Abstract
Sexual reproduction enables eukaryotic organisms to re-assort genetic diversity and purge deleterious mutations, producing better-fit progeny. Sex arose early and pervades eukaryotes. Fungal and parasite pathogens once thought asexual have maintained cryptic sexual cycles, including unisexual or parasexual reproduction. As pathogens become niche and host-adapted, sex appears to specialize to promote inbreeding and clonality yet maintain out-crossing potential. During self-fertile sexual modes, sex itself may generate genetic diversity de novo. Mating-type loci govern fungal sexual identity; how parasites establish sexual identity is unknown. Comparing and contrasting fungal and parasite sex promises to reveal how microbial pathogens evolved and are evolving.
Introduction
How microbial pathogens evolve, develop drug resistance, and interact with the host involves both genetic change and exchange. In bacteria, genetic exchange occurs largely via horizontal gene transfer; in the fungal and parasite eukaryotic microbial pathogens sexual reproduction drives genetic exchange. We review here how sexual identity is defined and modes and roles of sexual reproduction in virulence, production of infectious propagules, and generation of diversity. In fungi and parasites once thought asexual, genomics reveals they have retained machinery for sex, and laboratory studies uncovered extant sexual cycles that enable but limit genetic exchange, generating populations with clonal features.
In fungi, sex commonly involves two cells of opposite mating type (a, α) that secrete mating pheromones to trigger cell-cell fusion (heterothallism). Other fungi are self-fertile and a single isolate can undergo sexual reproduction (homothallism). Transitions between these sexual reproduction modes, outcrossing vs. selfing/inbreeding, commonly occur throughout the fungal kingdom. Recent studies of the three most common systemic human fungal pathogens (Cryptococcus neoformans, Candida albicans, Aspergillus fumigatus) reveal novel mating paradigms that differ substantially from model species like S. cerevisiae.
C. neoformans has an a-α opposite sex mating cycle that has been defined in the laboratory for more than 30 years, and yet the natural population is largely unisexual, predominantly α mating type, and had therefore been thought to be asexual. Instead, a novel form of self-fertility evolved in which cells of one mating type (α) can complete a unisexual cycle without an opposite partner, and population genetics studies suggest this may be the predominant form of sexual reproduction in nature (Bui et al., 2008; Lin et al., 2005; Lin et al., 2007; Lin et al., 2009). C. albicans was thought to be asexual for more than a century, but with the discovery of the MTL locus, isolation of a and α mating competent strains, and the discovery of genetic and host conditions that promote mating, we now appreciate that a parasexual cycle is extant (Bennett and Johnson, 2003). Recently, C. albicans was also discovered to reproduce unisexually illustrating a remarkable convergence in modes of sexual reproduction in two divergent successful human fungal pathogens (Alby et al., 2009). Studies of other pathogenic Candida sp. have converged to illustrate a marked plasticity in specification of sexual identity, meiotic machinery, and in the generation of aneuploid progeny with the capacity for phenotypic diversity (Butler et al., 2009; Reedy et al., 2009). Finally, an extant sexual cycle was uncovered for A. fumigatus, a species previously thought to be asexual, requiring specialized media and culture conditions (O’Gorman et al., 2009). Thus, rather than being asexual, pathogenic fungi have retained cryptic, modified sexual cycles that enable both inbreeding and out-crossing and generate diversity in novel ways. This includes the potential of sex itself to serve as a mutagen that generates genetic diversity de novo, analogous to the model fungus Aspergillus nidulans in which parasexual reproduction generates phenotypic plasticity during experimental evolution (Schoustra et al., 2007).
Our understanding of sexual reproduction in pathogenic fungi parallels recent advances in defining extant sexual cycles of human parasite pathogens. In the protozoan Giardia the discovery of a novel sexual reproduction mode involving nuclear fusion, meiotic gene expression, and genetic exchange illustrates a novel form of self-fertility, similar to unisexual mating of fungal pathogens (Poxleitner et al., 2008). In the protozoan parasite Leishmania co-infections in the sand fly vector reveal an extant capacity for genetic exchange with parallels to the C. albicans parasexual cycle (Akopyants et al., 2009). In the kinetoplastid Trypanosoma, an extant sexual cycle involving meiosis and intra-clonal and inter-clonal mating mirrors the out-crossing/self-fertile duality of human fungal pathogens (Heitman, 2006). In Plasmodium a well-defined sexual cycle is essential for vertebrate-insect transmission, and an outline for this developmental cascade is emerging from proteomic and molecular studies (Khan et al., 2005). Yet in all of the parasite pathogens, we know nothing about how their sexual identities are specified. Insights from fungal sex and MAT evolution might lay the foundation to approach this question, and suggests models by which parasite sexual identity is specified.
These studies of sex evolution and its impact throughout fungi and parasites illuminate paradigms for generating and maintaining genetic diversity in microbial pathogens with implications for multi-cellular eukaryotes. Reviews on fungal sex and the meiotic toolkit have recently appeared to which the interested reader is referred (Butler, 2010; Lee et al., 2010; Lin, 2009; Morrow and Fraser, 2009; Schurko and Logsdon, 2008; Schurko et al., 2009; Sherwood and Bennett, 2009). Parallels between fungal and parasite sexual reproduction were reviewed earlier (Heitman, 2006); advances since 2006 are the focus here.
Sex in Fungi
Cryptococcus neoformans
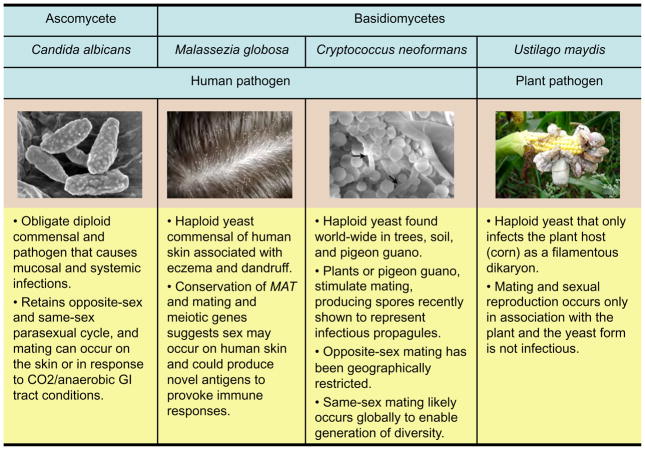
C. neoformans is a pathogenic basidiomycete yeast, more closely related to mushroom fungi and the plant pathogen Ustilago maydis than to the ascomycetes Saccharomyces cerevisiae and C. albicans (Figure 1). C. neoformans is ubiquitous and infects both immunocompromised and immunocompetent individuals, most frequently causing meningitis and pulmonary infections. It is responsible for >one million cases annually, > 600,000 mortalities, and ~one-third of all AIDS-associated deaths (Park et al., 2009). The closely-related species C. gattii infects immunocompetent hosts and is causing an outbreak on Vancouver Island and the Pacific Northwest (Byrnes et al., 2010; Fraser et al., 2005; Kidd et al., 2004). A laboratory defined a-α opposite sex mating cycle has been known for >30 years, and yet with a largely α unisexual population, it has been unclear if and how sex occurs in nature. Sexual reproduction of C. neoformans is central to virulence because it produces spores that serve as infectious propagules, generates genetic diversity, and the α-mating type has been linked to virulence.
