Abstract
Paecilomyces variotii is a commonly occurring species in air and food, but it is also associated with many types of human infections and is among the emerging causative agents of opportunistic mycoses in immunocompromised hosts. Paecilomyces can cause hyalohyphomycosis, and two species, Paecilomyces lilacinus and P. variotii, are the most frequently encountered organisms. In the present study, a set of 34 clinical isolates morphologically identified as P. variotii or P. lilacinus were formally identified by sequencing intergenic transcribed spacer regions 1 and 2 (including 5.8S rDNA) and a part of the β-tubulin gene. Three isolates were identified as P. lilacinus, and five of the presumptive P. variotii isolates did not belong to the genus Paecilomyces but were identified as Talaromyces eburneus (anamorph, Geosmithia argillacea) or Hamigera avellanea (anamorph, Merimbla ingelheimense). Applying the most recent taxonomy, we found that the clinical P. variotii isolates could be identified as P. variotii sensu stricto (14 strains), P. formosus (11 strains), and P. dactylethromorphus (1 strain). These data indicate that P. formosus occurs in clinical samples as commonly as P. variotii. Susceptibility tests showed that the antifungal susceptibility profiles of P. variotii, P. formosus, and P. dactylethromorphus are similar and that all strains tested were susceptible to amphotericin B in vitro. P. lilanicus, T. eburneus, and H. avellanea had different susceptibility profiles; and flucytosine and voriconazole were the least active of the antifungal drugs tested against these species. Our results indicate that correct species identification is important to help guide appropriate antifungal therapy.
Paecilomyces variotii is a commonly occurring species that has previously been isolated from various substrates, including (pasteurized) foods, soil, indoor air, and wood (23, 36, 41, 42, 43). However, it is also associated with many types of human infections and is listed among the emerging causative agents of opportunistic mycoses in immunocompromised hosts. Paecilomyces can cause hyalohyphomycosis (1), and two species, Paecilomyces lilacinus and P. variotii, are the most frequently encountered (20, 52). Both species are morphologically similar but can be differentiated on the basis of conidial color and growth rates (41). However, small-subunit ribosomal gene sequences showed that the two species are unrelated: P. variotii belongs to the order Eurotiales, while P. lilacinus is a member of the order Hypocreales (27). Although they are uncommon, Paecilomyces infections are associated with almost any organ or system of the human body (40). Most cases concern immunocompromised patients and are cutaneous or catheter related. However, dissemination, for example, that involving the central nervous system, has been observed in a number of cases (15, 24). Ocular infections associated with prolonged contact lens use or ocular surgery have also been reported (40), as has peritonitis in patients with continuous ambulatory peritoneal dialysis (24), which apparently responded well to antifungal therapy, with cures occurring in 11 of the 13 cases. However, the majority of these patients were treated for Paecilomyces peritonitis and were not severely immunocompromised. In vitro amphotericin B was active against clinical P. variotii isolates but not against P. lilacinus (8). Among the azoles, only itraconazole and posaconazole showed clinically relevant activity against P. variotii. The geometric mean MICs of voriconazole and ravuconazole were above 4 mg/liter, indicating that these azoles will not be effective in vivo. Interestingly, the echinocandins, especially micafungin and anidulafungin, were highly active against P. variotii, with MIC values being as low as 0.016 mg/liter. Conversely, P. lilacinus was not inhibited by the echinocandins, underscoring the importance of correct species identification (8).
Paecilomyces variotii grows rapidly on standard agar and forms velvety olive brown colonies. Conidiophores of P. variotii are irregularly branched, and the phialides have a broad base ending in a long and slender neck. Samson (41) noted that P. variotii is a morphologically variable species, and the taxonomy of P. variotii and the related Byssochlamys teleomorphs has recently been revised (45). This revision is based on morphology, extrolites, and molecules and shows that P. variotii sensu lato comprises five species, namely, Byssochlamys spectabilis (the sexual state of P. variotii), P. brunneolus, P. formosus, P. divaricatus, and P. dactylethromorphus. The last species was incorrectly named P. saturatus because P. dactylethromorphus was validly described in 1957 and has priority. The aim of the present study was to determine the prevalence of these species in clinical samples and settings. A total of 34 isolates originating from various clinical specimens and settings were identified by sequencing the intergenic transcribed spacer (ITS) regions, including the 5.8S rDNA, and a part of the β-tubulin gene. Furthermore, the antifungal susceptibility profiles of these species and ex-type strains are reported.
MATERIALS AND METHODS
Strains.
This study includes 34 strains isolated from clinical specimens and hospital environments. These strains were identified on the basis of macro- and microscopic characters and were maintained in various culture collections as P. variotii or P. lilacinus. In additional, ex-type strains and freshly isolated strains were also included in this study.
Morphological examination.
Isolates (Table 1) were grown for 3 days on malt extract agar (MEA) and were incubated in the dark at 25, 30, and 37°C. Furthermore, three-point inoculations were made on MEA, Czapek yeast agar (CYA), and creatine agar (CREA); and the isolates were incubated for 7 days at 25°C (the medium compositions are described by Samson et al. [43]). After incubation, the colony diameters were measured and the reactions on creatine agar recorded.
TABLE 1.
