Abstract
Definite and rapid diagnosis of rabies is required for individual case management as well as for public health. For the first time, a direct comparison of virus isolation with quantitative real-time reverse transcription (RT)-PCR on human rabies samples was conducted. RT-PCR was found to be more sensitive than virus isolation.
Rabies is an acute encephalomyelitis with fatal outcome. It is caused by rabies virus, one of at least seven species within the genus Lyssavirus, family Rhabdoviridae. In general, transmission to humans occurs by rabid animals (12). Although extremely rare, rabies has also been described upon transplantation of corneal or solid organ grafts (4, 8, 14). Since rabies is clinically indistiguishable from other neurological diseases, laboratory diagnosis is crucial for individual case management as well as for public health measures (7, 12). The current “gold standard” for the detection of rabies antigen remains the direct fluoresccent antibody (DFA) test on brain impression smears of postmortem samples (12). It is recommended by the World Health Organization and can be completed in a rather short time (<2 h) (17). However, false-negative results have been reported on decomposed samples, and it cannot be applied to liquid samples for intravitam diagnosis (15). Confirmatory assays for DFA comprise the rabies tissue culture infection test (RTCIT) and mouse inoculation test (MIT) but require days (RTCIT) to weeks (MIT) until final diagnosis. In addition to these classical methods, reverse transcription (RT)-PCR and real-time RT-PCR, which can be accomplished in less than 6 to 8 h, have been developed and used in pilot studies (1, 10, 13, 16). Although preliminary data suggest that sensitivity of RT-PCR seems to be higher than that of RTCIT or MIT, this issue has not been systematically analyzed with actual human rabies cases (3, 6, 16). Here we have compared heminested and real-time RT-PCR with RTCIT and DFA on a panel of human specimens from a recent transplantation-associated cluster of patients in Germany, in order to compare the sensitivity of classical and molecular detection (8).
Clinical intravitam as well as postmortem samples were derived from one patient. RNA was extracted from liquid samples using the viral RNA minikit (Qiagen, Hilden, Germany) and from tissue samples using the RNeasy kit (Qiagen) as recommended. Modified heminested RT-PCR was done as previously described (5). This assay was not specific for the virus detected in patients but covered a broad range of rabies virus strains. In brief, first-round PCR (Qiagen one-step RT-PCR kit) of 25 μl contained 3 μl of RNA, 1× buffer, 400 nmol of each deoxynucleoside triphosphate (dNTP), 600 nmol of primer RabiesF (ATGTAACACCYCTACAATG), 300 nmol of primer JW6AAS1 (CAATTCGCACACATTTTGTG), 300 nmol of primer JW6AAS2 (CAGTTAGCGCACATCTTATG), and 1 μl enzyme mix. Cycling was done on a Primus 25 advanced cycler (PeqLab, Erlangen, Germany) at 50°C for 30 min and 95°C for 15 min, followed by 10 cycles of 95°C for 20 s, 60°C for 30 s (1°C decrease/cycle), and 72°C for 30 s and 35 cycles of 95°C for 20 s, 52°C for 30 s, and 72°C for 30 s. The second-round PCR (Platinum Taq DNA polymerase; Invitrogen, Karlsruhe, Germany) with a 25-μl reaction volume included 2 μl of first-round PCR product, 1× buffer, 200 nmol of each dNTP, 1 mM MgCl, 600 nmol primer RabiesF, 300 nmol primer JW10IAS1 (GTCATCAATGTGTGATGTTC), 300 nmol primer JW10IAS2 (GTCATTAGAGTATGGTGTTC), and 0.1 μl enzyme. The second-round PCR comprised 94°C for 5 min and 35 cycles of 94°C for 20 s, 52°C for 20 s, and72°C for 30 s. A fragment of rabies nucleoprotein gene (positions 55 to 636; GenBank accession