Abstract
Frontotemporal lobar degeneration (FTLD) is a clinically and pathologically heterogeneous syndrome, characterized by progressive decline in behaviour or language associated with degeneration of the frontal and anterior temporal lobes. While the seminal cases were described at the turn of the 20th century, FTLD has only recently been appreciated as a leading cause of dementia, particularly in patients presenting before the age of 65 years. Three distinct clinical variants of FTLD have been described: (i) behavioural-variant frontotemporal dementia, characterized by changes in behaviour and personality in association with frontal-predominant cortical degeneration; (ii) semantic dementia, a syndrome of progressive loss of knowledge about words and objects associated with anterior temporal neuronal loss; and (iii) progressive nonfluent aphasia, characterized by effortful language output, loss of grammar and motor speech deficits in the setting of left perisylvian cortical atrophy.
The majority of pathologies associated with FTLD clinical syndromes include either tau-positive (FTLD-TAU) or TAR DNA-binding protein 43 (TDP-43)-positive (FTLD-TDP) inclusion bodies. FTLD overlaps clinically and pathologically with the atypical parkinsonian disorders corticobasal degeneration and progressive supranuclear palsy, and with amyotrophic lateral sclerosis. The majority of familial FTLD cases are caused by mutations in the genes encoding microtubule-associated protein tau (leading to FTLD-TAU) or progranulin (leading to FTLD-TDP). The clinical and pathologic heterogeneity of FTLD poses a significant diagnostic challenge, and in vivo prediction of underlying histopathology can be significantly improved by supplementing the clinical evaluation with genetic tests and emerging biological markers. Current pharmacotherapy for FTLD focuses on manipulating serotonergic or dopaminergic neurotransmitter systems to ameliorate behavioural or motor symptoms. However, recent advances in FTLD genetics and molecular pathology make the prospect of biologically driven, disease-specific therapies for FTLD seem closer than ever.
Frontotemporal lobar degeneration (FTLD) is a clinically and pathologically heterogeneous syndrome, characterized by a progressive decline in behaviour or language associated with degeneration of the frontal and anterior temporal lobes. The original cases described by Arnold Pick and Alois Alzheimer (new references #1, #2)[84] demonstrated neuronal inclusions that were later shown to be tau-positive at histopathology. The link between tau and FTLD was further strengthened by the discovery that mutations in the microtubule-associated protein tau (MAPT) gene cause familial FTLD.[126–128] In 2006, the TAR DNA-binding protein 43 (TDP-43) was identified as the major ubiquitinated protein associated with tau-negative FTLD,[96] and mutations in the progranulin (PGRN) gene were shown to be responsible for the majority of familial tau-negative cases. [129–131] These recent discoveries coincide with the publication of autopsy series from multiple centres that have helped elucidate clinicopathologic correlations in FTLD, and with the development of biological markers that can further improve in vivo prediction of underlying histopathology. Together, these advances have created the opportunity to develop biologically driven, disease-specific therapies for FTLD.
In this article, we review the epidemiology, clinical presentations and pathophysiology of the FTLD-spectrum disorders. We also discuss current evidence for symptomatic treatment, and speculate on future therapeutic strategies.
1. Terminology
Nomenclature in FTLD can be confusing, with different terminologies applied throughout the literature. In this review, we adopt the terminology used in the 1998 Consensus Criteria published by Neary and colleagues.[1] FTLD is used as an umbrella term for three clinical variants that can be distinguished based on the early and predominant symptoms: behavioural-variant frontotemporal dementia (bvFTD); semantic dementia (SD); and progressive nonfluent aphasia (PNFA). FTLD-associated pathologies are subclassified into disorders with tau-inclusion bodies (FTLD-TAU) and those with TDP-43 inclusions (FTLD-TDP). FTLD shows significant clinical and pathologic overlap with the atypical parkinsonian disorders corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP), and these are also considered in this review.
2. Epidemiology
The prevalence of FTLD in population-based studies has varied between 2.7/100,000 (with a peak of 9.4/100,000 in the 60- to 69-year age group) in the Zuid-Holland district in the Netherlands,[2] to 15.1/100,000 in adults aged <65 years in Cambridge, UK,[3] which is identical to the prevalence of Alzheimer’s disease in the latter population. Two studies have reported a similar incidence of FTLD and Alzheimer’s disease in adults with early age-of-onset dementia in Cambridge, UK (3.5/100,000 person-years for FTLD, 4.2/100,000 person-years for Alzheimer’s disease in 45- to 64-year-olds)[4] and Rochester, Minnesota, USA (3.3/100,000 person-years for both diseases in the 50- to 59-year age group).[5] The disease typically presents in the sixth decade, although the age of onset can vary widely from the third to the ninth decade.[3,6,7] Although FTLD is generally considered a presenile dementia, individuals over the age of 65 years account for 20–25% of all cases.[3,6,7] Gislason and colleagues[8] found a surprisingly high (3%) prevalence of bvFTD when screening a cohort of 85-year-olds in Gothenburg, Sweden. Autopsy studies based on consecutive, unselected cases have demonstrated that FTLD accounts for roughly 5% of all pathologic diagnoses in patients with dementia.[9,10] However, this is likely to represent an underestimate of the true prevalence, since many of the autopsies reported in these series predated the modern molecular techniques currently used to diagnose FTLD (see section 4). Taken together, these epidemiologic data suggest that FTLD is a common cause of early-onset (age <65 years) dementia, with an incidence and prevalence similar to Alzheimer’s disease, and is likely to be an underappreciated cause of dementia in older individuals.
Sex distribution in FTLD appears to vary by clinical syndrome, with most studies reporting a male preponderance in bvFTD, and a number of studies describing a male predominance in SD and a female predominance in PNFA.[3,6,7,11] Median survival in FTLD has been estimated at 6–11 years from symptom onset and 3–4 years from diagnosis.[7,11–13] In our centre, bvFTD is associated with the shortest survival (median 8.7 years from onset), SD with the longest survival (11.9 years) and PNFA with intermediate survival (9.4 years),[11] while the Cambridge group has reported the longest survival in PNFA (mean 10.6 years from onset) followed by bvFTD (8.2 years) and SD (6.9 years).[7] Across centres, the presence of motor neuron disease is associated with early mortality (2.4–4.9 years from onset and 1.2–1.4 years from diagnosis).[7,11] Overall, survival is shorter and cognitive and functional decline are more rapid than in Alzheimer’s disease.[11,12]
3. Clinical Syndromes
FTLD patients can be classified into three clinical syndromes depending on the early and predominant symptoms: a behavioural variant (bvFTD) and two language variants (SD and PNFA).[1] Each clinical variant is associated with a distinct regional pattern of brain atrophy and, to a varying degree, a characteristic histopathology (figure 1). Overlap between the syndromes can occur,[14] particularly later in the course as the disease spreads to involve the frontal and temporal lobes more diffusely.
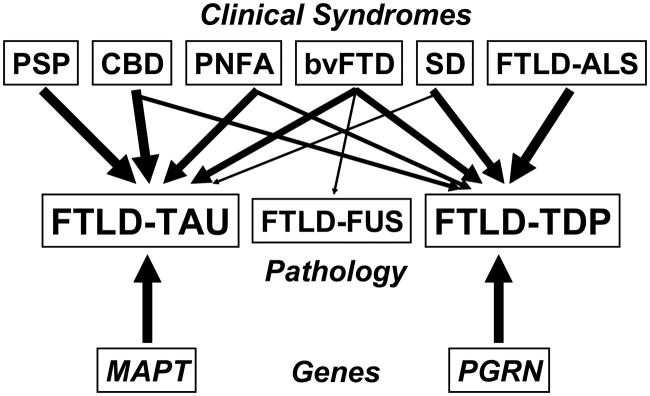
Fig. 1.
Clinical, pathologic and genetic spectrum of frontotemporal lobar degeneration (FTLD). Clinical syndromes (top row), pathologic subtypes (middle row) and common gene mutations (bottom row) in FTLD are shown. Arrows represent links between clinical syndromes, genes and underlying histopathology, with thicker arrows corresponding to stronger relationships. bvFTD = behavioural-variant frontotemporal dementia; CBD = corticobasal degeneration; FTLD-ALS = FTLD with amyotrophic lateral sclerosis; FTLD-TAU = FTLD with tau-positive inclusions; FTLD-FUS = FTLD with fused in sarcoma (FUS)-positive inclusions; FTLD-TDP = FTLD with TAR DNA-binding protein 43 (TDP-43)-positive inclusions; MAPT = microtubule-associated protein tau; PGRN = progranulin; PNFA = progressive nonfluent aphasia; PSP = progressive supranuclear palsy; SD = semantic dementia.
3.1 Behavioural-Variant Frontotemporal Dementia
Patients with this clinical variant present with marked changes in personality and behaviour, and often display a mixture of apathy and disinhibition.[1,15–21] Apathy is characterized by loss of interest in personal affairs and responsibilities, social withdrawal and, as the disease advances, loss of awareness of personal hygiene and sphincter control. Disinhibition is manifested by a multitude of socially inappropriate behaviours, including confrontation seeking, making hurtful or insensitive remarks to others, engaging in frankly sociopathic behaviours (e.g. shoplifting, traffic violations) or (rarely in our experience) physical assault.[22,23] Patients appear cold and unempathetic, showing little concern for the effect of their behaviour on loved ones.[24] Insight is dramatically impaired, with either frank denial of illness or very shallow recognition of a cognitive problem (often described as mild memory problems or word-finding difficulties).[1] Some patients develop dramatic changes in religious beliefs, political convictions, or dress and social style, personality changes that are so profound they have been described as a “change in self”.[25] Repetitive motor behaviours (e.g. rubbing, picking, throat clearing, pacing and wandering), idiosyncratic hoarding and collecting, changes in eating behaviour (e.g. overeating and weight gain, loss of table manners) and hyperorality (including oral exploration of inedible objects) are also common.[26–32]
Cognitive decline is typically less dramatic than the behavioural disturbance, and patients may require institutionalization despite normal performance on neuropsychometric tests.[33] The most common cognitive symptoms are poor judgment, inattentiveness and distractibility, loss of planning ability and disorganization. On cognitive testing, patients often show deficits on frontal/executive tasks, such as tests of set shifting, mental flexibility, response inhibition and abstract reasoning.[18,34–36] Attention and working memory may be impaired, while episodic memory is variably spared.[35,37–39] Visuospatial function is almost always preserved early in the disease (in contrast to Alzheimer’s disease).[40] The presence of rule violations, perseverative errors and confabulations during testing is highly characteristic, and can help differentiate bvFTD from other disorders.[35,41]
Amyotrophic lateral sclerosis (ALS) can co-occur with any of the FTLD clinical variants, but is most commonly associated with bvFTD.[42] When prospectively studied, approximately half of all bvFTD patients meet criteria for possible or probable ALS, while half of ALS patients show behavioural or cognitive deficits suggestive of bvFTD.[43,44] Patients with FTLD-ALS can present with either cognitive or motor dysfunction.[42] Bulbar-onset motor neuron disease is most common, although patients may present with either upper or lower motor neuron signs in any myotomal distribution.[44–46] Because of the strong association between FTLD and ALS, all FTLD patients should undergo a careful neuromuscular evaluation (with consideration for electromyography), while all ALS patients should be screened for behavioural and cognitive changes.
Structural and functional neuroimaging studies have highlighted frontal atrophy, hypometabolism and hypoperfusion in patients with bvFTD (figure 2a and b).[47–49] While the dorsolateral prefrontal cortex is often involved, the earliest changes occur in a medial paralimbic network that includes anterior cingulate, orbital frontal and frontoinsular cortices, and atrophy in these regions best distinguishes FTLD from Alzheimer’s disease.[47,50,51] The region of greatest atrophy within this network correlates with the clinical phenotype, with dorsomedial frontal atrophy associated with apathy and aberrant motor behaviour, and orbitofrontal atrophy associated with disinhibition.[52]
Fig. 2.
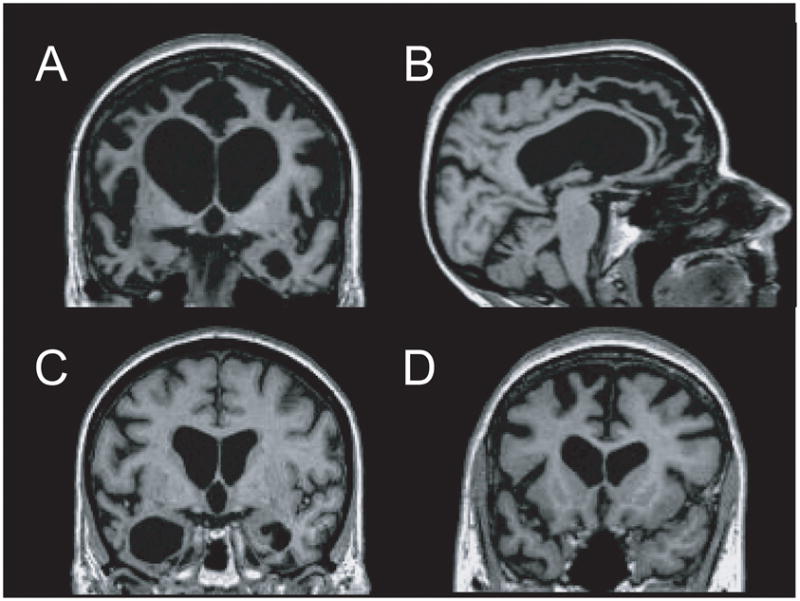
MRI findings in frontotemporal lobar degeneration (FTLD). T1-weighted images from representative patients with behavioural-variant frontotemporal dementia (bvFTD) [a and b], semantic dementia (SD) [c] and progressive nonfluent aphasia (PNFA) [d] are displayed in neurologic orientation. (a and b) bvFTD patient shows marked atrophy throughout the medial and lateral frontal cortex and the temporal poles, with striking relative preservation of the posterior brain regions on a sagittal view. (c) Patient with SD shows asymmetric degeneration of the temporal poles (left greater than right). (d) PNFA patient shows atrophy in the left inferolateral and dorsomedial frontal cortex and anterior insula.
