Abstract
The noninvasive diagnosis of amebic liver abscess is challenging, as most patients at the time of diagnosis do not have a concurrent intestinal infection with Entamoeba histolytica. Fecal testing for E. histolytica parasite antigen or DNA is negative in most patients. A real-time PCR assay was evaluated for detection of E. histolytica DNA in blood, urine, and saliva samples from amebic liver abscess as well as amebic colitis patients in Bangladesh. A total of 98 amebic liver abscess and 28 amebic colitis patients and 43 control subjects were examined. The real-time PCR assay detected E. histolytica DNA in 49%, 77%, and 69% of blood, urine, and saliva specimens from the amebic liver abscess patients. For amebic colitis the sensitivity of the real-time PCR assay for detection of E. histolytica DNA in blood, urine, and saliva was 36%, 61%, and 64%, respectively. All blood, urine, and saliva samples from control subjects were negative by the real-time PCR assay for E. histolytica DNA. When the real-time PCR assay results of the urine and saliva specimens were taken together (positive either in urine or saliva), the real-time PCR assay was 97% and 89% sensitive for detection of E. histolytica DNA in liver abscess and intestinal infection, respectively. We conclude that the detection of E. histolytica DNA in saliva and urine could be used as a diagnostic tool for amebic liver abscess.
Entamoeba histolytica is a protozoan parasite that causes amebic diarrhea, colitis, and amebic liver abscess (ALA), mostly in developing countries (5, 7, 22, 25). Eighty percent of infected individuals remain asymptomatic carriers, while the other 20% develop clinically overt disease (7, 9, 22, 25). About 50 million symptomatic cases of amebiasis occur worldwide each year, resulting in 40,000 to 100,000 deaths annually (25). Mortality from amebiasis is mainly due to extra-amebic colitis, of which ALA is the most common.
It is difficult to differentiate ALA from pyogenic liver abscess or other space-occupying lesions of the liver. Imaging techniques such as ultrasound, computed tomography, and magnetic resonance have excellent sensitivities for the detection of liver abscess arising from any cause, but there are no findings specific for ALA (13). Further complicating the diagnosis is the fact that most patients with an ALA do not have coexistent intestinal infection with E. histolytica (11). Therefore, detection of E. histolytica antigen or DNA in stool samples is not very helpful for the diagnosis of ALA (1, 6, 8, 12).
The current means for diagnosis of ALA is the detection of antiamebic antibody by serological tests combined with aspiration of the abscess. The presence of serum antibodies against E. histolytica and the absence of bacteria in the abscess fluid are consistent with an ALA. A drawback of serologic tests is that the serum antibody levels in people from areas of endemicity remain positive for years after infection with E. histolytica (3, 16, 23). Therefore, antiamebic antibodies in the serum may be due to amebiasis in the past, limiting their specificity for the diagnosis of ALA. A further limitation to the current approach to ALA diagnosis is that collection of liver abscess pus is an invasive procedure that requires technical expertise and can be done only in specialized hospitals.
Several groups have reported the detection of E. histolytica DNA in liver abscess pus, stool, and other clinical samples by PCR (14, 15, 18, 19, 21, 24, 26). A real-time PCR assay has also been used for detection of E. histolytica DNA in stool and liver abscess pus specimens (2, 10, 20). Real-time PCR has never been used for detection of E. histolytica DNA in urine, saliva, and blood specimens of ALA patients. In this study, we evaluated a real-time PCR assay to detect E. histolytica DNA in blood, urine, and saliva samples of amebic liver abscess and colitis patients in Bangladesh.
MATERIALS AND METHODS
Patients and controls.
The study included 98 ALA patients, 28 amebic colitis patients, and 43 control subjects. Of the 98 ALA patients, 39 were from Bangabandhu Seikh Mujib Medical University, Dhaka, and 59 were from Rajshahi Medical College Hospital, Rajshahi, Bangladesh. E. histolytica DNA was positive in liver abscess pus of all 98 ALA patients by real-time PCR assay. Most of the ALA patients were treated with antiamebic therapy before the collection of urine, saliva, and blood samples. The 28 amebic colitis patients were from the International Centre For Diarrheal Disease Research, Bangladesh (ICDDR, B) hospital, Dhaka. Forty-three unmatched control subjects from Rajshahi and Dhaka without amebic diseases were also enrolled. Informed consent was obtained from the patients and parents of the children. The Ethical Review Committee of the ICDDR, B and the Human Investigation Committee of the University of Virginia reviewed and approved the study design.
