Abstract
We report the first instance of macrolide resistance developing in vivo following appropriate antibiotic use in a healthy volunteer as a part of a controlled human infection with Campylobacter jejuni. In vivo development of macrolide resistance may be an important contributor to antibiotic resistance in C. jejuni.
CASE REPORT
As part of a well-controlled inpatient experimental human infection study, a 21-year-old healthy male was dosed with 8.5 × 104 CFU of Campylobacter jejuni CG8421 (21). This bacterial stock was prepared and stored under good manufacturing practices (cGMP), lacks ganglioside mimicry, is susceptible to macrolide and fluoroquinolone (FQ) antibiotics, and has been fully characterized (19). At 72 h postinoculation, the subject experienced the onset of diarrhea preceded by a high fever (maximal temperature, 103.1°F). Blood cultures were negative. The total episode of campylobacteriosis consisted of 12 loose/liquid stools (4 stools prior to antibiotics; total volume of all stools, 1,220 ml) without gross blood, accompanied by abdominal cramping and two episodes of vomiting. At 72 h after inoculation but before initiation of antibiotics (80 h), the subject shed C. jejuni, which was confirmed to be C. jejuni CG8421 (2.95 × 105 ± 1.8 × 105 CFU bacteria/gram of stool). Per protocol, azithromycin (500 mg orally once daily for 5 days) was administered. The subject's symptoms resolved within 48 h; stool cultures became negative and remained consecutively negative until discharge on day 9 postinoculation and at follow-up visits on days 10 and 14 postinoculation. On the day 21 follow-up visit, the subject was asymptomatic, but stool cultures were positive for C. jejuni, which was again confirmed to be CG8421 but now determined to be macrolide resistant. The subject was further treated with both ciprofloxacin (500 mg orally twice daily) and azithromycin (500 mg orally once daily, begun before antibiotic sensitivities were known). The subject remained asymptomatic, and all weekly stool cultures taken for 5 weeks post-antibiotic treatment were negative. HIV antibody, serum immunoglobulins and subsets, complement levels, and response to protein vaccination were all negative or normal. The subject mounted robust C. jejuni-specific immunologic responses postinfection, including serologic responses (8-, 16-, and 4-fold increases in IgM, IgA, and IgG, respectively), fecal IgA (8-fold rise), antibody-secreting cells (ASC) (maximal response, 129 IgA ASC/106 peripheral blood mononuclear cells), and in vitro C. jejuni-specific gamma interferon (IFN-γ) (4-fold rise).
After C. jejuni inoculation, the first two stools produced each day were cultured using validated standard operating procedures with a level of detection of 1.5 CFU/100 mg stool. Cultures were performed within 4 h of collection using Campylobacter-specific medium (Campy CVA or Campy blood agar plates) in chambers containing 5% O2, 10% CO2, and 85% N2 at 42°C for 48 h. Following administration of antibiotics, to maximize the detection of C. jejuni, stools were cultured within 2 h of specimen production and plated in sextuplicate.
The day 3 and day 21 C. jejuni isolates were confirmed as identical to the challenge strain, CG8421. Both isolates were evaluated by PCR, using previously described primers specific to CG8421 (4). These primers are complementary to genes located in a phage-like element unique to CG8421; C. jejuni strain 81-176, which lacks these sequences, was used as a negative control. As anticipated, the day 3, day 21, and CG8421 C. jejuni strains generated PCR products, while the negative control, C. jejuni 81-176, did not. Pulsed-field gel electrophoresis (PFGE) was also performed on the day 21 strain using a BssHII enzyme digestion and previously described parameters (4). Identical digestion patterns shown in Fig. 1 B and C confirmed the PCR results that the day 21 isolate was CG8421.
FIG. 1.
