Abstract
In New Caledonia, Wallis and Futuna, and French Polynesia, an active surveillance system was established to monitor pneumococcal serotype prevalence between 2000 and 2007. The most prevalent serotype was serotype 1, which belonged to the major clonal complex sequence type 306 (ST306) and was responsible for invasive pneumococcal disease outbreaks.
Invasive pneumococcal disease (IPD) is a significant cause of morbidity and mortality worldwide and affects principally the very young, the very old, and people with chronic diseases (19). While attack rates for pneumococcal disease are extremely high in developing countries (8), indigenous and disadvantaged minorities are also at particular risk (4, 10, 21, 22). Streptococcus pneumoniae serotype 1 has remained one of the most prevalent invasive serotypes (6). Its levels of importance differ among countries, and it is one of the few pneumococcal serotypes associated with disease outbreaks in small or closed communities (5, 9, 11, 13, 20). Its characteristics include a short duration of nasopharyngeal colonization and a propensity for the development of invasive infection (12). Of concern is that the heptavalent pneumococcal conjugate vaccine (PCV7, against serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) for children under 2 years of age does not cover this serotype, although new conjugate vaccines offer hope for the future. We describe the epidemiology of serotype 1 IPD outbreaks in a South Pacific region between 2000 and 2007.
(This study was presented at the 5th and 6th International Symposium on Pneumococci and Pneumococcal Diseases [ISPPD], Alice Springs, Australia, 2006, and Reykjavik, Iceland, 2008.)
All isolates of S. pneumoniae collected from any body site between January 2000 and December 2007 from a 285-bed, tertiary-care hospital in Noumea, New Caledonia, which serves the majority of the New Caledonian population, were studied. All invasive isolates of S. pneumoniae referred to the laboratory of the Hospital of Papeete, a 353-bed, tertiary-care hospital in French Polynesia, between 2002 and 2005 were also studied. IPD was defined as isolation of S. pneumoniae from a normally sterile body site, such as blood or cerebrospinal or pleural fluid. Identification of S. pneumoniae, serotyping by the Quellung reaction, and determination of susceptibilities to penicillin, amoxicillin, and cefotaxime were performed as previously described (2). Ninety-six serotype 1 S. pneumoniae isolates from New Caledonia (80 invasive, 15 noninvasive, and one carrier strain) and 40 invasive type 1 isolates from French Polynesia were collected. Other serotype 1 strains isolated between 2003 and 2005 from Wallis and Futuna (3 invasive and 3 noninvasive) and New South Wales, Australia (3 invasive and 3 noninvasive), were studied for molecular comparison. A total of 141 serotype 1 isolates (96 from New Caledonia, 6 from Australia, 6 from Wallis and Futuna, and 33 from French Polynesia) were analyzed by pulsed-field gel electrophoresis (PFGE) as previously described (15). Gel images were analyzed by Bionumerics (Applied Maths, Kortrijk, Belgium) (23). Thirty-two PFGE-selected serotype 1 Streptococcus pneumoniae strains were analyzed by multilocus sequence typing (MLST), as described elsewhere (7). Allelic profiles, or sequence types (ST), were analyzed using the software available on the MLST website (http://www.mlst.net). A prospective surveillance study of IPD was carried out from January 2000 to December 2007 in New Caledonia. Incidence rates were calculated using the 2004 census of the New Caledonian population (http://www.insee.fr). A retrospective analysis of medical files was undertaken to document the serotype 1 clinical characteristics. Statistical analysis was performed using the Student t test to compare mean ages and the χ2 test to determine odds ratios (ORs) with 95% confidence intervals (95% CI) (Epi-info, version 6.04; Centers for Disease Control and Prevention, Atlanta, GA).