The diagnosis of ALA was based on the following criteria: (i) a space-occupying lesion in the liver diagnosed by ultrasonography and suggestive of an hepatic abscess; (ii) clinical symptoms including fever and pain in the right hypochondrium; (iii) enlarged and/or tender liver without jaundice and/or right lower intercostal tenderness; (iv) raised right dome of the diaphragm on chest radiograph; (v) improvement after treatment with antiamebic drugs (metronidazole). All of the amebic colitis patients had diarrhea, and their stool samples had E. histolytica trophozoite-containing red blood cells (RBC) by microscopy and were positive by the antigen detection test (E. histolytica II enzyme-linked immunosorbent assay [ELISA]; TechLab). All stool samples from the amebic colitis patients were also positive by the real-time PCR assay. The stools of these control subjects were negative by the E. histolytica antigen detection test and real-time PCR assay.
Collection of specimens and DNA extraction.
Blood, urine, saliva, and stool samples were collected from all ALA, amebic colitis, and control subjects. Liver abscess pus was collected from only ALA patients. Liver abscess pus was aspirated only for clinical purposes as judged by the clinicians caring for the patients and not for the purpose of this study. Fecal or liver abscess pus specimens (0.2 g) were washed twice with sterile phosphate-buffered saline (PBS) and centrifuged for 5 min at 14,000 rpm. DNA was isolated from these specimens using the QIAamp DNA stool minikit (Qiagen, Hilden, Germany). Five milliliters of saliva and 10 ml of urine were collected from each patient and centrifuged at 14,000 rpm for 5 min. Saliva and urine specimens were washed two times with sterile PBS and centrifuged for 5 min at 14,000 rpm, and sediments were used for extraction of DNA. DNA was extracted from 200 μl of sediment of the saliva and urine samples by using the QIAamp DNA blood minikit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. A 200-μl volume of whole blood was also used for extraction of DNA by using the same kit as per the standardization protocol of the manufacturer of this DNA extraction kit. After extraction of DNA from liver abscesses, urine, saliva, stool, and whole blood, the concentration and purity of all DNA samples were measured by spectrophotometry at 260/280 nm. DNA yields were calculated on the basis of UV absorbance and dilution. The purity of the nucleic acid in the samples was considered sufficient if the ratio of the optical densities at 260 versus 280 nm was between 1.5 and 1.8.
Antigen detection.
The Techlab E. histolytica II test (designed to specifically detect E. histolytica) was performed on stool specimens according to the manufacturer's instructions. For detection of antigen in the serum, saliva, and urine samples, 100 μl of undiluted serum was added to the coated microtiter well of the kit. Liver abscess pus specimens were vortexed and centrifuged at 10,000 × g for 10 min, and 100 μl of the resulting undiluted supernatant was added to the microtiter well for antigen detection. A test was considered positive when the optical density reading of a sample at 450 nm was >0.150 (according to the manufacturer's instructions) (9).
ELISA for detection of anti-E. histolytica lectin antibodies.
The anti-lectin IgG ELISA was performed in 96-well microtiter plates. The plates were coated with purified E. histolytica Gal/GalNAc lectin. The serum samples were added at a 1:1,000 dilution in 0.9% PBS-0.05% Tween 20 (final volume, 100 μl) for 2 h at room temperature. Known serum samples were used as positive and negative controls. The wells were washed five times with PBS-Tween 20. The plates were incubated for 1 h with 100 μl of a 1:10,000 dilution of horseradish peroxidase-conjugated goat anti-human IgG with 1% bovine serum albumin. The wells were again washed five times with PBS-Tween 20, followed by the addition of tetramethylbenzidine substrate (final volume, 100 μl). After 10 min, 1 N H2SO4 was used as the stop solution. The optical density of the microtiter wells was measured at 450 nm with an ELISA plate reader. A sample was considered positive if the optical density reading was >0.5, as determined in a previous study (1, 8).
Real-time PCR assay.