3.2 Semantic Dementia
SD, also referred to as the temporal-variant of FTLD, is characterized by a fluent, anomic aphasia and behavioural changes in the setting of marked, often asymmetric degeneration of the anterior temporal lobes (figure 2c).[1,53] Patients with primarily left-sided atrophy present initially with progressive loss of ‘semantic’ knowledge about words, objects and concepts.[1,54–56] This is manifest as a fluent aphasia with impoverished speech content and semantic paraphasic errors, but intact syntax, prosody and motor speech.[57,58] Loss of meaning follows a hierarchical pattern; for example, patients may first lose their ability to differentiate between types of dogs, and later become unable to distinguish dogs from other animals. Eventually, all animals may be referred to as ‘things’. [57] With time, loss of knowledge extends beyond language, and patients develop features of a multimodal agnosia.[57,59] On cognitive testing, patients perform poorly on tests of confrontation naming, word-to-picture matching and category fluency, while episodic memory (particularly visual memory), spatial abilities and executive functions are spared.[56,57,60–62]
Patients with predominant right anterior temporal atrophy present with a behavioural syndrome that overlaps with bvFTD.[54,62] Patients develop emotional blunting with a flat, bizarre affect that has been described as ‘alien’. There is a marked loss of empathy and interest in others, and social behaviour is described by informants as awkward, tactless and impervious to social cues.[56,63] Rigidity is common and manifests with strict schedules and routines, clock watching and restrictive dieting or food fads. Prosopagnosia and associative agnosia are emphasized in the current diagnostic criteria,[1] but behavioural changes often precede these findings by years.[54] Compared with patients with bvFTD, patients with right-predominant SD tend to be more rigid, have distinct types of compulsions and eating disorders, and have a higher rate of constitutional symptoms such as sleep disorders, weight loss and sexual dysfunction.[15,54,64–66]
Patients with right-sided SD typically develop the semantic-loss characteristic of the left temporal variant after a mean of 3 years, as the disease spreads to the contralateral temporal pole, while patients with left temporal SD develop the behavioural changes associated with right temporal disease within a similar timeframe.[54] Compulsions are common in both patient groups, with left temporal patients developing visually oriented compulsions (e.g. collecting bright objects), while right temporal patients develop verbally oriented compulsions (e.g. word jumbles, punning).[54] Disinhibition and apathy reminiscent of bvFTD develop 5–7 years after symptom onset, perhaps reflecting spread of the disease into the frontoinsular network.[47] Left-sided SD patients outnumber right-sided patients 3:1 in most series, although this may reflect a referral bias to behavioural neurology centres, since right-sided patients may be misdiagnosed as having a primary psychiatric disorder.[54,56]
3.3 Progressive Nonfluent Aphasia
PNFA is a progressive disorder of language expression and motor speech associated with left perisylvian atrophy.[1,58] Patients present with slow, effortful speech, impaired production and comprehension of grammar (agrammatism), and motor speech deficits. Apraxia of speech, defined as difficulty initiating speech, a slow rate of speech or incorrect sequencing or omission of phonemes, is highly characteristic of PNFA, while dysarthria is more variably present.[58,67] Additional language features include phonemic paraphasic errors and mild anomia (without associated semantic loss). Comprehension is spared for single words and for all but the most complex syntactic structures. Reading is nonfluent and effortful, while writing is agrammatic and features phonemic paraphasias. The elemental neurologic examination may reveal supranuclear gaze palsies, parkinsonism and limb apraxia, reflecting a frequent association with CBD and PSP (see section 3.4). Neuropsychometric testing may show (in addition to the aphasia) mild deficits in working memory and executive function, with sparing of episodic memory and visuospatial function.[58] Behavioural disturbances can occur, but are less frequent and severe than in bvFTD and SD.[68] Anatomically, PNFA is associated with atrophy, hypometabolism and hypoperfusion of the left frontal operculum, premotor and supplementary motor areas and anterior insula (figure 2d).[58,69]
3.4 Frontotemporal Lobar Degeneration (FTLD) Overlap Syndromes: Corticobasal Degeneration and Progressive Supranuclear Palsy
FTLD clinical syndromes may show significant overlap with the atypical parkinsonian syndromes CBD and PSP, although both these syndromes were originally described as movement disorders.[14,70]
CBD was first described as a syndrome of asymmetric rigidity and apraxia.[71] Patients present with limb apraxia, axial and limb rigidity, dystonia, postural instability, myoclonus, supranuclear gaze palsies (primarily gaze apraxia and delayed saccades), the ‘alien limb phenomenon’ (spontaneous and at times antagonistic involuntary limb movements) and cortical sensory loss.[72] Cognitive features include executive and visuospatial dysfunction and, rarely, hemispatial neglect.[73,74] Although diagnostic criteria emphasize hemispheric asymmetry as a key feature, in our experience, symptoms and signs are often bilateral.
Patients with PSP show supranuclear gaze palsies (slowed and restricted saccades, with downgaze palsy being most specific), axial-predominant parkinsonism and profound retropulsion.[75] Pseudobulbar signs such as dysarthria, dysphagia and pseudobulbar affect often evolve. Cognitive dysfunction is referable to failure of frontal-subcortical circuits leading to executive dysfunction, psychomotor slowing and poor working memory.[76]
Patients with CBD and PSP may show behavioural changes similar to bvFTD or language changes reminiscent of PNFA.[70] Furthermore, patients who initially present with bvFTD or PNFA may, over time, evolve the movement disorders characteristic of CBD or PSP, with cognitive and behavioural changes preceding the movement disorder by years.[14,77,78]
4. Histopathology
On autopsy, FTLD patients show gross atrophy of the frontal and anterior temporal lobes.[79,80] Atrophy can be extreme and circumscribed, with marked sparing of posterior brain regions until the most advanced stages of disease (figures 2b and 3a).[81] On microscopic examination, there is loss of pyramidal neurons and microvacuolar degeneration in layers II and III of the frontal and temporal cortex, with a variable degree of cortical gliosis (figure 3b). Subjacent white matter shows both axonal and myelin loss and gliosis.[80,82,83] Patients with co-morbid motor neuron disease (FTLD-motor neuron disease) show further upper and lower motor neuron loss, corticospinal tract degeneration and Bunina bodies on gross and microscopic evaluation.[80] Most cases of FTLD can be subclassified into the following two major categories, which are based on the presence or absence of specific inclusion bodies: (i) FTLD with tau inclusions (FTLD-TAU, figure 3c); and (ii) FTLD with tau-negative, ubiquitin and TDP-43-positive inclusions (FTLD-TDP, figure 3d).[83]
Fig. 3.
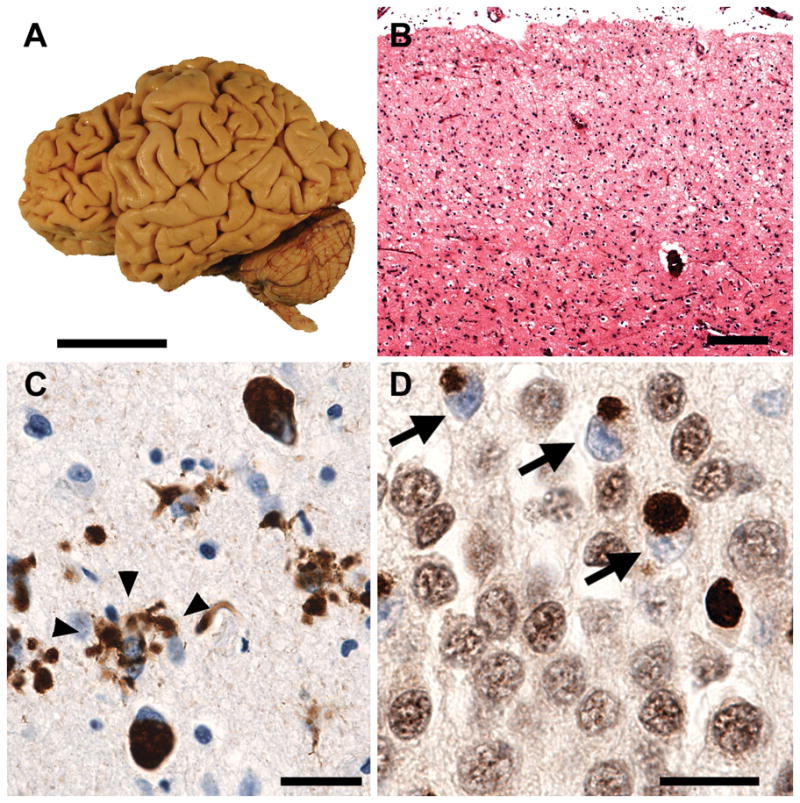
Frontotemporal lobar degeneration (FTLD) pathology. (a) Typical FTLD features circumscribed atrophy affecting anterior cortical and subcortical regions, as seen in this patient with progressive nonfluent aphasia due to underlying corticobasal degeneration. Scale bar = 5 cm. (b) Regardless of the associated disease protein, affected regions show neuronal loss, gliosis and microvacuolation, especially in superficial cortical layers. Hematoxylin and eosin stain. Scale bar = 200 microns. (c) Pick’s disease, a tau-positive form of FTLD, shows cytoplasmic hyperphosphorylated tau inclusions within neurons (Pick bodies) and in ramified astroglial processes (arrowheads). CP-13 antibody, courtesy of P. Davies. (d) In FTLD due to TAR DNA-binding protein 43 (TDP-43) proteinopathy, affected neurons like these dentate gyrus granule cells show cytoplasmic TDP-43 inclusions and clearing of TDP-43 from its normal nuclear location (arrows). TDP-43 antibody. Scale bars in c and d = 25 microns. Figure provided by W.W. Seeley, University of California, San Francisco.
4.1 Tau-Positive FTLD (FTLD-TAU)
FTLD-related tauopathies are classified based on both the morphologic features and the biochemical composition of the tau-positive inclusions. Pick’s disease, the prototypical tauopathy of FTLD, is characterized by the presence of Pick bodies, which are solitary, round or oval, argyrophilic inclusions found in the cytoplasm of neurons (figure 3c). These inclusions were first described by Alois Alzheimer,[84] but were later named after Arnold Pick by Onari and Spatz.[85] Pick bodies are most commonly found in the dentate gyrus of hippocampus, amygdala, and frontal and temporal neocortex.[82] They are stained by Bielschowsky but not Gallyas stains, and are most readily detected by tau immunohistochemistry.
CBD and PSP are at least as common as Pick’s disease in patients presenting with FTLD clinical syndromes.[86] The characteristic microscopic features of CBD are astrocytic plaques composed of tau-immunoreactive processes, and tau-positive gray and white matter threads, whereas PSP is distinguished by globose neurofibrillary tangles and tufted astrocytes.[75,82,87,88] The distribution of pathology and pattern of atrophy further distinguish CBD and PSP from each other and from Pick’s disease. CBD is characterized by frontal, parietal and striatal involvement, whereas in PSP the preponderance of pathology is in the brainstem (particularly midbrain), cerebellum and basal ganglia, with relative cortical sparing.[83,88–90]
Less common FTLD-related tauopathies include argyrophilic grain disease, sporadic multiple system tauopathy with dementia, neurofibrillary tangle dementia and ALS-parkinsonism-dementia complex of Guam.[83]
Patients with mutations in the MAPT gene (see section 5.1) may show the pathologic features of Pick’s disease, CBD or PSP. Human MAPT can be alternatively spliced to include either three or four repeated amino-acid sequences that serve as microtubule-binding sites. The fourth repeat sequence is encoded on exon 10, such that inclusion of this exon leads to 4-repeat (4R) tau isoforms, while exclusion leads to 3-repeat (3R) isoforms.[91] Similar amounts of 3R and 4R tau are present in normal brain, whereas pathologic tau may be predominantly composed of 4R, 3R or a mix of the two isoforms, as is seen in Alzheimer’s disease.[92] Tau inclusions in Pick’s disease are predominantly 3R; inclusions in CBD, PSP, argyrophilic grain disease and multiple system tauopathy with dementia are composed of 4R tau; and tau inclusions in neurofibrillary tangle dementia and the Guam complex include a mix of 3R and 4R isoforms.[83] Either or both isoforms may predominate in FTLD with MAPT mutations.