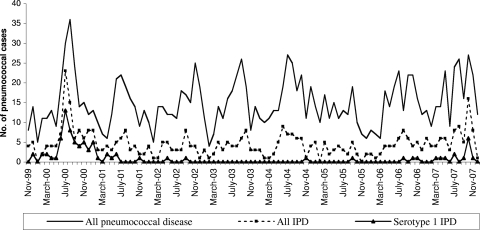
Of 1,436 isolates collected in New Caledonia between 2000 and 2007, 437 (30.4%) were considered invasive, with an estimated incidence rate of 24.0 IPD cases/100,000 persons/year. The absolute annual numbers of IPD cases and the estimated incidence rates are shown in Table 1. Of the total IPD isolates, cultures from blood accounted for 372 (85.1%), those from spinal fluid accounted for 50 (11.5%), and those from pleural fluid accounted for 15 (3.4%). In New Caledonia, the three most frequent pneumococcal serotypes of IPD isolated during this period were types 1 (80 cases, 18.3%), 4 (58, 13.3%), and 7F (55, 12.6%). A sharp increase in the number of IPD cases was noted from February 2000 to February 2001, with a peak in winter in the Southern Hemisphere, and in the winter of 2007, with the majority of isolates expressing the serotype 1 capsule (Fig. 1). Altogether, 56 of 80 cases of serotype 1 IPD were identified during the first outbreak; these affected all communities and all age groups, but a high percentage of cases (28/56, 50%) occurred in young adults aged 15 to 39 years old (significantly higher than other IPD serotypes, P < 0.01), and cases appeared with low frequency in adults up to 65 years old (P < 0.01) (data not shown). All patients were hospitalized, and the majority of cases (44/56, 78%) did not have recognized predisposing risk factors for IPD (compared to other serotypes, P < 0.01) (data not shown). However, none had to be transferred to an intensive care unit, and there were no reported deaths. The clinical presentations were 42 pneumonia cases with a positive blood culture for S. pneumoniae, 14 bacteremia cases, and no meningitis cases. The second outbreak, from July to November 2007, showed a lower incidence of IPD due to serotype 1 but revealed other characteristics, with almost 50% of infected individuals being children under 8 years of age (compared to other serotypes, P = 0.02). We also noted the first meningitis case during this outbreak.
TABLE 1.
Cases and annual estimated rates of IPD in New Caledonia
| Yr | No. of cases of: |
Total no. of cases | Case rate per 100,000 inhabitants/yra (95% CI) | |||
|---|---|---|---|---|---|---|
| Serotype 1 | Serotype 4 | Serotype 7F | Other serotype | |||
| 2000 | 50 | 5 | 1 | 31 | 87 | 41.0 |
| 2001 | 12 | 6 | 4 | 29 | 51 | 23.6 |
| 2002 | 2 | 3 | 9 | 28 | 42 | 19.1 |
| 2003 | 0 | 6 | 9 | 32 | 47 | 20.9 |
| 2004 | 1 | 11 | 9 | 31 | 52 | 22.5 |
| 2005 | 1 | 5 | 6 | 21 | 33 | 14.0 |
| 2006 | 2 | 10 | 6 | 31 | 49 | 20.4 |
| 2007 | 12 | 12 | 11 | 41 | 76 | 31.1 |
| Total | 80 | 58 | 55 | 244 | 437 | 24.0 |
According to the INSEE census, the population size in 1996 was 196,836, and that in 2004 was 230,789, with an increase of 1.9 % per annum.
FIG. 1.
Monthly data on numbers of laboratory-confirmed pneumococcal cases from 2000 to 2007 in New Caledonia, grouped by all cases, all invasive cases, and all invasive serotype 1 cases.
The prevalence of serotype 1 in invasive isolates (blood culture, spinal fluid, and pleural fluid) from the main hospital in French Polynesia, per year, was as follows: 25 of 48 isolates (52.1%) in 2002, 8 of 31 isolates (25.8%) in 2003, 3 of 29 isolates (10.3%) in 2004, and 4 of 30 isolates (13.3%) in 2005. These data indicate that there may have been an outbreak in this country in 2002 that gradually diminished.
All serotype 1 strains were completely susceptible to the antimicrobial agents tested.