Figure 1.
Sex and virulence of fungal pathogens. Four major human and plant fungal pathogens are illustrated to compare and contrast features of sexual reproduction and links to virulence.
The structure and evolution of a large, complex mating type (MAT) locus that governs sexual identity and promotes virulence has been defined (Fraser et al., 2004). Studies have also shown that a-α opposite sex occurs in environmental niches (pigeon guano, plants) and is extant in sub-Saharan Africa (Litvintseva et al., 2003; Nielsen et al., 2007; Xue et al., 2007), and document that sexually produced spores are infectious (Giles et al., 2009; Velagapudi et al., 2009). Moreover, an unusual sexual mode has been discovered involving only one mating type that generates infectious spores and diversity within unisexual populations (Lin et al., 2005).
C. neoformans mating involves two opposite haploid mating partners, a and α, which secrete pheromones that trigger cell-cell fusion (McClelland et al., 2004). The resulting dikaryon harbors two congressed, unfused nuclei, and undergoes a dimorphic transition from budding yeast to filamentous hyphae. The hyphae produce a specialized structure (basidia) where nuclear fusion and meiosis occur, and repeated rounds of mitosis/budding decorate the basidium surface with long spore chains. Spores are readily aerosolized, and their small size promotes alveolar deposition following inhalation. Similar to yeast cells, these sexually produced spores are infectious in murine inhalation models; unlike yeast cells, spores do not require opsonization for phagocytosis by alveolar macrophages (Giles et al., 2009; Velagapudi et al., 2009).
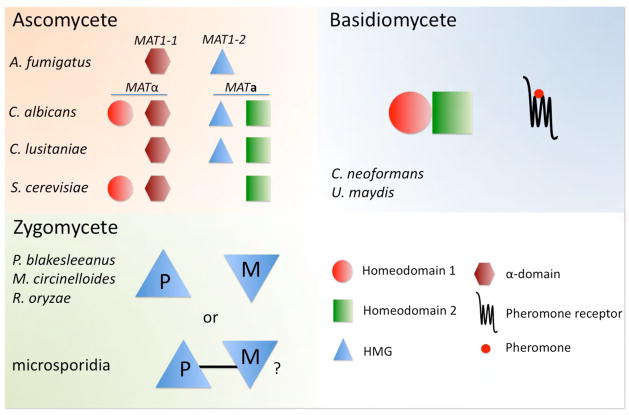
Sexual reproduction and identity are governed by the MAT locus, which occurs in two large, >100 kb idiomorphs encoding >20 genes, including many involved in mating/meiosis and encoding the Sxi1α/Sxi2a homeodomain regulators (HD1/HD2), pheromones, and pheromone receptors that define mating type and zygote identity (Figure 2) (Hull et al., 2002; Stanton et al., 2010). Based on comparative genomics, the bipolar MAT locus evolved from an ancestral tetrapolar system with unlinked homeodomain and pheromone/receptor loci via gene acquisition, fusion into a contiguous gene cluster, and additional rearrangements (Fraser et al., 2004; Lengeler et al., 2002). MAT also evolved via loss of allelic diversity and loss of one of the paired homeodomain genes (Metin et al., 2010). These transitions reduced out-crossing and promoted inbreeding, possibly as this pathogen became niche-adapted.
Figure 2.
MAT encoded cell identity determinants in pathogenic and model fungi. Genes resident within fungal MAT loci are illustrated for three phyla of the fungal kingdom. These genes encode transcription factors (homeodomain, HMG, α-domain) or pheromones or pheromone receptors that establish cell type identity, orchestrate sexual reproduction, and in some cases are linked to virulence.
The MAT locus α allele is linked to C. neoformans virulence. In some genetic backgrounds, α cells are more virulent than a cells (Kwon-Chung et al., 1992; Nielsen et al., 2005a). In other backgrounds, α and a cells are of equivalent virulence during solo infections, but during co-infection communicate via secreted mating pheromones that function in quorum sensing. Additionally, α cells preferentially cross the blood brain barrier to more effectively infect the CNS (Nielsen et al., 2005b; Okagaki et al., 2010). Several MAT genes are implicated in pathogenesis, including the PAK kinase Ste20.
While sex readily occurs in the lab, the natural population is almost exclusively α, and thus, whether a-α mating occurs in nature is unknown and has not been directly observed. Indirect proof for mating is that unusual environmental and clinical isolates are diploid (aADα, αADa isolates) products of a-α mating. a-α mating is stimulated by lab conditions mimicking environmental niches: culture on pigeon guano media and co-culture with or on plant seedlings (Nielsen et al., 2007; Xue et al., 2007). A unique C. neoformans lineage (VNB) restricted to sub-Saharan Africa harbors 25% a mating type isolates, exhibits evidence of recombination (an indirect measure of sex), and is robustly fertile in the laboratory. This unique African lineage is geographically restricted, and otherwise the a mating type is rare (~0.1%) or unknown in globally distributed serotype A C. neoformans lineages (VNI, VNII), precluding a-α mating opportunities in nature.
The discovery that C. neoformans can undergo a modified form of sexual reproduction involving only one mating type provides insight into how diversity is maintained and infectious spores produced in a largely unisexual global population (Figure 3) (Lin et al., 2005). This unisexual reproduction cycle occurs under similar conditions that support opposite-sex mating. This process involves ploidy changes to produce diploid intermediates, levels of recombination similar to that during a-α mating, and a requirement for the meiotic orthologs Spo11 and Dmc1 to generate and repair DNA double-strand breaks (DSBs) for recombination. However, whether unisexual mating occurred in nature was also unknown.
Figure 3.
