Abstract
Free-living amoebae that belong to the genus Acanthamoeba are widespread in the environment, including water. They are responsible for human infections and can host pathogenic microorganisms. Under unfavorable conditions, they form cysts with high levels of resistance to disinfection methods, thus potentially representing a threat to public health. In the present study we evaluated the efficacies of various biocides against trophozoites and cysts of several Acanthamoeba strains. We demonstrated that disinfectant efficacy varied depending on the strains tested, with environmental strains demonstrating greater resistance than collection strains. Trophozoites were inactivated by all treatments except those using glutaraldehyde as an active compound: for these treatments, we observed resistance even after 30 min exposure. Cysts resisted many treatments, including certain conditions with glutaraldehyde and other biocides. Moist heat at 55°C was not efficient against cysts, whereas exposure at 65°C was. Several chemical formulations containing peracetic acid, hydrogen peroxide, or ortho-phthalaldehyde presented greater efficacy than glutaraldehyde, as did ethanol and sodium hypochlorite; however, some of these treatments required relatively long incubation times to achieve cyst inactivation. Amoebal cysts can be highly resistant to some high-level disinfectants, which has implications for clinical practice. These results highlight the need to consider the effective disinfection of protozoa in their vegetative and resistant forms due to their intrinsic resistance. This is important not only to prevent the transmission of protozoa themselves but also due to the risks associated with a range of microbial pathogens that are found to be associated intracellularly with these microorganisms.
Free-living amoebae (FLA) are among the most prevalent protozoa found in the environment, being isolated from soil, air, and water, as well as from dust, sewage, and sediments (43). They can colonize water systems and have been isolated from drinking water plants (24, 57), hospital water networks (44, 56), domestic water networks (30), and cooling towers (3), to name but a few. Among FLA, Acanthamoeba species are, to date, the most frequently encountered in human infections. The life cycle of Acanthamoeba can be considered in two stages. During the first stage, the vegetative trophozoite form is feeding and replicating. The second stage develops not only under unfavorable environmental conditions, such as nutrient starvation, heat, cold, and desiccation, but also in the presence of elevated concentration of various chemical compounds, with the trophozoites differentiating into cysts (13, 40). During the encystment phase, amoebae become round and form two distinct layers: the endocyst, containing cellulose, and the ectocyst, containing various polysaccharides and proteins (27, 34). Cysts are metabolically inactive and can remain viable for more than 20 years under dry conditions (51) and 24 years at 4°C in water (38).
The most frequent infection associated with Acanthamoeba spp. is amoebic keratitis (AK), first described in 1974 (39). This is a particularly difficult to treat ocular infection that leads to trauma and corneal necrosis. The reports of AK have dramatically increased in parallel with the use of contact lenses, with incidence rates of 0.15 per million population in the United States and 1.4 per million population in the United Kingdom being reported (17). In a 2009 study, Acanthamoeba spp. were also encountered in the urine of critically ill patients in intensive care units, suggesting that FLA might play a role in hospital-acquired infections by facilitating transmission of bacteria to the patients (45). In fact, in addition to their intrinsic pathogenicity, Acanthamoeba can potentially harbor various bacterial, viral, and eukaryotic species pathogenic for human and animals (22). Thus, among 539 bacterial species listed as being pathogenic for humans and/or animals, 102 (including Acinetobacter, Enterobacter, Legionella, various mycobacteria, Pseudomonas, and Serratia spp.) were described in the literature as being able to resist and potentially proliferate after amoebal ingestion (59). Survival of bacteria within amoebal cysts has been demonstrated for several of these species, mainly mycobacteria (1), and cysts are thus considered potential reservoirs of pathogens in water networks.
Despite increasing health concerns over FLA, there is still a paucity of information concerning their resistance to various biocides and disinfectants. Trophozoites are considered relatively sensitive to most chemicals, but cysts have been shown to be more resistant. It has been reported in several studies that they can resist exposure to biguanides, quaternary ammonium compounds, chlorine, chlorine dioxide, and hydrogen peroxide (59). They have high levels of resistance to radiation, such as UV, X-ray, and gamma irradiation, but limited resistance to high temperatures (59). There is no official standard available to test the activities of disinfectant against amoebae. Amoebal strains, the media used to grow and encyst trophozoites, and the exposure and neutralization conditions, as well as methods to evaluate residual viability after treatments, vary from one study to another and might thus be responsible for the observed discrepancies (2, 12). Furthermore, there are few data available, if there are any data at all, on the activities of the various chemicals used for surface and medical device disinfection in health care settings, such as alcohols, aldehyde-based products, peracetic acid (PAA)-based products, and hydrogen peroxide-based products.
In the present work, we compared the efficacies of various disinfection treatments used in health care settings, including widely used high-level disinfectants, against reference and field isolate strains of Acanthamoeba.
MATERIALS AND METHODS
Amoebal strains.
Nine different strains were tested: Acanthamoeba castellanii ATCC 30010 and CCAP 1501/10, Acanthamoeba polyphaga CCAP 1501/18, and six environmental strains previously isolated from a hospital water network (56) and from Seine River water (57). All amoebal strains were grown in peptone yeast glucose (PYG) medium in tissue culture flasks at 28°C as described previously (60).
Further characterization of environmental amoebal strains.
Trophozoites were recovered from the PYG medium flasks and washed in phosphate-buffered saline. DNA was extracted using a Pure Link genomic minikit (Invitrogen), including digestion with lysozyme (20 mg/ml) for 30 min at 37°C and with proteinase K (20 mg/ml) for 2 h at 55°C. The diagnostic fragment DF3 was amplified using previously described primers JDP1 (5′-GGCCCAGATCGTTTACCGTGAA-3′) and JDP2 (5′-TCTCACAAGCTGCTAGGGGAGTCA-3′) (9). Sequencing was performed using primers JDP1 and JDP2, as well as internal primers 892CF (5′-GTCAGAGGTGAAATTCTTGG-3′) and 892CR (5′-CCAAGAATTTCACCTCTGAC-3′) (47).
Environmental Acanthamoeba strains were also screened for the presence of intracellular bacteria by performing PCR with DNA extracted from amoebae using universal primers FD1 (5′-AGAGTTTGATCATGGCTCAG-3′) and RP2 (5′-ACGGCTACCTTGTTACGACTT-3′), targeting the bacterial 16S rRNA gene (61).
Encystment.