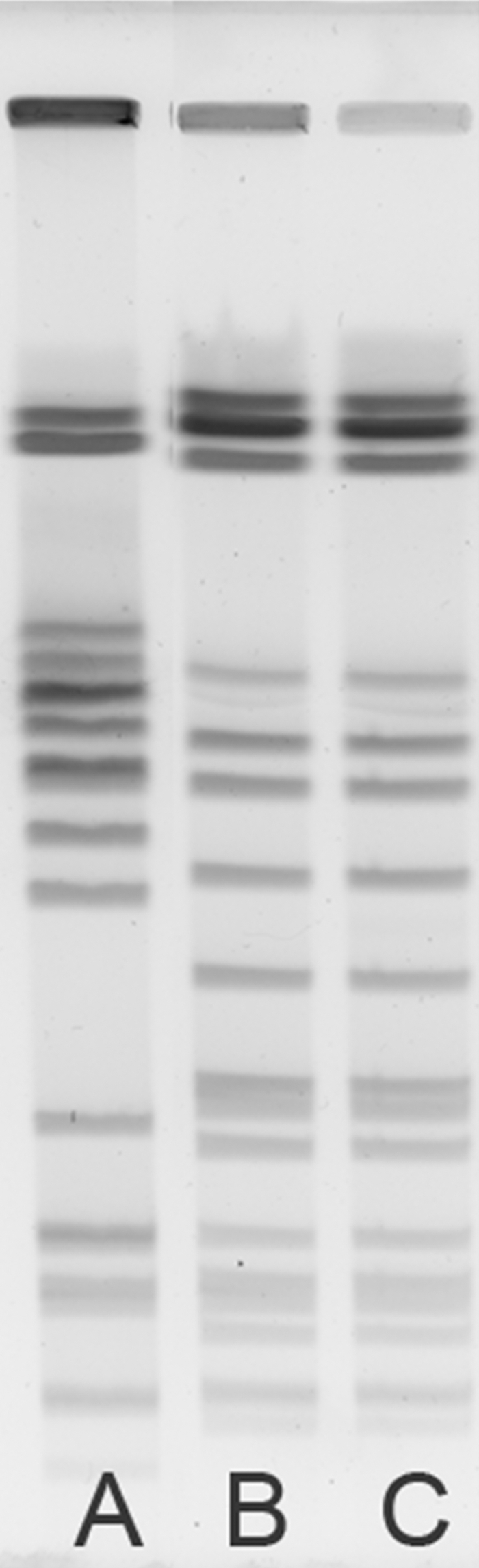
Pulsed-field gel electrophoresis. Genomic DNA was digested with BssHII enzyme (8 U; New England Biolabs, Beverly, MA) overnight at 37°C. Samples were run in a 1% agarose gel with a switch time of 2.2 to 17.6 s and a field strength of 6 V/cm for 23 h on a contour-clamped homogeneous electric field (CHEF) apparatus (Bio-Rad). Lane A, C. jejuni reference strain 81-176; lane B, inoculum strain C. jejuni CG8421; lane C, macrolide-resistant C. jejuni CG8421 isolate from day 21.
Antibiotic sensitivity tests for the day 21 isolate and the inoculum strain were performed in parallel by MIC testing (MICs) using Etests (AB Biodisk, Solna, Sweden). New, high-level macrolide resistance (azithromycin and erythromycin) was observed in the day 21 recrudescence strain (Table 1 ).
TABLE 1.
Etest MICs demonstrate macrolide resistance
| Antibiotic | Etest resulta for isolate and inoculum |
Resistance (μg/ml)b | |||
|---|---|---|---|---|---|
| Day 0 |
Day 21 |
||||
| Test 1 | Test 2 | Test 1 | Test 2 | ||
| Ciprofloxacin | 0.125 | 0.094 | 0.064 | 0.064 | 4 |
| Tetracyclined | NDc | 24 | 64 | 24 | ≥16 |
| Levofloxacin | 0.094 | 0.094 | 0.094 | 0.094 | |
| Trimethoprim-sulfamethoxazoled | >32 | >32 | >32 | >32 | |
| Imipenem | 0.125 | 0.094 | 0.094 | 0.094 | |
| Azithromycin | 0.125 | 0.125 | >256 | >256 | ≥8 |
| Ampicillin | 0.75 | 1.0 | 1.0 | 1.0 | |
| Erythromycin | 1.0 | 1.0 | >256 | >256 | ≥32 |
Etest MIC (μg/ml) for the C. jejuni CG8421 day 21 isolate, compared to the inoculum strain (Day 0) (cGMP lot), after 48 h of incubation.
