 | 3 | 1 | 6 | 1 | 2 | 0 |
Of all samples testing positive for coronavirus and another respiratory virus, 63.5% tested positive for HRSV. Coronavirus infection seasonality most closely resembles that of influenza (Fig. 2), and so the coinfection frequency of HRSV with coronaviruses is compared to the coinfection frequency of HRSV with influenza virus. Of 1,300 HRSV-positive samples, only 9 (0.682%) tested positive for influenza A or influenza B virus, whereas 54 (4.15%) tested positive for coronavirus (P < 0.0001, based on the assumption that influenza virus and coronaviruses have the same seasonality).
Samples from patients testing positive for a routine virus were more likely to have LRTI when coronavirus was also detected (52.2% compared to 43.2%), though this result did not reach significance (P = 0.231).
CT values were available for most coronavirus-positive samples (229/286; Table 4). Few differences in CT value were observed between singly infected coronavirus-positive samples and coronavirus-positive samples also testing positive for another virus (Table 4).
TABLE 4.
Coronavirus CT values for singly infected and coinfected samples
| CoV CT value | No. of samples with indicated result |
|||||||
|---|---|---|---|---|---|---|---|---|
| HCoV-229E |
HCoV-HKU1 |
HCoV-NL63 |
HCoV-OC43 |
|||||
| Single infection | Coinfection | Single infection | Coinfection | Single infection | Coinfection | Single infection | Coinfection | |
| <30 | 15 | 3 | 6 | 6 | 1 | 0 | 7 | 3 |
| 30-35 | 15 | 1 | 19 | 8 | 19 | 4 | 31 | 13 |
| >35 | 0 | 0 | 2 | 1 | 33 | 9 | 22 | 14 |
DISCUSSION
Human coronaviruses are increasingly recognized as respiratory pathogens associated with a broadening range of clinical outcomes, whereas they were once recognized as “common cold” viruses. Here we demonstrate that HCoV-OC43, HCoV-NL63, and HCoV-HKU1 were specifically associated with lower respiratory tract disease in the study population. Three of the four coronaviruses were more frequently detected as the sole pathogen in subjects with LRTI than in those with no respiratory symptoms and were detected as often as PIV-1 and PIV-2 (Fig. 3A). Samples representing monoinfections were frequently (26/184 [14.1%]) from subjects in intensive care and/or high-dependency units, with presentations that included severe lower respiratory tract disease and pyrexia, and case reports have associated all coronaviruses with high morbidity and/or fatal outcomes (23, 25, 26, 34).
HCoV-OC43 was the most commonly detected coronavirus, followed by HCoV-NL63 and HCoV-HKU1, with similar moderate detection frequencies, and HCoV-229E, with a comparatively low detection frequency; this observation has been previously reported (18). However, in contrast to the results of a previous study (18), we found that HCoV-HKU1 was detected as frequently in patients with LRTI as in those with URTI, and there was a lack of association between HCoV-229E infection and respiratory symptoms in otherwise healthy individuals. HCoV-NL63 detection peaked in winter and spring (January to April), which contrasts with the summer-to-autumn seasonality reported to occur in the tropics (4, 18), though the autumn-to-winter seasonality of HCoV-HKU1 and HCoV-OC43 infections and lack of a discernible seasonality of HCoV-229E infections were epidemiologically comparable features of both regions.
In 42% and 38% of infections with HCoV-OC43 and HCoV-HKU1, other respiratory viruses were codetected, most commonly HRSV. There were significantly more HRSV-positive samples that tested positive for coronavirus compared to the numbers of samples testing negative for all viruses included in routine diagnostics (one-tailed test; P = 1.34 × 10−5). The high rate of coinfection of coronaviruses with HRSV observed here was previously demonstrated (17). No differences in clinical outcome were observed for those coinfected with HRSV and coronavirus compared with those singly infected with either virus, and so HRSV presumably facilitates coronavirus infections (or vice versa) without exacerbating morbidity. HCoV-OC43 was significantly more frequently detected in hospitalized males than in females, an attribute that HCoV-OC43 shares with HRSV (8, 21, 24). No difference was observed between the sex distribution of HCoV-OC43 in singly infected subjects and the sex distribution in those coinfected with HRSV, indicating that the observed sex bias was coincidental and is not attributable to high coinfection rates.
The coronavirus load in singly positive samples was not different from the coronavirus load in samples in which copathogens were detected. This suggests that infection with another respiratory virus does not affect the ability of coronaviruses to establish infection and further demonstrates that, in mixed infections in respiratory patients, detection of coronaviruses should not be interpreted as representing an incidental infection that does not contribute to disease.