Cysts were prepared from trophozoites using Neff's encystment medium (0.1 M KCl, 0.02 M Tris, 8 mM MgSO4, 0.4 mM CaCl2, 1 mM NaHCO3). Briefly, adhering trophozoites were suspended in PYG medium in cell culture flasks, harvested by centrifugation at 2,700 × g for 10 min, and washed in encystment medium. Approximately 5 × 107 trophozoites were then added to 25 ml of encystment medium in a 150-cm2 culture flask and incubated at 33°C for 7 days. Cysts were then recovered from the flask, suspended for 5 min in sterile distilled water with 0.5% SDS to lyse nonmature cysts and dissociate aggregates, and then washed two times in 1/4-strength Ringer's solution and stored at 4°C for testing within 15 days.
Decontamination tests. (i) Trophozoites.
Confluent cell monolayers were harvested from the culture flasks and resuspended in 1/4-strength Ringer broth, with the cell concentrations adjusted to 107 to 108 cells/ml. Trophozoites were then diluted 1/10 in the products to be tested or in Page amoeba saline (PAS) for the controls. The disinfectants tested included various diluted biocides as well as formulated biocides, as their activities vary significantly. We tested various oxidizing biocides based on acids (hydrogen peroxide or peracetic acid) or chlorine (sodium hypochlorite), as well as fixative biocides based on various aldehydes and ethanol (Table 1). Once they were diluted in the disinfectants, trophozoites were incubated for 5 min (all products except glutaraldehyde and a glutaraldehyde-based product) to 25 min (glutaraldehyde and a glutaraldehyde-based product) at room temperature. They were then recovered by centrifugation at 2,700 × g for 5 min, resuspended in Dey-Engley (D/E) neutralizing broth (with 0.2% catalase for hydrogen-peroxide-based products) for 5 min, and vortexed at maximum speed for 30 s. After another centrifugation step at 2,700 × g for 5 min, trophozoites were resuspended in 1/4-strength Ringer broth and serial diluted in PYG medium in 48-well plates (6 wells per dilution). The plates were incubated for 21 days at 28°C, with the wells being regularly observed for trophozoite growth; log10 reductions were calculated using the Spearman-Karber method. Each test was performed at least four times.
TABLE 1.
Unformulated and formulated active compounds tested in this study and contact times used for cyst exposures
| Biocide | Final concn(s) of active compound | pH of solutiona | Contact time(s) (min) |
|---|---|---|---|
| Unformulated biocides | |||
| Sodium hypochlorite | 2,500 and 25,000 ppm | 11.3 and 12.1 | 10, 20, and 30 |
| Ethanol | 70% | 6.2 | 10 |
| Glutaraldehyde | 2% | 8.0 (adjusted pH) | 10 |
| Hydrogen peroxide | 7.5% | 3.4 | 10, 20, and 30 |
| ortho-Phthalaldehyde | 0.55% | 6.5 (adjusted pH) | 10 |
| Peracetic acid | 0.2% | 2.7 | 10 |
| Formulated biocides | |||
| Glutaraldehyde-based product | 2% glutaraldehyde | 5.6 | 10, 20, and 30 |
| ortho-phthalaldehyde-based product | 0.55% OPA | 7.5 | 10, 20, and 30 |
| PAA-based product STERIS-20 | 0.2% PAA | 6.2 | 10 |
| H2O2-based product Resert HLD | 2% H2O2 | 2.3 | 10 |
| H2O2/PAA-based product Sporklenz RTU | 1% H2O2/0.08% PAA | 1.9 | 10, 20, and 30 |
| Heat | |||
| 55°C | 7.2 | 10 | |
| 65°C | 7.2 | 10 |
Measured at 21°C.
(ii) Cysts.
Cysts were adjusted at 107 to 108 cysts/ml in 1/4-strength Ringer broth. Suspensions were diluted 1/10 in the product to be tested (PAS for controls) and incubated for 5, 15, or 25 min at the appropriate temperature (Table 1). They were then recovered by centrifugation at 12,000 × g for 5 min, resuspended in D/E neutralizing broth plus 0.5% SDS for 5 min, and vortexed at maximum speed for 30 s. After another centrifugation step at 12,000 × g, cysts were resuspended in 1/4-strength Ringer broth and serially diluted on a nickel-nitrilotriacetic acid-Escherichia coli ATCC 25922 lawn in 48-well plates (6 wells per dilution). After 7 days of incubation at 28°C, the wells were observed for trophozoite growth and the log10 reductions were calculated using the Spearman-Karber method.
Aggregation tests.
Cysts were observed at the end of the 7 days of incubation in Neff's medium to evaluate their capacity to form aggregates. The same cysts were directly examined after treatment with 0.5% SDS to confirm the dissociation of aggregates. Any effects of treatments on aggregation of SDS-treated cysts were monitored after incubation of cysts in 24-well plates in the presence of products for various times: (i) 10 min for 2.5% sodium hypochlorite, 70% ethanol, 2% glutaraldehyde, 0.55% ortho-phthalaldehyde (OPA), 0.2% peracetic acid (PAA), an H2O2-based product (Resert HLD; STERIS Corporation, St. Louis, MO), and a PAA-based product (STERIS-20; STERIS Corporation, Mentor, OH); (ii) 30 min for 0.25% sodium hypochlorite, a glutaraldehyde-based product, an OPA-based product, 7.5% hydrogen peroxide, and a further hydrogen peroxide/peracetic acid-based product (Sporklenz RTU; STERIS Corporation, St. Louis, MO).
TEM.
Cysts obtained after incubation of amoebal strains 1, 3, and 4 and Acanthamoeba polyphaga CCAP 1501/18 in Neff's medium were prepared for observation by transmission electron microscopy (TEM). The samples were fixed in 2.5% glutaraldehyde for 1 night, postfixed for 1 h in 0.1 M cacodylate buffer-1% OsO4 for 1 night, stained with uranyl acetate (0.25%) for 1 night, dehydrated, and embedded in epoxy resin. Thin sections (50 to 60 nm) were stained with uranyl acetate (5 min) and lead citrate (5 min), being then examined with a Philips EM 208 electron microscope. A minimum of 100 measurements were taken to evaluate the thickness of the ectocysts for each of the four isolates.
Statistical analysis.
Statistical analysis was performed using one-way analysis to compare the thickness of amoebal cysts or a Fisher's exact test to compare the numbers of wells positive for each isolate and each treatment. Statistical significance was set at an alpha level of 0.05.
Nucleotide sequence accession numbers.
Partial 18S rRNA gene sequences corresponding to the DF3 fragment of environmental strains were deposited in GenBank under accession numbers GU459317 to GU459322.
RESULTS
Further characterization of environmental amoebal strains.