Resistance levels according to 2007 NARMS report (6).
ND, not determined.
CG8421 is known to be resistant to tetracycline and trimethoprim-sulfamethoxazole.
The most common mechanism of macrolide resistance in C. jejuni is mutation of the 23S ribosomal gene (9, 23, 24). In C. jejuni, macrolide resistance is mostly due to a reduction in macrolide binding affinity to the clarithromycin-binding site located on the 23S rRNA genes. Such resistance is, in most cases, associated with a point mutation at position 2075 that replaces an adenine by a guanine residue (A2075G) (8, 23, 24). This mutation must occur in at least two of the three copies of the rRNA genes to confer the resistance phenotype (8, 23). A less frequent point mutation is also observed at position 2074, in which the adenine is replaced by a cytosine residue (A2074C) (8, 23, 24). For the day 3 and day 21 isolates, the 23S genes of C. jejuni were amplified by PCR and sequenced using previously described primers (23). A single transition mutation, A2075G, was identified in the C. jejuni strain isolated on day 21 compared to the starting challenge strain. The DNA sequence of the amplicons did not indicate any mixed signals at position 2075, which is consistent with mutations at all three sites. Importantly, the day 3 strain, which was shed prior to antibiotic treatment and characterized as CG8421 by PCR, was macrolide sensitive since it did not contain mutations at positions 2074 or 2075, suggesting that macrolide resistance developed in vivo following antibiotic use.
The mutation rate of the challenge strain was determined as follows to ensure that there was not a higher mutation rate in CG8421 resulting in antibiotic-resistant bacteria. A cGMP seed lot vial was plated on Mueller-Hinton (MH) agar plates at 42°C overnight as per the protocol for the inoculation. The cells were scraped off the plate and resuspended in phosphate-buffered saline. Aliquots of the suspensions were incubated on MH agar supplemented with 10 μg/ml erythromycin at 37°C for 6 to 7 days, and the total number of cells plated was enumerated on MH plates without selection and incubated for 48 h at 37°C. The mutation rate was compared to that of a second, well-characterized C. jejuni strain used in human studies, 81-176. The mutation rate for CG8421 (1.76 × 10−10) was similar to that of 81-176 (2.1 × 10−10).
We present the first clinical case of in vivo-acquired macrolide resistance causing recrudescent infection in a healthy subject infected with C. jejuni. Campylobacter is a leading cause of food-borne enteritis worldwide, causing an estimated 400 million cases of gastroenteritis annually. Although antibiotic use is usually reserved for moderate to severe infection, antibiotic options are limited, and increasing antibiotic resistance is a growing concern (2, 3, 9, 16, 20). Through randomized control trials, macrolide antibiotics have been recognized as efficacious against Campylobacter since the 1980s and are currently considered the antibiotic of choice for moderate to severe human infection (10, 15). In the United States, the current rate of macrolide resistance is approximately 2% in human C. jejuni isolates and has fluctuated from 0.6% to 5% since 1998 (6, 9). In addition to being used in humans, macrolides (such as tylosin) are widely used in animal husbandry as antibiotics and growth promoters. The contribution of macrolide use in animals to overall macrolide resistance trends is controversial, but the volume of animal use appears to exceed human use (1).
Macrolide resistance in C. jejuni strains has remained relatively stable, particularly compared to results with FQ antibiotics, in which Campylobacter resistance has rapidly increased in the United States from approximately 15 to 26% over a 10-year period (6). FQ resistance in clinical isolates was not seen on the Asian and African continents before 1991 and has rapidly increased in both regions, reaching over 80% in Thailand and Hong Kong (16). The highest rates of resistance are in Spain, where 99% resistance has been reported (16). The FQs are also heavily used in animal care, and in vivo Campylobacter resistance to FQ has been well described in humans (7).