The primers and probes for E. histolytica (accession no. X64142) were designed for the small subunit rRNA gene. The amplified target was 134 bp. Primers and TaqMan probes used in this study were purchased from Eurogentec (Seraing, Belgium). The E. histolytica-specific primers and probe set consisted of the forward primer (Eh-f), 5′-AAC AGT AAT AGT TTC TTT GGT TAG TAA AA-3′, and the reverse primer (Eh-r), 5′-CTT AGA ATG TCA TTT CTC AAT TCAT-3′. The TaqMan probe used in this assay was a double-labeled probe, YYT, 5′-ATT AGT ACA AAC TGG CCA ATT CAT TCA-3′ (Eclipse). A 0.4-μmol/liter concentration of each primer (Eh-f and Eh-r primers), 0.08 μmol/liter of Eh-YYT), and 3.0 μl of the extracted DNA were used in each reaction mixture. Amplification reactions were performed in a volume of 25 μl with Bio-Rad IQ supermix (100 mM KCl, 40 mM Tris-HCl [pH 8.4], 1.6 mM deoxynucleoside triphosphates, iTaq DNA polymerase [50 U/ml], 2 mM MgCl2) with an additional 3 mM MgCl2 added. Amplification consisted of 40 cycles of 3 min at 95°C, 30 s at 60°C, and 30 s at 72°C. The ramping of the machine was 3.3°C/second in every step. Amplification, detection, and data analysis were performed with the iCycler real-time detection system (Bio-Rad). Fluorescence was measured during the annealing step of each cycle. The ramping of the machine was 3.3°C/second in every step. Fluorescence was measured at 530 nm (10).
RESULTS
The age and sex distributions of the ALA, amebic colitis, and control subjects from whom samples were collected are given in Table 1. Eighty-eight and 60 percent of the ALA and amebic colitis patients were male, respectively. All 98 of the liver abscess pus specimens collected from the ALA patients were positive for E. histolytica DNA by real-time PCR assay. Positive and negative controls were included in each run of the real-time PCR assay. There were two types of positive controls, strong positive controls (10,000 trophozoites/ml) and weak positive controls (10 trophozoites/ml). The positive and negative controls always gave consistent results. The coefficient of variation of the strong positive control was 2.7%, while for the weak positive control it was 1.7% (based on threshold cycle [CT] values from 10 quantitative PCR runs). The mean CT value of the real-time PCR assay for the liver abscess pus specimens was 28.7 ± 3.3 (± standard deviation). Twenty-eight stool specimens collected from the amebic colitis patients were positive for E. histolytica DNA by real-time PCR assay as well as by the antigen detection test. The mean CT value of the real-time PCR assay for the stool specimens of these 28 amebic colitis patients was 35.7 ± 4.2. Fourty-three stool samples collected from the control subjects were negative for E. histolytica DNA by real-time PCR assay as well as by the antigen detection test of TechLab. Antilectin IgG antibody in serum was detected in 96% (94/98) of ALA patients but only 46% (13/28) of amebic colitis patients. Out of 43 control subjects, 5 were also positive for antilectin IgG in their serum samples. So, the antibody-based test was 96% sensitive and 88% specific for diagnosis of ALA patients but only 46% sensitive and 88% specific for amebic colitis patients in this study.
TABLE 1.
Age and sex distributions of ALA, amebic colitis, and control subjects
| Characteristic | ALA patients (n = 98) | Amebic colitis patients (n = 28) | Controls subjects (n = 43) |
|---|---|---|---|
| Age [mean yrs (SD)] | 42.2 (12.6) | 24.3 (17.0) | 36.3 (12.4) |
| Gender [n (%)] | |||
| Male | 86 (87.8) | 17 (60.7) | 39 (90.7) |
| Female | 12 (12.2) | 11 (39.3) | 04 (09.3) |
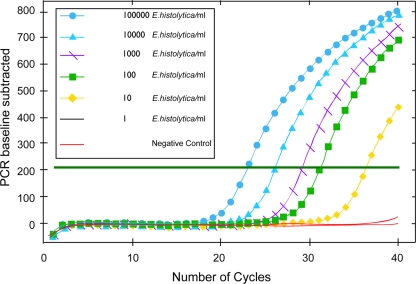
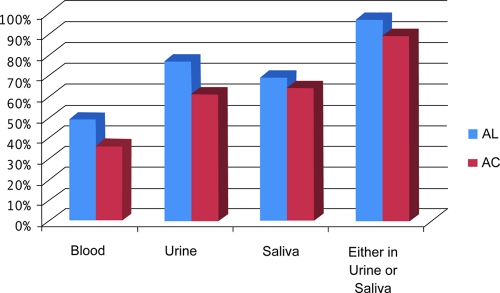
To establish a calibration curve for this real-time PCR assay, serial dilutions of cultured trophozoites of E. histolytica were spiked in urine and saliva samples before extraction of DNA and then the DNA was extracted. The real-time PCR assay used in this study detects 10 trophozoites of E. histolytica per milliliter (Fig. 1). This is comparable to our earlier results of a similar assay using a molecular beacon probe (21). The results of the real-time PCR assay for detection of E. histolytica DNA in blood, urine, and saliva samples of ALA, amebic colitis, and control subjects are presented in Fig. 2. Out of 98 ALA patients, saliva samples was not collected from 3 patients. The real-time PCR assay was 49%, 77%, and 69% sensitive for detection of E. histolytica DNA in blood, urine, and saliva specimens, respectively, for the 95 ALA patients where both samples were tested. For the 28 amebic colitis patients the sensitivity of the real-time PCR assay for detection of E. histolytica DNA in blood, urine, and saliva was 36%, 61%, and 64%, respectively. All blood, urine, and saliva samples from control subjects were negative by the real-time PCR assay for E. histolytica DNA. When the real-time PCR assay results of the urine and saliva specimens were taken together (positive either in urine or saliva), the real-time PCR assay was 97% and 89% sensitive for detection of E. histolytica DNA in ALA and amebic colitis patients, respectively (Fig. 2).