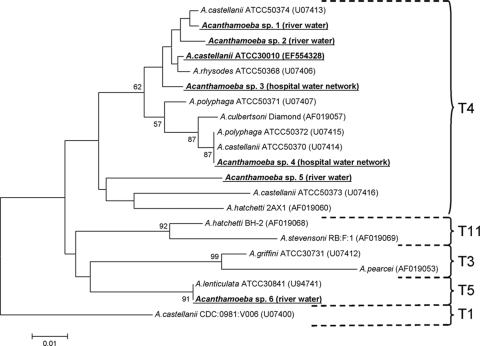
Partial sequencing of the 18S rRNA gene allowed classification of most isolates in the genotype T4 group (Fig. 1). Only one isolate was classified under the T5 genotype, being identified as Acanthamoeba lenticulata after sequencing of the 18S rRNA intron specific to this species (data not shown). Interestingly, we were not able to amplify any DNA with 16S ribosomal DNA (rDNA) universal primers, suggesting that all environmental isolates were free of bacteria.
FIG. 1.
Phylogenetic tree showing the relationship of FLA isolates used in disinfection tests. DF3 fragment sequences were aligned using Muscle software (19), and the tree was constructed using the neighbor-joining method. Bootstrap values resulting from 2,000 replications are presented at each node when the value was >50%.
Disinfection tests. (i) Trophozoites.
All products tested against trophozoites except 2% neat glutaraldehyde and a glutaraldehyde-based disinfectant formulation achieved complete kill for all strains after 10-min exposures. For the two glutaraldehyde treatments, no morphological modifications were observed after the treatment and the cells could still adhere to the bottom of the wells when the plates were seeded in PYG medium to count the survivors. The calculated log10 reductions were very similar after 30-min exposures to glutaraldehyde alone or in formulation and varied from approximately 1 log10 unit for strain 3 to 4.5 log10 units for A. castellanii CCAP 1501/10 (Table 2) .
TABLE 2.
Biocidal effects of glutaraldehyde alone or in formulation against trophozoites of nine Acanthamoeba strains
| Biocidea | Log10 reduction |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Acanthamoeba environmental isolates |
A. castellanii CCAP 1501/10 | A. castellanii ATCC 30010 | A. polyphaga CCAP 1501/18 | ||||||
| 1 (river water) | 2 (river water) | 3 (hospital) | 4 (hospital) | 5 (river water) | 6 (river water) | ||||
| Glutaraldehyde, 2% | 4.1 ± 0.2 | 1.9 ± 0.8 | 0.9 ± 0.7 | 1.9 ± 0.1 | 1.4 ± 0.3 | 3.3 ± 0.2 | 4.6 ± 0.2 | 3.6 ± 0.1 | 3.9 ± 0.4 |
| Glutaraldehyde-based product | 4.1 ± 0.2 | 1.8 ± 0.6 | 1.0 ± 0.7 | 2.2 ± 0.2 | 1.6 ± 0.1 | 3.4 ± 0.0 | 4.5 ± 0.1 | 3.9 ± 0.1 | 3.9 ± 0.3 |
The contact time was 30 min for each biocide.
(ii) Cysts.
Table 3 summarizes the efficacies of all biocides tested against cysts of the nine Acanthamoeba strains. As some formulated biocides tested in this study have recommended-use temperatures of >50°C, tests investigated the effect of elevated temperatures on cyst viability. Exposure at 55°C for 10 min was completely inefficient (≤1-log10-unit reduction for all strains), whereas exposure at 65°C for 10 min was completely efficient (>4-log10-unit reduction for all strains).
TABLE 3.
Cysticidal effects of biocidal treatments, including unformulated active compounds, active compounds in formulation, and moist heat, against cysts of nine Acanthamoeba strains
| Biocide | Contact time (min) | Log10 reduction |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Acanthamoeba environmental isolates |
A. castellanii CCAP 1501/10 | A. castellanii ATCC 30010 | A. polyphaga CCAP 1501/18 | |||||||
| 1 (river water) | 2 (river water) | 3 (hospital) | 4 (hospital) | 5 (river water) | 6 (river water) | |||||
| 55°C | 10 min | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.5 ± 0.0 | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.5 ± 0.2 | 0.6 ± 0.1 |
| 65°C | 10 min | >4.7 | >4.8 | >4.7 | >4.7 | >4.8 | >4.1 | >5.1 | >4.7 | >4.5 |
| Sodium hypochlorite, 2.5% | 10 min | >4.7 | >4.8 | >4.7 | >4.7 | >4.8 | >4.1 | >5.1 | >4.7 | >4.5 |
| Sodium hypochlorite, 0.25% | 10 min | 2.9 ± 0.1 | 3.5 ± 0.2 | 0.1 ± 0.1 | 0.2 ± 0.1 | 1.3 ± 0.2 | >4.1 | >5.1 | 2.9 ± 0.2 | 1.8 ± 0.2 |
| Sodium hypochlorite, 0.25% | 20 min | >4.7 | >4.8 | 1.4 ± 0.1 | 3.6 ± 0.1 | >4.8 | NDa | ND | >4.7 | >4.5 |
| Sodium hypochlorite, 0.25% | 30 min | ND | ND | 2.6 ± 0.2 | >4.7 | ND | ND | ND | ND | ND |
| Ethanol 70% | 10 min | >4.7 | >4.8 | 2.8 ± 0.1 | 4.3 ± 0.3 | 3.5 ± 0.2 | >4.1 | >5.1 | >4.7 | >4.5 |
| Glutaraldehyde 2% | 10 min | 1.6 ± 0.4 | 0.2 ± 0.2 | 0.0 ± 0.1 | 1.1 ± 0.6 | 4.6 ± 0.1 | >4.1 | 2.6 ± 0.1 | 1.5 ± 0.2 | 1.3 ± 0.3 |
| Glutaraldehyde-based product | 10 min | 1.3 ± 0.2 | 0.6 ± 0.1 | 0.2 ± 0.0 | 0.3 ± 0.8 | 0.5 ± 0.3 | 1.6 ± 0.2 | 2.8 ± 0.2 | 1.3 ± 0.3 | 1.2 ± 0.1 |
| Glutaraldehyde-based product | 20 min | 3.9 ± 0.1 | 0.7 ± 0.1 | 0.0 ± 0.0 | 1.9 ± 0.2 | 2.4 ± 0.1 | 2.2 ± 0.2 | 2.8 ± 0.2 | 2.4 ± 0.1 | >4.5 |