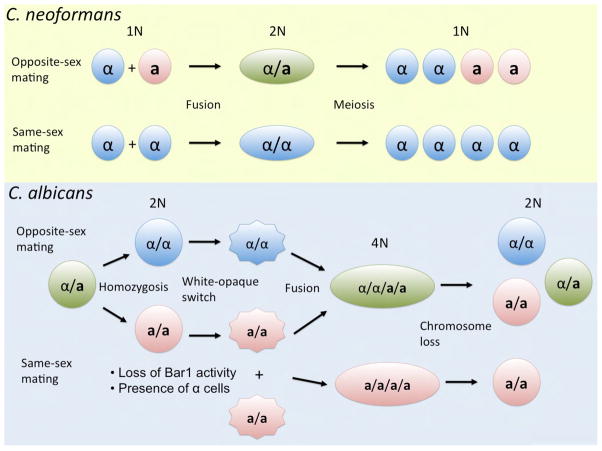
Unisexual reproduction of pathogenic fungi. The opposite and same-sex cycles for C. neoformans and C. albicans are depicted. These reproductive cycles involve meiosis in C. neoformans but are currently thought to be parasexual in C. albicans. These alternative sexual cycles contribute to generate and maintain population diversity and same-sex mating likely also contributes to features of clonality observed for both of these common fungal pathogens.
A series of studies provide compelling evidence for natural unisexual mating of C. neoformans serotypes A and D, and C. gattii. Natural αADα hybrids produced via cell-cell fusion between two parental α isolates of different serotypes were identified and characterized (Lin et al., 2007). A novel truncated allele of the mating-type-specific cell identity determinant SXI1α is present in all ADα diploid strains analyzed, including those produced via opposite-sex (aADα) and unisexual (αADα) mating, and may confer enhanced fertility (Lin et al., 2007).
Based on population genetics studies, sexual reproduction of C. neoformans serotype A (VNI) occurs in trees in India, apparently via unisexual mating in an exclusively α population (Hiremath et al., 2008). Previous studies of C. neoformans isolates from pigeon guano and human patients suggested clonal reproduction, but this analysis from a different niche (trees) found evidence of clonality and recombination. These studies of natural populations in trees argue for same-sex mating in nature in the predominant pathogenic form of the organism (serotype A, variety grubii). A challenge remaining is to recapitulate this unisexual cycle under lab-defined conditions for serotype A strains.
Two studies further support that unisexual mating occurs in the C. neoformans var. grubii serotype A isolates (Bui et al., 2008). First, analysis of veterinary isolates from Sydney, Australia defined an α-only population in which linkage equilibrium was apparent, consistent with genetic exchange. Four α/α diploid isolates identified under went sex-specific hyphal differentation; these self-filamentous diploids are intermediates of unisexual reproduction. These findings support that unisexual mating occurs and may produce spores that infect animals in this and other regions. In the second study, ~500 C. neoformans isolates were tested for ploidy and ~8% were diploid by FACS analysis (Lin et al., 2009). The majority of these were αAAα diploids, including intravarietal allodiploid hybrids produced by fusion of two genetically distinct α cells through same-sex mating and autodiploids harboring two seemingly identical copies of the genome that arose via endoreplication or clonal mating. Thus, unisexual mating produces C. neoformans diploids in nature, giving rise to populations of hybrids and mixed ploidy.
Based on population genetics approaches, the sibling species C. gattii has been found to undergo sexual reproduction in tree hollows in Australia, both within mixed mating populations and those exclusively of the α mating type (Saul et al., 2008). Eucalyptus trees are the environmental niche for C. gattii, but whether the organism has been clonally or sexually reproducing there is unclear. C. gattii is known to undergo a-α mating in the laboratory (Fraser et al., 2003; Kwon-Chung, 1976b), and yet the natural population is predominantly α. While a cells could be less fit than α giving rise to their disparity, congenic strains of opposite mating type appear equally fit under a broad range of different conditions (Nielsen et al., 2005b; Nielsen et al., 2005a). These studies show natural populations of C. gattii are undergoing both opposite-sex and same-sex mating in trees in nature, and the balance between the two sexual modes likely depends on the local availability of mating partners (Carter et al., 2007).
Population genetics and molecular studies implicate sexual reproduction in both the origin and ongoing outbreak of C. gattii on Vancouver Island and in the Pacific Northwest. All of the isolates associated with the outbreak are α, many are fertile in laboratory crosses, a diploid α/α mating intermediate was identified, and particles small enough to be spores are present in the air on Vancouver Island (Fraser et al., 2005; Fraser et al., 2003; Kidd et al., 2007). A detailed population genetic analysis of the outbreak and global VGII C. gattii molecular type revealed evidence for recombination based on MLST allele compatibility tests and the occurrence of hybrid recombinant alleles (Byrnes et al., 2010). Both the major outbreak VGIIa genotype present only on Vancouver Island and in the Pacific Northwest and the novel VGIIc genotype so far only present in Oregon are hypervirulent in both macrophage and animal models, and this may implicate novel roles for mitochondria in pathogenesis (Byrnes et al., 2010; Ma et al., 2009a). An altered tubular mitochondrial morphology has been associated with increased intracellular replication in macrophages (Ma et al., 2009a). The outbreak VGIIb minor genotype is identical at 30 MLST loci with isolates from Australia that are part of a fertile, recombining population (Campbell et al., 2005a; Campbell et al., 2005b) and may therefore have originated by transfer from Australia to the Pacific Northwest. Comparisons of the genome and MAT locus of the VGIIa/major and VGIIb/minor genotype suggest the two may be sexually related, either as parent-sibling, or siblings (Fraser et al., 2005). One hypothesis is that sex (opposite or same-sex mating) contributed to produce a novel hypervirulent genotype, and that ongoing same-sex mating is generating readily aerosolized infectious spores. All outbreak isolates are α mating type, thus α-α unisexual mating represents a parsimonious hypothesis by which diversity and infectious spores are produced (Byrnes et al., 2010; Fraser et al., 2005). Populations with a isolates have been identified in South America (Escandon et al., 2006), and a-α opposite sex mating might also occur there and contribute to diversity (Fraser et al., 2005). Infectious spores may be produced because of the association with plants in the environment; co-culture with Arabidopsis or eucalyptus seedlings stimulated C. gattii mating and spore production, and the filamentous dikaryon infects plants, similar to Ustilago maydis (Figure 1) (Xue et al., 2007).
While an organism maintaining extant opposite and same-sex mating cycles might seem unusual, previous studies of S. cerevisiae are illustrative. The natural S. cerevisiae population consists of Ho+ isolates that switch mating type and are self-fertile and ho-mutants that do not switch mating type but remain cross-fertile. Similarly C. neoformans has maintained both an a-α opposite-sex cycle promoting out-crossing and an α-α sexual cycle promoting inbreeding and clonality. This unisexual cycle may have arisen concomitant with emergence as a successful human pathogen well-adapted to particular environmental niches.
