Abstract
The prevalence of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli (ESBLEC) in Spain increased 8-fold from 2000 to 2006. ESBL type, clonal relationship, antimicrobial susceptibility, and clinical data about infections caused by ESBLEC are evaluated in a second nationwide study developed in 2006. From 1008 clinical isolates obtained over 2 months from 44 hospitals, 254 were used for further analysis. ESBL production was evaluated by synergy testing, PCR, and sequencing. Antimicrobial activity was evaluated by microdilution. The clonal relationship was evaluated by repetitive extragenic palindromic-PCR (REP-PCR). The O25b subtype and the new afa operon FM955459 were determined by triplex PCR in isolates producing CTX-M-15. Multilocus sequence typing was performed on these isolates. A total of 72% of all ESBLs were of the CTX-M type, 26.8% were of the SHV type, and 1.2% were of the TEM type. The most prevalent ESBLs were CTX-M-14 (119 isolates), SHV-12 (68 isolates), CTX-M-15 (37 isolates), and CTX-M-9 (21 isolates). By REP-PCR, 214 clones were detected. All but five CTX-M-15 ESBLEC isolates corresponded to the international O25b/ST131 clone. This clone had not been detected in the first study (published in 2000). Epidemiological and clinical features were studied in 304 representative patients. A total of 60% of the patients were older than 60 and had nonfatal underlying diseases, and 55% had recently received antibiotics. Urinary tract infections accounted for 71% of cases, and 9% were bacteremic. There has been a significant increase in the prevalence of ESBLEC in Spain, with most of these strains being CTX-M-producing isolates, including the pandemic O25b-ST131. SHV-12-producing E. coli remains an important cause of community-acquired infection.
Extended-spectrum β-lactamase (ESBL)-producing Escherichia coli (ESBLEC) has emerged worldwide as a significant cause of both community and healthcare-associated infections (13). Moreover, the role of this microorganism as a cause of nosocomial infection is also increasing (15). The type of ESBL expressed by this microorganism has changed in recent years. The classic SHV and TEM types have often been substituted by members of the CTX-M family (3).
The epidemiology of ESBLEC is a complex and evolving phenomenon. A few years ago most ESBLEC strains were clonally unrelated, and the rapid emergence of ESBL was related to the dissemination of mobile genetic elements (14). Nevertheless, both plasmid and bacterial transmission between humans has been demonstrated (17). Recently, the international spread of the O25b-ST131 clone producing CTX-M-15 and other β-lactamases has been described (6, 11). For these reasons, the development of studies directed at discovering the epidemiology of ESBLs in a specific area is recommended.
In 2000, the first nationwide study of ESBLEC was developed in Spain (GEIH-BLEE 2000) (8). The prevalence of ESBL production among E. coli isolates was determined to be 0.5%, with CTX-M-9, SHV-12, and CTX-M-14 being the most commonly found ESBLs. No CTX-M-15-producing E. coli strain was isolated. A nationwide study designed along similar lines was developed in 2006 (GEIH-BLEE 2006) because of perceived important changes in the epidemiology of ESBLEC. In 6 years, the prevalence of ESBLEC increased to 4.04% (range, 0.4 to 20.3%) in Spain (7). The distributions of origins of infection between community-acquired, healthcare-associated, and nosocomial strains were 32, 36, and 30%, respectively. The changes in ESBL type, clonal relationship, susceptibility to antimicrobial agents, and relevant clinical data pertaining to ESBLEC in Spain are discussed here.
MATERIALS AND METHODS
Bacterial isolates.
Forty-four hospitals from all Spanish regions participated in the GEIH-BLEE 2006 project. In the study period (from 1 February to 30 March 2006), 1,008 ESBLEC isolates were obtained from clinical samples (7). Identification of isolates to the species level was performed with the API 20E system (bioMérieux, Marcy l'Etoile, France). ESBL production was confirmed by broth microdilution according to CLSI guidelines (4). The first 254 ESBLEC isolates were included for further microbiologic study.