| Glutaraldehyde-based product | 30 min | >4.7 | 0.9 ± 0.1 | 0.9 ± 0.1 | >4.7 | 2.5 ± 0.1 | 2.3 ± 0.2 | 2.8 ± 0.1 | >4.7 | ND |
| OPA, 0.55% | 10 min | >4.7 | >4.8 | 3.7 ± 0.2 | 1.1 ± 0.3 | >4.8 | >4.1 | >5.1 | >4.7 | >4.5 |
| OPA-based product | 10 min | 2.8 ± 0.2 | 3.8 ± 0.1 | 2.6 ± 0.2 | 1.1 ± 0.1 | 4.6 ± 0.0 | >4.1 | 3.0 ± 0.1 | 2.6 ± 0.1 | 2.2 ± 0.1 |
| OPA-based product | 20 min | >4.7 | >4.8 | 2.9 ± 0.1 | 3.7 ± 0.1 | ND | ND | >5.1 | >4.7 | >4.5 |
| OPA-based product | 30 min | ND | ND | >4.7 | >4.7 | ND | ND | ND | ND | ND |
| Hydrogen peroxide, 7.5% | 10 min | 1.1 ± 0.5 | 0.3 ± 0.3 | 1.2 ± 0.1 | 1.1 ± 0.3 | 2.7 ± 0.2 | 1.6 ± 0.2 | 0.8 ± 0.6 | 0.4 ± 0.3 | 2.5 ± 0.4 |
| Hydrogen peroxide, 7.5% | 20 min | 3.3 ± 0.1 | 1.2 ± 0.2 | 0.5 ± 0.2 | 1.2 ± 0.2 | >4.8 | >4.1 | 2.7 ± 0.2 | 2.2 ± 0.1 | >4.5 |
| Hydrogen peroxide, 7.5% | 30 min | 3.8 ± 0.1 | 1.4 ± 0.2 | 0.9 ± 0.1 | 1.7 ± 0.2 | ND | ND | 3.1 ± 0.1 | 2.8 ± 0.2 | ND |
| Hydrogen peroxide-based Resert HLD | 10 min | >4.7 | >4.8 | >4.7 | 4.3 ± 0.4 | >4.8 | >4.1 | >5.1 | >4.7 | >4.5 |
| H2O2/PAA-based SporKlenz RTU | 10 min | >4.7 | 3.6 ± 0.5 | 1.6 ± 0.2 | 1.7 ± 0.8 | 4.1 ± 0.2 | >4.1 | >5.1 | >4.7 | >4.5 |
| H2O2/PAA-based SporKlenz RTU | 20 min | ND | >4.8 | >4.7 | 4.1 ± 0.8 | ND | ND | ND | ND | ND |
| H2O2/PAA-based SporKlenz RTU | 30 min | ND | ND | ND | >4.5 | ND | ND | ND | ND | ND |
| PAA, 0.2% | 10 min | >4.7 | 0.9 ± 0.3 | >4.7 | 0.2 ± 0.2 | >4.8 | >4.1 | >5.1 | 2.1 ± 0.5 | >4.5 |
| PAA-based STERIS-20 | 10 min | >4.7 | >4.8 | >4.7 | 4.0 ± 0.2 | >4.8 | >4.1 | >5.1 | >4.7 | >4.5 |
ND, not done.
With the chemical biocides tested, variability in strain resistance profiles was observed. With 10-min exposures, all strains resisted at least 2 of 12 chemical biocides (<4-log10-unit reduction of viable cysts), and the cysts of the most resistant strain (strain 4; P < 0.05) resisted exposure to 8 of 12 treatments. Sodium hypochlorite at 2.5% achieved complete kill of all tested strains. A PAA-based product (at the associated exposure temperature of 55°C, as recommended by the manufacturer) and one hydrogen peroxide-based product (Resert HLD at room temperature) demonstrated a >4-log10-unit reduction. Efficacy was observed with ethanol 70% (>4-log10-unit reduction for all strains except strains 3 and 4, for which only 2.8- and 3.5-log10-unit reductions were achieved) and ortho-phthalaldehyde alone (>4-log10-unit reductions for all strains except strain 3 [3.7-log10-unit reduction] and strain 4 [1.1-log10-unit]). A further hydrogen peroxide/peracetic acid-based product (Sporklenz RTU) was effective against six of the nine tested strains after a 10-min exposure, with the other three strains presenting moderate resistance (strain 2, 3.6-log10-unit reduction; strain 3, 1.6-log10-unit reduction; strain 4, 1.7-log10-unit reduction) and being inactivated after 20 min exposure (except for strain 4, for which the reduction was >4 log10 units with few survivors but complete inactivation after 30 min). Three strains presented moderate to high levels of resistance to 10 min exposure to PAA alone at room temperature: 0.2- to 2.1-log10-unit reductions for strains 2 and 4 and A. castellanii ATCC 30010. Sodium hypochlorite (0.25%) and an OPA-based product were moderately efficient at 10-min exposures, with greater efficacy at 20 min for seven of nine strains being noted; 30-min exposures were required to achieve the complete kill of strains 3 and 4 with the OPA-based product, whereas strain 3 resisted 30 min exposure to 0.25% sodium hypochlorite. Glutaraldehyde (2%) alone or in formulation had little efficacy, with five of the nine tested strains resisting 30-min exposures to the glutaraldehyde-based product (0.9- to 2.8-log10-unit reductions).
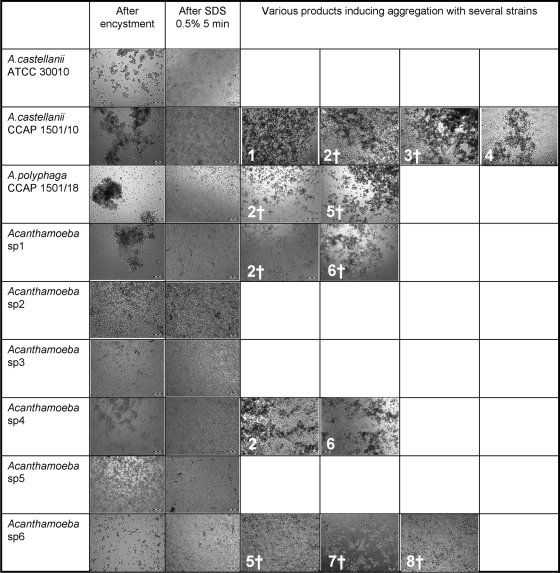
Aggregation tests.
Acanthamoeba castellanii strains ATCC 30010 and CCAP 1501/10, Acanthamoeba polyphaga strain CCAP 1501/18, and environmental isolates Acanthamoeba sp. strains 1 and 4 formed large aggregates during encystment in Neff's medium (Fig. 2). These aggregates were dissociated after treatment with 0.5% SDS for 5 min (Fig. 2). Several treatments induced aggregation, but it was limited only to several strains (Fig. 2); some of these treatments were still efficient at killing cysts (such as the hydrogen peroxide-based products, sodium hypochlorite, and ethanol), whereas others (PAA and hydrogen peroxide alone and a glutaraldehyde-based product) were not (Table 3).
FIG. 2.