In examples where two genetically divergent α cells mate, their resulting progeny exhibit genetic diversity. In contrast, when identical genomes engage in unisexual reproduction, how is a benefit conferred with no pre-existing diversity? One could argue that in the later case that the need to purge accumulating deleterious mutations and transposons may suffice to render sex beneficial. Alternatively, uniparental inheritance of mitochondria may drive unisexual mating of identical isolates to purge defective mitochondrial genomes. Finally, we propose a new model in which sex serves as a mutagen to generate genetic diversity de novo. This model is in part based on the finding that the diploid state can serve as a capacitor for evolution during parasexual fungal reproduction enabling multiple recessive mutations to accumulate that would be deleterious individually but are advantageous when combined (Schoustra et al., 2007). By analogy, sexual reproduction may produce diploids, yielding a sheltered state in which genetic diversity arises de novo, and then release this stored diversity by meiosis into haploid progeny subject to selective pressures. In this model, sex does not admix pre-existing diversity, but instead generates diversity de novo.
Candida albicans
The most common human fungal pathogen, C. albicans, is an obligate diploid thought to be asexual for >100 years until the discovery of the MAT locus and an extant parasexual cycle (Bennett and Johnson, 2003). In contrast, other pathogenic Candida species (C. lusitaniae, C. guilliermondii) are haploid with complete sexual cycles, including sporulation. Studies to define the structure and function of MAT in these species and genomic analyses revealed that many key meiotic genes were lost (Butler et al., 2009; Reedy et al., 2009). Meiosis still occurs during C. lusitaniae sex, yet generates a high level of aneuploidy, possibly as an additional route to genetic diversity. Aspects of this modified sexual cycle may provide insights into the true sexual nature of C. albicans.
The yeast C. albicans is a human microbiota resident and frequently causes mucosal and systemic infections. The MTL mating locus discovery, isolation of mating competent strains (i.e. a/a, α/α) from the sterile a/α state, and discovery that white-opaque cell-type switching enhances mating efficiency by one million - fold revolutionized our understanding (Hull and Johnson, 1999; Hull et al., 2000; Magee and Magee, 2000; Miller and Johnson, 2002; Slutsky et al., 1987). We now appreciate mating involves diploid cell-cell fusion producing a tetraploid cell that undergoes parasexual reduction to the diploid/aneuploid state. The discovery of host-specific conditions supporting mating suggests sex occurs in or on the host, and may d rive immune evasion, virulence, and d rug resistance.
Opaque cells are specialized formating, essentially functioning as gametes, and respond robustly to mating pheromones to produce con jugation tubes that enable cell-cell fusion. MTL heterozygous strains do not undergo white-opaque switching, which is repressed by a1/α2. Homozygosis of the MTL locus (gene conversion, chromosome loss/gain) to a/a or α/α occurs first occur (Wu et al., 2005). Expression of the Wor1 transcription factor then d rives a bistable positive feedback loop enhancing its own expression and that of other opaque-specific genes (Huang et al., 2006; Zordan et al., 2006). Mating efficiency of white cell populations is inefficient, yet white cells do respond transcriptionally to mating pheromone via unique signaling pathway specialization (Sahni et al., 2009; Yi et al., 2008). In biofilms, rare opaque cell minorities signal the white cell majority population, stimulating adhesiveness and enforcing pheromone gradients enabling distant mating partners to communicate and locate each other via conjugation tubes (Daniels et al., 2006).
Other host conditions enhance mating efficiency. Because opaque cells are unstable at 37°C, how mating might occur in the host was unknown. One possibility involves cooler niches, and mating readily occurs on skin of mice (31.5°C) (Lachke et al., 2003). C. albicans resides on oral, vaginal, and GI mucosa, and might mate on adjacent skin and return to the host or transmit to another host. An early C. albicans study detected mating in vivo following infection of mice with white cell populations (Hull et al., 2000). Later studies involving oral/intravenous delivery of opaque cells improved mating efficiency. Similarly, anaerobic conditions, elevated CO2, anaerobic/high CO2, and N-acetyl glucosamine enhance mating efficiency (Dumitru et al., 2007; Huang et al., 2009; Huang et al., 2010; Ramirez-Zavala et al., 2008). CO2 conversion to bicarbonate (by carbonic anhydrase) stimulates adenylyl cyclase (Klengel et al., 2005), increasing cAMP levels that may signal enhanced mating.
We now appreciate that most C. albicans strains are a/α diploids that, like S. cerevisiae, do not mate. No haploid C. albicans has ever been observed, and the sexual cycle involves diploid-tetraploid-diploid transitions in contrast to the diploid-haploid-diploid S. cerevisiae cycle. However, S. cerevisiae can complete a tetraploid sexual cycle, like C. albicans, and a/a/α/α tetraploids undergo meiosis to produce asci containing four diploid progeny. In contrast, C. albicans produces neither asci nor spores, and may have evolved to mate but not produce antigenic spores thereby avoiding stimulating immune responses. Also in contrast to S. cerevisiae, C. albicans mates but has no recognized meiosis; instead parasexual chromosome loss occurs stochastically, returning cells from 4N to 2N and generating considerable aneuploidy in the process (Bennett and Johnson, 2003). Recombination does occur in some progeny produced by the parasexual cycle, and requires Spo11 (Forche et al., 2008). Given that Spo11 is a meiosis-specific recombinase in all organisms in which it has been studied in detail, this could suggest that meiosis (or parameiosis, which connotes a meiosis-like process, by an alogy to “parasexual”)) occurs during the parasexual cycle, but it has also been proposed that Spo11 might have a novel mitotic role (Forche et al., 2008). A second C. albicans meiotic ortholog, Dlh1, is fully functional to support meiosis in S. cerevisiae dmc1 mutants and restores repair of DNA double-strand breaks and recombination (Diener and Fink, 1996); its roles in C. albicans remain to be studied.