FIG. 1.
Calibration curve of the E. histolytica real-time PCR assay. Shown are the numbers of E. histolytica trophozoites spiked in saliva or urine samples (number of organisms per milliliter of specimen).
FIG. 2.
Detection of E. histolytica DNA in blood, urine, and saliva of ALA and amebic colitis (AC) patients.
A comparison of the results of the real-time PCR results from urine with saliva for the ALA and amebic colitis patients is given in Table 2. Out of 22 ALA patients whose urine samples gave negative results by the urine real-time PCR assay, 19 were positive for E. histolytica DNA in their saliva samples. Similarly, of 29 ALA patients whose saliva samples were negative by the real-time PCR assay, 26 were positive based on urine. Similar findings were also obtained for the amebic colitis patients (Table 2). Two of three ALA patients whose urine and saliva specimens were negative for E. histolytica DNA by real-time PCR assay were positive for E. histolytica DNA in their blood by the real-time PCR assay. Combining the real-time PCR assay results from urine and saliva gave a positive predictive value of this real-time PCR assay for diagnosis of ALA of 100% and a negative predictive value of 93%.
TABLE 2.
Urine and saliva real-time PCR assay results in ALA and amebic colitis patients
| PCR result in saliva | PCR resulta in urine from subjects with: |
|||
|---|---|---|---|---|
| ALA |
Amebic colitis |
|||
| Negative | Positive | Negative | Positive | |
| Negative | 3 | 26 | 3 | 7 |
| Positive | 19 | 47 | 8 | 10 |
| Total | 22 | 73 | 11 | 17 |
Number of patients with indicated result.
The semiquantitative nature of the real-time PCR may allow for the estimation of parasite load in the different clinical samples. There were no significant differences between the CT values from blood, urine, and saliva specimens of ALA and amebic colitis patients (Table 3). However, the mean CT values of the positive blood, urine, and saliva specimens of ALA patients were significantly higher than the mean CT value of the liver abscess pus specimens (P < 0.001). Similarly, the mean CT values of the positive blood, urine, and saliva specimens of amebic colitis patients were higher than the mean CT value of the stool specimens from the same patients (P = 0.02).
TABLE 3.
Parasite loads in blood, urine, and saliva of ALA and amebic colitis patientsa
| Patient group and sample | No. of positive samples | Mean CT (95% CI) | Median CT |
|---|---|---|---|
| ALA patients | |||
| Blood | 48 | 38.0 (36.7-39.2) | 37.6 |
| Urine | 75 | 38.2 (37.3-39.0) | 38.0 |
| Saliva | 66 | 37.5 (36.8-38.2) | 37.3 |
| Liver abscess pus | 98 | 28.7 (28.0-29.3) | 28.2 |
| Amebic colitis patients | |||
| Blood | 10 | 36.2 (35.0-37.4) | 35.5 |
| Urine | 17 | 37.5 (36.3-38.8) | 36.7 |
| Saliva | 18 | 37.8 (36.4-39.1) | 37.4 |
| Stool | 28 | 35.8 (34.3-37.5) | 36.2 |
Parasite loads were based on the CT, determined using quantitative PCR. CI, confidence interval.