Self-aggregation of cysts and treatment-induced aggregation of cysts. Cysts were observed after 7 days of incubation in Neff's medium, after treatment with 0.5% SDS for 5 min, and after treatment with various biocides for various contact times (only treatments that induced visible aggregation are reported). 1, 7.5% hydrogen peroxide for 30 min; 2, hydrogen peroxide-based product Resert XL-HLD for 10 min; 3, hydrogen peroxide/peracetic acid-based product Sporklenz RTU for 10 min; 4, 2% glutaraldehyde-based product for 30 min; 5, 70% ethanol for 10 min; 6, 0.2% peracetic acid for 10 min; 7, 0.25% sodium hypochlorite for 10 min; 8, 2.5% sodium hypochlorite for 10 min; †, no growth observed after treatment. Magnification, ×100.
Transmission electron microscopy.
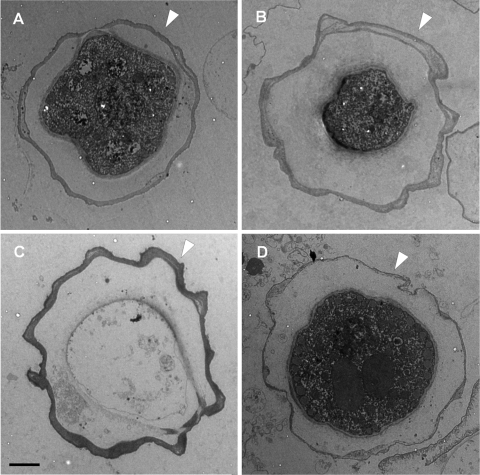
The measured thicknesses of the ectocysts were 0.4 ± 0.1 μm for strains 1 and 4, 0.6 ± 0.3 μm for strain 3, and 0.3 ± 0.1 μm for A. polyphaga CCAP 1501/18 (Fig. 3). Statistical analysis demonstrated that the ectocysts of strain 3 (Fig. 3B) were significantly thicker than the ectocysts of the three other strains and that the ectocysts of strains 1 and 4 (Fig. 3A and C) were significantly thicker than the ectocysts of A. polyphaga CCAP 1501/18 (Fig. 3D).
FIG. 3.
Cysts obtained from strains 1 (A), 3 (B), and 4 (C) and A. polyphaga strain CCAP 1501/18 (D). Arrows indicate ectocyst walls. Bar, 2 μm.
DISCUSSION
Free-living amoebae that belong to the Acanthamoeba genus represent a potential threat to human health due to not only their intrinsic pathogenicity (37) but also their role as protective and disseminating hosts for other pathogenic microorganisms (59). Due to their capacity to resist chemical and physical treatments used for drinking water production and distribution (36, 55, 57), they can colonize virtually any artificial water system. They are also widely distributed in moist areas, air, and dust (14, 42, 44). Despite increasing health concerns caused by these organisms, there is still a paucity of information concerning their inactivation by various physical and chemical biocides (59). In the present study we were mainly interested in testing the trophocidal and cysticidal activities of several chemical biocides used in health care settings to decontaminate medical instruments and surfaces. The amoebal strains tested included recent environmental isolates and culture collection strains. Characterization of six environmental strains using the 18S rDNA diagnostic fragment DF3 demonstrated that five of them belong to the genotype T4 group, which is also the group to which most isolates associated with keratitis and nonkeratitis infections belong (10, 33, 46). The other strain was identified as A. lenticulata, a species that belongs to the genotype T5 group. This species has rarely been associated with keratitis (50), with disseminated infection in an immunocompromised individual (5) and with acute granulomatous encephalitis in an immunocompetent individual (32) being noted. We also included collection strains in this representative set since it has been demonstrated that amoebae present markedly different properties after long-term axenic culture and have a decreased ability to encyst synchronously and a reduced temperature tolerance (31, 41).
Resistance of trophozoites to glutaraldehyde has already been documented with A. polyphaga strain Linc-Ap1 (21), which is identical to strain CCAP 1501/18 that was used in our study. The authors used trypan blue viability staining to demonstrate that a 2% glutaraldehyde-based product was not effective at killing trophozoites even after 3 h of exposure and that it actually induced encystment (21). We arrived at a similar conclusion using a different method to measure residual viability; however, the relatively shorter exposures did not allow demonstration of encystment. Aggregation tests with cysts did not reveal trophozoite aggregation induced by glutaraldehyde alone or in formulation (data not shown). Environmental isolates that belong to the T4 group presented significantly (P < 0.05) higher levels of resistance than collection strains that belong to the same group, except strain 1. Surprisingly, we observed better efficacy of glutaraldehyde against cysts than against trophozoites for strains 1, 4, and 5; A. castellanii ATCC 30010; and A. polyphaga CCAP 1501/18 (P < 0.05). A possible explanation is that cross-linking of cyst external structures impairs deencystment even if the amoebae remain viable; alternatively, the cyst structure may be more sensitive to the activity of this biocide. Viability staining of cysts treated with glutaraldehyde might be useful to understand these effects. Resistance of Acanthamoeba trophozoites to glutaraldehyde raises important questions about the potential association of glutaraldehyde-resistant Acanthamoeba with nontuberculous mycobacteria (NTM) that can also resist glutaraldehyde and grow within amoebae (18, 23, 58). It has been demonstrated that the resistance of NTM to glutaraldehyde is at least partially due to mutations in cell wall porins and that these mutations also increase the resistance of NTM to antibiotics commonly used to treat these infections (53). Furthermore, mutations in the same genes also increase the intra-amoebal survival capacity of NTM (49). In a critical scenario, glutaraldehyde could thus select for NTM strains that are resistant to antibiotics and that can multiply within Acanthamoeba trophozoites that also resist exposure to glutaraldehyde.
Previously described quantitative kill assays were used as references for the cyst inactivation assay used in this study (7, 29; see www.fda.gov/downloads/MedicalDevices/NewsEvents/WorkshopsConferences/UCM130752.ppt). Neff's encystment medium was chosen since it allows recovery of high numbers of cysts and it may better represent the conditions encountered by amoebae in water networks than other conditions used to produce cysts (25). SDS (0.5%) was used after encystment to kill immature cysts and dissociate aggregates (35), although these aggregates may need to be further considered, as they likely exist naturally in the environment. D/E broth was confirmed to adequately neutralize the various biocides and has been demonstrated to be nontoxic to A. castellanii (11). Seeding of treated cysts onto E. coli lawns was preferred to direct seeding into PYG medium since it leads to higher recovery rates.