Comparative genomics of the pathogenic Candida species complex has revealed considerable plasticity in mating type and meiosis configuration (Butler et al., 2009; Reedy et al., 2009) (Figure 2). The C. albicans genome revealed that while many meiotic orthologs were present, others were missing, potentially providing an explanation why meiosis hasn’t been observed (Tzung et al., 2001). To address this hypothesis, the genomes of other Candida species known to have sexual cycles involving sporulation, such as Candida lusitaniae were sequenced. The hypothesis was that these species would retain meiotic genes that had been lost in C. albicans. However, just the opposite was observed. Not only were these sexual Candida species missing all of the meiotic genes lost in C. albicans, but they were missing some two dozen other meiotic genes based on studies from S. cerevisiae, including loss of the MAT gene α2 (Figure 2). Because the historic definition of a complete sexual cycle including meiosis in C. lusitaniae and other sexual Candida species was based on observing mating and sporulation with the inference that meiosis was occurring, detailed analyses were conducted. Multiple criteria, including 1) changes in ploidy (1N-2N-1N), 2) a high rate of recombination on par with meiotic recombination in other fungi, and 3) a requirement for Spo11, support that meiosis occurs during the C. lusitaniae sexual cycle. Interestingly, ~2/3 of the progeny produced are euploid, haploid recombinant progeny. Equally interesting however is that ~1/3 of the progeny produced are aneuploid (1N+1) or diploid (2N, 2N-1). This high rate of aneuploid progeny may result from loss of meiotic components, or may reflect an evolutionary adaptation to generate genotypic and phenotypic plasticity. Recent studies provide evidence that aneuploidy plays adaptive roles, including in d rug resistance of C. albicans (Selmecki et al., 2006; Torres et al., 2007). Where else in biology do we observe such a sloppy version of meiosis? We need look no further than human biology, as aneuploidy frequently occurs in humans leading to trisomy and contributing to a significant proportion of miscarriages.
What are the implications for C. albicans sex? Given rampant aneuploidy resulting from the C. lusitaniae sexual cycle, aneuploidy observed during C. albicans parasex might result from meiosis rather than mitosis. Moreover, given that recombination during C. albicans parasex is Spo11-dependent, this might also reflect meiotic rather than mitotic roles.
There are several approaches to address if meiosis occurs in C. albicans. One approach is to examine roles of other meiotic orthologs such as Dmc1 (Dlh1) in C. albicans. Another is to look for pre-meiotic DNA synthesis that often precedes meiosis, and which would produce 8N cells from the 4N precursor. During the parasexual cycle, some cells may engage in meiosis and others lose chromosomes spontaneously, such that progeny are produced via concomitant parasexual/sexual pathways. If so, defining the subset of cells undergoing meiosis may provide insight (similar to white-opaque switching) into how and when meiosis occurs in C. albicans. Given that C. albicans and closely related species retain two dozen meiotic gene orthologs lost in sexual species (C. lusitaniae, C. guilliermondii), it suggests these meiotic genes are biologically functional. Finally, because S. cerevisiae meiosis produces tetrads, but C. lusitaniae produces dyads and Lodderomyces elongisporus produces monads, C. albicans meiosis may produce fewer nuclear products than canonical meiosis.
Mating in C. albicans involves an a-α opposite-sex mating process similar to other fungi, but recently an alternative mating route has been discovered with striking parallels to unisexual mating discovered earlier in C. neoformans (Figure 3) (Alby et al., 2009). C. albicans a/a cells express both the a- and α-mating pheromone genes, in contrast to S. cerevisiae in which a cells only express a-pheromone. Moreover, Alby et al observed that C. albicans a/a mutants lacking a protease that normally degrades α-pheromone (Bar1) produce unusual wrinkled colonies in which the cells appear morphologically to be responding to pheromone. They showed that when Bar1 is missing, a cells produce and respond to α factor via both autocrine and paracrine signaling, and as a consequence a cells are enabled to mate with other a cells via same-sex mating. Given that this a-a mating requires a bar1 mutation, it is uncertain whether and how this might occur in nature? It may be that naturally occurring bar1 mutants arise or are present in the population, or that conditions that inhibit Bar1 occur, such as the acidic pH of the vaginal mucosa since Bar1 is inhibited at acidic pH in vitro. An alternative way in which a-a mating is stimulated is by a minority of α cells as the α-pheromone source, analogous to ménage a trios mating in C. neoformans involving α-α unisex stimulated by a cells. The resulting a/a/a/a C. albicans tetraploid products can undergo parasexual reduction to return to the diploid state. One difference between the a/a/α/α and a/a/a/a tetraploids produced by opposite-sex and same-sex mating is that a/a/α/α tetraploids undergo an opaque-white transition (they express a1/α2 that inhibits opaque genes including Wor1), returning to the white self-sterile cell fate, whereas the opaque cell fate perdures in the a/a/a/a tetraploid state and might therefore play novel roles. In summary, like C. neoformans, we now appreciate that C. albicans has both opposite-sex and same-sex mating cycles. That both are maintained likely reflects that each contributes uniquely to generate genetic diversity, possibly in response to selective pressures in which higher or lower levels of diversity provide greater adaptive potential.
Unisexual mating in two divergent, successful human fungal pathogens
Taken together, these laboratory and environmental population genetics studies provide robust support that both opposite sex and unisexual reproduction occur to impact diversity and population structure for both of these pathogenic microbes. Unisexual mating may produce infectious propagules in nature, and thus further studies on genetic and genomic control of this novel sexual cycle, and conditions that support this in the lab and in nature, are warranted. Such studies are likely to generate general principles for understanding transitions between modes of sexual reproduction, both in pathogenic fungi and other pathogenic microbes (Heitman, 2006) and even extend to self-fertility in plants and animals.
Aspergillus fumigatus, extant sex of an infectious mold
Aspergillus fumigatus is a common human fungal pathogen long thought asexual. O’Gorman et al discovered an extant sexual cycle in this pathogen by successfully mating strains of opposite mating types (O’Gorman et al., 2009). The key innovations leading to success were pairing strains from a naturally occurring recombinant population and patience, as complete sexual reproduction required six months of incubation in the dark on specialized medium!
Together C. albicans, C. neoformans, and A. fumigatus are the triumvirate of systemic human fungal pathogens and which occur most commonly globally. We are all exposed to A. fumigatus as a ubiquitous environmental fungus associated with composting and other niches, yet infection occurs most commonly in severely immunocompromised hosts. Exposure and infection occur via inhalation of spores that, until recently, were thought to be asexual products. Whole genome sequencing and population studies revealed conserved mating and meiotic machinery, an equal distribution of the two mating types in nature, and possible genetic evidence for mating (Galagan et al., 2005; Paoletti et al., 2005). The A. fumigatus MAT genes are functional when heterologously expressed in the related model species A. nidulans (Grosse and Krappmann, 2008; Pyrzak et al., 2008). However, despite considerable effort, A. fumigatus had not been observed to mate under laboratory conditions.
Recently, O’Gorman et al. paired MAT1-1 and MAT1-2 strains from an environmental isolate collection from Dublin, discovering that A. fumigatus undergoes sexual reproduction under specific, defined conditions (O’Gorman et al., 2009). The Aspergillus sexual cycle produces viable spores that germinate into haploid, meiotically recombined progeny. These findings enable the use of classic genetic tools involving crosses to be applied, and have broad implications for generation of diversity in the population and virulence evolution. The discovery of a complete sexual cycle for this “asexual” fungus (Kwon-Chung and Sugui, 2009), together with accumulating genomic evidence that the vast majority of fungi retain mating/meiotic machinery, suggests there may be fewer truly asexual species than previously appreciated (Lee et al., 2010). The challenge remains to discover laboratory-defined sexual cycles and elucidate how these contribute to diversity and survival in nature and in the host.