Isolates used in this study
| Strain no.a | Species | Source |
|---|---|---|
| CBS 100.11NT** | Byssochlamys nivea | Unknown |
| CBS 146.48NT** | Byssochlamys fulva | Bottled fruit, UK |
| CBS 373.70T** | Byssochlamys lagunculariae | Wood of Laguncularia racemosa (mangue), Brazil |
| CBS 605.74HT** | Byssochlamys verrucosa | Nesting material of Leipoa ocellata, Australia |
| CBS 374.70isoT** | Byssochlamys zollerniae | Wood of Zollernia ilicifolia and Protium heptaphyllum, Brazil |
| UMCN V63-56, DTO 63F6 | Hamigera avellanea | Human, ear swab; Nijmegen, Netherlands (2007) |
| CBS 295.48isoT* | Hamigera avellanea | Soil; San Antonio, TX |
| CBS 370.70T** | P. brunneolus | Nonfat dry milk, Canada |
| CBS 110430* | P. divaricatum | Soil, Thailand |
| CBS 284.48T | P. divaricatum | Mucilage bottle with library paste, USA |
| UMCN V54-40, DTO 63F3 | P. formosus | Human, sputum; Nijmegen, Netherlands (2006) |
| CBS 296.93 | P. formosus | Human, bone marrow of patient; Taskent, Uzbekistan |
| CBS 297.93 | P. formosus | Human, blood of patient; Taskent, Uzbekistan |
| CBS 298.93 | P. formosus | Human, breast milk of patient; Taskent, Uzbekistan |
| CBS 990.73BT | P. formosus | Unknown |
| DTO 45H8 | P. formosus | Pseudo-outbreak in hospital, blood culture, United Kingdom |
| UMCN 1274, DTO 63E3 | P. formosus (lecythidis type) | Human, bronchoalveolar lavage fluid; Nijmegen, Netherlands |
| UMCN V49-58, DTO 63F1 | P. formosus (lecythidis type) | Human, sputum; Zwolle, Netherlands (2006) |
| UMCN V56-25, DTO 63F4 | P. formosus (lecythidis type) | Human, sputum; Nijmegen, Netherlands (2006) |
| CBS 372.70 | P. formosus (lecythidis type) | Type of P. lecythidis, Lecythis unsitata (Lecythidaceae), wood, Brazil |
| DTO 45I1 | P. formosus (lecythidis type) | Pseudo-outbreak in hospital, blood culture; UK |
| NCPF 2825, DTO 49D5 | P. formosus (lecythidis type) | Brain abscess, United Kingdom (1991) |
| NCPF 2837, DTO 49D6 | P. formosus (lecythidis type) | Brain abscess, same patient as NCPF 2825, United Kingdom (1991) |
| CBS 113247* | P. formosus (maximus type) | Soil, Thailand |
| CBS 371.70 | P. formosus (maximus type) | Type of P. maximus, Annona squamosa, Brazil |
| UMCN 1156,* DTO 63E1 | P. lilacinus | Unknown source |
| UMCN 2419,* DTO 63E5 | P. lilacinus | Human, skin swab; Nijmegen, Netherlands (1994) |
| CBS 284.36T* | P. lilacinus | Soil; Ithaca, NY |
| V52-21,* DTO 63F2 | P. lilacinus | Human, sputum; Nijmegen, Netherlands (2006) |
| UMCN V66-47, DTO 63F7 | P. dactylethromorphus | Human, cornea scrapings from keratomycosis; Nijmegen, Netherlands (2008) |
| CBS 323.34T | P. dactylethromorphus | Unknown source |
| CBS 492.84* | P. dactylethromorphus | Lepidium sativum, Denmark |
| CBS 110036, DTO 34C8 | P. variotii | Cerebrospinal fluid of 60-year-old female with diabetes and cancer; Istanbul, Turkey |
| UMCN 1157*, DTO 63E2 | P. variotii | Unknown source |
| UMCN 2266, DTO 63E4 | P. variotii | Human, feces; Nijmegen, Netherlands (1994) |
| UMCN 3796, DTO 63E6 | P. variotii | Human, mouthwash; Nijmegen, Netherlands (1995) |
| UMCN 45H9*, DTO 45H9 | P. variotii | Liver biopsy; London, UK |
| UMCN 577, DTO 63D6 | P. variotii | Human, mouthwash; Nijmegen, Netherlands |
| UMCN 654, DTO 63D7 | P. variotii | Human, feces, Nijmegen; Netherlands |
| UMCN 730, DTO 63D8 | P. variotii | Hospital environment, elevator shaft; Nijmegen, Netherlands |
| UMCN 731, DTO 63D9 | P. variotii | Human, mouthwash; Nijmegen, Netherlands |
| UMCN 7845, DTO 63E7 | P. variotii | Human, cerebrospinal fluid; Nijmegen, Netherlands (1998) |
| UMCN 8490, DTO 63E9 | P. variotii | Human, mouthwash; Nijmegen, Netherlands (1999) |
| UMCN V57-21, DTO 63F5 | P. variotii | Human, abscess; Zwolle, Netherlands (2007) |
| CBS 101075 | P. variotii | Type of B. spectabilis, heat-processed fruit beverage, Japan |
| CBS 102.74T | P. variotii | Unknown source |
| CBS 124.97 | P. variotii | Human, vitreous tumor left eye, neutropenic leukemic patient with acute endophthalmitis, Hong Kong |
| CBS 339.51 | P. variotii | Human, sputum, Netherlands |
| DTO 45I3 | Talaromyces eburneus | Pseudo-outbreak in hospital, blood culture, UK |
| NCPF 2801, DTO 49D4 | Talaromyces eburneus | Sputum, cystic fibrosis patient, UK (1991) |
| NCPF 7594, DTO 49D7 | Talaromyces eburneus | Blood culture, patient with peritonitis, UK (2002) |
| NCPF 7596, DTO 49D9 | Talaromyces eburneus | Peritoneal dialysis fluid (same patient source as NCPF 7594) |
Strains indicated with one asterisk are not included in the phylogenetic analysis; these strains are used only in the susceptibility tests. The strains labeled with two asterisks are included in the phylogenetic study but not in the susceptibility tests. NT, neotype; HT, holotype; isoT, isotype. CBS, culture collection of the CBS-Fungal Biodiversity Centre, Utrecht, Netherlands; UMCN, culture collection of Radboud University Nijmegen Medical Center; DTO, internal culture collection of CBS-Fungal Biodiversity Centre.