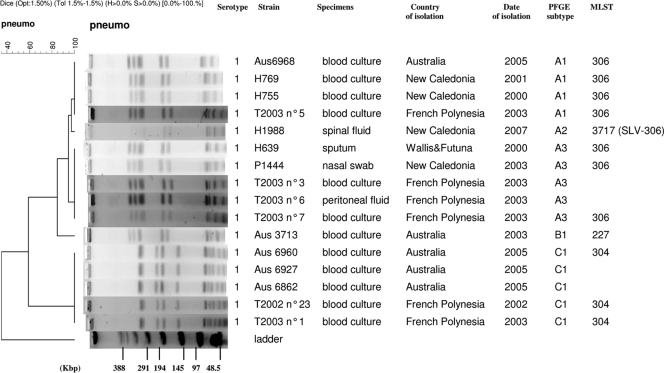
Of the total of 141 serotype 1 pneumococcal strains tested, only three distinct pulsotypes were found by PFGE, the major type A (130 strains, found in all of the 4 countries) and the minor types B (one strain) and C (10 strains, found in both Australia and French Polynesia) (Fig. 2). The major profile A was of ST306, except two “new ST” strains isolated in New Caledonia in 2007, which differed only in the locus aroE by a G/A nucleotide substitution at position 301. ST306, responsible for outbreaks in 2000 and 2007, was the exclusive clonal complex found across New Caledonia, and it encompassed epidemic and nonepidemic serotype 1 S. pneumoniae strains from all ethnic and age groups of patients, irrespective of year of isolation or sample type. The unique pulsotype B corresponded to an ST227 strain (from Australia), differing from ST306 by only two loci. The minor pulsotype C corresponded to an ST304 clone and was found in both Australia and French Polynesia but never in Polynesians living in New Caledonia.
FIG. 2.
Phylogenetic analysis of 5 representative PFGE profiles (A1, A2, A3, B1, and C1) obtained among 141 S. pneumoniae isolates. Serotypes, strain designations, types of specimen, countries and dates of isolation, PFGE types and subtypes, and STs are listed to the right of the patterns. SLV, single-locus variant.
New Caledonia is a developed French overseas territory located in the South Pacific, with 230,789 inhabitants. The country is a discrete epidemiologic entity with Pacific Island characteristics and a multicultural population. A previous study carried out between May 1999 and May 2001 investigated which serotypes were responsible for pneumococcal diseases in New Caledonia (18). The most frequent serotypes were types 1 (20%), 23F (10%), 12F (8%), 19F (8%), and 6B (5%) (18). This distinctive distribution led to the implementation of an active surveillance system for the serotypes responsible for IPD. We were thus able to reveal, retrospectively, that this high prevalence was in fact due to an outbreak where the majority of isolates expressed the serotype 1 capsule. The present serotype 1 outbreaks described in New Caledonia and French Polynesia are due to the limited clonal complex ST306. Interestingly, PFGE was not able to discriminate between ST306 strains, suggesting the high stability of this clone in the South Pacific from 2000 to 2007. This observation is in contrast to another recent report where evidence of a temporal switch was established between two lineages of S. pneumoniae serotype 1 in Brazil (3). A review of the MLST S. pneumoniae database suggests that ST306 is the most important serotype 1-associated sequence type; this sequence type has been recovered predominantly from continental Europe, the United States, and Canada (1) and is clearly now also important in the South Pacific.
In New Caledonia, this strain represents a lineage with a propensity to cause pneumonia and septicemia in healthy indigenous people (18). Furthermore, a study conducted in New Caledonia by Charveriat et al. in 2003, looking at the nasopharyngeal carriage of S. pneumoniae in healthy children aged 2 to 24 months old, showed that only a single serotype 1 isolate was identified out of 544 isolates (2). There were very few invasive cases of serotype 1 in the same year, which also supports the contention that serotype 1 occurs as outbreaks rather than as a strain endemic to the area, with high carriage rates (12).