The microbiological rationale for the differences in prevalence of C. jejuni resistance between FQ and macrolides has two likely explanations. C. jejuni strains gain macrolide resistance at the cost of biological fitness, whereas FQ resistance causes a gain in C. jejuni fitness (11, 12, 17). Second, only one of several mechanisms associated with macrolide resistance (mutations within 23S rRNA) is stably maintained whereas all mutations generating FQ resistance are stably maintained (5, 14, 17).
In this case, the close clinical and bacteriological monitoring of this study permitted the detection of new macrolide resistance that was selected following appropriate antibiotic use. Following infection with a well-characterized, known macrolide-sensitive strain, the subject shed the antibiotic-sensitive strain (day 3) prior to antibiotic use (19, 21). Recrudescence of infection with the newly macrolide-resistant strain occurred after a 15-day period of negative stool cultures. This observation illustrates the risk of antibiotic resistance even with appropriate use of antibiotics and in the most controlled clinical settings. Clinically asymptomatic shedding of macrolide-resistant Campylobacter infection is likely to be more common than recognized, particularly since stool cultures are generally not performed in the absence of gastrointestinal symptoms.
Our findings do not appear to be strain specific; no evidence of increased mutation rates was found in our inoculum strain, and no evidence exists to suggest that this strain (C. jejuni CG8421) has characteristics of increased clinical virulence. Indeed, compared to another strain used in clinical testing (C. jejuni 81-176), strain CG8421 appears clinically less virulent (21, 22). Clinical precautions implemented after recognition of recrudescence of Campylobacter in the clinical setting have permitted continued safe use of strain CG8421 in human studies; these include dual use of antibiotics and extended microbiological monitoring of subjects.
The mechanism of that recrudescence remains unknown, but luminal replication of the strain may have resumed as antibiotic levels dropped, permitting microbiologic relapse due to survival and growth of a now-resistant strain. Although Campylobacter has survived up to a week in macrophages in vitro, no gallbladder, diverticulum, or appendiceal carriage of Campylobacter has thus far been clinically identified (13). It is interesting to postulate that other enteric bacterial pathogens might follow the same pattern and to consider the clinical significance (or lack thereof) of asymptomatic shedding (18). Further research is clearly needed to clarify these issues.
Clinical Campylobacter model work, which uses very closely monitored subjects dosed with C. jejuni, has brought to light the incidence of recrudescent infection in otherwise healthy adults. We have observed recrudescence in ∼5% of antibiotic-treated persons, all following a period in which Campylobacter cannot be isolated from stool by optimized, standard microbiological techniques (4 and unpublished data). Our data suggest that following antibiotics, microbiologic recrudescence can occur 14 to 28 days after the initial infection, but it is not known if recrudescence is dependent upon antibiotic treatment. Although not seen in this case, recrudescence may occur in the setting of suboptimal immune responses to C. jejuni and may or may not be clinically apparent (4). Therefore, there may be several mechanisms by which recrudescence can occur during C. jejuni infections. Given the increasing Campylobacter resistance to FQs and the limited antibiotic treatment options for treating this infection, it will be important to monitor trends in resistance to the macrolides. Although the development of in vivo macrolide resistance is likely underrecognized, our description of the development of in vivo resistance after appropriate use of antibiotics is a reminder of the principles of careful antibiotic stewardship.
Acknowledgments
The opinions or assertions contained herein are private views of the authors and are not to be construed as official or as reflecting views of the Naval Medical Research Center, the Department of the Navy, the Department of Defense, or the U.S. Government. D.R.T., S.B., C.K.P., and P.G. are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. 101 defines U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
This work was supported by the University of Vermont General Clinical Research Center, NIH award MO1 RR000109, and the Military Infectious Diseases Research Program. ACE Biosciences work at NMRC was supported by U.S. Naval Research and Development Command Work Unit 6000.RAD1.DA3.A0308.