Phylogeny and molecular identification.
Strains were grown on MEA (Oxoid) for 4 to 7 days at 25°C. Genomic DNA was isolated using an Ultraclean microbial DNA isolation kit (MoBio), according to the manufacturer's instructions. Fragments containing ITS region 1 (ITS1) and ITS2, including 5.8S rDNA) and a part of the β-tubulin gene were amplified and subsequently sequenced and analyzed according to the procedure described previously (22). For parsimony analyses, PAUP (version 4.0) software was used (48) and Byssochlamys verrucosa CBS 605.74 was used as the outgroup.
Antifungal susceptibility tests.
The susceptibilities of the majority of the strains listed in Table 1 were tested; exceptions were the (ex type) strains of uncommon species in clinical environments, such as Byssochlamys nivea, B. fulva, B. verrucosa, B. lagunculariae, B. zollerniae, and P. brunneolus. Paecilomyces divaricatus is also uncommon, but it is included to provide a representative overview of the susceptibility of the members of the Paecilomyces variotii complex. Isolates were revived by subculturing twice on Sabouraud dextrose agar tubes for 5 to 7 days at 35°C. Conidial suspensions were adjusted spectrophotometrically and were further diluted in RPMI 1640 medium (with l-glutamine and without bicarbonate; Gibco BRL, Life Technologies, Woerden, Netherlands). Microtiter plates were inoculated with an initial concentration of 1 × 104 to 5 × 104 conidia/ml, as recommended by the CLSI (formerly the NCCLS) for mold testing (30).
The antifungal activities of amphotericin B (Bristol-Myers Squibb, Woerden, Netherlands), flucytosine (5FC; Valeant, Zoetermeer, Netherlands), itraconazole (Janssen Pharmaceutica BV, Tilburg, Netherlands), voriconazole (Pfizer, Capelle aan de IJssel, Netherlands), posaconazole (Schering-Plough, Maarssen, Netherlands), terbinafine (Novartis Pharma, Arnhem, Netherlands), and caspofungin (Merck, Sharpe, and Dohme, Haarlem, Netherlands) were determined in vitro using a broth microdilution method, according to CLSI guidelines (M38-A) (30). The concentration range for amphotericin B, terbinafine, itraconazole, voriconazole, and posaconazole was 0.016 to 16 mg/liter; a range of 0.062 to 64 mg/liter was used for 5FC and caspofungin. MICs were determined after 24 and 48 h of incubation. For amphotericin B and the azoles itraconazole, voriconazole, and posaconazole, the MIC was defined as the lowest concentration that showed no visible growth. For 5FC and terbinafine, the MIC was defined as the lowest concentration at which 50% inhibition of growth compared with that of the control was measured (32). For caspofungin, the minimum effective concentration was determined. All susceptibility tests were performed in duplicate.
Nucleotide sequence accession numbers.
The sequences newly generated in the present study are deposited in GenBank under accession numbers GU968650 to GU968703.
RESULTS
Identification.
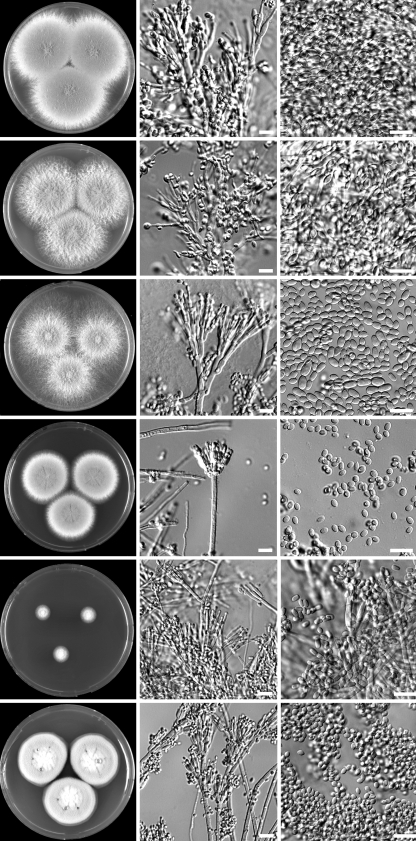
Identification of the strains was performed by combining phenotypic characteristics and the sequences of the ITS regions and part of the β-tubulin gene. The investigated clinical strains were maintained in various collections as P. variotii or P. lilacinus. Critical examination of the cultures showed that one Hamigera avellanea isolate and four Talaromyces eburneus isolates were present among the isolates which had previously been identified as P. variotii. The ITS sequences of three T. eburneus isolates (NCPF 7594, NCPF 7596, and DTO 45I3) were identical and had 99.8% homology with the type strain of T. eburneus (CBS 100538). Isolate DTO 49D4 was more divergent and shared 96.4% homology with the type strain of T. eburneus and 98.8% similarity with the type strain of Geosmithia argillaea (NRRL 5177). Although this strain is more closely related to G. argillaea, we identified this strain as T. eburneus, since both species are claimed to be conspecific (53). The T. eburneus strains were isolated from patient material in three separate cases: from the sputum of a patient with cystic fibrosis (NCPF 2801), from a blood culture (DTO 45I3), and from the peritoneal dialysis fluid and blood of a patient (NCPF 7594 and NCPF 7596). P. variotii superficially resembles T. eburneus in its olive brown conidial colors and thermophilic nature. However, it differs in growing very slowly at 25°C (attaining a diameter of between 10 and 25 mm) and having a Geosmithia anamorph. Geosmithia anamorphs are characterized by cylindrical phialides, ornamented conidiophores and phialides, and cylindrical conidia (Fig. 1). A further presumptive isolate of P. variotii was identified as H. avellanea. The ITS sequence of this strain had a similarity of 98.0% with the type strain of this species (CBS 295.48). This species macroscopically resembles P. variotii in many respects and also forms powdery olive brown colonies, and it has a high growth rate at 25°C and 37°C. However, H. avellanea can be distinguished from P. variotii by the presence of a Merimbla-type anamorph (Fig. 1). Three strains were identified as P. lilacinus, and the ITS sequences of these strains have 100% homology with the type strain of P. lilacinus (CBS 284.36).