HCoV-229E differed clinically from other coronaviruses and other respiratory viruses in general; 70% of detections were in samples from immunocompromised patients. HCoV-229E has previously been associated with infections of immunocompromised individuals (9, 26). The further association of HCoV-NL63 with this group was previously suggested in a report of a smaller epidemiologic study (9) and in a case report (23). Evidence indicating the role of these coronaviruses in disease etiology in immunocompromised individuals increasingly points to the conclusion that detection of infections by these viruses should be considered to require alternative diagnoses for immunocompromised individuals with respiratory tract symptoms.
Most individuals seroconvert for HCoV-229E, HCoV-NL63, and HCoV-OC43 in childhood (5, 13, 15, 20). No cell culture system is currently available for HCoV-HKU1 analyses, and so seroprevalence studies rely on alternative techniques (1). Reinfection with coronaviruses HCoV-229E and HCoV-OC43 is a common occurrence (22). Epidemiologically, HCoV-NL63 and HCoV-HKU1 were similar to other coronaviruses in that they were detected in all age groups and equally frequently, and so it is likely that HCoV-HKU1 and HCoV-NL63 cause repeated infections throughout life also.
Human coronaviruses divide serologically into two groups, though the extent of serologic cross-reactivity within and between groups is mostly evaluated in comparison with severe acute respiratory syndrome (SARS)-associated coronavirus infections (2, 3, 25, 36). Coronaviruses may independently fluctuate in circulation rates, or epidemiologic interference within or between Alphacoronavirinae (HCoV-229E and HCoV-NL63) and Betacoronavirinae (HCoV-HKU1 and HCoV-OC43) species may occur. HCoV-HKU1, HCoV-NL63, and HCoV-OC43 all significantly differed in detection frequencies throughout the study period, and variability in the detection frequency of HCoV-229E has been previously reported (9, 12, 20).
Development of a novel pan-coronavirus multiplex PCR allows unique insights into the epidemiology and clinical implications of infections by the four respiratory coronaviruses. Fluctuating circulation frequencies of coronaviruses between respiratory seasons are reported here for the first time. All coronaviruses are associated with high coinfection rates, and detection of HCoV-229E infection should be particularly considered for an alternative diagnosis for immunocompromised patients with respiratory tract disease.
Acknowledgments
We are very grateful to Peter McCullough, Mary Notman, Julie White, and Carol Thomson for providing samples, data, and other virus testing results from the respiratory sample archive.
This research was supported by a studentship awarded to E.R.G. by the BBSRC. This work represents no conflicts of interest. Ethical approval was provided by the Lothian Regional Ethics Committee (08/S11/02/2).
Footnotes
Published ahead of print on 16 June 2010.
REFERENCES
- 1.Chan, C. M., H. Tse, S. S. Y. Wong, P. C. Y. Woo, S. K. P. Lau, L. Chen, B. J. Zheng, J. D. Huang, and K. Y. Yuen. 2009. Examination of seroprevalence of coronavirus HKU1 infection with S protein-based ELISA and neutralization assay against viral spike pseudotyped virus. J. Clin. Virol. 45:54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan, K. H., V. C. C. Cheng, P. C. Y. Woo, S. K. P. Lau, L. L. M. Poon, Y. Guan, W. H. Seto, K. Y. Yuen, and J. S. M. Peiris. 2005. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229E, OC43, and NL63. Clin. Diagn. Lab. Immunol. 12:1317-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Che, X. Y., L. W. Qiu, Z. Y. Liao, Y. D. Wang, K. Wen, Y. X. Pan, W. Hao, Y. B. Mei, V. C. Cheng, and K. Y. Yuen. 2005. Antigenic cross-reactivity between severe acute respiratory syndrome associated coronavirus and human coronaviruses 229E and OC43. J. Infect. Dis. 191:2033-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu, S. S., K. Hung Chan, K. Wing Chu, S. Wai Kwan, Y. Guan, L. Lit Man Poon, and J. S. M. Peiris. 2005. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin. Infect. Dis. 40:1721-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dijkman, R., M. F. Jebbink, N. B. El Idrissi, K. Pyrc, M. A. Muller, T. W. Kuijpers, H. L. Zaaijer, and L. van der Hoek. 2008. Human coronavirus NL63 and 229E seroconversion in children. J. Clin. Microbiol. 46:2368-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esper, F., C. Weibel, D. Ferguson, M. L. Landry, and J. S. Kahn. 2006. Coronavirus HKU1 infection in the United States. Emerg. Infect. Dis. 12:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fields, B. N., D. M. Knipe, and P. M. Howley (ed.). 1996. Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, PA.