4.2 TAR DNA-Binding Protein 43 (TDP-43)-Positive FTLD (FTLD-TDP)
Tau-negative FTLD pathology has been more elusive to define and, as a result, has been described in the literature using various terms. The initial impression was that many cases of tau-negative FTLD were not associated with distinguishing protein inclusions, and were thus described as dementia lacking distinctive histology (DLDH).[93] With advances in immunohistochemistry, it became apparent that a large majority of DLDH cases showed immunoreactivity to ubiquitin, leading to the term FTLD with ubiquitin-positive inclusions (FTLD-U).[94,95] In 2006, the ubiquitinated protein in the vast majority of FTLD-U brains was found to be TDP-43.[96] Thus, the term FTLD-TDP is preferred in this review.
TDP-43 is a ubiquitously expressed, highly conserved nuclear protein involved in DNA transcription and splicing.[97] Under pathologic conditions, TDP-43 is displaced from the cell nucleus to the cytoplasm, hyperphosphorylated, ubiquitinated and cleaved to produce C-terminal fragments.[96,98,99] Neuronal and glial TDP-43 inclusions are found in the vast majority of cases previously classified as FTLD-U with and without motor neuron disease, and in familial cases associated with PGRN and valosin-containing protein (VCP) gene mutations (see section 5.3).[100–103] Inclusions are most abundant in the dentate gyrus of hippocampus (figure 3d) and in layer II neurons in the frontotemporal cortex, and may also be found in cranial nerve nuclei and in the anterior horn cells in the spinal cord.[83,96] TDP-43 inclusions are also found in patients with sporadic ALS, and mutations in TDP-43 have been linked to autosomal dominant ALS.[104,105] These observations support the concept of an FTLD-ALS clinical and pathologic continuum, and provide compelling evidence for a pathogenic role for TDP-43 in this disease spectrum. Nevertheless, there are a number of unresolved questions regarding the primary role of TDP-43 in FTLD pathogenesis. Mutations in TDP-43 have thus far been very rarely linked to FTLD clinical presentations,[106,107] [add new reference #3 Benajiba et al. Annals of Neurology 2009 and #4 Kovacs et al. Movement Disorders 2009) and the mechanisms by which TDP-43 accumulation leads to neurodegeneration have not been delineated. Furthermore, TDP-43 inclusions are not specific to FTLD, and can be been found in many other degenerative dementias, including Alzheimer’s disease, dementia with Lewy bodies, Pick’s disease, CBD and ALS-parkinsonism complex of Guam.[108–112] Whether the role of TDP-43 in these disorders differs from its role in FTLD remains to be determined.
4.3 Fused in Sarcoma (FUS)-Positive FTLD and other Rare Variants
A total of 5–20% of FTLD-U cases stain negative for TDP-43.[113–115] The inclusions in many of these cases were recently found to consist of the protein fused in sarcoma (FUS) (new references #5, #6). Like TDP-43, FUS is a ubiquitously expressed DNA/RNA binding protein that regulates gene expression, and chromosomal translocations of FUS lead to human sarcomas and hematological malignancies (new reference #7). As with TDP-43, mutations in the FUS gene are associated with familial ALS (new reference #8), while FTLD-FUS cases are nearly always sporadic. The clinical phenotype is distinct and characterized by very early age-at-onset (mean age 38 years in the largest series to date) (new reference #5), severely disturbed behaviour, and profound caudate atrophy on MRI (new references #5, #6). FTLD-FUS inclusions are similar in morphology and distribution to TDP-43 inclusions, though unlike TDP-43, some of the normal nuclear staining pattern is retained in affected neurons (new reference #5). FUS-positive inclusions have now been demonstrated in other rare pathologic variants of FTLD including basophilic inclusion body disease (new reference #9) and neuronal intermediate filament disease (new reference #10), [117,118] though not in the rare familial cases associated with chromatin-modifying protein 2B (CHMP2B) gene mutations (see section 5.3)[116] (new reference #11). Finally, even applying modern immunohistochemical techniques, a minority of FTLD patients fail to show discernable inclusions and thus continue to meet criteria for DLDH.[119,120]
5. Genetics
Up to 40% of FTLD patients have a history that is suggestive of familial transmission, with roughly 10% of patients showing an autosomal dominant inheritance pattern.[121–123] When obtaining the family history, in addition to FTLD and ALS, clinicians should also inquire about mid- or late-life psychiatric disease, Alzheimer’s disease and parkinsonian disorders, since FTLD is often mistaken for these conditions. Familial FTLD is most common in patients with bvFTD and FTLD-ALS and least common in patients with SD. FTLD was first linked to chromosome 17q21–22 in 1994, prompting the designation frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17).[124,125] In 1998, MAPT was identified as the causal gene in FTDP-17 families with tau-positive histopathology[126–128] and, in 2006, mutations in the PGRN gene were found to account for FTDP-17 families with tau-negative histopathology.[129–131] Over 40 mutations in MAPT and nearly 70 mutations in PGRN have been linked to FTLD thus far.[234] Taken together, MAPT and PGRN mutations account for the majority of familial FTLD, although the frequency of mutations varies considerably depending on the population that is being studied.[122,123] In our centre, PGRN mutations are more common than MAPT mutations; however, in European cohorts, the frequency of MAPT and PGRN mutations appears to be similar.[123,129,132]
5.1 FTLD Associated with Microtubule-Associated Protein Tau Gene Mutations
The tau protein in neurons binds to axonal microtubules, promotes microtubule assembly and stabilization and is also involved in signal transduction. MAPT mutations lead to neurodegeneration via a variety of mechanisms.[133–138] Mutant protein may have a decreased binding affinity to microtubules, leading to impaired microtubule assembly, stability and, ultimately, disruption of axonal transport. Alternatively, mutant tau may have an increased affinity to self aggregate into filamentous, insoluble inclusions that are neurotoxic. These mechanisms are not mutually exclusive and often act synergistically. Many MAPT mutations are located in or adjacent to the alternatively spliced exon 10 (see section 4.3), altering the normal 1:1 ratio between 3R and 4R isoforms in favour of 4R, which binds poorly to microtubules and shows an increased affinity for self-aggregation.[127,128,139] Regardless of the mechanism, MAPT mutations are highly penetrant and lead to disease onset between the ages of 40 and 60 years (mean 55 years), with disease duration ranging from 8–10 years.[122,123,140] The clinical phenotype can be quite variable even within a single pedigree, and may include clinical features of bvFTD, SD, PNFA, CBD, PSP and even ALS.[141,142] Presymptomatic gene carriers may show cognitive deficits decades before the predicted onset of dementia, suggesting a possible neurodevelopmental component.[143] The pathologic features are also variable and can range the gamut of FTLD-TAU (see section 4.1).
In addition to causative mutations, normal genetic variation in MAPT can modify the risk for developing a tauopathy. MAPT has two extended haplotypes, H1 and H2, which are in complete disequilibrium with each other and show no recombination.[144] These two haplotypes were likely to have been created by an inversion event involving 200 noncoding polymorphisms. The H1/H1 genotype is consistently over-represented in CBD and PSP,[144,145] suggesting there is an increased vulnerability to developing 4R tauopathies in H1 homozygotes.
5.2 FTLD Associated with Progranulin Gene Mutations
Progranulin is a secreted growth factor that plays an important role in inflammation, wound healing and tumour growth in non-brain tissue.[146,147] In the brain, PGRN RNA is highly expressed in cortical and hippocampal pyramidal cells and cerebellar Purkinje cells.[148] Putative roles include regulation of sexual differentiation in the embryonic brain, promotion of neuronal survival and stimulation of neuritic outgrowth.[149,150] PGRN knockout mice show behavioural changes (increased anxiety and aggression), perhaps as a result of alterations in expression of the serotonin 5-HT1A receptor.[151] Interestingly, expression patterns of large numbers of genes in the frontal cortex are altered in patients with PGRN mutations compared with non-demented controls.[152] Nearly all mutations described thus far are nonsense or missense mutations that lead to absent or nonfunctional protein.[129,153] (new reference #12) Thus, in contrast to MAPT mutations that lead to disease by toxic gain of function, PGRN mutations appear to lead to neurodegeneration via haploinsufficiency.[129] Putative mechanisms by which loss of PGRN function may lead to neurodegeneration include chronic depletion of neurotrophic support and inadequate response to neuronal injury. As in MAPT, a subtle developmental abnormality rendering the brain more vulnerable to neurodegeneration is also possible. Compared with MAPT, PGRN mutations are associated with more variable age of onset (range 35–89 years, mean 60 years) and penetrance (estimated at only 50% by age 60 years, but 90% by age 70 years).[123] PGRN mutations were found in 3.2% of sporadic FTLD cases in one study,[153] in contrast to MAPT mutations, which are exceedingly rare in sporadic FTLD.
Similar to MAPT, the clinical phenotype in patients with PGRN mutations can be variable even within a pedigree, and there are no clear genotype/phenotype relationships.[154,155] Hallucinations and delusions are more common than in sporadic or MAPT-related FTLD, occurring in up to 25% of patients.[154] A total of 20–25% of PGRN patients present with a progressive aphasia similar to PNFA, although apraxia of speech is often absent.[156] bvFTD and CBD clinical phenotypes are also common. Parietal involvement may be more frequent than in other FTLD types, and 10–30% may have an Alzheimer’s disease-like amnestic presentation.[154,157,158] Parkinsonism is common and may be the predominant feature,[159] whereas motor neuron disease is rare.[129] The mechanistic link between PGRN and TDP-43 is under active investigation: one group has reported that PGRN suppression leads to caspase-dependant cleavage and translocation of TDP-43 from the nucleus to the cytoplasm,[160] but this has result has not been replicated in subsequent studies [new reference #13].
5.3 Additional Genes and Linkages
Although mutations in MAPT and PGRN account for the majority of cases of autosomal dominant FTLD, additional rare mutations have been described. A single Danish family with an FTLD phenotype has been found to have a mutation in the CHMP2B gene on chromosome 3p11.[161,162] CHMP2B is part of the endosomal sorting complex involved in cargo sorting into multivesicular bodies in the endosomal/lysosomal system. Interestingly, an additional CHMP2B mutation has recently been linked to ALS.[163] Mutations in the VCP gene at 9p13 are linked to an autosomal dominant FTLD syndrome associated with Paget’s disease and inclusion body myositis.[164] VCP is an adenosine triphosphatase that is involved in protein degradation in the endoplasmic reticulum. VCP mutation carriers show variable penetrance and phenotypic variation, with myopathy developing in the majority of carriers, while FTLD and Paget’s disease occur in fewer than 50%, sometimes decades after onset of the myopathy.[165] Despite the presence of TDP-43 or FUS-positive inclusions in FTLD, mutations in these genes are associated with familial ALS but so far only very rarely with clinical FTLD (new references #3, #4, #14). Up to 10% of autosomal dominant forms of FTLD are not accounted for by currently known genes. An important gene is likely to reside at 9p21.3–13.3, a linkage site found in a number of families with familial FTLD-ALS, although the associated gene has yet to be identified.[166,167] International genome-wide association studies are underway in an attempt to identify additional causative and risk-modifying genes.[168]
6. Differential Diagnosis
As in any case of progressive dementia, the clinician must first exclude treatable conditions that can mimic FTLD, such as metabolic disturbances, nutritional deficiencies, CNS infections, substance abuse, vascular disease, heavy metal toxicity, and primary neoplastic and paraneoplastic conditions. These can often be excluded by the combination of a careful medical history, laboratory testing and neuroimaging, preferably with magnetic resonance imaging (MRI). A subset of patients presenting with bvFTD may have a primary psychiatric disorder, usually major depression or bipolar affective disorder.[169,170] These patients show little progression over time and do not have frontotemporal atrophy on MRI. However, in our experience, it is far more common for patients with degenerative FTLD to be misdiagnosed with a psychiatric disorder than vice versa.[171]
Once the FTLD syndrome is determined to be degenerative, the next challenge is to predict the underlying histopathology. Recent clinicopathologic studies have demonstrated consistent relationships between the FTLD syndrome and the most likely underlying pathology.[14,78,172–174] In patients with sporadic FTLD, SD is most often associated with TDP-43 inclusions, PNFA is frequently associated with one of the tau-inclusion disorders (in particular CBD), whereas patients with bvFTD are split between FTLD-TAU and FTLD-TDP (figure 1). Very early age of onset in a sporadic case of bvFTD suggests FTLD-FUS. The presence of motor neuron disease strongly predicts FTLD-TDP pathology, whereas parkinsonism is suggestive of FTLD-TAU. However, in familial cases, PNFA and parkinsonism are commonly found in patients with either MAPT or PGRN mutations. The PSP clinical syndrome is highly predictive of FTLD-TAU, while clinical CBD can be caused by either FTLD-TAU or FTLD-TDP, the latter usually in association with a PGRN mutation. Aside from the link between motor neuron disease and FTLD-TDP, which is nearly 100% specific, these clinicopathologic correlations are probabilistic: they are reproducible across centres at the group level, but do not always hold true for individual patients.