FIG. 1.
Macro- and micromorphological features of various species related to P. variotii. Columns, from left to right, MEA, conidiophores, and conidia, respectively; rows, from top to bottom, P. variotii, P. formosus, P. dactylethromorphus, Hamigera avellanea, Talaromyces eburneus, and Paecilomyces lilacinus, respectively. Bars, 10 μm.
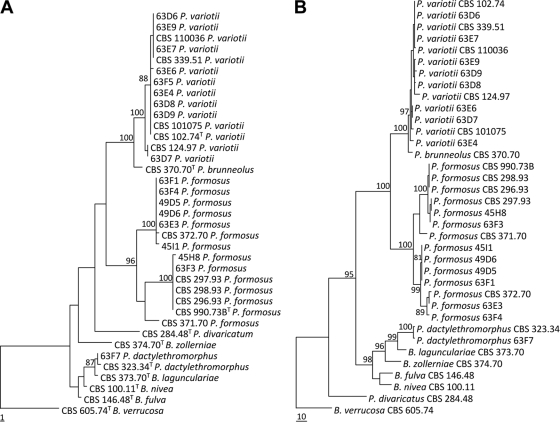
In the present study, we have adopted the taxonomy of Paecilomyces variotii and related species proposed by Samson et al. (45), which uses morphological characteristics, in combination with extrolite data and sequences. Combined molecular and morphological examination of the remaining P. variotii sensu lato isolates showed that three different species were present, namely, P. variotii, P. formosus, and P. dactylethromorphus. P. variotii was the species encountered the most frequently (12), 11 isolates were identified as P. formosus, and 1 isolate was identified as P. dactylethromorphus. These three species can be differentiated on the basis of morphological criteria. P. variotii morphologically resembles P. formosus, but the latter produces acid components on creatine agar and grows faster at 30°C than at 37°C. P. dactylethromorphus can be differentiated from the other two species by its cylindrical conidia and regular branched conidiophores (Fig. 1). Figure 2 shows the results of the phylogenetic analysis of the ITS and partial tubulin sequences. Both sequenced regions gave similar identification results, and all the species of the P. variotii complex can be differentiated by either their ITS or partial tubulin sequences. A high degree of variation was present in the ITS and tubulin sequences of the P. formosus isolates. Two distinct groups, with high bootstrap support, were observed. A proportion of the isolates (5) formed a group together with the type strain of P. formosus, and the other group clustered together with the type strain of P. lecythidis. These two groups could represent two cryptic species but were not treated as such since they are morphologically similar and produce the same pattern of extrolites (45). Three strains (CBS 296.93, CBS 297.93, and CBS 298.93) received as P. variotii var. zaaminella and claimed to be the causal agent of zaaminellosis (11) were identified as P. formosus.
FIG. 2.
One of the most parsimonious trees from each of the two analyzed loci sequenced. (A) ITS1, ITS2, and 5.8S rDNA (consistency index = 0.796; retention index = 0.938, rescaled consistency index = 0.747); (B) partial beta-tubulin data (consistency index = 0.745; retention index = 0.909; rescaled consistency index = 0.677).
Susceptibility testing.
Susceptibility data, i.e., the geometric mean (GM) of the MIC and the MIC range, are shown in Table 2. For those isolates that were not inhibited by the highest drug concentration, the next higher concentration was used to calculate the GM MIC; if no growth was observed in the well with the lowest drug concentration, the next lower concentration was used. The activities of most of the antifungal agents differed between the seven different species. Posaconazole and terbinafine showed good in vitro activity against all the species tested. Posaconazole showed the lowest MICs, as all isolates except T. eburneus were inhibited by a concentration of 0.25 mg/liter; T. eburneus had slightly higher MIC values (MIC range, 0.25 to 1 mg/liter). All isolates (except one P. formosus isolate) were inhibited by terbinafine at a concentration of 1 mg/liter or lower. Itraconazole was the second most active azole, having in vitro activity against all Paecilomyces species except P. lilanicus and T. eburneus. The P. lilanicus isolates were not inhibited by itraconazole, which was also true for three of four of T. eburneus isolates. H. avellanea was moderately susceptible to itraconazole, but only two isolates were available for testing. Voriconazole was the least active azole and had in vitro activity only against P. lilanicus and H. avellanea. Amphotericin B was active in vitro against all species tested with the exception of P. lilanicus and T. eburneus. Flucytosine was also active against most of the species tested; the exceptions were P. lilanicus and H. avellanea. For amphotericin B, itraconazole, posaconazole, voriconazole, and flucytosine, little intraspecific variation in antifungal susceptibility was noted. This was not the case for caspofungin, where minimal effective concentration values varied between 0.063 and 4 mg/liter for different isolates of the same species.
TABLE 2.