no. AY956319) was amplified directly from a patient sample and sequenced. Sequencing was done with a CEQ 8000 genetic analysis system (Beckman Coulter, Krefeld, Germany). Sequences were analyzed with the Lasergene software package (DNASTAR, Madison, WI). Hereafter, a patient-specific real-time RT-PCR targeting the nucleoprotein gene amplicon was developed. All oligonucleotides were used in the following assay: 25-μl reaction volume, 3 μl of RNA extract, Qiagen OneStep RT-PCR kit, 700 nM primer RSS1 (AGAAGGGAATTGGGCTTTGAC), 700 nM primer RSAs1 (AGATGCATGCTCGGGAACA), and 200 nM probe RSP (6-carboxyfluorescein [FAM]-AATGGAACTGACGAGGGACCCCAT-6-carboxytetramethylrhodamine [TAMRA]). Cycling was performed at 50°C for 30 min and 95°C for 15 min, followed by 40 cycles of 95°C for 10 s and 61°C for 30 s on a 1.2 LightCycler (Roche Diagnostics, Mannheim, Germany). For RTCIT, N2a neuroblastoma cells were inoculated with saliva, sputum, and corneal swab samples or postmortem samples comprising a 20% suspension of tissue homogenates of brain, peripheral nerve, heart, lung, small intestine, liver, spleen, kidney, lymph node, and skin biopsy specimens, respectively. All cultures were passaged at least five times. Virus antigen in cell culture was detected by fluorescent antibody staining after each passage. In addition, detection of virus antigen by DFA was done on all postmortem samples (2).
Rabies was confirmed in the recipient as described previously (8). After sequencing part of the nucleoprotein gene, a patient-derived rabies virus strain-specific real-time RT-PCR was developed. In the first step, the analytical sensitivity of the real-time PCR was determined. The 95% limit of detection (LOD) as assessed by probit analysis was 4.5 RNA copies/RT-PCR (95% confidence interval, 3.2 to 11.4 RNA copies/RT-PCR). This LOD corresponded to 73 RNA copies/mg tissue or 535 RNA RNA copies/ml fluid. The linear range of the assay was from 1.8 × 102 to 1.8 × 108 RNA copies/PCR.
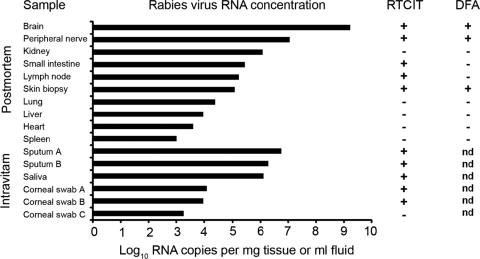
Rabies viral RNA concentrations were measured in various specimens. The highest intravitam concentrations were measured in a sputum sample with 5.7 × 106 RNA copies/ml. In postmortem samples, the highest concentration of 1.6 × 109 RNA copies/mg tissue was detected in a brain sample, followed by 1.1 × 107 RNA copies/mg in a peripheral nerve sample (Fig. 1). Viral RNA at lower concentrations could be demonstrated in kidney, small intestine, skin biopsy, lung, liver, heart, and spleen tissue samples. Concomitantly heminested RT-PCR was positive in all real-time RT-PCR-positive samples, demonstrating the high clinical sensitivity of the assay. In parallel, virus isolation by RTCIT was attempted from the same specimens. In intravitam samples, RTCIT was successful in all but one sample containing 1.3 × 103 RNA copies/ml (Fig. 1). In postmortem samples, RTCIT was only successful in brain, peripheral nerve, small intestine, lymph node, and skin biopsy samples. Interestingly, a postmortem kidney sample remained negative upon RTCIT, although high viral RNA concentrations were measured. In most samples, RTCIT-positive results were obtained at day 4 postinoculation. However, a peripheral nerve sample and all corneal swab samples required up to 4 passages, until definite virus isolation. DFA on postmortem samples was the least-sensitive method, with positive results only for brain, peripheral nerve, and one skin biopsy sample (Fig. 1). In summary, all RTCIT-positive intravitam samples yielded rabies viral RNA concentrations of >1 × 104 RNA copies/ml, whereas RTCIT-positive postmortem samples yielded RNA concentrations of >1 × 105 RNA copies/mg tissue.
FIG. 1.