A total of 10–30% of patients presenting with an FTLD clinical syndrome are found to have Alzheimer’s disease on autopsy.[172–174] Identifying these patients with high accuracy during life is of paramount importance because they may be candidates for emerging therapies directed against β-amyloid. Patients presenting with a progressive dysexecutive syndrome in the absence of behavioural changes or a movement disorder are more likely to have the frontal-variant of Alzheimer’s disease[175] than FTLD. Patients with logopenic aphasia, a progressive aphasia characterized by slow, hesitant speech, frequent word-finding pauses and profound deficits in sentence repetition, are also more likely to have underlying Alzheimer’s disease.[176,177] Even accounting for these clinical distinctions, a significant minority of patients with typical FTLD clinical presentations are ultimately found to have underlying Alzheimer’s disease pathology.[172] Dementia with Lewy bodies can be ruled out clinically by the absence of visual hallucinations (which are uncommon in FTLD), fluctuations and rapid eye movement sleep behaviour.[178] Vascular dementia can be excluded by the absence of cortical and subcortical vascular lesions on MRI.
Neuroimaging studies can help improve diagnostic accuracy by identifying signature anatomic patterns that distinguish FTLD from Alzheimer’s disease. The presence of posterior temporal and parietal brain atrophy on MRI, hypometabolism on fluoro-deoxy-glucose positron emission tomography (FDG-PET) or hypoperfusion on single proton emission computerized tomography are predictive of a pathologic diagnosis of Alzheimer’s disease, whereas medial prefrontal lesions are specific for FTLD.[21,50,179–182] These characteristic anatomic patterns are again reproducible at the group level, but do not always hold true for individual patients.
Much effort in recent years has focused on developing and testing biological markers that directly measure the pathologic changes associated with each disease. CSF levels of the 42 amino acid β-amyloid polypeptide (Aβ42) and tau are a sensitive and specific marker of Alzheimer’s disease,[183,184] and the ratio of tau to Aβ42 can help discriminate between pathologically confirmed cases of Alzheimer’s disease and FTLD.[185] Amyloid PET imaging with the Aβ-specific ligand 11C-labeled Pittsburgh Compound-B (PIB) can detect the fibrillar Aβ plaques of Alzheimer’s disease with high sensitivity and specificity and can help exclude the presence of Alzheimer’s disease pathology in patients with FTLD clinical presentations.[177,186,187] Although these biomarkers are not yet available for clinical practice, they hold great promise as diagnostic tools that will improve in vivo diagnostic accuracy in the future. Accurately differentiating FTLD-TAU from FTLD-TDP in individual patients on clinical grounds can be challenging, particularly in patients with bvFTD, and the development of specific imaging and CSF markers for FTLD pathologic subtypes is an important area of active investigation.[188–190]
7. Treatment
7.1 Behavioural and Cognitive Symptoms
Current pharmacotherapy for FTLD focuses primarily on symptomatic, neurotransmitter-based treatments.[191] None of the drugs were developed specifically for FTLD, and the rationale for their use is their efficacy in treating similar symptoms in primary psychiatric conditions or in other neurodegenerative disorders.[27] Most reports of FTLD treatments in the literature are based on single cases or small case series, and the few clinical trials that have been published have largely been open-label studies with small sample sizes.[192] Two recent systematic literature reviews identified a total of six double-blind, placebo-controlled trials for FTLD, with sample sizes ranging from 8 to 36 participants.[192,193] Evaluating the efficacy of FTLD treatments is further complicated by the absence of standardized clinical scales. Clinical instruments used to measure efficacy in Alzheimer’s disease, such as the Mini-Mental State Examination, Alzheimer Disease Assessment Scale-Cognitive, or Clinical Dementia Rating (CDR) scale, emphasize memory loss and do not adequately capture the executive and language deficits seen in FTLD.[194] Even instruments used to measure behavioural disturbances in Alzheimer’s disease such as the Neuropsychiatric Inventory (NPI) may not be applicable to FTLD, since longitudinal improvement on the NPI in FTLD patients may reflect worsening apathy due to disease progression.
Of all neurotransmitter-based therapies for FTLD, drugs that modify serotonin have the strongest biological rationale, since there is strong evidence for a selective serotonergic deficit in this disorder.[191] Patients with FTLD show alterations in the levels of serotonin metabolites in the CSF,[195] and significant neuronal loss in the serotonergic dorsal raphe nuclei on autopsy.[196] A decrease in 5-HT1A and 5-HT2A receptors in the frontal cortex has been demonstrated both in pathological specimens[196–199] and in vivo using PET.[200] Furthermore, many of the behavioural symptoms of FTLD, such as depression, compulsions, repetitive behaviours, disinhibition, stereotypical movements and dysregulated eating, respond to selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs) in patients with primary psychiatric disease.
The efficacy of SSRIs in FTLD has been demonstrated in relatively small, often uncontrolled trials.[201] Open-label studies (n = 11–18; study duration = 12 weeks to 6 months) of fluoxetine,[202] fluvoxamine,[202,203] sertraline[28,202] and paroxetine,[202] and one randomized controlled trial (n = 16; duration = 14 months) of paroxetine,[204] have demonstrated efficacy in controlling behavioural disturbances and stereotypical movements in FTLD, whereas one very brief (n = 10; duration = 6 weeks) randomized study of paroxetine failed to show any benefit.[205] Trazodone, which has serotonin reuptake and mixed agonist/antagonist activity, lowered NPI scores in a 12-week, crossover, placebo-controlled study,[206] although fatigue, dizziness and hypotension were common and limiting adverse effects at the high dosages used in this study (up to 300 mg/day). A recent meta-analysis suggested that the use of serotonergic drugs in FTLD is associated with a mean reduction of 15.4 points on the NPI, although the absence of a placebo control in many of the included studies, the small sample sizes and publication bias limit the reliability of this estimate.[191] In our practice, we prescribe SSRIs or SNRIs as first-line drug therapy for behavioural disturbances in FTLD. The preferred agents used in our clinic are listed in table I. When apathy is prominent, we may try venlafaxine for its activating properties; when parkinsonism is present, bupropion is considered because of its dopaminergic tone. It should be emphasized that our choices are based on adverse effect profiles and clinical experience and are not based on the (very limited) clinical trial data.
Table I.
Symptomatic therapy in frontotemporal lobar degeneration
| Symptom | Therapy |
|---|---|
| Behavioural disturbances | Caregiver education |
| Environmental, physical and behavioural modifications | |
| Antidepressants | |
| escitalopram, citalopram, sertraline | |
| bupropion (with parkinsonism) | |
| venlafaxine (with prominent apathy) | |
| Antipsychoticsb | |
| quetiapine | |
| Aphasia | Speech therapy |
| Augmentative communication devices | |
| Parkinsonism | Physical, occupational and speech therapy |
| Levodopa/carbidopa | |
| Pramipexole, ropinirole | |
| Motor neuron disease | Multidisciplinary treatment |
| neurologic | |
| nutritional | |
| pulmonary | |
| physical, occupational and speech therapy | |
| Riluzolec | |
| Bladder dysfunction | |
| upper motor neuron | Trospium chloride, darifenacin |
| lower motor neuron | Intermittent catheterization |
Nonpharmacological therapies are paramount, and drug therapy in isolation is unlikely to be successful. Listed pharmacological agents are based on the clinical experience of the authors, and all recommendations represent off-label use unless otherwise specified. Medications should always be started at low doses and titrated slowly.
Should be used as last resort and with extreme caution because of increased mortality risk.
US FDA-approved for the treatment of amyotrophic lateral sclerosis.
In patients with severe behavioural disturbances (agitation, psychosis) that are refractory to SSRIs, treatment with atypical antipsychotics may be considered. This practice is supported by a single open-label study of olanzapine (n = 17 ; duration = 24 months), which showed similar efficacy in lowering the NPI score to that reported with SSRIs.[207] Single cases reporting benefit for risperidone[208] and aripiprazole[209] have also been published. This class of medications should be used with great caution for two reasons. First, FTLD patients are particularly vulnerable to extrapyramidal adverse effects.[210] Second, atypical antipsychotics are associated with an increased (1.6- to 1.7-fold) mortality risk in elderly patients with dementia, prompting the US FDA to issue a ‘black box warning’ on their use.[235] Increased mortality appears to be related to an increase in cardiovascular events and infections, and may also reflect a nonspecific effect of sedation in this vulnerable population. Our clinical practice is to use atypical antipsychotics only as a last resort, after first trying behavioural modifications (see section 7.3) and SSRIs or SNRIs. We prefer quetiapine to other antipsychotic agents because of its relatively low dopamine D2 receptor antagonism. The increased mortality risk and concerning potential adverse effects are discussed with patients and families, and the need for continued use is re-evaluated at every clinic visit. Often, atypical antipsychotics are necessary only as a temporizing measure, and can be tapered as patients become more apathetic (and thus less agitated and disinhibited) as the disease progresses.
Acetylcholinesterase inhibitors have an important role in treating cognitive and behavioural symptoms in Alzheimer’s disease and dementia with Lewy bodies, and have thus generated interest as a potential treatment in FTLD. However, in contrast to Alzheimer’s disease and dementia with Lewy bodies, there is no cholinergic deficit in FTLD.[211–213] Three studies have examined acetylcholinesterase inhibitor use in FTLD with mixed results. One open-label study (n = 20; duration = 12 months) found that rivastigmine improved neuropsychiatric symptoms and caregiver burden but did not halt cognitive decline.[214] A trial of galantamine in FTLD and primary progressive aphasia (PPA), which included an 18-week, open-label phase followed by an 8-week, randomized, double-blind, placebo-controlled withdrawal (n = 36), found a nonsignificant trend for benefit in language function and global severity in the PPA subgroup.[215] However, in retrospect, many of the patients in the PPA group may have had the logopenic variant of progressive aphasia that is associated with underlying Alzheimer’s disease. In a third open-label trial (n = 24; duration = 6 months), donepezil was associated with a reversible worsening of behaviour and showed no benefit on cognitive measures.[216] Our clinical experience is more consistent with the latter study, and we do not prescribe acetylcholinesterase inhibitors for FTLD unless we have reason to suspect the patient truly has underlying Alzheimer’s disease (e.g. positive PIB-PET study).
Memantine, a noncompetitive NMDA antagonist, effectively treats agitation in moderate-to-severe Alzheimer’s disease, and may exert a neuroprotective effect by preventing chronic neuronal depolarization that can lead to excitotoxicity.[217] A case series reporting three FTLD patients treated with memantine showed improved NPI scores over 3 months of follow-up.[218] Two open label studies (n = 16 and 43, trial durations = 26 weeks) have demonstrated good tolerability,[219] [220] and larger randomized, placebo-controlled studies of memantine in FTLD are currently in progress.
7.2 Motor and Sphincter Symptoms
Patients with parkinsonism should receive a trial of levodopa/carbidopa, although the response to levodopa may be poor or transient, particularly in CBD and PSP.[221,222] Dopamine agonists such as pramipexole or ropinirole should be considered in patients who do not respond to levodopa. Unlike levodopa, these medications act at the postsynaptic terminal, and do not rely on the presynaptic conversion of levodopa to dopamine in the substantia nigra, which can suffer severe neuronal loss in CBD and PSP. Patients with FTLD-ALS are candidates for riluzole treatment,[223] and should be referred to a multidisciplinary ALS centre where their pulmonary and nutritional status can be closely monitored.[224]
Urinary incontinence is common in FTLD and may occur through a variety of mechanisms, including loss of voluntary sphincter control due to degeneration of medial frontal cortex, and both upper and lower motor neuron bladder dysfunction (particularly in CBD and PSP). Patients should be referred for urodynamic studies to determine the cause and optimal treatment of the bladder dysfunction. Upper motor neuron bladder dysfunction may respond to anticholinergic medications, although these should be used sparingly because they may exacerbate cognitive and neuropsychiatric deficits. When necessary, drugs with lower CNS penetration such as trospium chloride or darifenacin are preferred. Intermittent catheterization should be considered in patients with lower motor neuron bladder dysfunction, given the risk of infection associated with indwelling catheters (these are also poorly tolerated in FTLD patients). Constipation is common and responds in most cases to a daily bowel regimen.
7.3 Nonpharmacological Interventions
While this report focuses on pharmacological treatment, the first-line therapy for behavioural disturbances in FTLD should be nonpharmacological,[27,225,226] since current drug therapies for FTLD are modestly effective at best and have serious potential adverse effects. An FTLD treatment plan that does not include nonpharmacological interventions is unlikely to be successful. The cornerstones of nonpharmacological treatment in FTLD include caregiver and family education, and environmental, behavioural and physical interventions designed to minimize the occurrence and consequences of undesired behaviours.[225] Caregiver and patient support groups can be invaluable. Additional helpful interventions include physical, occupational and speech therapy, home safety evaluations and the implementation of augmentative communication devices. We universally suggest a structured exercise programme for physically able patients and caregivers because, in our anecdotal experience, regular physical activity may delay or slow the progression of motor symptoms. A frank and pre-emptive discussion about end-of-life decisions and goals of care is imperative.[226]
7.4 Future Directions
Efforts to develop specific, disease-modifying therapies for FTLD are focused on the critical proteins implicated in pathogenesis, namely tau, progranulin and TDP-43. Tau-directed therapies have a significant head start in terms of identifying pathogenic mutations, generating animal models and elucidating molecular mechanisms. Transgenic mice expressing human pathogenic MAPT mutations show behavioural and motor deficits, with an age- and gene dose-dependent accumulation of tau inclusions (reviewed by Rademakers and colleagues).[227] Mice expressing the human P301L mutation, the most studied transgenic model of FTLD-TAU, show impaired performance on memory tasks, increased docility, decreased weight, vocalization and grooming behaviours, and severe motor deficits leading to paralysis.[228] Pathologic inclusions of hyperphosphorylated 4R tau are found in neurons and glial cells (similar to those seen in human pathologic specimens), and motor neuron loss is seen in the anterior horn of the spinal cord.[228,229] Mice expressing a variety of other pathogenic MAPT mutations all show a range of similar behavioural and pathologic changes, although the morphology of tau inclusions and the degree of neurodegeneration varies between models.[227] Expression of MAPT mutations in non-rodent models such as Drosophila melanogaster[230] and Caenorhabditis elegans[231] similarly leads to behavioural changes, tau accumulation and neurodegeneration.