DISCUSSION
In this study we have shown that E. histolytica DNA can be detected either in urine or saliva in 97% (92/95) of ALA cases by real-time PCR. We have also shown that urine and saliva are more suitable specimens than blood for detection of E. histolytica DNA in ALA patients.
There are several reports on the use of saliva and urine for the detection of DNA for the diagnosis of infectious diseases, including amebiasis (4, 17, 18, 26). However, to our knowledge this is the first report on the use of a real-time PCR assay for detection of E. histolytica DNA in both urine and saliva of ALA patients. Earlier studies had attempted to detect E. histolytica DNA in urine or saliva in ALA patients by using conventional PCR methods, and the sensitivity was low compared to our study (14, 19).
In the present study, we have also shown that by the real-time PCR assay E. histolytica DNA can also be detected in blood, urine, and saliva of amebic colitis patients. And as in the case of ALA, urine and saliva were more suitable specimens than blood for detection of E. histolytica DNA. But the overall sensitivity for diagnosis of amebic colitis by real-time PCR on urine and saliva was far less than that of antigen detection or real-time PCR on stool samples.
In summary, detection of parasite DNA in urine and saliva provides a sensitive and noninvasive means for the diagnosis of ALA.
Acknowledgments
This work was supported by NIH grant R01 AI043596.
Footnotes
Published ahead of print on 9 June 2010.
REFERENCES
- 1.Abd-Alla, M. D., T. F. H. G. Jackson, S. Reddy, and J. I. Ravdin. 2000. Diagnosis of invasive amebiasis by enzyme-linked immunosorbent assay of saliva to detect amebic lectin antigen and anti-lectin immunoglobulin G antibodies. J. Clin. Microbiol. 38:2344-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blessmann, J., H. Buss, P. A. Nu, B. T. Dinh, Q. T. Ngo, A. L. Van, M. D. Alla, T. F. Jackson, J. I. Ravdin, and E. Tannich. 2002. Real-time PCR for detection and differentiation of Entamoeba histolytica and Entamoeba dispar in fecal samples. J. Clin. Microbiol. 40:4413-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britten, D., S. M. Wilson, R. McNerney, A. H. Moody, P. L. Chiodini, and J. P. Ackers. 1997. An improved colorimetric PCR-based method for detection and differentiation of Entamoeba histolytica and Entamoeba dispar in feces. J. Clin. Microbiol. 35:1108-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crepin, P., L. Aurdy, Y. Rotivel, A. Gacoin, C. Caroff, and H. Bourhy. 1998. Intravitam diagnosis of human rabies by PCR using saliva and cerebrospinal fluid. J. Clin. Microbiol. 36:1117-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haque, R., I. K. M. Ali, and W. A. Petri, Jr. 1999. Prevalence and immune response of Entamoeba histolytica infection in preschool children in Bangladesh. Am. J. Trop. Med. Hyg. 60:1031-1034. [DOI] [PubMed] [Google Scholar]
- 6.Haque, R., I. K. M. Ali, S. Akther, and W. A. Petri, Jr. 1998. Comparison of PCR, isoenzyme analysis, and antigen detection for diagnosis of Entamoeba histolytica infection. J. Clin. Microbiol. 36:449-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haque, R., C. D. Huston, M. Hughes, E. Houpt, and W. A. Petri, Jr. 2003. Current concepts: amebiasis. N. Engl. J. Med. 348:1565-1573. [DOI] [PubMed] [Google Scholar]
- 8.Haque, R., N. U. Mollah, I. K. M. Ali, K. Alam, A. Eubanks, D. Lyerly, and W. A. Petri, Jr. 2000. Diagnosis of amebic liver abscess and intestinal infection with the TechLab Entamoeba histolytica II antigen detection and antibody tests. J. Clin. Microbiol. 38:3235-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haque, R., L. M. Neville, P. Hahn, and W. A. Petri, Jr. 1995. Rapid diagnosis of Entamoeba infection by using Entamoeba and Entamoeba histolytica stool antigen detection kits. J. Clin. Microbiol. 33:2558-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haque, R., S. Roy, A. Siddique, U. Mondal, S. M. M. Rahman, D. Mondal, E. Houpt, and W. A. Petri, Jr. 2007. Multiplex real time PCR assay for the diagnosis of Entamoeba histolytica, Giardia intestinalis, and Cryptosporidium spp. Am. J. Trop. Med. Hyg. 76:713-717. [PubMed] [Google Scholar]
- 11.Juniper, K., C. L. Worrell, M. C. Minshew, L. S. Roth, H. Cypert, and R. E. Lloyd. 1972. Serologic diagnosis of amebiasis. Am. J. Trop. Med. Hyg. 21:157-167. [DOI] [PubMed] [Google Scholar]
- 12.Kasper, D. L., E. Braunwald, A. S. Fauci, S. L. Hauser, D. L. Longo, and J. L. Jameson. 2005. Harrison's principles of internal medicine, 16th ed., p. 1215-1216. McGraw Hill, New York, NY.