Susceptibility results for Paecilomyces species, Hamigera avellanea, and Talaromcyes eburneus strains, by species and antifungal agent
| Species | No. of isolates | Geometric mean (range) MIC (mg/liter)a |
||||||
|---|---|---|---|---|---|---|---|---|
| AMB | 5FC | ITZ | VCZ | POS | TB | CAS | ||
| P. variotii | 16 | 0.11 (0.03-0.5) | 0.03 | 0.04 (0.008-4) | 11.3 (1-32) | 0.02 (0.008-0.125) | 0.08 (0.031-0.5) | 0.52 (0.063-4) |
| P. lilanicus | 4 | 32 | 128 | 5.7 (0.063-32) | 0.15 (0.063-0.25) | 0.2 (0.008-0.25) | 0.04 (0.031-0.063) | 0.59 (0.5-1) |
| P. formosus | 14 | 0.13 (0.063-0.25) | 0.04 (0.031-0.25) | 0.13 (0.063-1) | 26.25 (16-32) | 0.08 (0.031-0.25) | 0.12 (0.031-2) | 1.16 (0.063-4) |
| P. dactylethromorphus | 3 | 0.16 (0.125-0.25) | 0.05 (0.031-0.125) | 0.08 (0.031-0.125) | 32 | 0.03 | 0.31 (0.125-1) | 0.25 (0.125-0.5) |
| P. divaricatus | 2 | 0.25 (0.125-0.5) | 0.008 | 0.5 (0.25-1) | 32 | 0.18 (0.125-0.25) | 0.06 (0.031-0.125) | 0.71 (0.25-2) |
| H. avellanea | 2 | 0.5 | 2 (1-4) | 0.031 | 0.09 (0.063-0.125) | 0.031 | 0.04 (0.031-0.063) | 0.063 |
| T. eburneus | 4 | 3.3 (1-8) | 0.032 | 12.5 (1-32) | 28 (16-32) | 0.69 (0.25-1) | 0.032 | 0.31 (0.25-0.5) |
For caspofungin, the minimum effective concentration was determined. AMB, amphotericin B; 5FC, flucytosine; ITZ, itraconazole; VCZ, voriconazole; POS, posaconazole; TB, terbinafine; CAS, caspofungin.
DISCUSSION
Of 32 isolates which were identified as P. variotii by their phenotypic characteristics, 5 were shown here not to belong to the genus Paecilomyces and instead proved to be Talaromyces eburneus (anamorph, Geosmithia argillacea) or Hamigera avellanea (anamorph, Merimbla ingelheimense). These two species superficially resemble P. variotii, but the micromorphology is distinct from that of Paecilomyces (35). The occurrence of these two species in clinical environments might be more common than has been noted to date. Screening of the StrainInfo bioportal (www.straininfo.net) identified two other Hamigera isolates that have been reported from clinical environments. One isolate (CBS 128.90) originates from continuous ambulatory peritoneal dialysis liquid of a dialysis patient, and the other (UAMH 2531), maintained under the anamorphic name M. ingelheimense, was isolated from skin between the toes of a man. Multiple isolates of G. argillacea originating from bronchial washings (UAMH 7717, UAMH 8639, UAMH 9714, UAMH 9854, UAMH 10232), a brain abscess (UAMH 9833), and a disseminated infection in a German shepherd dog (UAMH 10932, UAMH 10933 [21]) are also present in the UAMH culture collection. In addition, this species has recently been proposed to be a potential new pathogen that colonizes patients with cystic fibrosis lung disease (6, 19). The remaining isolates belonged to three different Paecilomyces species. P. variotii and P. formosus predominated, although one isolate of P. dactylethromorphus was also detected. The presence of these species in the clinical samples might be explained by the fact that these species occur more commonly in food and indoor environments than the other members of the P. variotii complex (J. Houbraken, unpublished results).
Species identification of fungi in the past has primarily been based on morphological features. However, identification solely on the basis of morphology appears to be difficult, and trained staff is required for correct identification. Identification of fungi from clinical samples might even encounter the problem that isolates grow atypically on inappropriate agars or become atypical if antimycotics are used (31). Therefore, molecular-based methods, such as sequencing, appear to be more reliable and are a robust alternative to discriminate fungal species (4, 9, 17, 39). Sequencing data are objective and fast, and reliable identification of uncommon species can be obtained. The ITS regions are recommended for use for identification of species in a clinical setting, since they are easy to amplify and large data sets are present in various databases, such as GenBank and European Molecular Biology Laboratory Nucleotide Sequence Database. These databases will expand dramatically in the near future since the ITS region has become the prime bar coding region (5, 46; U. Eberhardt, personal communication). The disadvantage of the ITS region is that it does not have sufficient discriminatory power in various genera, for example, the genera Aspergillus, Penicillium, and Fusarium (5, 18, 33, 47). In this study, the ITS regions and part of the β-tubulin gene were used, and both loci were shown to exhibit sufficient interspecific variation for identification purposes.