We demonstrated significant strain-specific variability in resistance of the cysts to treatments, with strain 3 being the most resistant (P < 0.05), followed by strains 2 and 4 (P < 0.001). Of note, both strains 3 and strain 4 were isolated from a hospital water network (56), thus demonstrating that strains isolated in health care settings can potentially resist the disinfection treatments used in these facilities. Other strains presented intermediate resistance profiles. The observed differences in resistance may partly be due to the ectocyst structure, since it was significantly (P < 0.001) thicker in the more resistant strain than in the others. This cyst wall structure has been demonstrated to contain large amounts of cellulose (15), and an increase in cyst wall cellulose content during encystment has been demonstrated to coincide with increasing resistance to biocides (60). Other mechanisms may also play a role, since the cysts of strain 4 presented a thickness similar to those of strain 1 but were significantly (P < 0.001) more resistant to treatments.
We confirmed that the minimal cysticidal temperature is 65°C for most Acanthamoeba sp. isolates, with the cysticidal efficacy being from almost no effect to complete kill when the temperature is increased from 55°C to 65°C. These differences confirm previous reports from studies with cysts of A. polyphaga (28). It should be noted that the cysts of some thermotolerant isolates could resist exposure at 80°C for 10 min (52). Hydrogen peroxide (7.5%) showed limited activity even after 30 min exposure for resistant strains. Conversely, a hydrogen peroxide-based product, despite having lower concentrations of peroxide (2%), showed greater efficacy (P < 0.001). Differences in the cysticidal activities of lens care solutions containing 3% hydrogen peroxide have already been demonstrated (26), thus highlighting the critical importance of nonactive ingredients, notably surfactants, for the microbicidal activities of formulations. The same remark applies to peracetic acid alone compared to peracetic acid in formulation: two strains resisted very well exposure to 0.2% PAA alone for 10 min, whereas only one strain showed slight resistance after exposure to STERIS-20 for 10 min at 55°C (identical PAA concentration; recommended contact time, 12 min). In this case, increased activity (P < 0.001) might also be due to the synergistic effects of surfactants and elevated temperature, since it has been reported that a PAA-based product containing a higher biocide concentration was not fully active against A. polyphaga cysts after 10 min exposure at room temperature (21). We were surprised by the relatively good efficacy of ethanol, since this chemical shows limited activity against various microorganisms, including bacteria, viruses, and protozoa (4, 8, 20); further viability studies may also be interesting in this case due to the fixing mechanism of action associated with this biocide. Sodium hypochlorite was fully efficient at 2.5% (2,500 ppm) but required longer exposures when it was diluted 1/10, and one strain (strain 3) resisted this concentration even after 30 min exposure. Chlorine is considered ineffective against Acanthamoeba cysts, notably at the low residual concentrations (2 to 5 ppm) used to control microbial flora in drinking water networks. Surprisingly, ortho-phthalaldehyde alone showed better efficacy than an OPA-based product after 10 min exposure for strains 1, 2, and 3 and the three collection strains. This might be due to additional compounds (phosphate buffering salts, corrosion inhibitors, chelating agents) in the formulation, changing the biocidal activity or availability of the active molecule against cysts (16). The OPA-based product showed good efficacy after 20 min exposure but required 30 min exposure to become fully efficient against resistant strains 3 and 4. The limited efficacy of glutaraldehyde against other dormant forms has already been reported, requiring more than 10 h of contact time for the complete inactivation of Cryptosporidium oocysts (63). Furthermore, aldehydes are known to have fixative properties, which could lead to the accumulation of organic soils and render inactivation even more difficult (62). As for OPA, glutaraldehyde alone showed better efficacy against cysts than the glutaraldehyde-based product after 10 min exposure for strains 5 and 6.
As already mentioned, amoebal cysts are ubiquitous in drinking water networks but can also contaminate various surfaces and travel through the air in droplets as well as in dry forms. Their potential role as vehicles for various pathogenic microbial species and their intrinsic resistance to high-level disinfectants highlight the need to better evaluate and understand the actions of these treatments against these Trojan horses of the microbial world (6). Standardized procedures for determining the efficacies of contact lens solutions are under investigation by various working groups (26); such procedures should also be investigated for testing the disinfection treatments used for other purposes.
Footnotes
Published ahead of print on 2 June 2010.
REFERENCES
- 1.Adekambi, T., S. Ben Salah, M. Khlif, D. Raoult, and M. Drancourt. 2006. Survival of environmental mycobacteria in Acanthamoeba polyphaga. Appl. Environ. Microbiol. 72:5974-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anger, C., and J. M. Lally. 2008. Acanthamoeba: a review of its potential to cause keratitis, current lens care solution disinfection standards and methodologies, and strategies to reduce patient risk. Eye Contact Lens 34:247-253. [DOI] [PubMed] [Google Scholar]
- 3.Barbaree, J. M., B. S. Fields, J. C. Feeley, G. W. Gorman, and W. T. Martin. 1986. Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl. Environ. Microbiol. 51:422-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbee, S. L., D. J. Weber, M. D. Sobsey, and W. A. Rutala. 1999. Inactivation of Cryptosporidium parvum oocyst infectivity by disinfection and sterilization processes. Gastrointest. Endosc. 49:605-611. [DOI] [PubMed] [Google Scholar]
- 5.Barete, S., A. Combes, J. F. de Jonckheere, A. Datry, S. Varnous, V. Martinez, S. G. Ptacek, E. Caumes, F. Capron, C. Frances, C. Gibert, and O. Chosidow. 2007. Fatal disseminated Acanthamoeba lenticulata infection in a heart transplant patient. Emerg. Infect. Dis. 13:736-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker, J., and M. R. Brown. 1994. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140(Pt 6):1253-1259. [DOI] [PubMed] [Google Scholar]