Dermatophytes/dimorphic fungal pathogens, host-associated mating?
Studies on the MAT locus and sexual reproduction of dimorphic fungal pathogens and dermatophytes contribute experimental advances and examples in which mating may occur on the host. Koch ’s postulates w ere first satisfied in studies of fungi infecting humans. Dr. David Gruby, working in Paris in the 1840s, implicated the dermatophyte fungus Microsporum audouini with ringworm/Tinea capitas in humans. This work included potato slice culture and transfer to uninfected individuals causing disease. The dermatophyte and dimorphic human fungal pathogens encompass more than a dozen phylogenetically related ascomycete species, and dermatophytes are arguably the most common causes of skin infection in humans whereas the dimorphic fungi are environmental and cause pulmonary infections following inhalation.
Genomics is rapidly advancing our understanding of these pathogenic microbes. The dimorphic species Blastomyces dermatitidis and Histoplasma capsulatum have classically defined extant sexual cycles (Kwon-Chung, 1972; McDonough and Lewis, 1967) and yet with laboratory passage often lose fecundity via unknown mechanisms. Recent studies define the organization and evolutionary trajectory of the MAT locus for these two groups of species, including both sexual species and others currently classified as asexual (Bubnick and Smulian, 2007; Li et al., 2010; Mandel et al., 2007; Torres et al., 2010) and genetics has begun to be applied to define why sex is frequently lost. The presence of the MAT locus and meiotic machinery suggests extant sexual cycles remain to be discovered.
The dermatophyte fungal pathogens are uniquely specialized to live on human skin, where they may undergo sexual reproduction, analogous to C. albicans mating on mice (Lachke et al., 2003). Fungal sex could produce antigens provoking skin immune/allergic responses. Similarly, Malessezia spp. are ubiquitous fungal commensals of human skin associated with eczema and dandruff that may also undergo sex on human skin (Figure 1). These fungi are basidiomycetes closely related to the plant pathogen Ustilago maydis, which undergoes sexual reproduction to form invasive hyphae necessary to infect its host plant (maize) (Figure 1). The M. restricta and M. globosa genomes revealed why they associate with human skin: they lack fatty acid synthase and thus must scavenge lipids for growth from sebaceous secretions (Xu et al., 2007). Their genomes further reveal conserved meiotic/mating machinery and a bipolar MAT locus related to those of Cryptococcus and U. hordei (Xu et al., 2007). Whether these fungi mate on human skin remains to be explored.
Zygomycetes and microsporidia, sexual identity of basal fungi
The fungal kingdom is diverse, encompassing more than 1.5 million species in as many as 8 to 10 divergent phyla. The fungal pathogens discussed thus far are ascomycetes and basidiomycetes, a monophyletic group (the dikarya). At the base of the fungal kingdom lies a radiation of earlier branching phyla, including the polyphyletic zygomycota and chytridiomycota super-phyla. These basal species share features with the last common ancestor to the animal and fungal kingdoms lost in the more derived fungi, including the flagella that is extant in metazoans and chytridiomycetes. Several pathogens of humans and other animals populate these lineages, including the human pathogens Rhizopus and Mucor in the Mucorales/zygomycota, and the aquatic pathogen Batrachochytrium dendrobatidis, a chytrid causing amphibian decline and extinctions. Rhizopus and Mucor cause devastating infections difficult to manage with current antifungal therapies, and often require surgical debridement.
Available genomes for these species reveal conserved meiotic machinery consistent with extant sex (Ma et al., 2009b). No sexual cycle is known for B. dendrobatidis; however, population genetics has provided evidence for clonal expansion with limited genetic exchange, possibly reflecting covert sex (Morgan et al., 2007). Rhizopus and Mucor are sexual, and their sex locus encodes divergent HMG domain proteins (SexM/P) that govern sexual identity analogos to the mammalian sex determinant Sry, with implications for how sex determination evolved (Idnurm et al., 2008; Lee et al., 2008).
The microsporidia are a group of unusual obligate eukaryotic microbial pathogens with more than 1200 known species including 13 that infect humans commonly causing diarrhea in AIDS patients (Keeling, 2009). Microsporidia are evolutionarily closely related to fungi, either as true fungi or their sisters. Molecular phylogenetics and whole genome comparisons support the microsporidia as true fungi that may share a more recent common ancestor with the basal zygomycete fungi. This is also based on the presence of a sex-related locus with an architecture similar to the zygomycete sex locus (Lee et al., 2008). Little is known about microsporidia sex but the presence of meiotic gene orthologs and a sex-related locus suggest they may undergo a sexual cycle during host infection. They also have a capacity for horizontal gene transfer (HGT). Encephalitazoon cuniculi has acquired four genes encoding ATP transporters localized to the plasma or mitochondrial membrane that enable it to procure ATP from the host cell. These genes were acquired via HGT from a co-resident species of Chlamydiae, a bacterial obligate intracellular pathogen, allowing metabolic energy production genes to be jettisoned (Tsaousis et al., 2008). Both mechanisms (sex, HGT) might have operated in fomenting the dramatic genomic compaction that enabled this unusual, highly successful clade of obligate intracellular pathogenic fungi to emerge.
Sex in parasites
Giardia
The protozoan parasite Giardia intestinalis had long been thought to be asexual, but the finding that its genome encodes meiotic gene homologs and evidence for genetic exchange based on population genetics studies suggested that a sexual cycle might be extant (Cooper et al., 2007; Ramesh et al., 2005). These studies culminated in the discovery of an unusual form of sexual reproduction involving nuclear fusion, genetic exchange, and meiosis-specific gene homolog expression (Poxleitner et al., 2008).