Antimicrobial susceptibility testing.
The MICs of cefotaxime (alone or with clavulanic acid [4 mg/liter]), ceftazidime (alone or with clavulanic acid [4 mg/liter]), cefepime, cefoxitin, cefotetan, imipenem, and meropenem were determined with MicroScan ESBL Plus ESBL confirmation panels (Siemens Healthcare Diagnostics, Sacramento, CA). In addition, broth microdilution using Mueller-Hinton broth according to CLSI recommendations (5) was used to determine susceptibility to the following antimicrobials: piperacillin (Sigma-Aldrich, Madrid, Spain)-tazobactam (4 mg/liter, fixed concentration; Wyeth-Lederle, Madrid, Spain), amoxicillin (Sigma-Aldrich) and clavulanic acid (GSK, Madrid, Spain) (amoxicillin-clavulanate proportion, 2:1), ertapenem (Merck, Sharp, & Dohme, Madrid, Spain), amikacin (Sigma-Aldrich), gentamicin (Sigma-Aldrich), tobramycin (Sigma-Aldrich), ciprofloxacin (Sigma-Aldrich), cotrimoxazole (Sigma-Aldrich), tigecycline (Wyeth), and nitrofurantoin (Sigma-Aldrich). E. coli ATCC 25922 and ATCC 35218 were used as control strains.
Molecular study.
Clonal relationship of ESBL-producing strains was assessed by repetitive extragenic palindromic-PCR (REP-PCR), as previously described (14). Strains showing more than two different bands after electrophoresis of the PCR product and ethidium bromide staining were considered unrelated. ESBL-encoding genes were characterized by PCR using specific primers for TEM (forward, 5′-ATG AGT ATT CAA CAT TTC CG; reverse, 5′-CTG ACA GTT ACC AAT GCT TA), SHV (forward, 5′-GGG TTA TTC TTA TTT GTC GC; reverse, 5′-TTA GCG TTG CCA GTG CTC), CTX-M-1 (forward, 5′-GTT AAA AAA TCA CTG CG; reverse, 5′-CAT TCC GTT TCC GCT ATT AC; forward 2, 5′-GCG GCC GCG CTA CAG TAC), and CTX-M-9 (forward, 5′-GTG ACA AAG AGA GTG CAA CGG; reverse, 5′-ATG ATT CTC GCC GCT GAA GCC) groups (8). Bacterial DNA was obtained by boiling a suspension of one or two fresh colonies in distilled water for 10 min. Then, 10 μl of supernatant was added to a master mix containing PCR buffer (1×), MgCl2 (2 mM), deoxynucleoside triphosphates (200 μM), primer (0.5 μM), and Taq polymerase (2 U). A Techne TC-132 thermal cycler was used for amplification: a denaturation cycle of 4 min (95°C) was followed by 35 amplification cycles of 30 s (95°C), 30 s (58°C for TEM/SHV, 62°C for CTX-M-9, and 60°C for CTX-M-1), and 1 min 15 s (72°C), with a final extension cycle of 7 min (72°C). For sequencing the corresponding ESBL-encoding gene, the products of specific ESBL-PCR were purified with the Real Clean Spin kit (Durviz) purification kit for direct sequencing. ESBL sequences were developed in an external center (Newbiotechnic S.A., Seville, Spain) equipped with an ABI Prism 377 (Applied Biosystems/Perkin-Elmer) sequencer. Sequences were analyzed by using the Chromas-Pro application and BLAST Internet services (www.ncbi.nlm.nih.gov/BLAST). For CTX-M-15-producing isolates, the O25b subtype and the presence of the new afa operon FM955459 were determined by triplex PCR according to the method of Blanco et al. (2). Furthermore, multilocus sequence typing (MLST) was used to confirm that CTX-M-15-producing E. coli O25b belonged to the international clone ST131 (11).