- 13.Katzenstein, D., V. Rickerson, and A. Braude. 1982. New concepts of amebic liver abscess derived from hepatic imaging, serodiagnosis, and hepatic enzymes in 67 consecutive cases in San Diego. Medicine (Baltimore) 68:237-246. [DOI] [PubMed] [Google Scholar]
- 14.Khairnar, K., and S. C. Parija. 2008. Detection of E. histolytica DNA in the saliva of amebic liver abscess patients who received prior treatment with metronidazole. J. Health Popul. Nutr. 26:418-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan, U., B. R. Mirdha, J. C. Samantray, and M. P. Sharma. 2006. Detection of Entamoeba histolytica using polymerase chain reaction in pus samples from amebic liver abscess. Indian J. Gastroentrol. 25:55-57. [PubMed] [Google Scholar]
- 16.Krupp, I. M., and S. J. Powell. 1971. Comparative study of the antibody response in amebiasis: persistence after successful treatment. Am. J. Trop. Med. Hyg. 20:421-424. [DOI] [PubMed] [Google Scholar]
- 17.Li, C., P. R. Musich, T. Ha, D. A. Ferguson, Jr., N. R. Patel, D. S. Chi, et al. 1995. High prevalence of Helicobacter pylori in saliva demonstrated by a novel PCR assay. J. Clin. Pathol. 48:662-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunez, Y. O., M. A. Fernandez, D. Torres-Nunez, J. A. Silva, I. Montano, J. L. Maestre, et al. 2001. Multiplex polymerase chain reaction amplification and differentiation of Entamoeba histolytica and Entamoeba dispar DNA from stool samples. Am. J. Trop. Med. Hyg. 64:293-297. [DOI] [PubMed] [Google Scholar]
- 19.Parija, S. C., and K. Khairnar. 2007. Detection of excretory Entamoeba histolytica DNA in the urine, and detection of E. histolytica DNA and lectin antigen in the liver abscess pus for the diagnosis of amoebic liver abscess. BMC Microbiol. 7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qvarnstrom, Y. C., C. James, M. Xayavong, B. P. Holloway, G. S. Visvesvera, R. Sriram, and A. J. Silva. 2005. Comparison of real-time PCR protocols for differential laboratory diagnosis of amebiasis. J. Clin. Microbiol. 43:5491-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy, S., M. Kabir, D. Mondal, I. K. M. Ali, W. A. Petri, Jr., and R. Haque. 2005. Real-time PCR assay for the diagnosis of Entamoeba histolytica infection. J. Clin. Microbiol. 43:2168-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanley, S. L., Jr. 2003. Amoebiasis. Lancet 361:1025-1034. [DOI] [PubMed] [Google Scholar]
- 23.Stanley, S. L., Jr., T. F. G. H. Jackson, L. Foster, and S. Singh. 1998. Longitudinal study of the antibody response to recombinant E. histolytica antigen in patients with amebic liver abscess. Am. J. Trop. Med. Hyg. 58:414-416. [DOI] [PubMed] [Google Scholar]
- 24.Troll, H., H. Marti, and N. Weiss. 1997. Simple differential detection of Entamoeba histolytica and Entamoeba dispar in fresh stool specimens by sodium acetate-acetic acid formalin concentration and PCR. J. Clin. Microbiol. 35:1701-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. 1997. Amoebiasis. Wkly. Epidemiol. Rec. 72:97-100.9100475 [Google Scholar]
- 26.Zaman, S., J. Khoo, S. W. Ng, R. Ahmed, M. A. Khan, R. Hussain, and V. Zaman. 2000. Direct amplification of Entamoeba histolytica DNA from amoebic liver abscess pus using polymerase chain reaction. Parasitol. Res. 86:724-728. [DOI] [PubMed] [Google Scholar]