Giardia is a diplomonad that harbors two diploid nuclei that were found to undergo karyogamy during the encystment phase of growth, based on plasmid exchange and observations by transmission electron microscopy (Poxleitner et al., 2008). Analysis by fluorescent in situ hybridization (FISH) revealed that stably introduced episomes present in only one nucleus in the trophozoite stage are frequently observed in two or three of the four cyst nuclei, consistent with plasmid exchange via nuclear fusion. Moreover, morphological analysis by transmission electron microscopy documented nuclear envelope fusion. Previous inspection of the Giardia genome had identified meiotic gene homologs, including Spo11, Dmc1a and Dmc1b (two forms), Mnd1, and Hop1 (Ramesh et al., 2005). Spo11-, Dmc1a-, and Hop1-GFP fusions were expressed and nuclear - localized during encystation and no expression was observed in trophozoites, consistent with potential meiosis-specific roles (Poxleitner et al., 2008). Dmc1b and Mnd1 were expressed in nuclei of both trophozoites and cysts, and may serve broader roles. These findings support a novel sexual process, termed diplomixis, during which two of the four cyst nuclei fuse leading to meiosis, genome reduction, and production of recombinant progeny nuclear genotypes. Alternatively, meiosis could precede nuclear fusion producing haploid intermediate “gamete” nuclei that fuse to restore diploid y. No evidence has been presented yet for cell-cell fusion of different isolates, which would be required for genetic exchange within the population; however, population studies provide evidence for exchange in the population and thus both selfing and out-crossing may occur. These findings share features with the diploid-tetraploid-diploid parasexual cycle of C. albicans, and the dikaryotic feature of Giardia also mirrors the dikaryon intermediate during Cryptococcus sexual reproduction. When and where Giardia sexual reproduction occurs in nature or the infected host, and how it impacts genetic exchange in the population, remains to be explored.
Leishmania
Leishmania are protozoan parasites that cause vector-borne diseases in humans and domesticated animals globally, and are a serious cause of morbidity and mortality with >two million new cases annually. Population studies revealed extensive linkage disequilibrium potentially consistent with largely clonal reproduction (Tibayrenc and Ayala, 2002), yet the observation of naturally occurring hybrid isolates and the presence of conserved meiotic orthologs in the genome suggest the capacity for extant sex (Heitman, 2006). A direct demonstration of hybridization and genetic exchange was recently reported for L. major (Akopyants et al., 2009).
Co-infection of the sand fly vector (Phlebotomus duboscqi) with parental strains genetically marked with dominant d rug resistance markers yielded doubly d rug-resistant progeny that could be bite-transmitted to mice (Akopyants et al., 2009). Hybrids only formed in the sand fly vector, not during co-culture in vitro or murine co-infection. Analysis of single-nucleotide polymorphisms (SNPs) that distinguish the homozygous parents revealed that all of 18 progeny analyzed were heterozygous, consistent with hybridization. When DNA content was analyzed by flow cytometry, the parental isolates and 11 of 18 hybrids were diploid, but 7 of 18 hybrid progeny were triploid (intermediate ploidy was not observed). The maxicircle kinetoplastid DNA was inherited from one or the other parent (uniparental inheritance). Finally, parental phenotypic differences were inherited in a dominant (antibody reactivity, clumping) or semi-dominant (slow or fast virulence) pattern.
Taken together, these findings reveal a mechanism for genetic exchange in Leishmania, consistent with cell-cell fusion to produce hybrid progeny (Akopyants et al., 2009). The diploid progeny produced appear to be heterozygous at all markers that are homozygous in the diploid parents. This may be consistent with meiotic generation of haploid gametes that fuse to produce hybrid progeny. Because triploid progeny are also observed suggests models in which one parental diploid isolate skips meiotic reduction and fuses with a haploid gamete produced by the other parent. Alternatively, similar to C. albicans, Leishmania may undergo a diploid-tetraploid-diploid sexual/parasexual cycle in which triploid isolates are an intermediate. Triploids might undergo parasexual reduction to diploidy, play novel roles in the life cycle, or be less fit and selected against. In summary, these studies demonstrate that genetic exchange involving hybridization can occur in Leishmania, and lay the foundation to test if a complete, meiotic sexual cycle impacts evolution and virulence.
Trypanosoma
Trypanosomes are kinetoplastid protozoan parasites transmitted by insect vectors (tsetse flies, reduvid bugs) that cause African sleeping sickness in humans (T. brucei), devastating infections in African livestock (T. congolense), and Chagas disease in humans prevalent in South America (T. cruzi). Sex is best understood in T. brucei, but a mechanism for genetic exchange in T. cruzi involving co-culture in Vero cells has been established in the laboratory. The sexual cycle consists of fusion of diploid isolates to produce a tetraploid intermediate that may undergo parasexual/sexual reduction to aneuploid/diploid progeny, like C. albicans (Gaunt et al., 2003). Population genetic studies of the T. cruzi TcIIc lineage disclosed a higher level of homozygosity in sub-populations, consistent with either sexual reproduction or genome-wide mitotic gene conversion and loss of heterozygosity (Llewellyn et al., 2009). Recent population genetic studies also adduce evidence formating in the cattle pathogen T. congolense (Morrison et al., 2009). In this later study, the T. congolense genome sequence enabled identification of polymorphic microsatellite reporters that were analyzed from Gambian livestock isolates. In one of four cryptic sub-populations, the level of genetic diversity observed was interpreted as consistent with sexual reproduction and genetic exchange in the population.
An extant sexual cycle involving both meiosis and largely Mendelian inheritance patterns is well-established in T. brucei, enabling generation of a high-resolution meiotic map and application of genetic approaches for laboratory investigations of virulence (Cooper et al., 2008). Studies of differentially fluorescent labeled parasites provide direct evidence for cell-cell fusion events during sexual reproduction in the insect host salivary glands (Gibson et al., 2006, 2008; Peacock et al., 2007). Co-infection of tsetse flies with two parental T. brucei strains expressing GFP (green) or RFP (red) yielded yellow hybrid products, and implicate the unattached epimastigote as the sexual stage of the parasite (Gibson et al., 2008). Analysis of co-infection dynamics of two differentially marked strains reveals that rather than competing for infection, co-infection was more frequently observed in all salivary gland compartments examined. These findings reveal a lack of competition, and may even provide evidence for stimulation of co-infection, which could facilitate sex and genetic exchange. How the two co-infecting isolates interact is unknown, but could involve secreted factors analogous to fungal mating pheromones.
During the T. brucei sexual cycle, polyploid hybrids consistent with triploids, tetraploids, and aneuploids are frequently detected (Gibson et al., 2008). How these arise is not known, but may entail: 1) sexual intermediates (similar to tetraploids produced during C. albicans parasex), 2) mating of haploid gametes with unreduced or partially reduced diploid/aneuploid gametes, or 3) defects during meiotic segregation similar to the rampant diploid/aneuploid progeny produced by sexual reproduction of C. lusitaniae (Reedy et al., 2009). Finally, when GFP- and RFP-expressing clones from a single T. brucei strain co-infect, intraclonal mating also occurs (Peacock et al., 2009). Flies infected with RFP- and GFP-expressing clones were found to harborred, green, and yellow parasites in the salivary glands, and both red and non-fluorescent clones were recovered in which recombination and altered DNA content were apparent. These findings reveal the capacity for selfing/inbreeding, in addition to out - crossing modes of sexual reproduction, and raise the issue that lab passage and natural transmission of T. brucei clones could lead to sex-induced genomic alterations.
