Abstract
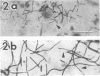
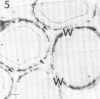
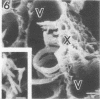
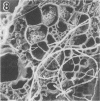
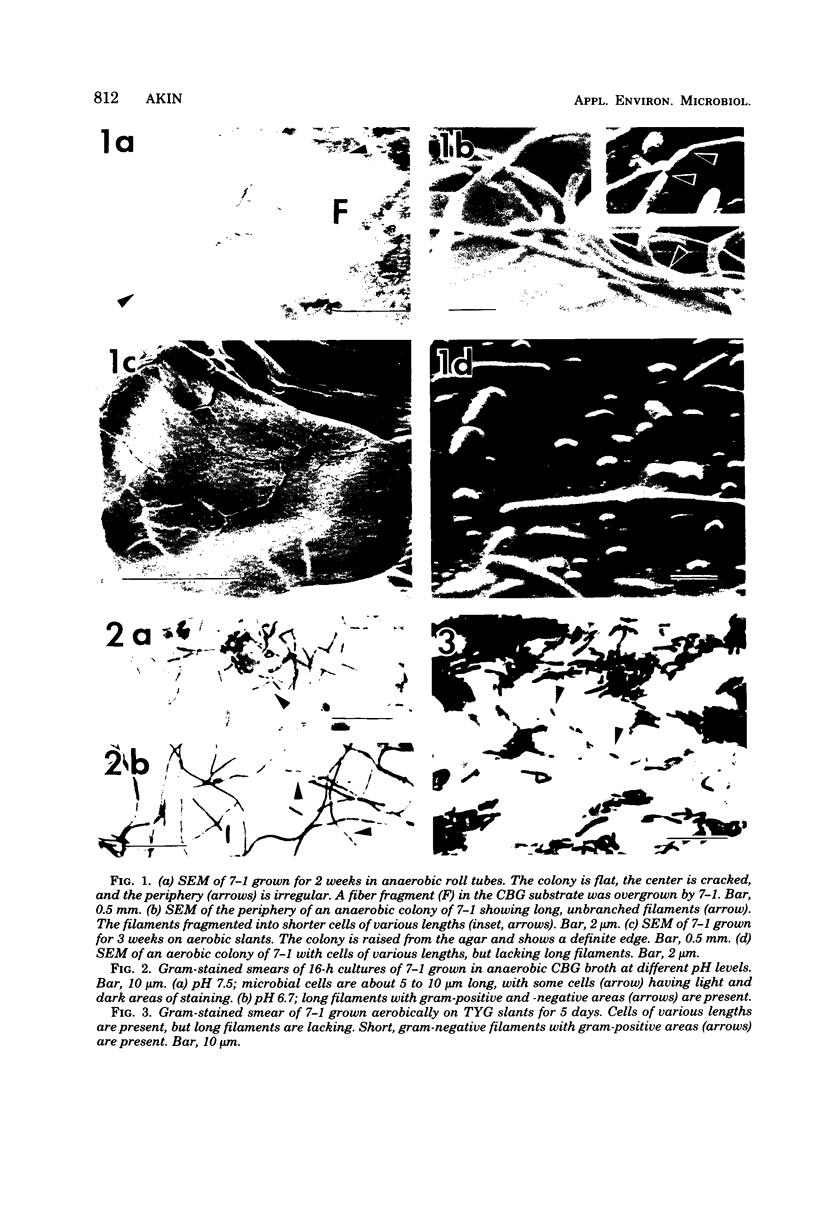
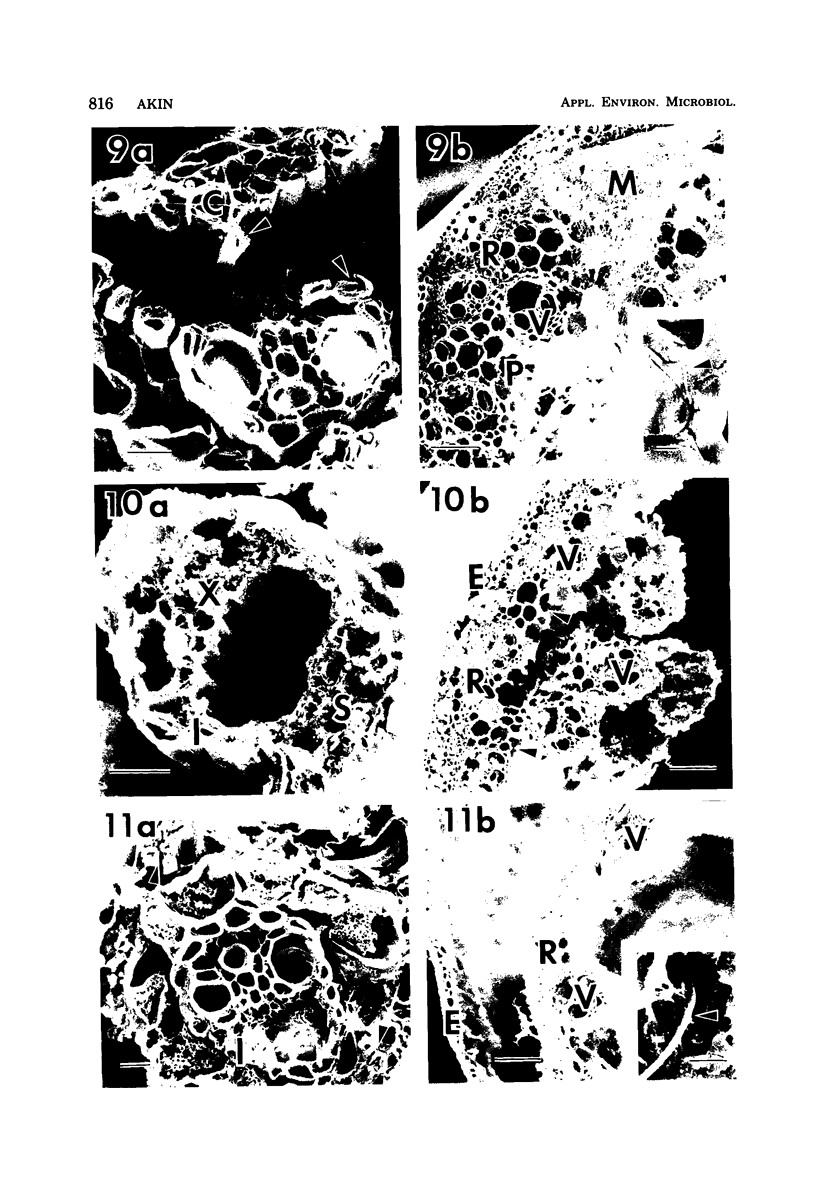
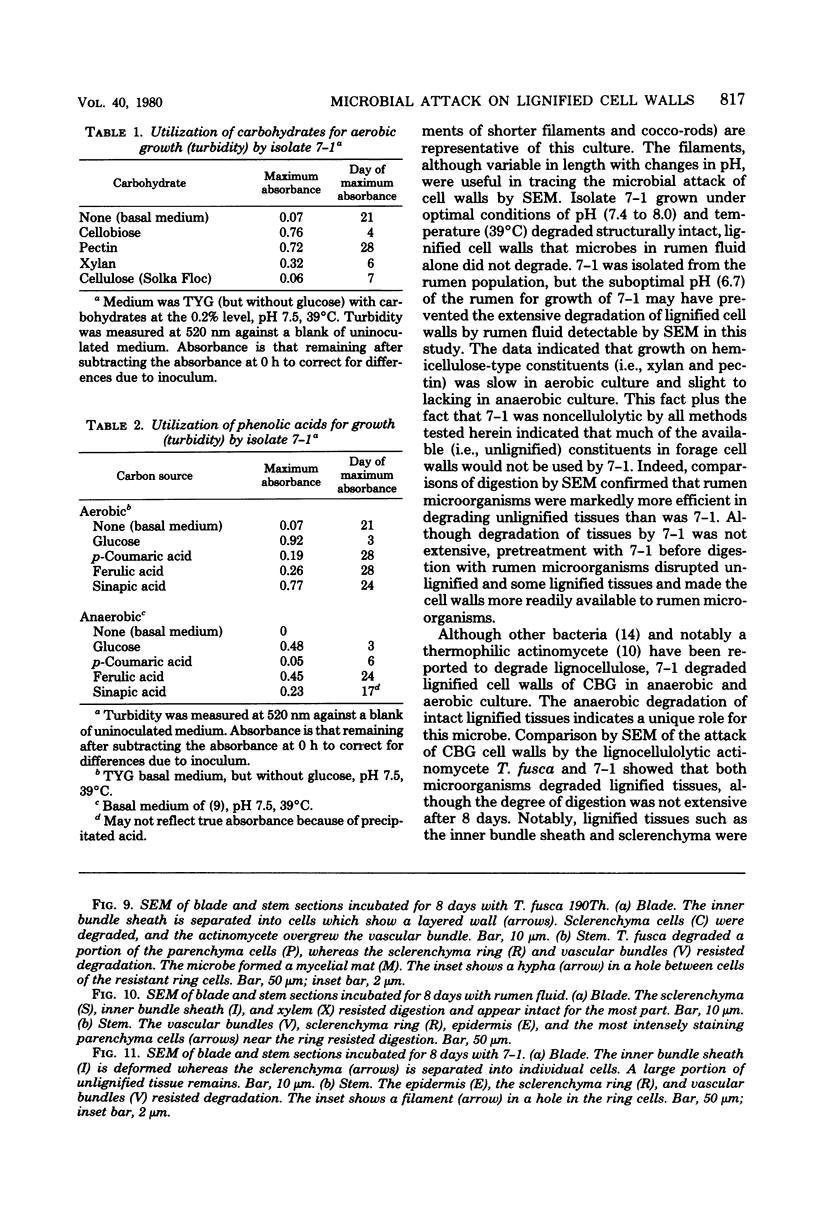
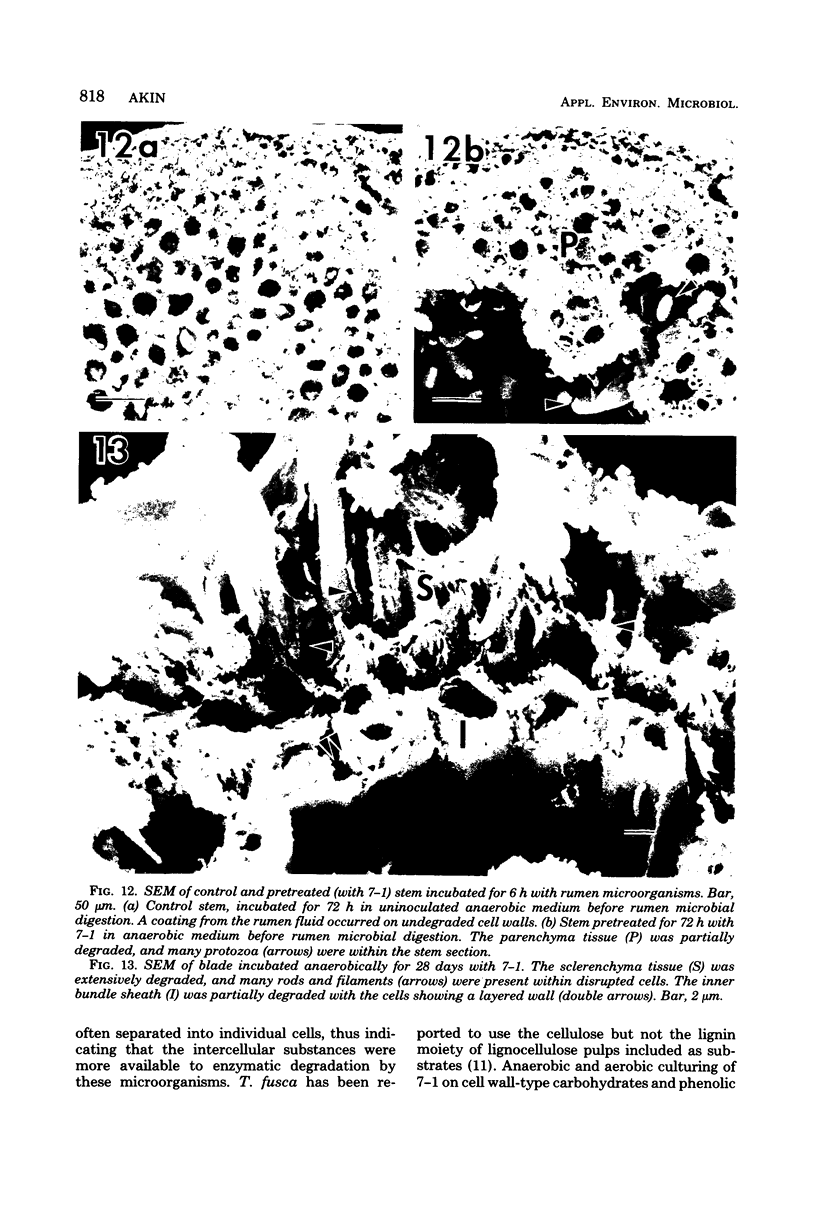
A filamentous, facultatively anaerobic microorganism that attacked lignified tissue in forage grasses was isolated from rumen fluid with a Bermuda grass-containing anaerobic medium in roll tubes. The microbe, designated 7-1, demonstrated various colony and cellular morphologies under different growth conditions. Scanning electron microscopy revealed that 7-1 attacked lignified cell walls in aerobic and anaerobic culture. 