- 7.Beattie, T. K., D. V. Seal, A. Tomlinson, A. K. McFadyen, and A. M. Grimason. 2003. Determination of amoebicidal activities of multipurpose contact lens solutions by using a most probable number enumeration technique. J. Clin. Microbiol. 41:2992-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Best, M., S. A. Sattar, V. S. Springthorpe, and M. E. Kennedy. 1990. Efficacies of selected disinfectants against Mycobacterium tuberculosis. J. Clin. Microbiol. 28:2234-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Booton, G. C., D. J. Kelly, Y. W. Chu, D. V. Seal, E. Houang, D. S. Lam, T. J. Byers, and P. A. Fuerst. 2002. 18S ribosomal DNA typing and tracking of Acanthamoeba species isolates from corneal scrape specimens, contact lenses, lens cases, and home water supplies of Acanthamoeba keratitis patients in Hong Kong. J. Clin. Microbiol. 40:1621-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booton, G. C., G. S. Visvesvara, T. J. Byers, D. J. Kelly, and P. A. Fuerst. 2005. Identification and distribution of Acanthamoeba species genotypes associated with nonkeratitis infections. J. Clin. Microbiol. 43:1689-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buck, S. L., and R. A. Rosenthal. 1996. A quantitative method to evaluate neutralizer toxicity against Acanthamoeba castellanii. Appl. Environ. Microbiol. 62:3521-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buck, S. L., R. A. Rosenthal, and B. A. Schlech. 2000. Methods used to evaluate the effectiveness of contact lens care solutions and other compounds against Acanthamoeba: a review of the literature. CLAO J. 26:72-84. [PubMed] [Google Scholar]
- 13.Byers, T. J., B. G. Kim, L. E. King, and E. R. Hugo. 1991. Molecular aspects of the cell cycle and encystment of Acanthamoeba. Rev. Infect. Dis. 13(Suppl. 5):S373-S384. [DOI] [PubMed] [Google Scholar]
- 14.Carlesso, A. M., G. L. Artuso, K. Caumo, and M. B. Rott. 2010. Potentially pathogenic Acanthamoeba isolated from a hospital in Brazil. Curr. Microbiol. 60:185-190. [DOI] [PubMed] [Google Scholar]
- 15.Chavez-Munguia, B., M. Omana-Molina, M. Gonzalez-Lazaro, A. Gonzalez-Robles, P. Bonilla, and A. Martinez-Palomo. 2005. Ultrastructural study of encystation and excystation in Acanthamoeba castellanii. J. Eukaryot. Microbiol. 52:153-158. [DOI] [PubMed] [Google Scholar]
- 16.Critchley, M., and R. Bentham. 2009. The efficacy of biocides and other chemical additives in cooling water systems in the control of amoebae. J. Appl. Microbiol. 106:784-789. [DOI] [PubMed] [Google Scholar]
- 17.Dart, J. K., V. P. Saw, and S. Kilvington. 2009. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am. J. Ophthalmol. 148:487-499. [DOI] [PubMed] [Google Scholar]
- 18.Duarte, R. S., M. C. Lourenco, S. Fonseca Lde, S. C. Leao, L. Amorim Ede, I. L. Rocha, F. S. Coelho, C. Viana-Niero, K. M. Gomes, M. G. da Silva, N. S. Lorena, M. B. Pitombo, R. M. Ferreira, M. H. Garcia, G. P. de Oliveira, O. Lupi, B. R. Vilaca, L. R. Serradas, A. Chebabo, E. A. Marques, L. M. Teixeira, M. Dalcolmo, S. G. Senna, and J. L. Sampaio. 2009. Epidemic of postsurgical infections caused by Mycobacterium massiliense. J. Clin. Microbiol. 47:2149-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edgar, R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eterpi, M., G. McDonnell, and V. Thomas. 2009. Disinfection efficacy against parvoviruses in comparison to reference viruses. J. Hosp. Infect. 73:64-70. [DOI] [PubMed] [Google Scholar]
- 21.Greub, G., and D. Raoult. 2003. Biocides currently used for bronchoscope decontamination are poorly effective against free-living amoebae. Infect. Control Hosp. Epidemiol. 24:784-786. [DOI] [PubMed] [Google Scholar]
- 22.Greub, G., and D. Raoult. 2004. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17:413-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffiths, P. A., J. R. Babb, C. R. Bradley, and A. P. Fraise. 1997. Glutaraldehyde-resistant Mycobacterium chelonae from endoscope washer disinfectors. J. Appl. Microbiol. 82:519-526. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann, R., and R. Michel. 2001. Distribution of free-living amoebae (FLA) during preparation and supply of drinking water. Int. J. Hyg. Environ. Health 203:215-219. [DOI] [PubMed] [Google Scholar]
- 25.Hughes, R., W. Heaselgrave, and S. Kilvington. 2003. Acanthamoeba polyphaga strain age and method of cyst production influence the observed efficacy of therapeutic agents and contact lens disinfectants. Antimicrob. Agents Chemother. 47:3080-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston, S. P., R. Sriram, Y. Qvarnstrom, S. Roy, J. Verani, J. Yoder, S. Lorick, J. Roberts, M. J. Beach, and G. Visvesvara. 2009. Resistance of Acanthamoeba cysts to disinfection in multiple contact lens solutions. J. Clin. Microbiol. 47:2040-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khunkitti, W., D. Lloyd, J. R. Furr, and A. D. Russell. 1998. Acanthamoeba castellanii: growth, encystment, excystment and biocide susceptibility. J. Infect. 36:43-48. [DOI] [PubMed] [Google Scholar]
- 28.Kilvington, S. 1991. Moist-heat disinfection of Acanthamoeba cysts. Rev. Infect. Dis. 13(Suppl. 5):S418. [DOI] [PubMed] [Google Scholar]
- 29.Kilvington, S., and C. Anger. 2001. A comparison of cyst age and assay method of the efficacy of contact lens disinfectants against Acanthamoeba. Br. J. Ophthalmol. 85:336-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilvington, S., T. Gray, J. Dart, N. Morlet, J. R. Beeching, D. G. Frazer, and M. Matheson. 2004. Acanthamoeba keratitis: the role of domestic tap water contamination in the United Kingdom. Invest. Ophthalmol. Vis. Sci. 45:165-169. [DOI] [PubMed] [Google Scholar]
- 31.Kohsler, M., D. Leitsch, U. Furnkranz, M. Duchene, H. Aspock, and J. Walochnik. 2008. Acanthamoeba strains lose their abilities to encyst synchronously upon prolonged axenic culture. Parasitol. Res. 102:1069-1072. [DOI] [PubMed] [Google Scholar]
- 32.Lackner, P., R. Beer, G. Broessner, R. Helbok, B. Pfausler, C. Brenneis, H. Auer, J. Walochnik, and E. Schmutzhard. 2010. Acute granulomatous Acanthamoeba encephalitis in an immunocompetent patient. Neurocrit. Care 12:91-94. [DOI] [PubMed] [Google Scholar]