Epidemiological and clinical features.
Epidemiological and clinical data from a representative sample comprising 304 (30%) of all 1,008 patients in the study were analyzed, a sample size that allowed us to performed comparisons in subgroups according to acquisition. Cases included for analysis were chosen by a random stratified procedure, using acquisition type and geographical area as the stratification variables. Epidemiological and clinical features were collected by using a structured questionnaire based on the following data: age, sex, healthcare relation, underlying conditions, invasive procedures performed during the preceding week, antimicrobial use during the preceding month, type of infection, and outcome. An ESBLEC was classified as nosocomially acquired (NA), healthcare associated (HCA), or community acquired (CA) according to the following criteria: an ESBLEC isolate obtained 48 h after hospital admission was considered NA; of the rest, an ESBLEC isolate was considered HCA if the patient had been admitted to an acute or long-term care center, received hemodialysis, specialized home care, or day hospital care during the preceding 3 months. Qualitative variables were compared by using the chi-square test or the Fisher exact test, as appropriate. The study was approved by the Ethic Committee of the participating centers.
RESULTS
Microbiological results.
A total of 254 strains were characterized. The antimicrobial susceptibility data of ESBL-producing strains are presented in Table 1 . All of the strains included in the present study were considered resistant to cefotaxime, ceftazidime, cefpodoxime, cefepime, and aztreonam. We found that 88 and 100% of E. coli isolates tested were susceptible to cefoxitin and cefotetan, respectively. All isolates were susceptible to imipenem, meropenem, ertapenem, and tigecycline.
TABLE 1.
In vitro activity of several antimicrobial agents against ESBLEC
| Agent | MIC (μg/ml) |
%Sa | ||
|---|---|---|---|---|
| Range | MIC50 | MIC90 | ||
| Cefotaxime | 8->128 | >128 | >128 | 0 |
| Ceftazidime | ≤0.5->128 | 64 | >128 | 0 |
| Cefepime | ≤1->32 | >32 | >32 | 0 |
| Cefoxitin | ≤2->32 | 4 | 16 | 88.2 |
| Cefotetan | ≤1-4 | <1 | <1 | 100 |
| Amoxicillin-clavulanate (2:1) | ≤0.25-128 | 8 | 32 | 69.3 |
| Piperacillin-tazobactam (4 μg/ml) | ≤0.5->64 | 4 | 32 | 88.6 |
| Imipenem | ≤0.5-1 | <0.5 | <0.5 | 100 |
| Meropenem | ≤0.5 | ≤0.5 | ≤0.5 | 100 |
| Ertapenem | ≤0.007-2 | 0.03 | 0.125 | 100 |
| Amikacin | 0.125-128 | 2 | 16 | 98 |
| Gentamicin | ≤0.06->128 | 0.5 | 64 | 78.3 |
| Tobramycin | 0.125-128 | 1 | 32 | 76 |
| Ciprofloxacin | ≤0.06->128 | 16 | 128 | 29.1 |
| Cotrimoxazole | 0.05/0.013->512/27 | >512/27 | >512/27 | 36.1 |
| Tigecycline | 0.06-2 | 0.125 | 0.25 | 100 |
| Nitrofurantoin | 2->512 | 16 | 64 | 87 |
%S, the percent susceptibility determined according to CLSI guidelines (4).
The most active β-lactam/β-lactamase inhibitor combination against E. coli was piperacillin-tazobactam (88.6% susceptible strains), followed by amoxicillin-clavulanic acid (69.3% susceptible strains). The susceptibility percentages for other antimicrobials evaluated were as follows: 98%, amikacin; 78.3%, gentamicin; 76%, tobramycin; 29.1%, ciprofloxacin; and 36.1%, cotrimoxazole.