Comparative analysis of quantitative real-time RT-PCR results and results of RTCIT and DFA on a panel of patient specimens. Solid black bars indicate rabies viral RNA concentrations per mg in solid postmortem samples and per ml in fluid intravitam samples, respectively, as measured by real-time RT-PCR. Quantative real-time RT-PCR results have been transformed to log10 RNA copies per mg and ml, respectively. Results of virus isolation by RTCIT for the respective postmortem and intravitam samples are indicated by + for a positive result and − for a negative result. Results of DFA are indicated by + for a positive result, − for a negative result, and nd for material not appropriate for DFA analysis.
In this study we could demonstrate that detection of rabies viral RNA by RT-PCR was possible in intravitam as well as postmortem specimens with high sensitivity, supporting previous reports (1, 13). RT-PCR might therefore be a feasible approach to support intravitam diagnosis of rabies in a timely fashion. In selected cases, organ donors with a history of acute progressive encephalitis and a possible exposure might be subjected to testing even after organ extraction (8). Importantly, RT-PCR proved to be 1.6× more sensitive than RTCIT, suggesting that RT-PCR can complement RTCIT as a confirmatory assay. Given the severe consequences of a positive rabies diagnosis intravitam, however, confirmation by RTCIT should never remain unattempted. Notably, this should not delay the initiation of therapeutic interventions but is due to the fact molecular assays may fail upon primer base pair mismatches (11). Interestingly in our study, a modified generic heminested RT-PCR proved to be as sensitive as the newly developed rabies virus strain-specific real-time RT-PCR. Although nested RT-PCR may be less sensitive than real-time RT-PCR in selected cases (16), it provides simultaneous detection of different rabies virus genotypes and high confidence in exclusion screening for rabies. In selected situations, quantitative real-time RT-PCR data might also prove beneficial for follow-up of treatment (8, 10). In addition it can be used for assessment of risks of transmission in the nosocomial setting (9). In spite of the many challenges that PCR-based diagnostics pose, especially in less-affluent settings (15), our report strongly encourages the application of RT-PCR methodology to complement classical approaches of rabies virus detection.
Acknowledgments
We appreciate the excellent technical assistance of Britta Liedigk, Ulrike Krause, Heide Hilbig-Hanl, and Gundula Müseler.
This study was supported by the German Ministry of Health as part of funding of the National Reference Centre for Imported Infections at the Bernhard-Nocht-Institute.
Footnotes
Published ahead of print on 16 June 2010.
REFERENCES
- 1.Crepin, P., L. Audry, Y. Rotivel, A. Gacoin, C. Caroff, and H. Bourhy. 1998. Intravitam diagnosis of human rabies by PCR using saliva and cerebrospinal fluid. J. Clin. Microbiol. 36:1117-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dean, D. J., M. K. Abelseth, and P. Athanasiu. 1996. The fluorescence antibody test, 4th ed. World Health Organization, Geneva, Switzerland.
- 3.Franka, R., S. Svrcek, M. Madar, M. Kolesarova, A. Ondrejkova, R. Ondrejka, Z. Benisek, J. Suli, and S. Vilcek. 2004. Quantification of the effectiveness of laboratory diagnostics of rabies using classical and molecular-genetic methods. Vet. Med.-Czech. 49:259-267. [Google Scholar]
- 4.Gode, G. R., and N. K. Bhide. 1988. Two rabies deaths after corneal grafts from one donor. Lancet ii:791. [DOI] [PubMed] [Google Scholar]