Animal models of FTLD-TAU do not show the anatomic selectivity seen in human disease, and the human symptoms of impaired comportment and aphasia cannot be recapitulated in an animal model. Nevertheless, transgenic models have helped elucidate the molecular mechanisms involved in human tauopathies, and have identified a number of potential therapeutic strategies. These include inhibition of tau kinases (particularly the glycogen synthase kinase 3β [GSK3β] and cyclin-dependant kinase 5 pathways), inhibition of tau fibrillization, manipulation of tau-processing pathways (e.g. ubiquitination), inhibition of tau expression, tau-independent microtubule stabilization and immunosuppression.[193,232] Lithium and valproic acid are GSK3β inhibitors that are approved for other indications and are being considered for FTLD clinical trials. Tau drug discovery is also a major focus of the pharmaceutical industry given the apparent role of tau in the pathogenesis of Alzheimer’s disease.
The roles of progranulin and TDP-43 in FTLD have only recently been described. The molecular mechanisms that surround their function in normal brain and disease are still being elucidated, and transgenic animals expressing disease-causing PGRN mutations are currently being developed.[123] The haploinsufficiency mechanism that appears to underlie PGRN-associated neurodegeneration is unique and will require novel strategies to increase expression of wild-type PGRN. The relevance of progranulin to sporadic FTLD with TDP-43 inclusions is still not clear, although a recent study suggests that PGRN haplotype can modify the risk for developing sporadic FTLD-TDP.[233] Based on the changes in TDP-43 localization and biochemistry seen in autopsy specimens, the steps involved in pathologic TDP-43 processing are hypothesized to include hyperphosphorylation, ubiquitination, cleavage and translocation from nucleus to cytoplasm,[96] suggesting a number of potential therapeutic targets. Although CHMP2B and VCP mutations are rare, the mechanisms by which these mutations lead to frontotemporal degeneration may also be relevant to sporadic disease and remain areas of active investigation.
The capacity to effectively test candidate disease-modifying drugs in FTLD will depend greatly on our ability to identify biologically homogeneous patient populations for clinical trials. Based on our current understanding of clinicopathologic correlations, patients with PNFA, CBD, PSP and MAPT mutations would be preferred candidates for a tau-specific drug, while patients with PGRN mutations, SD or FTLD-ALS would be the preferred population in which to test a progranulin/TDP-43-based therapy. The detection of Aβ pathology by CSF or imaging biomarkers should exclude patients from participation in FTLD clinical trials. The development of tau- and TDP-43-specific biomarkers will be necessary to further improve in vivo prediction of histopathology, particularly in patients with bvFTD who are equally likely to have FTLD-TAU or FTLD-TDP.
The efficacy of candidate drugs will need to be tested with clinical outcome measures that are sensitive to the changes seen with disease progression in FTLD. In a recently published ‘virtual clinical trial’, Knopman and colleagues[194] tested the utility of new cognitive and functional instruments to measure longitudinal change in FTLD. These included an FTLD-specific CDR scale that added ‘behaviour, comportment and personality’ and ‘language’ to the existing CDR domains, and a composite cognitive measure based on tests of executive function and language. These measures performed more favourably as prospective outcome variables for FTLD clinical trials than the ‘traditional’ cognitive, behavioural and functional measures used to measure outcomes in Alzheimer’s disease trials. In addition to piloting these new tools, the study by Knopman et al.[194] demonstrated the feasibility of conducting a collaborative, multicentre trial in FTLD. Such a collaborative effort will certainly be necessary to recruit the cohort of over 200 FTLD patients per trial that may be needed to demonstrate treatment effects in FTLD.[194]
8. Conclusions
FTLD is increasingly recognized as a leading cause of early-onset dementia. The proclivity of the disease for the social and language networks of the brain make it a particularly devastating illness, because it robs patients of these uniquely human functions, often during the prime of their lives. The past two decades of research have led to major advances in our understanding of the genetics and molecular mechanisms of FTLD. Nevertheless, the remarkable anatomic selectivity of the disease remains a mystery. As with other neurodegenerative disorders, a major goal of future FTLD research is to translate our increasing understanding of pathogenesis into effective treatments that will slow, halt or ultimately prevent this devastating disease.
Acknowledgments
The authors are supported by the National Institute on Aging grants K23-AG031861 (Gil D. Rabinovici), P01-AG1972403 (Bruce L. Miller), P50-AG023501 (Bruce L. Miller), Alzheimer’s Association grant NIRG-07-59422 (Gil D. Rabinovici) and the John Douglas French Alzheimer’s Association (Gil D. Rabinovici).
Footnotes
The authors have no conflicts of interest that are directly relevant to the content of this review. Dr. Rabinovici has received personal compensation for serving on scientific advisory boards for General Electric Healthcare and Novartis Diagnostics. Dr Miller has received personal compensation for participating in the speaker’s bureau for Novartis Pharmaceuticals and Pfizer.
References
- 1.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 2.Rosso SM, Donker Kaat L, Baks T, et al. Frontotemporal dementia in the Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain. 2003;126:2016–22. doi: 10.1093/brain/awg204. [DOI] [PubMed] [Google Scholar]
- 3.Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–21. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 4.Mercy L, Hodges JR, Dawson K, et al. Incidence of early-onset dementias in Cambridgeshire, United Kingdom. Neurology. 2008;71:1496–9. doi: 10.1212/01.wnl.0000334277.16896.fa. [DOI] [PubMed] [Google Scholar]
- 5.Knopman DS, Petersen RC, Edland SD, et al. The incidence of frontotemporal lobar degeneration in Rochester, Minnesota, 1990 through 1994. Neurology. 2004;62:506–8. doi: 10.1212/01.wnl.0000106827.39764.7e. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JK, Diehl J, Mendez MF, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol. 2005;62:925–30. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- 7.Hodges JR, Davies R, Xuereb J, et al. Survival in frontotemporal dementia. Neurology. 2003;61:349–54. doi: 10.1212/01.wnl.0000078928.20107.52. [DOI] [PubMed] [Google Scholar]
- 8.Gislason TB, Sjogren M, Larsson L, et al. The prevalence of frontal variant frontotemporal dementia and the frontal lobe syndrome in a population based sample of 85 year olds. J Neurol Neurosurg Psychiatry. 2003;74:867–71. doi: 10.1136/jnnp.74.7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203–12. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Brunnstrom H, Gustafson L, Passant U, et al. Prevalence of dementia subtypes: a 30-year retrospective survey of neuropathological reports. Arch Gerontol Geriatr. 2009;49:146–149. doi: 10.1016/j.archger.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Roberson ED, Hesse JH, Rose KD, et al. Frontotemporal dementia progresses to death faster than Alzheimer disease. Neurology. 2005;65:719–25. doi: 10.1212/01.wnl.0000173837.82820.9f. [DOI] [PubMed] [Google Scholar]
- 12.Rascovsky K, Salmon DP, Lipton AM, et al. Rate of progression differs in frontotemporal dementia and Alzheimer disease. Neurology. 2005;65:397–403. doi: 10.1212/01.wnl.0000171343.43314.6e. [DOI] [PubMed] [Google Scholar]
- 13.Kertesz A, Blair M, McMonagle P, et al. The diagnosis and course of frontotemporal dementia. Alzheimer Dis Assoc Disord. 2007;21:155–63. doi: 10.1097/WAD.0b013e31806547eb. [DOI] [PubMed] [Google Scholar]
- 14.Kertesz A, McMonagle P, Blair M, et al. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Miller BL, Kramer JH, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62:742–8. doi: 10.1212/01.wnl.0000113729.77161.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustafson L. Frontal lobe degeneration of non-Alzheimer type: II. Clinical picture and differential diagnosis. Arch Gerontol Geriatr. 1987;6:209–23. doi: 10.1016/0167-4943(87)90022-7. [DOI] [PubMed] [Google Scholar]
- 17.Levy ML, Miller BL, Cummings JL, et al. Alzheimer disease and frontotemporal dementias: behavioral distinctions. Arch Neurol. 1996;53:687–90. doi: 10.1001/archneur.1996.00550070129021. [DOI] [PubMed] [Google Scholar]
- 18.Neary D, Snowden JS, Northen B, et al. Dementia of frontal lobe type. J Neurol Neurosurg Psychiatry. 1988;51:353–61. doi: 10.1136/jnnp.51.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1994;57:416–8. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustafson L. Clinical picture of frontal lobe degeneration of non-Alzheimer type. Dementia. 1993;4:143–8. doi: 10.1159/000107313. [DOI] [PubMed] [Google Scholar]
- 21.Miller BL, Cummings JL, Villanueva-Meyer J, et al. Frontal lobe degeneration: clinical, neuropsychological, and SPECT characteristics. Neurology. 1991;41:1374–82. doi: 10.1212/wnl.41.9.1374. [DOI] [PubMed] [Google Scholar]
- 22.Miller BL, Darby A, Benson DF, et al. Aggressive, socially disruptive and antisocial behaviour associated with fronto-temporal dementia. Br J Psychiatry. 1997;170:150–4. doi: 10.1192/bjp.170.2.150. [DOI] [PubMed] [Google Scholar]
- 23.Mendez MF, Chen AK, Shapira JS, et al. Acquired sociopathy and frontotemporal dementia. Dement Geriatr Cogn Disord. 2005;20:99–104. doi: 10.1159/000086474. [DOI] [PubMed] [Google Scholar]
- 24.Rankin KP, Baldwin E, Pace-Savitsky C, et al. Self awareness and personality change in dementia. J Neurol Neurosurg Psychiatry. 2005;76:632–9. doi: 10.1136/jnnp.2004.042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller BL, Seeley WW, Mychack P, et al. Neuroanatomy of the self: evidence from patients with frontotemporal dementia. Neurology. 2001;57:817–21. doi: 10.1212/wnl.57.5.817. [DOI] [PubMed] [Google Scholar]
- 26.Swartz JR, Miller BL, Lesser IM, et al. Behavioural phenomenology in Alzheimer’s disease, frontotemporal dementia, and late-life depression: a retrospective analysis. J Geriatr Psychiatry Neurol. 1997;10:67–74. doi: 10.1177/089198879701000206. [DOI] [PubMed] [Google Scholar]
- 27.Perry RJ, Miller BL. Behavior and treatment in frontotemporal dementia. Neurology. 2001;56 (Suppl 4):S46–51. doi: 10.1212/wnl.56.suppl_4.s46. [DOI] [PubMed] [Google Scholar]
- 28.Mendez MF, Shapira JS, Miller BL. Stereotypical movements and frontotemporal dementia. Mov Disord. 2005;20:742–5. doi: 10.1002/mds.20465. [DOI] [PubMed] [Google Scholar]
- 29.Miller BL, Darby AL, Swartz JR, et al. Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dementia. 1995;6:195–9. doi: 10.1159/000106946. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda M, Brown J, Holland AJ, et al. Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;73:371–6. doi: 10.1136/jnnp.73.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woolley JD, Gorno-Tempini ML, Seeley WW, et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69:1424–33. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
- 32.Gustafson L. Frontal lobe degeneration of non-Alzheimer type: II. Clinical picture and differential diagnosis. Arch Gerontol Geriatr. 1987;6:209–23. doi: 10.1016/0167-4943(87)90022-7. [DOI] [PubMed] [Google Scholar]
- 33.Gregory CA, Serra-Mestres J, Hodges JR. Early diagnosis of the frontal variant of frontotemporal dementia: how sensitive are standard neuroimaging and neuropsychologic tests? Neuropsychiatry Neuropsychol Behav Neurol. 1999;12:128–35. [PubMed] [Google Scholar]
- 34.Neary D, Snowden J, Mann D. Frontotemporal dementia. Lancet Neurol. 2005;4:771–80. doi: 10.1016/S1474-4422(05)70223-4. [DOI] [PubMed] [Google Scholar]
- 35.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–8. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Rosen HJ, Narvaez JM, Hallam B, et al. Neuropsychological and functional measures of severity in Alzheimer disease, frontotemporal dementia, and semantic dementia. Alzheimer Dis Assoc Disord. 2004;18:202–7. [PubMed] [Google Scholar]
- 37.Elfgren C, Brun A, Gustafson L, et al. Neuropsychological tests as discriminators between dementia of Alzheimer type and frontotemporal dementia. Int J Geriatr Psychiatry. 1994;9:635–42. [Google Scholar]
- 38.Rascovsky K, Salmon DP, Ho GJ, et al. Cognitive profiles differ in autopsy-confirmed frontotemporal dementia and AD. Neurology. 2002;58:1801–8. doi: 10.1212/wnl.58.12.1801. [DOI] [PubMed] [Google Scholar]
- 39.Mendez MF, Cherrier M, Perryman KM, et al. Frontotemporal dementia versus Alzheimer’s disease: differential cognitive features. Neurology. 1996;47:1189–94. doi: 10.1212/wnl.47.5.1189. [DOI] [PubMed] [Google Scholar]