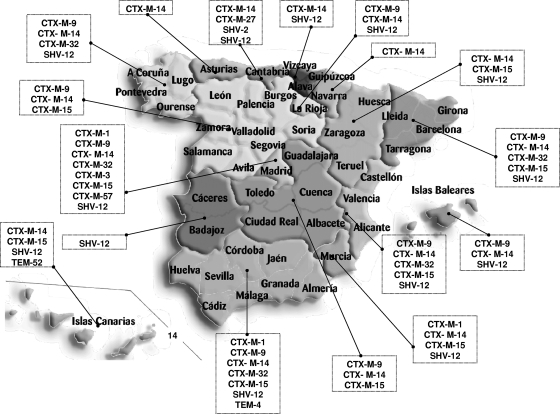
blaTEM, blaSHV, and blaCTX-M genes were detected by using specific PCR in all cases. Of all identified ESBLs, 72% belonged to the CTX-M type, 26.8% belonged to the SHV type, and 1.2% belonged to the TEM type. The most prevalent ESBLs were CTX-M-14 (119 isolates), SHV-12 (68 isolates), CTX-M-15 (37 isolates), and CTX-M-9 (21 isolates). All other ESBLs were identified in five isolates or fewer (Fig. 1). Two ESBLs were obtained in seven isolates, with the CTX-M-9 plus SHV-12 combination (four isolates) being the most prevalent.
FIG. 1.
Distribution of ESBLEC isolates in Spain. (Modified from reference 8.)
A total of 214 unrelated REP-PCR profiles were found; 7 profiles included two isolates each, and 1 profile included three isolates. Isolates from these clusters came from a single hospital, and no transmission between centers was detected. Finally, 32 of 37 isolates of CTX-M-15-producing E. coli were determined to be positive by triplex PCR for the O25b molecular subtype. All 32 O25b isolates were found by MLST to belong to ST131, indicating that they all belonged to the international clonal group O25b-ST131. These isolates were obtained in 15 participating centers.
The geographical distribution of ESBLs in Spain is shown in Fig. 1. Of note is the broad distribution of CTX-M-14, CTX-M 9, CTX-M-15, and SHV-12, found in several regions of Spain.
Epidemiological and clinical features.
Of the 1,008 patients included, the source of ESBLEC acquisition was classified as NA in 312 (31%) cases, HCA in 363 (36%) cases, and CA in 333 (33%) cases. As explained in Materials and Methods, we further analyzed the data of 304 representative patients. Apart from the source of acquisition and the geographical area, these patients showed no significant differences with regard to the total population in terms of age distribution, gender, and sample yielding ESBLEC (data not shown). The features of the patients according to origin of acquisition are shown in Table 2. Of the 112 patients with healthcare-associated ESBLEC, 17 (15%) were nursing home residents and 18 (16%) transplant recipient patients. The majority of these patients were more than 60 years old. Diabetes mellitus and recurrent urinary tract infections were the most common underlying conditions. Community-acquired cases more frequently had a nonfatal underlying condition. Previous receipt of antimicrobials (particularly fluoroquinolones) was common, more significantly for patients with nosocomial and healthcare-associated infections. The most common source of infection was the urinary tract; 52 of the 215 (24%) urinary tract infection episodes were asymptomatic bacteriuria; 10 of the 25 episodes of intra-abdominal infections were biliary tract infections, and 8 of the 15 respiratory tract infections were pneumonia (7 being nosocomially acquired). Overall, bacteremia (either primary or secondary) occurred in 27 (9%) cases, and 16 cases (5%) were classified as surgical-site infections. Crude mortality was 9% (27 patients). Types of ESBLs produced by isolates from patients with NA, HCA, and CA ESBLEC were similar (data not shown).
TABLE 2.