The study protocol was approved by the Naval Medical Research Center and University of Vermont Institutional Review Boards in compliance with all applicable federal regulations governing the protection of human subjects.
The members of the Campylobacter study team were as follows: at UVM, Cathy Larsson, Cassandra Ventrone, Ginger Corrada, Patrick Daunais, Caroline Lyon, and Ann Fingar; at NMRC, Theron Gillilan, Jr., Erika Jones, Cheryl P. Ewing, Sandra Dadouk, Stephanie Sincock, and Lanfong Lee; and at ACE Biosciences, Charlotte Buchwaldt, Pascale Puel, Robert Miller, and Dennis Matiesen.
We acknowledge the hard work and dedication of the study subjects, UVM/FAHC General Clinical Research Center nursing staff, and DSMB.
Footnotes
Published ahead of print on 16 June 2010.
REFERENCES
- 1.Anderson, A. D., J. M. Nelson, S. Rossiter, and F. J. Angulo. 2003. Public health consequences of use of antimicrobial agents in food animals in the United States. Microb. Drug Resist. 9:373-379. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 2006. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, United States, 2005. MMWR Morb. Mortal. Wkly. Rep. 55:392-395. [PubMed] [Google Scholar]
- 3.Anonymous. 2007. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, 2006. MMWR Morb. Mortal. Wkly. Rep. 56:336-339. [PubMed] [Google Scholar]
- 4.Baqar, S., D. R. Tribble, M. Carmolli, K. Sadigh, F. Poly, C. Porter, C. J. Larsson, K. K. Pierce, P. Guerry, M. Darsley, and B. Kirkpatrick. 2010. Recrudescent Campylobacter jejuni infection in an immunocompetent adult following experimental infection with a well-characterized organism. Clin. Vaccine Immunol. 17:80-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell, D. B., Y. Wang, and J. Lin. 2008. Development, stability, and molecular mechanisms of macrolide resistance in Campylobacter jejuni. Antimicrob. Agents Chemother. 52:3947-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. 2009. National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS): human isolates final report, 2007. U.S. Department of Health and Human Services, CDC, Atlanta, GA.
- 7.Engberg, J., F. M. Aarestrup, D. E. Taylor, P. Gerner-Smidt, and I. Nachamkin. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 7:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibreel, A., V. N. Kos, M. Keelan, C. A. Trieber, S. Levesque, S. Michaud, and D. E. Taylor. 2005. Macrolide resistance in Campylobacter jejuni and Campylobacter coli: molecular mechanism and stability of the resistance phenotype. Antimicrob. Agents Chemother. 49:2753-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibreel, A., and D. E. Taylor. 2006. Macrolide resistance in Campylobacter jejuni and Campylobacter coli. J. Antimicrob. Chemother. 58:243-255. [DOI] [PubMed] [Google Scholar]
- 10.Guerrant, R. L., T. Van Gilder, T. S. Steiner, N. M. Thielman, L. Slutsker, R. V. Tauxe, T. Hennessy, P. M. Griffin, H. DuPont, R. B. Sack, P. Tarr, M. Neill, I. Nachamkin, L. B. Reller, M. T. Osterholm, M. L. Bennish, and L. K. Pickering. 2001. Practice guidelines for the management of infectious diarrhea. Clin. Infect. Dis. 32:331-351. [DOI] [PubMed] [Google Scholar]
- 11.Han, F., S. Pu, F. Wang, J. Meng, and B. Ge. 2009. Fitness cost of macrolide resistance in Campylobacter jejuni. Int. J. Antimicrob. Agents 34:462-466. [DOI] [PubMed] [Google Scholar]
- 12.Hao, H., M. Dai, Y. Wang, D. Peng, Z. Liu, and Z. Yuan. 2009. 23S rRNA mutation A2074C conferring high-level macrolide resistance and fitness cost in Campylobacter jejuni. Microb. Drug Resist. 15:239-244. [DOI] [PubMed] [Google Scholar]