7-1 predominately degraded tissues reacting positively for lignin with the chlorine-sulfite stain (i.e., sclerenchyma in leaf blades and parenchyma in stems) rather than the more resistant acid phloroglucinol-positive tissues (i.e., lignified vascular tissue and sclerenchyma ring in stems), although the latter tissues were occasionally attacked. Turbidimetric tests showed that 7-1 in anaerobic culture grew optimally at 39°C at a pH of 7.4 to 8.0. Tests for growth on plant cell wall carbohydrates showed that 7-1 grew on xylan and pectin slowly in aerobic cultures but not with pectin and only slightly with xylan in anaerobic culture. 7-1 was noncellulolytic as shown by filter paper tests. The microbe used the phenolic acids sinapic, ferulic, and p-coumaric acids as substrates for growth; the more highly methoxylated acids were used more effectively.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Burdick D., Michaels G. E. Rumen bacterial interrelationships with plant tissue during degradation revealed by transmission electron microscopy. Appl Microbiol. 1974 Jun;27(6):1149–1156. doi: 10.1128/am.27.6.1149-1156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E. Ultrastructure of rigid and lignified forage tissue degradation by a filamentous rumen microorganism. J Bacteriol. 1976 Mar;125(3):1156–1162. doi: 10.1128/jb.125.3.1156-1162.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy W. D. Biotechnology report: single cell proteins from cellulosic wastes. Biotechnol Bioeng. 1974 Jul;16(7):869–880. doi: 10.1002/bit.260160702. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L., Crawford R. L. Microbial degradation of lignocellulose: the lignin component. Appl Environ Microbiol. 1976 May;31(5):714–717. doi: 10.1128/aem.31.5.714-717.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L. Growth of Thermomonospora fusca on lignocellulosic pulps of varying lignin content. Can J Microbiol. 1974 Jul;20(7):1069–1072. doi: 10.1139/m74-167. [DOI] [PubMed] [Google Scholar]
- Gaillard B. D., Richards G. N. Presence of soluble lignin-carbohydrate complexes in the bovine rumen. Carbohydr Res. 1975 Jun;42(1):135–145. doi: 10.1016/s0008-6215(00)84106-3. [DOI] [PubMed] [Google Scholar]
- Stafford H. A. Histochemical & Biochemical Differences Between Lignin-Like Materials in Phleum pratense L. Plant Physiol. 1962 Sep;37(5):643–649. doi: 10.1104/pp.37.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]