- 33.Ledee, D. R., A. Iovieno, D. Miller, N. Mandal, M. Diaz, J. Fell, M. E. Fini, and E. C. Alfonso. 2009. Molecular identification of T4 and T5 genotypes in isolates from Acanthamoeba keratitis patients. J. Clin. Microbiol. 47:1458-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd, D., N. A. Turner, W. Khunkitti, A. C. Hann, J. R. Furr, and A. D. Russell. 2001. Encystation in Acanthamoeba castellanii: development of biocide resistance. J. Eukaryot. Microbiol. 48:11-16. [DOI] [PubMed] [Google Scholar]
- 35.Lorenzo-Morales, J., J. Kliescikova, E. Martinez-Carretero, L. M. De Pablos, B. Profotova, E. Nohynkova, A. Osuna, and B. Valladares. 2008. Glycogen phosphorylase in Acanthamoeba spp.: determining the role of the enzyme during the encystment process using RNA interference. Eukaryot. Cell 7:509-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loret, J. F., M. Jousset, S. Robert, C. Anselme, G. Saucedo, F. Ribas, A. Martinez, and V. Catalan. 2008. Elimination of free-living amoebae by drinking water treatment processes. Eur. J. Water Quality 39:37-50. [Google Scholar]
- 37.Marciano-Cabral, F., and G. Cabral. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazur, T., E. Hadas, and I. Iwanicka. 1995. The duration of the cyst stage and the viability and virulence of Acanthamoeba isolates. Trop. Med. Parasitol. 46:106-108. [PubMed] [Google Scholar]
- 39.Nagington, J., P. G. Watson, T. J. Playfair, J. McGill, B. R. Jones, and A. D. M. Steele. 1974. Amoebic infection of the eye. Lancet 304:1537-1540. [DOI] [PubMed] [Google Scholar]
- 40.Neff, R. J., S. A. Ray, W. F. Benton, and M. Wilborn. 1964. Induction of synchronous encystation (differentiation) in Acanthamoeba spp., p. 55-83. In D. M. Prescott (ed.), Methods in cell physiology, vol. 1. Academic Press, New York, NY. [Google Scholar]
- 41.Pumidonming, W., M. Koehsler, and J. Walochnik. 2010. Acanthamoeba strains show reduced temperature tolerance after long-term axenic culture. Parasitol. Res. 106:553-559. [DOI] [PubMed] [Google Scholar]
- 42.Rivera, F., P. Bonilla, E. Ramirez, A. Calderon, E. Gallegos, S. Rodriguez, R. Ortiz, D. Hernandez, and V. Rivera. 1994. Seasonal distribution of air-borne pathogenic and free-living amoebae in Mexico City and its suburbs. Water Air Soil Pollution 74:65-87. [Google Scholar]
- 43.Rodriguez-Zaragoza, S. 1994. Ecology of free-living amoebae. Crit. Rev. Microbiol. 20:225-241. [DOI] [PubMed] [Google Scholar]
- 44.Rohr, U., S. Weber, R. Michel, F. Selenka, and M. Wilhelm. 1998. Comparison of free-living amoebae in hot water systems of hospitals with isolates from moist sanitary areas by identifying genera and determining temperature tolerance. Appl. Environ. Microbiol. 64:1822-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos, L. C., M. S. Oliveira, R. D. Lobo, H. R. Higashino, S. F. Costa, I. M. van der Heijden, M. C. Giudice, A. R. Silva, and A. S. Levin. 2009. Acanthamoeba spp. in urine of critically ill patients. Emerg. Infect. Dis. 15:1144-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schroeder, J. M., G. C. Booton, J. Hay, I. A. Niszl, D. V. Seal, M. B. Markus, P. A. Fuerst, and T. J. Byers. 2001. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J. Clin. Microbiol. 39:1903-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroeder-Diedrich, J. M., P. A. Fuerst, and T. J. Byers. 1998. Group-I introns with unusual sequences occur at three sites in nuclear 18S rRNA genes of Acanthamoeba lenticulata. Curr. Genet. 34:71-78. [DOI] [PubMed] [Google Scholar]
- 48.Reference deleted.
- 49.Sharbati-Tehrani, S., J. Stephan, G. Holland, B. Appel, M. Niederweis, and A. Lewin. 2005. Porins limit the intracellular persistence of Mycobacterium smegmatis. Microbiology 151:2403-2410. [DOI] [PubMed] [Google Scholar]
- 50.Spanakos, G., K. Tzanetou, D. Miltsakakis, E. Patsoula, E. Malamou-Lada, and N. C. Vakalis. 2006. Genotyping of pathogenic acanthamoebae isolated from clinical samples in Greece—report of a clinical isolate presenting T5 genotype. Parasitol. Int. 55:147-149. [DOI] [PubMed] [Google Scholar]
- 51.Sriram, R., M. Shoff, G. Booton, P. Fuerst, and G. S. Visvesvara. 2008. Survival of Acanthamoeba cysts after desiccation for more than 20 years. J. Clin. Microbiol. 46:4045-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Storey, M. V., J. Winiecka-Krusnell, N. J. Ashbolt, and T. A. Stenstrom. 2004. The efficacy of heat and chlorine treatment against thermotolerant acanthamoebae and legionellae. Scand. J. Infect. Dis. 36:656-662. [DOI] [PubMed] [Google Scholar]
- 53.Svetlikova, Z., H. Skovierova, M. Niederweis, J. L. Gaillard, G. McDonnell, and M. Jackson. 2009. The role of porins in the susceptibility of Mycobacterium smegmatis and Mycobacterium chelonae to aldehyde-based disinfectants and drugs. Antimicrob. Agents Chemother. 53:4015-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reference deleted.
- 55.Thomas, V., T. Bouchez, V. Nicolas, S. Robert, J. F. Loret, and Y. Lévi. 2004. Amoebae in domestic water systems: resistance to disinfection treatments and implication in Legionella persistence. J. Appl. Microbiol. 97:950-963. [DOI] [PubMed] [Google Scholar]
- 56.Thomas, V., K. Herrera-Rimann, D. S. Blanc, and G. Greub. 2006. Biodiversity of amoebae and amoebae-resisting bacteria in a hospital water network. Appl. Environ. Microbiol. 72:2428-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas, V., J. F. Loret, M. Jousset, and G. Greub. 2008. Biodiversity of amoebae and amoebae-resisting bacteria in a drinking water treatment plant. Environ. Microbiol. 10:2728-2745. [DOI] [PubMed] [Google Scholar]
- 58.Thomas, V., and G. McDonnell. 2007. Relationship between mycobacteria and amoebae: ecological and epidemiological concerns. Lett. Appl. Microbiol. 45:349-357. [DOI] [PubMed] [Google Scholar]
- 59.Thomas, V., G. McDonnell, S. P. Denyer, and J. Y. Maillard. 2010. Free-living amoebae and their intracellular pathogenic microorganisms: risks for water quality. FEMS Microbiol. Rev. 34:231-259. [DOI] [PubMed] [Google Scholar]
- 60.Turner, N. A., A. D. Russell, J. R. Furr, and D. Lloyd. 2000. Emergence of resistance to biocides during differentiation of Acanthamoeba castellanii. J. Antimicrob. Chemother. 46:27-34. [DOI] [PubMed] [Google Scholar]
- 61.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson, J., and A. B. Margolin. 2003. Efficacy of glutaraldehyde disinfectant against Cryptosporidium parvum in the presence of various organic soils. J. AOAC Int. 86:96-100. [PubMed] [Google Scholar]
- 63.Wilson, J. A., and A. B. Margolin. 1999. The efficacy of three common hospital liquid germicides to inactivate Cryptosporidium parvum oocysts. J. Hosp. Infect. 42:231-237. [DOI] [PubMed] [Google Scholar]