Features of 304 selected patients with ESBLC
| Parameter | No. of patients (%) with ESBLEC |
|||
|---|---|---|---|---|
| Total (n = 304) | Community-acquired (n = 99)a | Healthcare associated (n = 112)b | Nosocomially acquired (n = 93) | |
| Age (yr) | ||||
| <15 | 16 (5) | 9 (9)† | 2 (2) | 5 (5) |
| 15-60 | 98 (32) | 29 (20) | 37 (33) | 32 (34) |
| >60 | 190 (63) | 61 (62) | 73 (65) | 56 (60) |
| Males | 124 (41) | 32 (32)‡ | 47 (42) | 45 (48) |
| McCabe classification | ||||
| Nonfatal | 204 (67) | 79 (80)†‡ | 71 (63) | 54 (58) |
| Ultimately fatal | 90 (30) | 19 (19)‡ | 37 (33) | 34 (37) |
| Rapidly fatal | 10 (3) | 1 (1) | 4 (4) | 5 (5) |
| Underlying conditions | ||||
| Diabetes mellitus | 61 (20) | 16 (16)† | 35 (31)* | 10 (11) |
| Chronic renal insufficiency | 26 (9) | 2 (2)†‡ | 14 (13) | 10 (11) |
| Chronic pulmonary disease | 40 (13) | 8 (8)† | 17 (15) | 15 (16) |
| Solid cancer | 32 (11) | 5 (5) | 15 (13) | 12 (13) |
| Hematologic cancer | 11 (4) | 0‡ | 4 (4) | 7 (8) |
| Liver cirrhosis | 10 (3) | 0‡ | 4 (4) | 6 (7) |
| Urinary tract structural disease | 39 (13) | 9 (9)‡ | 24 (21)* | 6 (7) |
| Recurrent urinary tract infection | 63 (21) | 21 (21) | 36 (32)* | 6 (7) |
| Invasive procedures | ||||
| Urinary catheter | 94 (31) | 8 (8)†‡ | 26 (23)* | 60 (64) |
| Vascular catheter | 76 (25) | 0†‡ | 14 (13)* | 56 (60) |
| Mechanical ventilation | 23 (8) | 0‡ | 0* | 19 (20) |
| Previous antimicrobials | 166 (55) | 35 (35)†‡ | 71 (63) | 60 (65) |
| Fluoroquinolones | 80 (26) | 13 (13)†‡ | 37 (33) | 30 (32) |
| Oxyimino β-lactams | 46 (16) | 7 (7)†‡ | 19 (17) | 20 (22) |
| Amoxicillin-clavulanate | 46 (15) | 8 (8)‡ | 19 (17) | 19 (20) |
| Types of infection | ||||
| Urinary tract infection | 215 (71) | 70 (80)‡ | 88 (79) | 48 (52) |
| Skin and soft tissue infection | 26 (9) | 4 (4)‡ | 9 (8) | 13 (14) |
| Intra-abdominal infection | 25 (8) | 10 (10) | 5 (5) | 10 (11) |
| Respiratory tract infection | 15 (5) | 1 (1)‡ | 2 (2)* | 12 (13) |
| Primary bacteremia | 15 (5) | 2 (2) | 6 (5) | 7 (8) |
| Others | 8 (3) | 2 (2) | 3 (3) | 3 (3) |
†, P < 0.05 for comparisons between community-acquired and healthcare-associated cases; ‡, P < 0.05 for comparisons between community-acquired and nosocomially acquired cases.
*, P < 0.05 for comparison between healthcare-associated and nosocomially acquired cases.
DISCUSSION
The prevalence and epidemiology of ESBLEC is changing very rapidly in several countries (13). CTX-M is currently the most prevalent ESBL family, particularly in community-acquired infections (16). Nevertheless, other ESBLs, such as SHV-12, are also relevant in the community (18). Furthermore, specific clones, such as the CTX-M-15-producing O25b-ST131 clone, have emerged and been disseminated on different continents. This clone has recently been described as present in different areas in Spain (2, 12) and, to monitor the phenomenon in Spain, a second prevalence study was developed, one similar in design to that carried out in 2000. The prevalence of ESBLEC in Spain has increased 8-fold, from 0.5% in 2000 to 4.04% in 2006 (7). In 2006, the incidence of infections caused by ESBLEC ranged from 0.12 to 12.0/100,000 population/month. This increase has been even greater in other European countries (6).