- 40.Varma AR, Snowden JS, Lloyd JJ, et al. Evaluation of the NINCDS-ADRDA criteria in the differentiation of Alzheimer’s disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1999;66:184–8. doi: 10.1136/jnnp.66.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nedjam Z, Devouche E, Dalla Barba G. Confabulation, but not executive dysfunction discriminate AD from frontotemporal dementia. Eur J Neurol. 2004;11:728–33. doi: 10.1111/j.1468-1331.2004.00981.x. [DOI] [PubMed] [Google Scholar]
- 42.Lomen-Hoerth C. Characterization of amyotrophic lateral sclerosis and frontotemporal dementia. Dement Geriatr Cogn Disord. 2004;17:337–41. doi: 10.1159/000077167. [DOI] [PubMed] [Google Scholar]
- 43.Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59:1077–9. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- 44.Lomen-Hoerth C, Murphy J, Langmore S, et al. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology. 2003;60:1094–7. doi: 10.1212/01.wnl.0000055861.95202.8d. [DOI] [PubMed] [Google Scholar]
- 45.Neary D, Snowden JS, Mann DM. Cognitive change in motor neurone disease/amyotrophic lateral sclerosis (MND/ALS) J Neurol Sci. 2000;180:15–20. doi: 10.1016/s0022-510x(00)00425-1. [DOI] [PubMed] [Google Scholar]
- 46.Strong MJ, Lomen-Hoerth C, Caselli RJ, et al. Cognitive impairment, frontotemporal dementia, and the motor neuron diseases. Ann Neurol. 2003;54 (Suppl 5):S20–3. doi: 10.1002/ana.10574. [DOI] [PubMed] [Google Scholar]
- 47.Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 48.Boccardi M, Sabattoli F, Laakso MP, et al. Frontotemporal dementia as a neural system disease. Neurobiol Aging. 2005;26:37–44. doi: 10.1016/j.neurobiolaging.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 49.Schroeter ML, Raczka K, Neumann J, et al. Neural networks in frontotemporal dementia: a meta-analysis. Neurobiol Aging. 2008;29:418–26. doi: 10.1016/j.neurobiolaging.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 50.Rabinovici GD, Seeley WW, Kim EJ, et al. Distinct MRI atrophy patterns in autopsy-proven Alzheimer’s disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen. 2007;22:474–88. doi: 10.1177/1533317507308779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seeley WW, Crawford R, Rascovsky K, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65:249–55. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosen HJ, Allison SC, Schauer GF, et al. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–25. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hodges JR. Frontotemporal dementia (Pick’s disease): clinical features and assessment. Neurology. 2001;56:6S–10. doi: 10.1212/wnl.56.suppl_4.s6. [DOI] [PubMed] [Google Scholar]
- 54.Seeley WW, Bauer AM, Miller BL, et al. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64:1384–90. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snowden J, Goulding P, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol. 1989;2:167–82. [Google Scholar]
- 56.Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61:1196–203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- 57.Hodges JR, Patterson K, Oxbury S, et al. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115 (Pt 6):1783–806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 58.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howard D, Patterson K. Pyramids and Palm trees: a test of semantic access from pictures and words. Bury St Edmunds: Thames Valley Publishing Company; 1992. [Google Scholar]
- 60.Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer’s disease. Neurology. 2000;54:2277–84. doi: 10.1212/wnl.54.12.2277. [DOI] [PubMed] [Google Scholar]
- 61.Hodges JR, Patterson K, Ward R, et al. The differentiation of semantic dementia and frontal lobe dementia (temporal and frontal variants of frontotemporal dementia) from early Alzheimer’s disease: a comparative neuropsychological study. Neuropsychology. 1999;13:31– 40. doi: 10.1037//0894-4105.13.1.31. [DOI] [PubMed] [Google Scholar]
- 62.Edwards-Lee T, Miller BL, Benson DF, et al. The temporal variant of frontotemporal dementia. Brain. 1997;120:1027–40. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- 63.Mychack P, Kramer JH, Boone KB, et al. The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology. 2001;56:S11–5. doi: 10.1212/wnl.56.suppl_4.s11. [DOI] [PubMed] [Google Scholar]
- 64.Gorno-Tempini ML, Rankin KP, Woolley JD, et al. Cognitive and behavioral profile in a case of right anterior temporal lobe neurodegeneration. Cortex. 2004;40:631–44. doi: 10.1016/s0010-9452(08)70159-x. [DOI] [PubMed] [Google Scholar]
- 65.Rankin KP, Kramer JH, Mychack P, et al. Double dissociation of social functioning in frontotemporal dementia. Neurology. 2003;60:266–71. doi: 10.1212/01.wnl.0000041497.07694.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol. 2005;18:28–36. doi: 10.1097/01.wnn.0000152225.05377.ab. [DOI] [PubMed] [Google Scholar]
- 67.Duffy JR. Motor speech disorders: substrates, differential diagnosis, and management. 1. xi. St. Louis (MO): Mosby; 1995. p. 467. [Google Scholar]
- 68.Rosen HJ, Allison SC, Ogar JM, et al. Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology. 2006;67:1752–6. doi: 10.1212/01.wnl.0000247630.29222.34. [DOI] [PubMed] [Google Scholar]
- 69.Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kertesz A, Martinez-Lage P, Davidson W, et al. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology. 2000;55:1368–75. doi: 10.1212/wnl.55.9.1368. [DOI] [PubMed] [Google Scholar]
- 71.Rebeiz JJ, Kolodny EH, Richardson EP., Jr Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol. 1968;18:20–33. doi: 10.1001/archneur.1968.00470310034003. [DOI] [PubMed] [Google Scholar]
- 72.Litvan I, Bhatia KP, Burn DJ, et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18:467–86. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- 73.Belfor N, Amici S, Boxer AL, et al. Clinical and neuropsychological features of corticobasal degeneration. Mech Ageing Dev. 2006;127:203–7. doi: 10.1016/j.mad.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 74.Murray R, Neumann M, Forman MS, et al. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology. 2007;68:1274–83. doi: 10.1212/01.wnl.0000259519.78480.c3. [DOI] [PubMed] [Google Scholar]
- 75.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 76.Grafman J, Litvan I, Stark M. Neuropsychological features of progressive supranuclear palsy. Brain Cogn. 1995;28:311–20. doi: 10.1006/brcg.1995.1260. [DOI] [PubMed] [Google Scholar]
- 77.Gorno-Tempini ML, Murray RC, Rankin KP, et al. Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: a case report. Neurocase. 2004;10:426–36. doi: 10.1080/13554790490894011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66:41–8. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- 79.Brun A. Frontal lobe degeneration of the non-alzheimer type: I. Neuropathology Arch Gerontol Geriatr. 1987;6:193–208. doi: 10.1016/0167-4943(87)90021-5. [DOI] [PubMed] [Google Scholar]
- 80.McKhann GM, Albert MS, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803–9. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 81.Broe M, Hodges JR, Schofield E, et al. Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology. 2003;60:1005–11. doi: 10.1212/01.wnl.0000052685.09194.39. [DOI] [PubMed] [Google Scholar]
- 82.Dickson DW. Neuropathology of Pick’s disease. Neurology. 2001;56:S16–20. doi: 10.1212/wnl.56.suppl_4.s16. [DOI] [PubMed] [Google Scholar]
- 83.Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alzheimer A. Uber eigenartige Krankheitsfalle des sparteren Alters. Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1911;4:356–85. [Google Scholar]
- 85.Onari K, Spatz H. Anatomische Beitragezur Lehre von der Pickschen umschriebene-Grosshirnriden-Atrophie (‘Picksche Krankheit’) Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1926;101:470–511. [Google Scholar]
- 86.Roberson ED. Frontotemporal dementia. Curr Neurol Neurosci Rep. 2006;6:481–9. doi: 10.1007/s11910-006-0050-7. [DOI] [PubMed] [Google Scholar]
- 87.Dickson DW, Bergeron C, Chin SS, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–46. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- 88.Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol. 1999;246(Suppl 2):II6–5. doi: 10.1007/BF03161076. [DOI] [PubMed] [Google Scholar]
- 89.Boxer AL, Geschwind MD, Belfor N, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol. 2006;63:81–6. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- 90.Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29:280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goedert M, Spillantini MG, Potier MC, et al. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. Embo J. 1989;8:393–9. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munoz DG, Dickson DW, Bergeron C, et al. The neuropathology and biochemistry of frontotemporal dementia. Ann Neurol. 2003;54 (Suppl 5):S24–8. doi: 10.1002/ana.10571. [DOI] [PubMed] [Google Scholar]
- 93.Knopman DS. Overview of dementia lacking distinctive histology: pathological designation of a progressive dementia. Dementia. 1993;4:132–6. doi: 10.1159/000107354. [DOI] [PubMed] [Google Scholar]
- 94.Lipton AM, White CL, 3rd, Bigio EH. Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathol (Berl) 2004;108:379–85. doi: 10.1007/s00401-004-0900-9. [DOI] [PubMed] [Google Scholar]
- 95.Josephs KA, Holton JL, Rossor MN, et al. Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol. 2004;30:369–73. doi: 10.1111/j.1365-2990.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- 96.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 97.Buratti E, Baralle FE. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front Biosci. 2008;13:867–78. doi: 10.2741/2727. [DOI] [PubMed] [Google Scholar]
- 98.Igaz LM, Kwong LK, Xu Y, et al. Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Am J Pathol. 2008;173:182–94. doi: 10.2353/ajpath.2008.080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neumann M, Kwong LK, Truax AC, et al. TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J Neuropathol Exp Neurol. 2007;66:177–83. doi: 10.1097/01.jnen.0000248554.45456.58. [DOI] [PubMed] [Google Scholar]
- 100.Cairns NJ, Neumann M, Bigio EH, et al. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171:227–40. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hasegawa M, Arai T, Nonaka T, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64:60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mackenzie IR. The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol. 2007;114:49–54. doi: 10.1007/s00401-007-0223-8. [DOI] [PubMed] [Google Scholar]
- 103.Weihl CC, Temiz P, Miller SE, et al. TDP-43 accumulation in inclusion body myopathy muscle suggests a common pathogenic mechanism with frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2008;79:1186–9. doi: 10.1136/jnnp.2007.131334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sreedharan J, Blair IP, Tripathi VB, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–72. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuhnlein P, Sperfeld AD, Vanmassenhove B, et al. Two German kindreds with familial amyotrophic lateral sclerosis due to TARDBP mutations. Arch Neurol. 2008;65:1185–9. doi: 10.1001/archneur.65.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schumacher A, Friedrich P, Diehl-Schmid J, et al. No association of TDP-43 with sporadic frontotemporal dementia. Neurobiol Aging. 2009;30:157–159. doi: 10.1016/j.neurobiolaging.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 107.Gijselinck I, Sleegers K, Engelborghs S, et al. Neuronal inclusion protein TDP-43 has no primary genetic role in FTD and ALS. Neurobiol Aging. 2009;30:1329–1331. doi: 10.1016/j.neurobiolaging.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 108.Uryu K, Nakashima-Yasuda H, Forman MS, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67:555–64. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Josephs KA, Whitwell JL, Knopman DS, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–7. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu WT, Josephs KA, Knopman DS, et al. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol. 2008;116:215–20. doi: 10.1007/s00401-008-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miklossy J, Steele JC, Yu S, et al. Enduring involvement of tau, beta-amyloid, alpha-synuclein, ubiquitin and TDP-43 pathology in the amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam (ALS/PDC) Acta Neuropathol. 2008;116:625–637. doi: 10.1007/s00401-008-0439-2. [DOI] [PubMed] [Google Scholar]
- 112.Higashi S, Iseki E, Yamamoto R, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–94. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 113.Josephs KA, Lin WL, Ahmed Z, et al. Frontotemporal lobar degeneration with ubiquitin-positive, but TDP-43-negative inclusions. Acta Neuropathol. 2008;116:159–67. doi: 10.1007/s00401-008-0397-8. [DOI] [PubMed] [Google Scholar]
- 114.Roeber S, Mackenzie IR, Kretzschmar HA, et al. TDP-43-negative FTLD-U is a significant new clinico-pathological subtype of FTLD. Acta Neuropathol. 2008;116:147–57. doi: 10.1007/s00401-008-0395-x. [DOI] [PubMed] [Google Scholar]
- 115.Mackenzie IR, Foti D, Woulfe J, et al. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain. 2008;131:1282–93. doi: 10.1093/brain/awn061. [DOI] [PubMed] [Google Scholar]