Paecilomyces variotii is a commonly occurring species and has previously been isolated from various substrates. Immunosuppression is the critical risk factor for infection; and cases of pneumonia (7), sinusitis (13, 34, 50), endophthalmitis (25, 49), otitis media (12), wound infection in a transplant recipient (26), cutaneous hyalohyphomycoses (3, 29), onychomycosis (2), osteomyelitis in a patient with granulomatous disorder (10), and dialysis-related peritonitis (38) have all been reported to be caused by this fungus. This species can be considered extremotolerant and is able to grow at high temperatures, on decaying wood, and on creosote treated wooden utility poles (E. de Meyere et al., unpublished data). The ability to grow on creosote-treated wooden poles suggests that this species is able to break down aromatics and is able to grow under stressful conditions (very hot conditions, dry conditions, conditions very low in micronutrients). Additionally, P. formosus has also been isolated from toluene gas biofilters. However, these isolates were misidentified as P. sinensis and P. variotii, and the correct name for these isolates is P. formosus (14, 16, 37). The extremotolerant nature is suggested to contribute to the pathogenic potential of fungi. Prenafeta-Boldú et al. (37) speculated that there might be a link between neurotropism and assimilation of aromatic substrates, and this might be one of the factors that enable fungi to grow in the human brain, with its unique chemical properties. This suggested link is also found in our study, as we have also encountered four strains of three independent cases originating from brain or cerebrospinal fluid.
Correlations of species identities with susceptibility profiles.
Major differences in in vitro antifungal susceptibility profiles were found between the investigated species. In general, voriconazole is not active against members of the Paecilomyces variotii complex but is active against P. lilanicus and H. avellanea. Treatment of infections due to P. lilanicus may be complicated, as amphotericin B also showed no activity in vitro. Posaconazole may be the only appropriate alternative agent, although the lack of an intravenous formulation and limited penetration into the cerebrospinal fluid might limit its use. Amphotericin B showed good activity against all other species tested, as was also the case for flucytosine. The combination of amphotericin B and flucytosine may therefore be an option in complicated infections due to Paecilomyces species other than P. lilanicus. Flucytosine was recently shown to be active in vitro and in vivo against A. fumigatus, with the MIC measured at pH 5.0 being found to correlate better with the outcome in a murine model of disseminated aspergillosis than that determined at pH 7.0 (51). It would be of interest to determine the activity of flucytosine at pH 5.0 against other molds, including Paecilomyces species. As published previously, terbinafine also shows potent activity against all species tested (8). However, the clinical use of this drug for the treatment of invasive fungal infections remains limited due to its pharmacological properties. The role of the echinocandins remains unclear, as the in vitro activity of caspofungin was variable, with the MIC ranges within species being broad. This indicates that the activity of the drug is not easily predictable, thereby precluding a prominent role in the first-line therapy of Paecilomyces infections. In general, the antifungal susceptibility profiles of P. variotii, P. formosus, P. dactylethromorphus, and P. divaricatus appeared to be similar, although a limited number of species have been tested. The profiles of P. lilanicus, T. eburneus, and H. avellanea are different. This is in agreement with the phylogeny, since T. eburneus and H. avellanea are not related to Paecilomyces or Byssochlamys (45, 53) and P. lilacinus will shortly be accommodated in a new genus because it is only distantly related to P. variotii and the other species hitherto placed in the genus Paecilomyces (27, 28). In summary, it is clear that correct species identification of Paecilomyces isolates is important to help guide appropriate antifungal therapy. The correlation between the in vitro activity and the in vivo efficacy of these agents against Paecilomyces species remains to be investigated further.
Acknowledgments
Andrew Borman thanks Elizabeth Johnson for her interest in the study and permitting him to collaborate in this study.
Footnotes
Published ahead of print on 2 June 2010.
REFERENCES
- 1.Ajello, L. 1986. Hyalohyphomycosis and phaeohyphomycosis: two global disease entities of public health importance. Eur. J. Epidemiol. 2:243-251. [DOI] [PubMed] [Google Scholar]
- 2.Arenas, R., M. Arce, H. Munoz, and J. Ruiz-Esmenjaud. 1998. Onychomycosis due to Paecilomyces variotii. Case report and review. J. Mycol. Med. 8:32-33. [Google Scholar]
- 3.Athar, M. A., A. S. Sekhon, J. V. Mcgrath, and R. M. Malone. 1996. Hyalohyphomycosis caused by Paecilomyces variotii in an obstetrical patient. Eur. J. Epidemiol. 12:33-35. [DOI] [PubMed] [Google Scholar]
- 4.Balajee, S. A., J. Gribskov, M. Brandt, J. Ito, A. Fothergill, and K. A. Marr. 2005. Mistaken identity: Neosartorya pseudofischeri and its anamorph masquerading as Aspergillus fumigatus. J. Clin. Microbiol. 43:5996-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balajee, S. A., A. M. Borman, M. E. Brandt, J. Cano, M. Cuenca-Estrella, E. Dannaoui, J. Guarro, G. Haase, C. C. Kibbler, W. Meyer, K. O'Donnell, C. A. Petti, J. L. Rodriguez-Tudela, D. Sutton, A. Velegraki, and B. L. Wickes. 2009. Sequence-based identification of Aspergillus, Fusarium and the Mucorales in the clinical mycology laboratory: where are we and where should we go from here? J. Clin. Microbiol. 47:877-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton, R. C., A. M. Borman, E. M. Johnson, J. Houbraken, R. P. Hobson, M. Denton, S. P. Conway, K. G. Brownlee, D. Peckham, and T. W. R. Lee. 26 April 2010, posting date. Isolation of the fungus Geosmithia argillacea in the sputum of people with cystic fibrosis. J. Clin. Microbiol. doi: 10.1128/JCM. [DOI] [PMC free article] [PubMed]
- 7.Byrd, R. P., Jr., T. M. Roy, C. L. Fields, and J. A. Lynch. 1992. Paecilomyces variotii pneumonia in a patient with diabetes mellitus. J. Diabetes Complicat. 6:150-153. [DOI] [PubMed] [Google Scholar]
- 8.Castelli, M. V., A. Alastruey-Izquierdo, I. Cuesta, A. Monzon, E. Mellado, J. L. Rodriguez-Tudela, and M. Cuenca-Estrella. 2008. Susceptibility testing and molecular classification of Paecilomyces spp. Antimicrob. Agents Chemother. 52:2926-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2008. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing: guideline. CLSI document MM18-A. Clinical and Laboratory Standards Institute, Wayne, PA.