- 5.Heaton, P. R., P. Johnstone, L. M. McElhinney, R. Cowley, E. O'Sullivan, and J. E. Whitby. 1997. Heminested PCR assay for detection of six genotypes of rabies and rabies-related viruses. J. Clin. Microbiol. 35:2762-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes, G. J., I. V. Kuzmin, A. Schmitz, J. Blanton, J. Manangan, S. Murphy, and C. E. Rupprecht. 2006. Experimental infection of big brown bats (Eptesicus fuscus) with Eurasian bat lyssaviruses Aravan, Khujand, and Irkut virus. Arch. Virol. 151:2021-2035. [DOI] [PubMed] [Google Scholar]
- 7.Jackson, A. C., M. J. Warrell, C. E. Rupprecht, H. C. Ertl, B. Dietzschold, M. O'Reilly, R. P. Leach, Z. F. Fu, W. H. Wunner, T. P. Bleck, and H. Wilde. 2003. Management of rabies in humans. Clin. Infect. Dis. 36:60-63. [DOI] [PubMed] [Google Scholar]
- 8.Maier, T., A. Schwarting, D. Mauer, R. S. Ross, A. Martens, V. Kliem, J. Wahl, M. Panning, S. Baumgarte, T. Muller, S. Pfefferle, H. Ebel, J. Schmidt, K. Tenner-Racz, P. Racz, M. Schmid, M. Struber, B. Wolters, D. Gotthardt, F. Bitz, L. Frisch, N. Pfeiffer, H. Fickenscher, P. Sauer, C. E. Rupprecht, M. Roggendorf, A. Haverich, P. Galle, J. Hoyer, and C. Drosten. 2010. Management and outcomes after multiple corneal and solid organ transplantations from a donor infected with rabies virus. Clin. Infect. Dis. 50:1112-1119. [DOI] [PubMed] [Google Scholar]
- 9.Mattner, F., C. Henke-Gendo, A. Martens, C. Drosten, T. F. Schulz, A. Heim, S. Suerbaum, S. Kuhn, J. Bruderek, P. Gastmeier, and M. Strueber. 2007. Risk of rabies infection and adverse effects of postexposure prophylaxis in healthcare workers and other patient contacts exposed to a rabies virus-infected lung transplant recipient. Infect. Control Hosp. Epidemiol. 28:513-518. [DOI] [PubMed] [Google Scholar]
- 10.Nadin-Davis, S. A., M. Sheen, and A. I. Wandeler. 2009. Development of real-time reverse transcriptase polymerase chain reaction methods for human rabies diagnosis. J. Med. Virol. 81:1484-1497. [DOI] [PubMed] [Google Scholar]
- 11.Panning, M., P. Emmerich, S. Ölschläger, S. Bojenko, L. Koivogui, A. Marx, P. C. Lugala, S. Günther, D. G. Bausch, and C. Drosten. 2010. Laboratory diagnosis of Lassa fever, Liberia. Emerg. Infect. Dis. 16:1041-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rupprecht, C. E., C. A. Hanlon, and T. Hemachudha. 2002. Rabies re-examined. Lancet Infect. Dis. 2:327-343. [DOI] [PubMed] [Google Scholar]
- 13.Smith, J., L. McElhinney, G. Parsons, N. Brink, T. Doherty, D. Agranoff, M. E. Miranda, and A. R. Fooks. 2003. Case report: rapid ante-mortem diagnosis of a human case of rabies imported into the UK from the Philippines. J. Med. Virol. 69:150-155. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan, A., E. C. Burton, M. J. Kuehnert, C. Rupprecht, W. L. Sutker, T. G. Ksiazek, C. D. Paddock, J. Guarner, W. J. Shieh, C. Goldsmith, C. A. Hanlon, J. Zoretic, B. Fischbach, M. Niezgoda, W. H. El-Feky, L. Orciari, E. Q. Sanchez, A. Likos, G. B. Klintmalm, D. Cardo, J. LeDuc, M. E. Chamberland, D. B. Jernigan, and S. R. Zaki. 2005. Transmission of rabies virus from an organ donor to four transplant recipients. N. Engl. J. Med. 352:1103-1111. [DOI] [PubMed] [Google Scholar]
- 15.Wacharapluesadee, S., and T. Hemachudha. 2010. Ante- and post-mortem diagnosis of rabies using nucleic acid-amplification tests. Expert Rev. Mol. Diagn. 10:207-218. [DOI] [PubMed] [Google Scholar]
- 16.Wakeley, P. R., N. Johnson, L. M. McElhinney, D. Marston, J. Sawyer, and A. R. Fooks. 2005. Development of a real-time, TaqMan reverse transcription-PCR assay for detection and differentiation of lyssavirus genotypes 1, 5, and 6. J. Clin. Microbiol. 43:2786-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. 2005. WHO expert consultation on rabies. World Health Organization, Geneva, Switzerland.