- 116.Holm IE, Englund E, Mackenzie IR, et al. A reassessment of the neuropathology of frontotemporal dementia linked to chromosome 3. J Neuropathol Exp Neurol. 2007;66:884–91. doi: 10.1097/nen.0b013e3181567f02. [DOI] [PubMed] [Google Scholar]
- 117.Josephs KA, Holton JL, Rossor MN, et al. Neurofilament inclusion body disease: a new proteinopathy? Brain. 2003;126:2291–303. doi: 10.1093/brain/awg231. [DOI] [PubMed] [Google Scholar]
- 118.Cairns NJ, Grossman M, Arnold SE, et al. Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology. 2004;63:1376–84. doi: 10.1212/01.wnl.0000139809.16817.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hatanpaa KJ, Bigio EH, Cairns NJ, et al. TAR DNA-binding protein 43 immunohistochemistry reveals extensive neuritic pathology in FTLD-U: a midwest-southwest consortium for FTLD study. J Neuropathol Exp Neurol. 2008;67:271–9. doi: 10.1097/NEN.0b013e31816a12a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mackenzie IR, Shi J, Shaw CL, et al. Dementia lacking distinctive histology (DLDH) revisited. Acta Neuropathol. 2006;112:551–9. doi: 10.1007/s00401-006-0123-3. [DOI] [PubMed] [Google Scholar]
- 121.Goldman JS, Farmer JM, Wood EM, et al. Comparison of family histories in FTLD subtypes and related tauopathies. Neurology. 2005;65:1817–9. doi: 10.1212/01.wnl.0000187068.92184.63. [DOI] [PubMed] [Google Scholar]
- 122.Goldman JS, Adamson J, Karydas A, et al. New genes, new dilemmas: FTLD genetics and its implications for families. Am J Alzheimers Dis Other Demen. 2007;22:507–15. doi: 10.1177/1533317507306662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van Swieten JC, Heutink P. Mutations in progranulin (GRN) within the spectrum of clinical and pathological phenotypes of frontotemporal dementia. Lancet Neurol. 2008;7:965–974. doi: 10.1016/S1474-4422(08)70194-7. [DOI] [PubMed] [Google Scholar]
- 124.Lynch T, Sano M, Marder KS, et al. Clinical characteristics of a family with chromosome 17-linked disinhibition-dementia-parkinsonism-amyotrophy complex. Neurology. 1994;44:1878–84. doi: 10.1212/wnl.44.10.1878. [DOI] [PubMed] [Google Scholar]
- 125.Wilhelmsen K, Lynch T, Pavlou E, et al. Localization of disinhibition-dementia-parkinsonism-amyotrophy complex to 17q21–22. Am J Hum Genet. 1994;6:1159–65. [PMC free article] [PubMed] [Google Scholar]
- 126.Wilhelmsen KC. Frontotemporal dementia is on the MAPtau. Ann Neurol. 1997;41:139–40. doi: 10.1002/ana.410410202. [DOI] [PubMed] [Google Scholar]
- 127.Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 128.Spillantini MG, Murrell JR, Goedert M, et al. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci U S A. 1998;95:7737– 41. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gass J, Cannon A, Mackenzie IR, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet. 2006;15:2988–3001. doi: 10.1093/hmg/ddl241. [DOI] [PubMed] [Google Scholar]
- 130.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 131.Cruts M, Kumar-Singh S, Van Broeckhoven C. Progranulin mutations in ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Curr Alzheimer Res. 2006;3:485–91. doi: 10.2174/156720506779025251. [DOI] [PubMed] [Google Scholar]
- 132.Pickering-Brown SM, Rollinson S, Du Plessis D, et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: comparison with patients with MAPT and no known mutations. Brain. 2008;131:721–31. doi: 10.1093/brain/awm331. [DOI] [PubMed] [Google Scholar]
- 133.Pickering-Brown SM, Baker M, Nonaka T, et al. Frontotemporal dementia with Pick-type histology associated with Q336R mutation in the tau gene. Brain. 2004;127:1415–26. doi: 10.1093/brain/awh147. [DOI] [PubMed] [Google Scholar]
- 134.Rizzini C, Goedert M, Hodges JR, et al. Tau gene mutation K257T causes a tauopathy similar to Pick’s disease. J Neuropathol Exp Neurol. 2000;59:990–1001. doi: 10.1093/jnen/59.11.990. [DOI] [PubMed] [Google Scholar]
- 135.Neumann M, Schulz-Schaeffer W, Crowther RA, et al. Pick’s disease associated with the novel Tau gene mutation K369I. Ann Neurol. 2001;50:503–13. doi: 10.1002/ana.1223. [DOI] [PubMed] [Google Scholar]
- 136.Hayashi S, Toyoshima Y, Hasegawa M, et al. Late-onset frontotemporal dementia with a novel exon 1 (Arg5His) tau gene mutation. Ann Neurol. 2002;51:525–30. doi: 10.1002/ana.10163. [DOI] [PubMed] [Google Scholar]
- 137.Hogg M, Grujic ZM, Baker M, et al. The L266V tau mutation is associated with frontotemporal dementia and Pick-like 3R and 4R tauopathy. Acta Neuropathol (Berl) 2003;106:323–36. doi: 10.1007/s00401-003-0734-x. [DOI] [PubMed] [Google Scholar]
- 138.Hasegawa M, Smith MJ, Goedert M. Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett. 1998;437:207–10. doi: 10.1016/s0014-5793(98)01217-4. [DOI] [PubMed] [Google Scholar]
- 139.D’Souza I, Poorkaj P, Hong M, et al. Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc Natl Acad Sci U S A. 1999;96:5598–603. doi: 10.1073/pnas.96.10.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Seelaar H, Kamphorst W, Rosso SM, et al. Distinct genetic forms of frontotemporal dementia. Neurology. 2008;71:1220–1226. doi: 10.1212/01.wnl.0000319702.37497.72. [DOI] [PubMed] [Google Scholar]
- 141.Bird T, Nochlin D, Poorkaj P, et al. A clinical pathological comparison of three families with frontotemporal dementia and identical mutations in the tau gene (P301L) Brain. 1999;122 ( Pt 4):741–756. doi: 10.1093/brain/122.4.741. [DOI] [PubMed] [Google Scholar]
- 142.Bugiani O, Murrell JR, Giaccone G, et al. Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J Neuropathol Exp Neurol. 1999;58:667–77. doi: 10.1097/00005072-199906000-00011. [DOI] [PubMed] [Google Scholar]
- 143.Geschwind DH, Robidoux J, Alarcon M, et al. Dementia and neurodevelopmental predisposition: cognitive dysfunction in presymptomatic subjects precedes dementia by decades in frontotemporal dementia. Ann Neurol. 2001;50:741–6. doi: 10.1002/ana.10024. [DOI] [PubMed] [Google Scholar]
- 144.Baker M, Litvan I, Houlden H, et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8:711–5. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- 145.Houlden H, Baker M, Morris HR, et al. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology. 2001;56:1702–6. doi: 10.1212/wnl.56.12.1702. [DOI] [PubMed] [Google Scholar]
- 146.He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med. 2003;81:600–12. doi: 10.1007/s00109-003-0474-3. [DOI] [PubMed] [Google Scholar]
- 147.He Z, Ong CH, Halper J, et al. Progranulin is a mediator of the wound response. Nat Med. 2003;9:225–9. doi: 10.1038/nm816. [DOI] [PubMed] [Google Scholar]
- 148.Ahmed Z, Mackenzie IR, Hutton ML, et al. Progranulin in frontotemporal lobar degeneration and neuroinflammation. J Neuroinflammation. 2007;4:7. doi: 10.1186/1742-2094-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Suzuki M, Nishiahara M. Granulin precursor gene: a sex steroid-inducible gene involved in sexual differentiation of the rat brain. Mol Genet Metab. 2002;75:31–7. doi: 10.1006/mgme.2001.3274. [DOI] [PubMed] [Google Scholar]
- 150.Van Damme P, Van Hoecke A, Lambrechts D, et al. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol. 2008;181:37–41. doi: 10.1083/jcb.200712039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kayasuga Y, Chiba S, Suzuki M, et al. Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav Brain Res. 2007;185:110–8. doi: 10.1016/j.bbr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 152.Chen-Plotkin AS, Geser F, Plotkin JB, et al. Variations in the progranulin gene affect global gene expression in frontotemporal lobar degeneration. Hum Mol Genet. 2008;17:1349– 62. doi: 10.1093/hmg/ddn023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Le Ber I, van der Zee J, Hannequin D, et al. Progranulin null mutations in both sporadic and familial frontotemporal dementia. Hum Mutat. 2007;28:846–55. doi: 10.1002/humu.20520. [DOI] [PubMed] [Google Scholar]
- 154.Le Ber I, Camuzat A, Hannequin D, et al. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain. 2008;131:732–46. doi: 10.1093/brain/awn012. [DOI] [PubMed] [Google Scholar]
- 155.Rademakers R, Baker M, Gass J, et al. Phenotypic variability associated with progranulin haploinsufficiency in patients with the common 1477C-->T (Arg493X) mutation: an international initiative. Lancet Neurol. 2007;6:857–68. doi: 10.1016/S1474-4422(07)70221-1. [DOI] [PubMed] [Google Scholar]
- 156.Snowden JS, Pickering-Brown SM, Mackenzie IR, et al. Progranulin gene mutations associated with frontotemporal dementia and progressive non-fluent aphasia. Brain. 2006;129:3091–102. doi: 10.1093/brain/awl267. [DOI] [PubMed] [Google Scholar]
- 157.Whitwell JL, Jack CR, Jr, Baker M, et al. Voxel-based morphometry in frontotemporal lobar degeneration with ubiquitin-positive inclusions with and without progranulin mutations. Arch Neurol. 2007;64:371–6. doi: 10.1001/archneur.64.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rohrer JD, Warren JD, Omar R, et al. Parietal lobe deficits in frontotemporal lobar degeneration caused by a mutation in the progranulin gene. Arch Neurol. 2008;65:506–13. doi: 10.1001/archneur.65.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Beck J, Rohrer JD, Campbell T, et al. A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain. 2008;131:706–20. doi: 10.1093/brain/awm320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang YJ, Xu YF, Dickey CA, et al. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci. 2007;27:10530–4. doi: 10.1523/JNEUROSCI.3421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Skibinski G, Parkinson NJ, Brown JM, et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–8. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 162.Gydesen S, Brown JM, Brun A, et al. Chromosome 3 linked frontotemporal dementia (FTD-3) Neurology. 2002;59:1585–94. doi: 10.1212/01.wnl.0000034763.54161.1f. [DOI] [PubMed] [Google Scholar]
- 163.Parkinson N, Ince PG, Smith MO, et al. ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B) Neurology. 2006;67:1074–7. doi: 10.1212/01.wnl.0000231510.89311.8b. [DOI] [PubMed] [Google Scholar]
- 164.Watts GD, Wymer J, Kovach MJ, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–81. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 165.Kimonis VE, Mehta SG, Fulchiero EC, et al. Clinical studies in familial VCP myopathy associated with Paget disease of bone and frontotemporal dementia. Am J Med Genet A. 2008;146A:745–57. doi: 10.1002/ajmg.a.31862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Morita M, Al-Chalabi A, Andersen PM, et al. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology. 2006;66:839–44. doi: 10.1212/01.wnl.0000200048.53766.b4. [DOI] [PubMed] [Google Scholar]
- 167.Vance C, Al-Chalabi A, Ruddy D, et al. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2–21.3. Brain. 2006;129:868–76. doi: 10.1093/brain/awl030. [DOI] [PubMed] [Google Scholar]
- 168.Van Deerlin V, Martinez-Lage M, Hakonarson H, et al. Genome-wide association study of frontotemporal lobar degeneration with or without concomitant motor neuron disease and TDP-43 neuropathology. 6th International Conference on Frontotemporal Dementias; Sept 3–5 2008; Rotterdam. [Google Scholar]
- 169.Davies RR, Kipps CM, Mitchell J, et al. Progression in frontotemporal dementia: identifying a benign behavioral variant by magnetic resonance imaging. Arch Neurol. 2006;63:1627–31. doi: 10.1001/archneur.63.11.1627. [DOI] [PubMed] [Google Scholar]
- 170.Kipps CM, Nestor PJ, Dawson CE, et al. Measuring progression in frontotemporal dementia: implications for therapeutic interventions. Neurology. 2008;70:2046–52. doi: 10.1212/01.wnl.0000313366.76973.8a. [DOI] [PubMed] [Google Scholar]
- 171.Woolley JD, Wilson MR, Hung E, et al. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164:1811–6. doi: 10.1176/appi.ajp.2007.07061001. [DOI] [PubMed] [Google Scholar]
- 172.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59:952–62. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130:2636–45. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 174.Knibb JA, Xuereb JH, Patterson K, et al. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59:156–65. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- 175.Johnson J, Head E, Kim R, et al. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–9. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- 176.Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–19. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rabinovici GD, Jagust WJ, Furst AJ, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 179.Boxer AL, Rankin KP, Miller BL, et al. Cinguloparietal atrophy distinguishes Alzheimer disease from semantic dementia. Arch Neurol. 2003;60:949–56. doi: 10.1001/archneur.60.7.949. [DOI] [PubMed] [Google Scholar]
- 180.Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA. 2001;286:2120–7. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 181.Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain. 2007;130:2616–35. doi: 10.1093/brain/awm177. [DOI] [PubMed] [Google Scholar]
- 182.Nestor P, Hodges J. Non-Alzheimer dementias. Semin Neurol. 2000;20:439–46. doi: 10.1055/s-2000-13176. [DOI] [PubMed] [Google Scholar]