- 10.Cohen-Abbo, A., and K. M. Edwards. 1995. Multifocal osteomyelitis caused by Paecilomyces variotii in a patient with chronic granulomatous disease. Infection 23:55-57. [DOI] [PubMed] [Google Scholar]
- 11.Dekhkan-Khodzhayeva, N. 1992. Newly identified fungal disease zaaminellosis. Meditsinskaya Gazeta 3:8-9. [Google Scholar]
- 12.Dhindsa, M. K., J. Naidu, S. M. Singh, and S. K. Jain. 1995. Chronic suppurative otitis media caused by Paecilomyces variotii. J. Med. Vet. Mycol. 33:59-61. [PubMed] [Google Scholar]
- 13.Eloy, P., B. Bertrand, P. Rombeaux, M. Delos, and J. P. Trigaux. 1997. Mycotic sinusitis. Acta Otorhinolaryngol. Belg. 51:339-352. [PubMed] [Google Scholar]
- 14.Estevez, E., M. C. Veiga, and C. Kennes. 2005. Biodegradation of toluene by the new fungal isolates Paecilomyces variotii and Exophiala oligosperma. J. Ind. Microbiol. Biotechnol. 32:33-37. [DOI] [PubMed] [Google Scholar]
- 15.Fagerburg, R., B. Suh, H. R. Buckley, B. Lorber, and J. Karian. 1981. Cerebrospinal fluid shunt colonization and obstruction by Paecilomyces variotii. J. Neurosurg. 54:257-260. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Pena, I., S. Hernandez, R. Auria, and S. Revah. 2005. Correlation of biological activity and reactor performance in biofiltration of toluene with the fungus Paecilomyces variotii CBS 115145. Appl. Environ. Microbiol. 71:4280-4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geiser, D. M., J. I. Pitt, and J. W. Taylor. 1998. Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus. Proc. Natl. Acad. Sci. U. S. A. 95:388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geiser, D. M., M. A. Klich, J. C. Frisvad, S. W. Peterson, J. Varga, and R. A. Samson. 2007. The current status of species recognition and identification in Aspergillus. Stud. Mycol. 59:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraud, S., M. Pihet, B. Razafimandimby, J. Carrère, N. Degand, L. Mely, L. Favennec, E. Dannaoui, J.-P. Bouchara, and A. Calenda. 12 May 2010, posting date. Geosmithia argillacea: an emerging pathogen in cystic fibrosis patients? J. Clin. Microbiol. doi: 10.1128/JCM. [DOI] [PMC free article] [PubMed]
- 20.Groll, A. H., and T. J. Walsh. 2001. Uncommon opportunistic fungi: new nosocomial threats. Clin. Microbiol. Infect. 7(Suppl. 2):8-24. [DOI] [PubMed] [Google Scholar]
- 21.Grant, D. C., D. A. Sutton, A. Sandberg, R. D. Tyler, E. H. Thompson, A. M. Romanelli, and B. L. Wickes. 2009. Disseminated Geosmithia argillacea infection in a German shepherd dog. Med. Mycol. 47:221-226. [DOI] [PubMed] [Google Scholar]
- 22.Houbraken, J., M. Due, J. Varga, M. Meijer, J. C. Frisvad, and R. A. Samson. 2007. Polyphasic taxonomy of Aspergillus section Usti. Stud. Mycol. 59:107-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houbraken, J., J. Varga, E. Rico-Munoz, S. Johnson, and R. Samson. 2008. Sexual reproduction as the cause of heat resistance in the food spoilage fungus Byssochlamys spectabilis (anamorph Paecilomyces variotii). Appl. Environ. Microbiol. 74:1613-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantarcioglu, A. S., G. Hatemi, A. Yucel, G. S. De Hoog, and N. M. Mandel. 2003. Paecilomyces variotii central nervous system infection in a patient with cancer. Mycoses 46:45-50. [DOI] [PubMed] [Google Scholar]
- 25.Lam, D. S., A. P. Koehler, D. S. Fan, W. Cheuk, A. T. Leung, and J. S. Ng. 1999. Endogenous fungal endophthalmitis caused by Paecilomyces variotii. Eye 13:113-116. [DOI] [PubMed] [Google Scholar]
- 26.Lee, J., W. W. Yew, C. S. Chiu, P. C. Wong, C. F. Wong, and E. P. J. Wang. 2002. Delayed sternotomy wound infection due to Paecilomyces variotii in a lung transplant recipient. Heart Lung Transplant. 21:1131-1134. [DOI] [PubMed] [Google Scholar]
- 27.Luangsa-ard, J. J., N. L. Hywel-Jones, and R. A. Samson. 2004. The polyphyletic nature of Paecilomyces sensu lato based on 18S-generated rDNA phylogeny. Mycologia 96:773-780. [DOI] [PubMed] [Google Scholar]
- 28.Luangsa-Ard, J., N. L. Hywel-Jones, L. Manoch, and R. A. Samson. 2005. On the relationships of Paecilomyces sect. Isarioidea species. Mycol. Res. 109:581-589. [DOI] [PubMed]
- 29.Naidu, J., and S. M. Singh. 1992. Hyalohyphomycosis caused by Paecilomyces variotii: a case report, animal pathogenicity and ‘in vitro’ sensitivity. Antonie Van Leeuwenhoek 62:225-230. [DOI] [PubMed] [Google Scholar]
- 30.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 31.Nosanchuk, J. D., W. Cleare, S. P. Franzot, and A. Casadevall. 1999. Amphotericin B and fluconazole affect cellular charge, macrophage phagocytosis, and cellular morphology of Cryptococcus neoformans at subinhibitory concentrations. Antimicrob. Agents Chemother. 43:233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odds, F. C., F. van Gerven, A. Espinel-Ingroff, M. S. Bartlett, M. A. Ghannoum, M. V. Lancaster, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, and T. J. Walsh. 1998. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob. Agents Chemother. 42:282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Donnell, K., D. A. Sutton, A. Fothergill, D. McCarthy, M. G. Rinaldi, M. E. Brandt, N. Zhang, and D. M. Geiser. 2008. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J. Clin. Microbiol. 46:2477-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otcenasek, M., Z. Jirousek, Z. Nozicka, and K. Mencl. 1984. Paecilomycosis of the maxillary sinus. Mykosen 27:242-251. [DOI] [PubMed] [Google Scholar]
- 35.Pitt, J. I., and A. D. Hocking. 1979. Merimbla gen. nov. for the anamorphic state of Talaromyces avellaneus. Can. J. Bot. 57:2394-2398. [Google Scholar]
- 36.Pitt, J. I., and A. D. Hocking. 2009. Fungi and food spoilage, 3rd ed. Springer, Berlin, Germany.