In 2005, the Advisory Committee on Immunization Practices of New Caledonia recommended PCV7 for all children aged <2 years. Since then, the rate of IPD among children aged <2 years has decreased from 86.9 to 38 annual cases per 100,000 children (personal data), but it remains more frequent than the rate of IPD among children in the general French population, which is 29.8 annual cases per 100,000 children (2001 and 2002) (17). Like observations that were made among closed communities (14, 21), we observed no statistically significant increase in the rate of IPD due to non-vaccine-type strains among children aged <2 years since the implementation of PCV in 2005, except serotype 1 IPD in 2007.
When we looked at the serotype distribution among IPDs of this study, we noted a high prevalence of serotypes such as 1 and 7F. These serotypes had consistently ranked among invasive serotypes causing outbreaks (6, 9, 12, 13, 16). This observation impacts the assessment of the pneumococcal disease burden in New Caledonia and probably in the entire South Pacific. The predominance of ST306 serotype 1 among invasive Streptococcus pneumoniae isolates in the South Pacific contributes to the lower protective impact of vaccines designed for limited serotypes. Our study highlights the importance that the next generation of conjugate pneumococcal vaccines must reduce the worldwide disparity in the rate of IPD due to non-PCV7 serotypes.
Acknowledgments
We acknowledge the use of the pneumococcal multilocus sequence typing database, which is located at Imperial College London. We thank Nathalie Michel, Marie-Ange Charveriat, Francoise Charavay, and Olivia O'Connor for excellent technical assistance. We are grateful to the network of all microbiological laboratories of New Caledonia for isolate processing and Centre National de Reference des Pneumocoques for serotyping some isolates. We thank Eric Bonnet, Wyeth Vaccine France, for his financial support in taking charge of the serogroup kits.
We declare that we have no conflict of interest in relation to this work.
Footnotes
Published ahead of print on 9 June 2010.
REFERENCES
- 1.Brueggemann, A. B., and B. G. Spratt. 2003. Geographic distribution and clonal diversity of Streptococcus pneumoniae serotype 1 isolates. J. Clin. Microbiol. 41:4966-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charveriat, M. A., M. Chomarat, M. Watson, and B. Garin. 2005. Nasopharyngeal carriage of Streptococcus pneumoniae in healthy children, 2 to 24 months of age in New Caledonia. Med. Mal. Infect. 35:500-506. [DOI] [PubMed] [Google Scholar]
- 3.Chiou, A. C., S. S. Andrade, S. C. G. Almeida, R. C. Zanella, A. L. Andrade, and M. C. C. Brandileone. 2008. Molecular assessment of invasive Streptococcus pneumoniae serotype 1 in Brazil: evidence of clonal replacement. J. Med. Microbiol. 57:839-844. [DOI] [PubMed] [Google Scholar]
- 4.Cortese, M. M., M. Wolff, J. Almeido-Hill, R. Reid, J. Ketcham, and M. Santosham. 1992. High incidence rates of invasive pneumococcal disease in the White Mountain Apache population. Arch. Intern. Med. 152:1-6. [PubMed] [Google Scholar]
- 5.Dagan, R. S., S. Gradstein, I. Belmaker, N. Porat, Y. Siton, G. Weber, J. Janco, and P. Yagupsky. 2000. An outbreak of Streptococcus pneumoniae serotype 1 in a closed community in southern Israel. Clin. Infect. Dis. 30:319-321. [DOI] [PubMed] [Google Scholar]
- 6.Dochez, A. R., and L. J. Gillespie. 1913. A biologic classification of pneumococci by means of immunity reactions. JAMA 61:727-730. [Google Scholar]
- 7.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 8.Fedson, S. D., J. Anthony, and G. Scott. 1999. The burden of pneumococcal disease among adults in developed and developing countries: what is and is not known. Vaccine 17:S11-S18. [DOI] [PubMed] [Google Scholar]