- 8.Gaunt, E., E. C. McWilliam-Leitch, K. Templeton, and P. Simmonds. 2009. Incidence, molecular epidemiology and clinical presentations of human metapneumovirus; assessment of its importance as a diagnostic screening target. J. Clin. Virol. 46:318-324. [DOI] [PubMed] [Google Scholar]
- 9.Gerna, G., G. Campanini, F. Rovida, E. Percivalle, A. Sarasini, A. Marchi, and F. Baldanti. 2006. Genetic variability of human coronavirus OC43-, 229E-, and NL63-like strains and their association with lower respiratory tract infections of hospitalized infants and immunocompromised patients. J. Med. Virol. 78:938-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerna, G., N. Passarani, M. Battaglia, and E. G. Rondanelli. 1985. Human enteric coronaviruses: antigenic relatedness to human coronavirus OC43 and possible etiologic role in viral gastroenteritis. J. Infect. Dis. 151:796-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerna, G., E. Percivalle, A. Sarasini, G. Campanini, A. Piralla, F. Rovida, E. Genini, A. Marchi, and F. Baldanti. 2007. Human respiratory coronavirus HKU1 versus other coronavirus infections in Italian hospitalised patients. J. Clin. Virol. 38:244-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamre, D., and M. Beem. 1972. Virologic studies of acute respiratory disease in young adults. V. Coronavirus 229E infections during six years of surveillance. Am. J. Epidemiol. 96:94-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasony, H. J., and M. R. Macnaughton. 1982. Prevalence of human coronavirus antibody in the population of southern Iraq. J. Med. Virol. 9:209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heim, A., C. Ebnet, G. Harste, and P. Pring-Akerblom. 2003. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J. Med. Virol. 70:228-239. [DOI] [PubMed] [Google Scholar]
- 15.Kaye, H. S., H. B. Marsh, and W. R. Dowdle. 1971. Seroepidemiologic survey of coronavirus (strain OC 43) related infections in a children's population. Am. J. Epidemiol. 94:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koetz, A., P. Nilsson, M. Lindén, L. van der Hoek, and T. Ripa. 2006. Detection of human coronavirus NL63, human metapneumovirus and respiratory syncytial virus in children with respiratory tract infections in south-west Sweden. Clin. Microbiol. Infect. 12:1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuypers, J., E. T. Martin, J. Heugel, N. Wright, R. Morrow, and J. A. Englund. 2007. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics 119:e70-e76. [DOI] [PubMed] [Google Scholar]
- 18.Lau, S. K. P., P. C. Y. Woo, C. C. Y. Yip, H. Tse, H. W. Tsoi, V. C. C. Cheng, P. Lee, B. S. F. Tang, C. H. Y. Cheung, R. A. Lee, L. Y. So, Y. L. Lau, K. H. Chan, and K. Y. Yuen. 2006. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J. Clin. Microbiol. 44:2063-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung, T. F., C. Y. Li, W. Y. Lam, G. W. K. Wong, E. Cheuk, M. Ip, P. C. Ng, and P. K. S. Chan. 2009. Epidemiology and clinical presentations of human coronavirus NL63 infections in Hong Kong children. J. Clin. Microbiol. 47:3486-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntosh, K., A. Z. Kapikian, H. C. Turner, J. W. Hartley, R. H. Parrott, and R. M. Chanock. 1970. Seroepidemiologic studies of coronavirus infection in adults and children. Am. J. Epidemiol. 91:585-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monto, A. S., E. R. Bryan, and L. M. Rhodes. 1974. The Tecumseh study of respiratory illness. VII. Further observations on the occurrence of respiratory syncytial virus and mycoplasma pneumoniae infections. Am. J. Epidemiol. 100:458-468. [DOI] [PubMed] [Google Scholar]
- 22.Monto, A. S., and S. K. Lim. 1974. The Tecumseh study of respiratory illness. VI. Frequency of and relationship between outbreaks of coronavirus infection. J. Infect. Dis. 129:271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oosterhof, L., C. B. Christensen, and H. Sengelov. 2010. Fatal lower respiratory tract disease with human corona virus NL63 in an adult haematopoietic cell transplant recipient. Bone Marrow Transplant. 45:1115-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parrott, R. H., H. W. Kim, J. O. Arrobio, D. S. Hodes, B. R. Murphy, C. D. Brandt, E. N. A. Camargo, and R. M. Chanock. 1973. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am. J. Epidemiol. 98:289-300. [DOI] [PubMed] [Google Scholar]