In terms of antimicrobial resistance, there have been no major relevant changes in ESBLEC between 2000 and 2006. The most active antimicrobial agents against ESBLEC were carbapenems, cefotetan, and tigecycline (the latter was not included in the first survey), followed by amikacin. The actual mechanisms involved in the higher activity of cefotetan in comparison with cefoxitin have not been investigated specifically but could be related to altered permeability or increased efflux affecting the two cephamycins in different ways. Ciprofloxacin susceptibility decreased from 37.5% in 2000 to 29.1% in 2006. This fact could be partially due to the dissemination of the international clone O25b-ST131, which is resistant to ciprofloxacin. Besides chromosomal quinolone resistance mechanisms, isolates belonging to this clone commonly expressed the enzyme Aac(6′)-lb-cr that affects susceptibility to ciprofloxacin and some other fluoroquinolones, increasing their MIC values. Among the β-lactam/β-lactamase inhibitor combinations, piperacillin-tazobactam continues to be the most active agent against this microorganism, probably due to the higher intrinsic activity of piperacillin compared to amoxicillin. The potential use of these combinations in infections caused by ESBLEC is still controversial (9).
A molecular study of the selected strains in the present study revealed important differences between the two periods evaluated. In 2000, a highly diverse population structure was observed with a low clonal relationship among ESBLEC strains (170 strains/137 clones), even for those isolated in the same institution (8). In only three centers did more than one E. coli isolate (n = 2 to 14) present the same REP-PCR pattern. In 2006, a similar situation was observed, except for the dissemination of CTX-M-15-producing E. coli O25b-ST131, isolated in 15 different centers.
The ESBL type has also changed very rapidly. TEM-type-producing E. coli have decreased very rapidly in Spain from 2000 (19%) to 2006 (1.2%). Ten different TEM-type ESBLs were detected in 88 ESBLEC strains isolated in 2000. In the present study, only TEM-4 and TEM-52 were detected in a total of three isolates. Nevertheless, the prevalence and variability of the CTX-M-type ESBL have increased in E. coli from 52 to 72% in 6 years. CTX-M-14 remains the most prevalent, followed by CTX-M-15 (not found in the first nationwide study) and then CTX-M-9. It has been pointed out that in this short period of time, the O25b-ST131 clone producing CTX-M-15 was introduced and disseminated in Spain, as described for other countries (6, 11). CTX-M-15-producing ESBLEC was not confined to northern regions, and some local studies on clinical isolates obtained after 2006 have also detected an increase in these strains, particularly the international clone O25b-ST131, in the northwest of Spain (2). Whether the spread of this international clone will displace other clones in the future remains uncertain, although local data point to this hypothesis (2, 10).
The percentage of the SHV-type ESBL remains similar in both studies. Nevertheless, the proportion of SHV-12 increased in the 2006 study. Our group has remarked on the important role of SHV-12-producing E. coli as a cause of infection in the community, which has probably been underestimated as a consequence of the worldwide CTX-M explosion (18). In a similar study developed in 2006 in the French community setting, the percentage of CTX-M-producing ESBLEC was much higher (83%). SHV-12 was expressed only in 1 of 48 isolates (1).
ESBL distribution in Spain is different from that observed in other countries, underlining the great epidemiological variability of these resistance determinants and the need to study them under different epidemiological conditions. Nevertheless, the dissemination of the O25b-ST131 clone producing CTX-M-15 seems to be very similar in different European countries (1, 11).