- 9.Gratten, M. F., F. Morey, J. Dixon, K. Manning, P. Torzillo, R. Matters, J. Erlich, J. Hanna, V. Asche, and I. Riley. 1993. An outbreak of serotype 1 Streptococcus pneumoniae infection in central Australia. Med. J. Aust. 158:340-342. [DOI] [PubMed] [Google Scholar]
- 10.Gratten, M., P. Torzillo, F. Morey, J. Dixon, J. Erlich, J. Hagger, and J. Henrichsen. 1996. Distribution of capsular types and antibiotic susceptibility of invasive Streptococcus pneumoniae isolated from Aborigines in Central Australia. J. Clin. Microbiol. 34:338-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hausdorff, W. P., J. Bryant, P. R. Paradiso, and G. R. Siber. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin. Infect. Dis. 30:100-121. [DOI] [PubMed] [Google Scholar]
- 12.Hausdorff, W. P., D. R. Feikin, and K. P. Klugman. 2005. Epidemiological differences among pneumococcal serotypes. Lancet Infect. Dis. 5:83-93. [DOI] [PubMed] [Google Scholar]
- 13.Invasive Bacterial Infection Surveillance (IBIS) Group and International Clinical Epidemiology Network (INCLEN). 1999. Prospective multicentre hospital surveillance of Streptococcus pneumoniae disease in India. Lancet 353:1216-1221. [PubMed] [Google Scholar]
- 14.Lacapa, R., S. J. Bliss, F. Larzelere-Hinton, K. J. Eagle, D. J. McGinty, A. J. Parkinson, M. Santosham, M. J. Craig, and K. L. O'Brien. 2008. Changing epidemiology of invasive pneumococcal disease among White Mountain Apache persons in the era of the pneumococcal conjugate vaccine. Clin. Infect. Dis. 47:476-484. [DOI] [PubMed] [Google Scholar]
- 15.Lefevre, J. C., G. Faucon, A. M. Sicard, and M. A. Gasc. 1993. DNA fingerprinting of Streptococcus pneumoniae strains by pulsed-field gel electrophoresis. J. Clin. Microbiol. 31:2724-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leimkugel, J., A. A. Forgor, S. Gagneux, V. Pluger, C. Flierl, E. Awine, M. Naegeli, J. P. Dangy, T. Smith, A. Hodgson, and G. Pluschke. 2005. An outbreak of serotype 1 Streptococcus pneumoniae meningitis in Northern Ghana with features that are characteristic of Neisseria meningitidis meningitis epidemics. J. Infect. Dis. 192:192-199. [DOI] [PubMed] [Google Scholar]
- 17.Lepoutre, A., E. Varon, S. Georges, L. Gutmann, and D. Levy-Bruhl. 28 August 2008. Impact of infant pneumococcal vaccination on invasive pneumococcal diseases in France, 2001-2006. Euro Surveill. 13:pii18962. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18962. [DOI] [PubMed] [Google Scholar]
- 18.Michel, N., M. Watson, F. Baumann, P. Perolat, and B. Garin. 2005. Distribution of Streptococcus pneumoniae serotypes responsible for penicillin resistance and the potential role of new conjugate vaccines in New Caledonia. J. Clin. Microbiol. 43:6060-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musher, D. M. 1992. Infections cause by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 14:801-809. [DOI] [PubMed] [Google Scholar]
- 20.Proulx, J. F., S. Dery, L. P. Jette, J. Ismael, M. Libman, and P. De Wals. 2002. Pneumonia epidemic caused by a virulent strain of Streptococcus pneumoniae serotype 1 in Nunavik, Quebec. Can. Commun. Dis. Rep. 28:129-131. [PubMed] [Google Scholar]
- 21.Romney, M. G., M. W. Hull, R. Gustafson, J. Sandhu, S. Champagne, T. Wong, A. Nematallah, S. Forsting, and P. Daly. 2008. Large community outbreak of Streptococcus pneumoniae serotype 5 invasive infection in an impoverished, urban population. Clin. Infect. Dis. 47:768-774. [DOI] [PubMed] [Google Scholar]
- 22.Rudolph, K. M., A. J. Parkinson, A. L. Reasonover, L. R. Bulkow, D. J. Parks, and J. C. Butler. 2000. Serotype distribution and antimicrobial resistance patterns of invasive isolates of Streptococcus pneumoniae: Alaska, 1991-1998. J. Infect. Dis. 182:490-496. [DOI] [PubMed] [Google Scholar]
- 23.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulse-field electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]