- 25.Patrick, D. M., M. Petric, D. M. Skowronski, R. Guasparini, T. F. Booth, M. Krajden, P. McGeer, N. Bastien, L. Gustafson, J. Dubord, D. Macdonald, S. T. David, L. F. Srour, R. Parker, A. Andonov, J. Isaac-Renton, N. Loewen, G. McNabb, A. McNabb, S. H. Goh, S. Henwick, C. Astell, J. P. Guo, M. Drebot, R. Tellier, F. Plummer, and R. C. Brunham. 2006. An outbreak of human coronavirus OC43 infection and serological cross-reactivity with SARS coronavirus. Can. J. Infect. Dis. Med. Microbiol. 17:330-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pene, F., A. Merlat, A. Vabret, F. Rozenberg, A. Buzyn, F. Dreyfus, A. Cariou, F. Freymuth, and P. Lebon. 2003. Coronavirus 229E related pneumonia in immunocompromised patients. Clin. Infect. Dis. 37:929-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resta, S., J. P. Luby, C. R. Rosenfeld, and J. D. Siegel. 1985. Isolation and propagation of a human enteric coronavirus. Science 229:978-981. [DOI] [PubMed] [Google Scholar]
- 28.Sloots, T. P., P. McErlean, D. J. Speicher, K. E. Arden, M. D. Nissen, and I. M. Mackay. 2006. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J. Clin. Virol. 35:99-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki, A., M. Okamoto, A. Ohmi, O. Watanabe, S. Miyabayashi, and H. Nishimura. 2005. Detection of human coronavirus-NL63 in children in Japan. Pediatr. Infect. Dis. J. 24:645-646. [DOI] [PubMed] [Google Scholar]
- 30.Templeton, K. E., S. A. Scheltinga, M. F. C. Beersma, A. C. M. Kroes, and E. C. J. Claas. 2004. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J. Clin. Microbiol. 42:1564-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vabret, A., T. Mourez, S. Gouarin, J. Petitjean, and F. Freymuth. 2003. An outbreak of coronavirus OC43 respiratory infection in Normandy, France. Clin. Infect. Dis. 36:985-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Hoek, L., P. Krzysztof, and B. Berkhout. 2006. Human coronavirus NL63, a new respiratory virus. FEMS Microbiol. Rev. 30:760-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Hoek, L., K. Sure, G. Ihorst, A. Stang, K. Pyrc, M. F. Jebbink, G. Petersen, J. Forster, B. Berkhout, and K. Ãœberla. 2005. Croup is associated with the novel coronavirus NL63. PLoS Med. 2:e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woo, P. C. Y., S. K. P. Lau, C. M. Chu, K. H. Chan, H. W. Tsoi, Y. Huang, B. H. L. Wong, R. W. S. Poon, J. J. Cai, W. K. Luk, L. L. M. Poon, S. S. Y. Wong, Y. Guan, J. S. M. Peiris, and K. Y. Yuen. 2005. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 79:884-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo, P. C. Y., S. K. P. Lau, H. Tsoi, Y. Huang, R. W. S. Poon, C. M. Chu, R. A. Lee, W. K. Luk, G. K. M. Wong, B. H. L. Wong, V. C. C. Cheng, B. S. F. Tang, A. K. L. Wu, R. W. H. Yung, H. Chen, Y. Guan, K. H. Chan, and K. Y. Yuen. 2005. Clinical and molecular epidemiological features of coronavirus HKU1 associated community acquired pneumonia. J. Infect. Dis. 192:1898-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woo, P. C. Y., S. K. P. Lau, B. H. L. Wong, K. H. Chan, W. T. Hui, G. S. W. Kwan, J. S. M. Peiris, R. B. Couch, and K. Y. Yuen. 2004. False-positive results in a recombinant severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid enzyme-linked immunosorbent assay due to HCoV-OC43 and HCoV-229E rectified by Western blotting with recombinant SARS-CoV spike polypeptide. J. Clin. Microbiol. 42:5885-5888. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Wu, P. S., L. Y. Chang, B. Berkhout, L. van der Hoek, C. Y. Lu, C. L. Kao, P. I. Lee, P. L. Shao, C. Y. Lee, F. Y. Huang, and L. M. Huang. 2008. Clinical manifestations of human coronavirus NL63 infection in children in Taiwan. Eur. J. Pediatr. 167:75-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao, Q., S. Li, F. Xue, Y. Zou, C. Chen, M. Bartlam, and Z. Rao. 2008. Structure of the main protease from a global infectious human coronavirus, HCoV-HKU1. J. Virol. 82:8647-8655. [DOI] [PMC free article] [PubMed] [Google Scholar]