- 37.Prenafeta-Boldú, F. X., R. Summerbell, and G. Sybren de Hoog. 2006. Fungi growing on aromatic hydrocarbons: biotechnology's unexpected encounter with biohazard? FEMS Microbiol. Rev. 30:109-130. [DOI] [PubMed] [Google Scholar]
- 38.Rinaldi, S., E. Fiscarelli, and G. Rizzoni. 2000. Paecilomyces variotii peritonitis in an infant on automated peritoneal dialysis. Pediatr. Nephrol. 14:365-366. [DOI] [PubMed] [Google Scholar]
- 39.Rinyu, E., J. Varga, and L. Ferenczy. 1995. Phenotypic and genotypic analysis of variability in Aspergillus fumigatus. J. Clin. Microbiol. 33:2567-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salle, V., E. Lecuyer, T. Chouaki, F. X. Lescure, A. Smail, A. Vaidie, C. Dayen, J. L. Schmit, J. P. Ducroix, and Y. Douadi. 2005. Paecilomyces variotii fungemia in a patient with multiple myeloma: case report and literature review. J. Infect. 51:e93-e95. [DOI] [PubMed] [Google Scholar]
- 41.Samson, R. A. 1974. Paecilomyces and some allied hyphomycetes. Stud. Mycol. 6:1-119. [Google Scholar]
- 42.Samson, R. A., J. Houbraken, R. C. Summerbell, B. Flannigan, and J. D. Miller. 2001. Common and important species of fungi and actinomycetes in indoor environment, p. 287-474. In B. Flannigan, R. A. Samson, and J. Miller (ed.), Microorganisms in home and indoor work environments. CRC Press LLC, Boca Raton, FL.
- 43.Samson, R. A., E. S. Hoekstra, and J. C. Frisvad. 2004. Introduction to food- and airborne fungi, 7th ed. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands.
- 44.Reference deleted.
- 45.Samson, R. A., J. Houbraken, J. Varga, and J. C. Frisvad. 2009. Polyphasic taxonomy of the heat resistant ascomycete genus Byssochlamys and its Paecilomyces anamorphs. Persoonia 22:14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seifert, K. A. 2009. Barcoding fungi. Progress towards DNA barcoding of fungi. Mol. Ecol. Resource 9:83-89. [DOI] [PubMed] [Google Scholar]
- 47.Skouboe, P., J. C. Frisvad, J. W. Taylor, D. Lauritsen, M. Boysen, and L. Rossen. 1999. Phylogenetic analysis of nucleotide sequences from the ITS region of terverticillate Penicillium species. Mycol. Res. 103:873-881. [Google Scholar]
- 48.Swofford, D. L. 2000. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b4a. Sinauer Associates, Sunderland, MA.
- 49.Tarkkanen, A., V. Raivio, V. J. Anttila, P. Tommila, R. Ralli, L. Merenmies, and I. Immonen. 2004. Fungal endophthalmitis caused by Paecilomyces variotii following cataract surgery: a presumed operating room air-conditioning system contamination. Acta Ophthalmol. Scand. 82:232-235. [DOI] [PubMed] [Google Scholar]
- 50.Thompson, R. F., R. B. Bode, J. C. Rhodes, and J. L. Gluckman. 1988. Paecilomyces variotii. An unusual cause of isolated sphenoid sinusitis. Arch. Otolaryngol. Head Neck Surg. 114:567-569. [DOI] [PubMed] [Google Scholar]
- 51.Verweij, P. E., D. T. A. Te Dorsthorst, W. H. P. Janssen, J. F. G. M. Meis, and J. W. Mouton. 2008. In vitro activity at pH 5.0 and pH 7.0 and in vivo efficacy of flucytosine against Aspergillus fumigatus. Antimicrob. Agents Chemother. 52:4483-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walsh, T. J., A. Groll, J. Hiemenz, R. Fleming, E. Roilides, and E. Anaissie. 2004. Infections due to emerging and uncommon medically important fungal pathogens. Clin. Microbiol. Infect. 10:48-66. [DOI] [PubMed] [Google Scholar]
- 53.Yaghuchi, T., S. Udagawa, and K. Nishimura. 2005. Geosmithia argillacea is the anamorph of Talaromyces eburneus as a heat resistant fungus. Cryptogamie Mycol. 26:133-141. [Google Scholar]