In contrast to many other studies of ESBL-producing organisms in different countries, in which only microbiological aspects have usually been considered, we have been able to include, in this analytical study, epidemiologic and clinical features of ESBLEC patients. We cannot make comparisons with the 2000 study since the earlier one did not include such information. However, the data from the present study show largely similar results to a previous multicenter study in Spain performed during 2002 and 2003, evaluating community-onset infections due to ESBLEC (16), but with one significant difference: the percentage of patients coming from nursing homes doubled from 7% of patients with healthcare-associated infections to 15%. This might be related to the incipient spread in Spain of strains of the international O25b-ST131 clone producing CTX-M-15, which has been found to reside in nursing homes in Spain (2, 12) and other countries (19).
In summary, the present study emphasizes the significant increase in ESBLEC in Spain over a 6-year period. Although there was great variability in terms of clones and ESBL types, most were CTX-M-producing E. coli, including the pandemic CTX-M-15-producing clone O25b-ST131. It is also remarkable that SHV-12-producing E. coli remains an important cause of community-acquired infections.
Acknowledgments
This study was partially supported by an unrestricted grant from Wyeth Laboratories (Spain); by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III-FEDER, Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008), FIS (PI070190); and by the Junta de Andalucía (0048/2008 and CTS-5259).
The authors declare no conflict of interest related to this study.
Footnotes
Published ahead of print on 2 June 2010.
REFERENCES
- 1.Arpin, C., C. Quentin, F. Grobost, E. Cambau, J. Robert, V. Dubois, L. Coulange, C. André, and the Scientific Committee of ONERBA. 2009. Nationwide survey of extended-spectrum β-lactamase-producing Enterobacteriaceae in the French community setting. J. Antimicrob. Chemother. 63:1205-1214. [DOI] [PubMed] [Google Scholar]
- 2.Blanco, M., M. P. Alonso, M. H. Nicolas-Chanoine, G. Dahbi, A. Mora, J. E. Blanco, C. López, P. Cortés, M. Llagostera, V. Leflon-Guibout, B. Puentes, R. Mamani, A. Herrera, M. A. Coira, F. García-Garrote, J. M. Pita, and J. Blanco. 2009. Molecular epidemiology of Escherichia coli producing extended-spectrum β-lactamases in Lugo (Spain): dissemination of clone O25b:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 63:1135-1141. [DOI] [PubMed] [Google Scholar]
- 3.Canton, R., and T. M. Coque. 2006. The CTX-M β-lactamase pandemic. Curr. Opin. Microbiol. 9:466-475. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing: 19th informational supplement. CLSI document M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard, 7th ed. CLSI document M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Coque, T. M., A. Novais, A. Carattoli, L. Poirel, J. Pitout, L. Peixe, F. Baquero, R. Cantón, and P. Nordmann. 2008. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum beta-lactamase CTX-M-15. Emerg. Infect. Dis. 14:195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Díaz, M. A., J. R. Hernández, L. Martínez-Martínez, J. Rodríguez-Baño, and A. Pascual. 2009. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Spanish hospitals: second multicenter study (GEIH-BLEE project, 2006). Enferm. Infecc. Microbiol. Clin. 27:503-510. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez, J. R., L. Martinez-Martinez, R. Canton, T. M. Coque, A. Pascual, and the Spanish Group for Nosocomial Infections (GEIH). 2005. Nationwide study of Escherichia coli and Klebsiella pneumoniae producing extended-spectrum beta-lactamases in Spain. Antimicrob. Agents Chemother. 49:2122-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Cerero, L., E. Picón, C. Morillo, J. R. Hernández, F. Docobo, J. Pachón, J. Rodríguez-Baño, and A. Pascual. 2009. Comparative assessment of inoculum effects on the antimicrobial activity of amoxicillin-clavulanate and piperacillin-tazobactam with extended-spectrum beta-lactamase-producing and extended-spectrum beta-lactamase-non-producing Escherichia coli isolates. Clin. Microbiol. Infect. doi: 10.1111/j.1469-0691.2009.02893. [DOI] [PubMed]