- 13.Kiehlbauch, J. A., R. A. Albach, L. L. Baum, and K. P. Chang. 1985. Phagocytosis of Campylobacter jejuni and its intracellular survival in mononuclear phagocytes. Infect. Immun. 48:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, J. S., D. K. Carver, and S. Kathariou. 2006. Natural transformation-mediated transfer of erythromycin resistance in Campylobacter coli strains from turkeys and swine. Appl. Environ. Microbiol. 72:1316-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuschner, R. A., A. F. Trofa, R. J. Thomas, C. W. Hoge, C. Pitarangsi, S. Amato, R. P. Olafson, P. Echeverria, J. C. Sadoff, and D. N. Taylor. 1995. Use of azithromycin for the treatment of Campylobacter enteritis in travelers to Thailand, an area where ciprofloxacin resistance is prevalent. Clin. Infect. Dis. 21:536-541. [DOI] [PubMed] [Google Scholar]
- 16.Luangtongkum, T., B. Jeon, J. Han, P. Plummer, C. M. Logue, and Q. Zhang. 2009. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol. 4:189-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo, N., S. Pereira, O. Sahin, J. Lin, S. Huang, L. Michel, and Q. Zhang. 2005. Enhanced in vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antibiotic selection pressure. Proc. Natl. Acad. Sci. U. S. A. 102:541-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parry, C. M., T. T. Hien, G. Dougan, N. J. White, and J. J. Farrar. 2002. Typhoid fever. N. Engl. J. Med. 347:1770-1782. [DOI] [PubMed] [Google Scholar]
- 19.Poly, F., T. D. Read, Y. H. Chen, M. A. Monteiro, O. Serichantalergs, P. Pootong, L. Bodhidatta, C. J. Mason, D. Rockabrand, S. Baqar, C. K. Porter, D. Tribble, M. Darsley, and P. Guerry. 2008. Characterization of two Campylobacter jejuni strains for use in volunteer experimental infection studies. Infect. Immun. 76:5655-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz-Palacios, G. M. 2007. The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clin. Infect. Dis. 44:701-703. [DOI] [PubMed] [Google Scholar]
- 21.Tribble, D. R., S. Baqar, M. P. Carmolli, C. Porter, K. K. Pierce, K. Sadigh, P. Guerry, C. J. Larsson, D. Rockabrand, C. H. Ventone, F. Poly, C. E. Lyon, S. Dakdouk, A. Fingar, T. Gilliland, P. Daunais, E. Jones, S. Rymarchyk, C. Huston, M. Darsley, and B. D. Kirkpatrick. 2009. Campylobacter jejuni strain CG8421: a refined model for the study of campylobacteriosis and evaluation of Campylobacter vaccines in human subjects. Clin. Infect. Dis. 49:1512-1519. [DOI] [PubMed] [Google Scholar]
- 22.Tribble, D. R., S. Baqar, D. A. Scott, M. L. Oplinger, F. Trespalacios, D. Rollins, R. I. Walker, J. D. Clements, S. Walz, P. Gibbs, E. F. Burg III, A. P. Moran, L. Applebee, and A. L. Bourgeois. 2010. Assessment of the duration of protection in Campylobacter jejuni experimental infection in humans. Infect. Immun. 78:1750-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vacher, S., A. Menard, E. Bernard, and F. Megraud. 2003. PCR-restriction fragment length polymorphism analysis for detection of point mutations associated with macrolide resistance in Campylobacter spp. Antimicrob. Agents Chemother. 47:1125-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vacher, S., A. Menard, E. Bernard, A. Santos, and F. Megraud. 2005. Detection of mutations associated with macrolide resistance in thermophilic Campylobacter spp. by real-time PCR. Microb. Drug Resist. 11:40-47. [DOI] [PubMed] [Google Scholar]