- 183.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–13. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 184.Sunderland T, Linker G, Mirza N, et al. Decreased beta-amyloid1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289:2094–103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 185.Bian H, Van Swieten JC, Leight S, et al. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology. 2008;70:1827–35. doi: 10.1212/01.wnl.0000311445.21321.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 187.Rabinovici GD, Furst AJ, O’Neil JP, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68:1205–12. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- 188.Kim EJ, Rabinovici GD, Seeley WW, et al. Patterns of MRI atrophy in tau positive and ubiquitin positive frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2007;78:1375–8. doi: 10.1136/jnnp.2006.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Whitwell JL, Josephs KA, Rossor MN, et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol. 2005;62:1402–8. doi: 10.1001/archneur.62.9.1402. [DOI] [PubMed] [Google Scholar]
- 190.Grossman M, Libon DJ, Forman MS, et al. Distinct antemortem profiles in patients with pathologically defined frontotemporal dementia. Arch Neurol. 2007;64:1601–9. doi: 10.1001/archneur.64.11.1601. [DOI] [PubMed] [Google Scholar]
- 191.Huey ED, Putnam KT, Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology. 2006;66:17–22. doi: 10.1212/01.wnl.0000191304.55196.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Boxer AL, Boeve BF. Frontotemporal dementia treatment: current symptomatic therapies and implications of recent genetic, biochemical, and neuroimaging studies. Alzheimer Dis Assoc Disord. 2007;21:S79–87. doi: 10.1097/WAD.0b013e31815c345e. [DOI] [PubMed] [Google Scholar]
- 193.Vossel KA, Miller BL. New approaches to the treatment of frontotemporal lobar degeneration. Curr Opin Neurol. 2008;21:708–16. doi: 10.1097/WCO.0b013e328318444d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Knopman DS, Kramer JH, Boeve BF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131:2957–2968. doi: 10.1093/brain/awn234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Engelborghs S, Vloeberghs E, Maertens K, et al. Evidence for an association between the CSF HVA:5-HIAA ratio and aggressiveness in frontotemporal dementia but not in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1080. [PMC free article] [PubMed] [Google Scholar]
- 196.Yang Y, Schmitt HP. Frontotemporal dementia: evidence for impairment of ascending serotoninergic but not noradrenergic innervation. Immunocytochemical and quantitative study using a graph method. Acta Neuropathol. 2001;101:256–70. doi: 10.1007/s004010000293. [DOI] [PubMed] [Google Scholar]
- 197.Sparks D, Markesbery WR. Altered serotonergic and cholinergic synaptic markers in Pick’s disease. Arch Neurol. 1991;48:796–9. doi: 10.1001/archneur.1991.00530200032014. [DOI] [PubMed] [Google Scholar]
- 198.Procter AW, Qurne M, Francis PT. Neurochemical features of frontotemporal dementia. Dement Geriatr Cogn Disord. 1999;10 (Suppl 1):80–4. doi: 10.1159/000051219. [DOI] [PubMed] [Google Scholar]
- 199.Bowen DM, Procter AW, Mann DM, et al. Imbalance of a serotonergic system in frontotemporal dementia: implication for pharmacotherapy. Psychopharmacology (Berl) 2008;196:603–10. doi: 10.1007/s00213-007-0992-8. [DOI] [PubMed] [Google Scholar]
- 200.Franceschi M, Anchisi D, Pelati O, et al. Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Ann Neurol. 2005;57:216–25. doi: 10.1002/ana.20365. [DOI] [PubMed] [Google Scholar]
- 201.Huey ED, Grafman J, Wassermann EM, et al. Characteristics of frontotemporal dementia patients with a Progranulin mutation. Ann Neurol. 2006;60:374–80. doi: 10.1002/ana.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Swartz JR, Miller BL, Lesser IM, et al. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors [published erratum appears in J Clin Psychiatry 1997 Jun; 58 (6): 275] J Clin Psychiatry. 1997;58:212–6. [PubMed] [Google Scholar]
- 203.Ikeda M, Shigenobu K, Fukuhara R, et al. Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement Geriatr Cogn Disord. 2004;17:117–21. doi: 10.1159/000076343. [DOI] [PubMed] [Google Scholar]
- 204.Moretti R, Torre P, Antonello RM, et al. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49:13–9. doi: 10.1159/000067021. [DOI] [PubMed] [Google Scholar]
- 205.Deakin JB, Rahman S, Nestor PJ, et al. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl) 2004;172:400–8. doi: 10.1007/s00213-003-1686-5. [DOI] [PubMed] [Google Scholar]
- 206.Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17:355–9. doi: 10.1159/000077171. [DOI] [PubMed] [Google Scholar]
- 207.Moretti R, Torre P, Antonello RM, et al. Olanzapine as a treatment of neuropsychiatric disorders of Alzheimer’s disease and other dementias: a 24-month follow-up of 68 patients. Am J Alzheimers Dis Other Demen. 2003;18:205–14. doi: 10.1177/153331750301800410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Curtis RC, Resch DS. Case of pick’s central lobar atrophy with apparent stabilization of cognitive decline after treatment with risperidone. J Clin Psychopharmacol. 2000;20:384–5. doi: 10.1097/00004714-200006000-00018. [DOI] [PubMed] [Google Scholar]
- 209.Fellgiebel A, Muller MJ, Hiemke C, et al. Clinical improvement in a case of frontotemporal dementia under aripiprazole treatment corresponds to partial recovery of disturbed frontal glucose metabolism. World J Biol Psychiatry. 2007;8:123–6. doi: 10.1080/15622970601016538. [DOI] [PubMed] [Google Scholar]
- 210.Pijnenburg YA, Sampson EL, Harvey RJ, et al. Vulnerability to neuroleptic side effects in frontotemporal lobar degeneration. Int J Geriatr Psychiatry. 2003;18:67–72. doi: 10.1002/gps.774. [DOI] [PubMed] [Google Scholar]
- 211.Hansen LA, Deteresa R, Tobias H, et al. Neocortical morphometry and cholinergic neurochemistry in Pick’s disease. Am J Pathol. 1988;131:507–18. [PMC free article] [PubMed] [Google Scholar]
- 212.Meier-Ruge W, Iwangoff P, Reichlmeier K. Neurochemical enzyme changes in Alzheimer’s and Pick’s disease. Arch Gerontol Geriatr. 1984;3:161–5. doi: 10.1016/0167-4943(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 213.Yates CM, Simpson J, Maloney AFJ, et al. Neurochemical observations in a case of Pick’s disease. Neurol Sci. 1980;48:257–63. doi: 10.1016/0022-510x(80)90205-1. [DOI] [PubMed] [Google Scholar]
- 214.Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21:931–7. doi: 10.2165/00002512-200421140-00003. [DOI] [PubMed] [Google Scholar]
- 215.Kertesz A, Morlog D, Light M, et al. Galantamine in frontotemporal dementia and primary progressive aphasia. Dement Geriatr Cogn Disord. 2008;25:178–85. doi: 10.1159/000113034. [DOI] [PubMed] [Google Scholar]
- 216.Mendez MF, Shapira JS, McMurtray A, et al. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry. 2007;15:84–7. doi: 10.1097/01.JGP.0000231744.69631.33. [DOI] [PubMed] [Google Scholar]
- 217.Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348:1333–41. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 218.Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord. 2007;21:164–6. doi: 10.1097/WAD.0b013e318047df5d. [DOI] [PubMed] [Google Scholar]
- 219.Diehl-Schmid J, Forstl H, Perneczky R, et al. A 6-month, open-label study of memantine in patients with frontotemporal dementia. Int J Geriatr Psychiatry. 2008;23:754–9. doi: 10.1002/gps.1973. [DOI] [PubMed] [Google Scholar]
- 220.Boxer AL, Lipton AM, Womack K, et al. An open-label study of memantine treatment in 3 subtypes of frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord. 2009 Jul–Sep;23(3):211–7. doi: 10.1097/WAD.0b013e318197852f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.van Balken I, Litvan I. Current and future treatments in progressive supranuclear palsy. Curr Treat Options Neurol. 2006;8:211–23. doi: 10.1007/s11940-006-0012-z. [DOI] [PubMed] [Google Scholar]
- 222.Litvan I, Grimes DA, Lang AE, et al. Clinical features differentiating patients with postmortem confirmed progressive supranuclear palsy and corticobasal degeneration. J Neurol. 1999;246(Suppl 2):II1–5. doi: 10.1007/BF03161075. [DOI] [PubMed] [Google Scholar]
- 223.Bensimon G, Lacomblez L, Meininger V on behalf of the ALS/Riluzole Study Group. A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med. 1994;330:585–91. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 224.Lomen-Hoerth C. Amyotrophic lateral sclerosis from bench to bedside. Semin Neurol. 2008;28:205–11. doi: 10.1055/s-2008-1062265. [DOI] [PubMed] [Google Scholar]
- 225.Merrilees J. A model for management of behavioral symptoms in frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord. 2007;21:S64–9. doi: 10.1097/WAD.0b013e31815bf774. [DOI] [PubMed] [Google Scholar]
- 226.Talerico KA, Evans LK. Responding to safety issues in frontotemporal dementias. Neurology. 2001;56:S52–5. doi: 10.1212/wnl.56.suppl_4.s52. [DOI] [PubMed] [Google Scholar]
- 227.Rademakers R, Cruts M, van Broeckhoven C. The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum Mutat. 2004;24:277–95. doi: 10.1002/humu.20086. [DOI] [PubMed] [Google Scholar]
- 228.Lewis J, McGowan E, Rockwood J, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–5. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 229.Lin WL, Lewis J, Yen SH, et al. Ultrastructural neuronal pathology in transgenic mice expressing mutant (P301L) human tau. J Neurocytol. 2003;32:1091–105. doi: 10.1023/B:NEUR.0000021904.61387.95. [DOI] [PubMed] [Google Scholar]
- 230.Wittmann CW, Wszolek MF, Shulman JM, et al. Tauopathy in drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–4. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- 231.Kraemer BC, Zhang B, Leverenz JB, et al. Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proc Natl Acad Sci U S A. 2003;100:9980–5. doi: 10.1073/pnas.1533448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Trojanowski JQ, Duff K, Fillit H, et al. New directions for frontotemporal dementia drug discovery. Alzheimers Dement. 2008;4:89–93. doi: 10.1016/j.jalz.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rademakers R, Eriksen JL, Baker M, et al. Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet. 2008;17:3631–3642. doi: 10.1093/hmg/ddn257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Alzheimer disease and frontotemporal dementia mutation database [online] Available from URL: http://www.molgen.ua.ac.be/FTDMutations.
- 235.USFDA. Available from URL: http://www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm053171.htm.
New references
- 1.Pick A. Uber die Beziehungen der senilen Hirnatrophie zur Aphasie. Prager Medizinische Wochenschrift. 1892;17:165–167. [Google Scholar]
- 2.Pick A. Zur symptomatologie der linksseitigen schlafenappenatrophie. Monatsschrift fur Psychiatrie und Neurologie. 1904;16:378–388. [Google Scholar]
- 3.Benajiba L, Le Ber I, Camuzat A, et al. TARDBP mutations in motoneuron disease with frontotemporal lobar degeneration. Ann Neurol. 2009;65(4):470–3. doi: 10.1002/ana.21612. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs GG, Murrell JR, Horvath S, et al. TARDBP variation associated with frontotemporal dementia, supranuclear gaze palsy, and chorea. Mov Disord. 2009;24(12):1843–7. doi: 10.1002/mds.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neumann M, Rademakers R, Roeber S, et al. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009 Nov;132(Pt 11):2922–31. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seelaar H, Klijnsma KY, de Koning I, et al. Frequency of uniquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration. J Neurol. doi: 10.1007/s00415-009-5404-z. Epub Nov 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagier-Tourenne C, Cleveland DW. Rethinking ALS: the FUS about TDP-43. Cell. 2009 Mar 20;136(6):1001–4. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009 Feb 27;323(5918):1205–8. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 9.Munoz DG, Neumann M, Kusaka H, et al. FUS pathology in basophilic inclusion body disease. Acta Neuropathol. 2009 Nov;118(5):617–27. doi: 10.1007/s00401-009-0598-9. [DOI] [PubMed] [Google Scholar]
- 10.Neumann M, Roeber S, Kretzschmar HA, et al. Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease. Acta Neuropathol. 2009 Nov;118(5):605–16. doi: 10.1007/s00401-009-0581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holm IE, Isaacs AM, Mackenzie IR. Absence of FUS-immunoreactive pathology in frontotemporal dementia linked to chromosome 3 (FTD-3) caused by mutation in the CHMP2B gene. Acta Neuropathol. 2009 Nov;118(5):719–20. doi: 10.1007/s00401-009-0593-1. [DOI] [PubMed] [Google Scholar]
- 12.Yu CE, Bird TD, Bekris LM, et al. The spectrum of mutations in progranulin. Arch Neurol. 2010;67(2):161–170. doi: 10.1001/archneurol.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dormann D, Capell A, Carlson AM, et al. Proteolytic processing of TAR DNA binding protein-43 by caspases produces C-terminal fragments with disease defining properties independent of progranulin. J Neurochem. 2009;110:1082–1094. doi: 10.1111/j.1471-4159.2009.06211.x. [DOI] [PubMed] [Google Scholar]
- 14.Blair IP, Williams KL, Warraich ST, et al. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J Neurol Neurosurg Psychiatry. 2009 Dec 3; doi: 10.1136/jnnp.2009.194399. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]