- 10.Mora, A., C. López, G. Dabhi, M. Blanco, J. E. Blanco, M. P. Alonso, A. Herrera, R. Mamani, S. Bonacorsi, M. Moulin-Schouleur, and J. Blanco. 2009. Extraintestinal pathogenic Escherichia coli O1:K1:H7/NM from human and avian origin: detection of clonal groups B2 ST95 and D ST59 with different host distribution. BMC Microbiol. doi: 10.1186/1471-2180-9-132. [DOI] [PMC free article] [PubMed]
- 11.Nicolas-Chanoine, M. H., J. Blanco, V. Leflon-Guibout, R. Demarty, M. P. Alonso, M. M. Caniça, Y. J. Park, J. P. Lavigne, J. Pitout, and J. R. Johnson. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 61:273-281. [DOI] [PubMed] [Google Scholar]
- 12.Oteo, J., C. Navarro, E. Cercenado, A. Delgado-Iribarren, I. Wilhelmi, B. Orden, C. García, S. Miguelañez, M. Pérez-Vázquez, S. García-Cobos, B. Aracil, V. Bautista, and J. Campos. 2006. Spread of Escherichia coli strains with high-level cefotaxime and ceftazidime resistance between the community, long-term care facilities, and hospital institutions. J. Clin. Microbiol. 44:2359-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitout, J. D., and K. B. Laupland. 2008. Extended-spectrum β-lactamase producing Enterobacteriaceae: an emerging public-health problem. Lancet Infect. Dis. 8:150-166. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Baño, J., M. D. Navarro, L. Romero, L. Martínez, M. Muniain, E. J. Perea, R. Pérez-Cano, and A. Pascual. 2004. Extended-spectrum beta-lactamase-producing Escherichia coli as a cause of community-acquired infections. J. Clin. Microbiol. 42:1089-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez-Baño, J., M. D. Navarro, L. Romero, M. A. Muniain, E. J. Perea, R. Pérez-Cano, J. R. Hernández, and A. Pascual. 2006. Clinical and molecular epidemiology of extended spectrum β-lactamases producing Escherichia coli as a cause of nosocomial infections or colonization: implications for control. Clin. Infect. Dis. 42:37-45. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez-Baño, J., J. Alcalá, J. M. Cisneros, F. Grill, A. Oliver, J. P. Horcajada, T. Tórtola, B. Mirelis, G. Navarro, M. Cuenca, M. Esteve, C. Peña, C. Llanos, R. Cantón, and A. Pascual. 2008. Community infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Arch. Intern. Med. 168:1897-1902. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Baño, J., L. López-Cerero, M. D. Navarro, P. Díaz de Alba, and A. Pascual. 2008. Faecal carriage of extended-spectrum β-lactamase-producing Escherichia coli: prevalence, risk factors and molecular epidemiology. J. Antimicrob. Chemother. 62:1142-1149. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez-Baño, J., J. Alcalá, J. M. Cisneros, F. Grill, A. Oliver, J. P. Horcajada, T. Tórtola, B. Mirelis, G. Navarro, M. Cuenca, M. Esteve, C. Peña, A. C. Llanos, R. Cantón, and A. Pascual. 2009. Escherichia coli producing SHV-type extended-spectrum beta-lactamase is a significant cause of community-acquired infection. J. Antimicrob. Chemother. 63:781-784. [DOI] [PubMed] [Google Scholar]
- 19.Rooney, P. J., M. C. O'Leary, A. C. Loughrey, M. McCalmont, B. Smyth, P. Donaghy, M. Badri, N. Woodford, E. Karisik, and D. M. Livermore. 2009. Nursing homes as a reservoir of extended-spectrum β-lactamase (ESBL)-producing ciprofloxacin-resistant Escherichia coli. J. Antimicrob. Chemother. 64:635-641. [DOI] [PubMed] [Google Scholar]