In summary, while it is clear that T. brucei undergoes sexual reproduction, the molecular details remain to be elucidated. This includes resolving whether the sexual cycle involves diploid-tetraploid-diploid transitions or a diploid parental isolate that produces haploid gametes to undergo cell-cell fusion, and whether and how cell identity is established by a genomic region analogous to a MAT locus or sex chromosome.
Plasmodium
Malaria is caused by the unicellular haploid protozoan parasite Plasmodium that is transmitted by mosquitoes to humans. Plasmodium has a well-established sexual cycle in which gametocytes are produced in humans and then transmitted to mosquitoes where the sexual cycle is completed (Alano, 2007; Dixon et al., 2008). The only form of the parasite transmitted from humans to mosquitoes is the gametocyte, and thus sexual reproduction is an obligate life cycle step, in contrast to most other parasite pathogens that are facultatively sexual. Therefore, a molecular understanding of differentiation and sexual identity are critical in enabling improved control of parasite transmission. This unique Plasmodium life cycle constraint increases the impact of sex on evolution and emergence/spread of d rug resistance.
During infection of the vertebrate host, a single malaria parasite clone can undergo intraerythrocytic differentiation to produce both male and female gametocytes (gametocytogenesis). How this key event is initially specified at a molecular level is unknown. In P. falciparum schizonts are known to undergo commitment to either asexual or sexual development, and all merozoites derived from a sexually-committed schizont produce only male or female gametocytes (Silvestrini et al., 2000; Smith et al., 2000). Thus, events at commitment direct both sexual development and specify male or female identity. Moreover, in Plasmodium the molecular pathways governing sexual commitment and male/female identity are unknown and separate events dictated by distinct pathways. Moreover, the number committing to sexual development and the male: female ratio are not genetically fixed and are known to be influenced by environmental factors. Because a single clone produces both male and female gametocytes, self-fertility and out-crossing occur. No Plasmodium sex chromosomes or MAT loci are known, but deletions in Ch. 9 and 12 in several parasite strains lead to defects in gametocytogenesis. These genetic changes during long-term passage of asexual parasite lines are similar to sterility in passaged fungi and associated with defects in gametocyte or male gametocyte production but the molecular basis remains elusive (Dixon et al., 2008; Furuya et al., 2005; Vaidya et al., 1995). However, these parasite strains with sex-specific gametocytogenesis defects may serve as genetic models to further dissect the molecular processes governing differentiation and molecular identity of Plasmodium. It is also possible to use genetic linkage and association analyses to map the precise loci determining the different sexual stages among various parasite strains (Mu et al., 2010).
Following uptake of gametocytes from the vertebrate host to the mosquito in its blood meal, gamete differentiation occurs. Male gametocytes undergo exflagellation to produce as many as eight gametes with flagella; female macrogametocytes produce a single female gamete. Male and female gametes (selfing or out-crossing) fuse in the midgut producing a diploid zygote. The zygote then matures into a ookinete, escapes the midgut, matures into an oocyst in which meiosis occurs, and oocyst rupture releases myriad haploid sporozoites that migrate to the salivary glands for transmission to the next vertebrate host bitten by the carrier mosquito.
Proteomic analysis of purified P. berghei male and female gametocytes reveals marked differences between the two sexes and outlines a molecular differentiation cascade (Khan et al., 2005). Male-specific proteins include those for flagellar motility and DNA replication, whereas ribosomal and mitochondrial proteins abound in female gametocytes. Several proteins necessary for gamete formation have been defined. The calcium-dependent protein kinase CDPK4 expressed by both gametocytes (Billker et al., 2004), and the male gametocyte-specific mitogen-activated protein kinase MAP2, are both required for male gamete formation (Khan et al., 2005). In contrast, mutants lacking the female-gametocyte specific NIMA kinase NEK4 produce male and female gametes that fuse normally, but the zygote to ookinete maturation fails. The female-gametocyte-specific RNA helicase DOZI sequesters and translationally represses messages stored for deployment following fertilization (Mair et al., 2006). DOZI mutants produce gametocytes normally, but transcript profiles are markedly perturbed, and zygotes again fail to mature into ookinetes (Mair et al., 2006). The importance of post-transcriptional regulation is further demonstrated by a conserved U-rich region and Puf-family of RNA-binding proteins that function in parasite sexual differentiation (Braks et al., 2008). Studies have also defined a protein NAP2 that is male-specific and involved in male-female gamete fusion (Hirai et al., 2008; Liu et al., 2008). Hap2 is a homologue of a male-specific sterility protein of Arabidopsis and homologs are present in rice, Chlamydomonas, alga, slime mold, and Leishmania. The presence of conserved gamete-specific proteins in these diverse organisms might provide insights into not only the evolutionary origin of proteins specifying Plasmodium gametes but also the mechanisms specifying male-female identity is established. Moreover, a family of plant-like transcription factors (ApiAP2) have been discovered in Plasmodium and other Apicomplexa. These proteins appear to govern stage-specific genes and gene expression cascades evoking developmental switches (De Silva et al., 2008). These observations suggest that beyond the fungi in the Ophisthokonts, exploration of the Archaeplastida may reveal how sexual development is established. In summary, these studies reveal there are differences between male and female gametocytes/gametes that determine sexual cycle progression, but do not yet reveal insights into how male/female sexual identity is first established.
How is sex determined in Plasmodium?
How might the male and female gametocyte fate be specified from a single parasite clone? If Plasmodium were a fungus, we would call it homothallic/self-fertile and consider models by which other species exhibit self-fertile behavior (Lin and Heitman, 2007). One approach is to look for parasite homologs of fungal MAT genes (homeodomain, α-box, or HMG domain proteins) (Figure 2) as candidate sex determinants. The established molecular bases for fungal self-fertility include: 1) mating-type switching from silent to active mating-type cassettes (S. cerevisiae, Schizosaccharomyces pombe), 2) two opposite MAT alleles in the same genome (fused or unlinked) (A. nidulans, Cochliobolus sp.), and 3) unisexual reproduction (C. neoformans, C. albicans). Any of these models could apply to Plasmodium, and in all a defined genetic locus determines mating-type. Moreover, in each self-fertile mode a single clone can give rise to gametes that behave as mating partners. Alternatively, we might look to advances in understanding sexual reproduction in the alveolate phylum of eukaryotes, including apicomplexan parasites and ciliates, i.e. Tetrahymena. However, while the Tetrahymena sexual cycle is known and involves multiple sexes, an understanding of the molecular nature is still rudimentary (Phadke and Zufall, 2009).
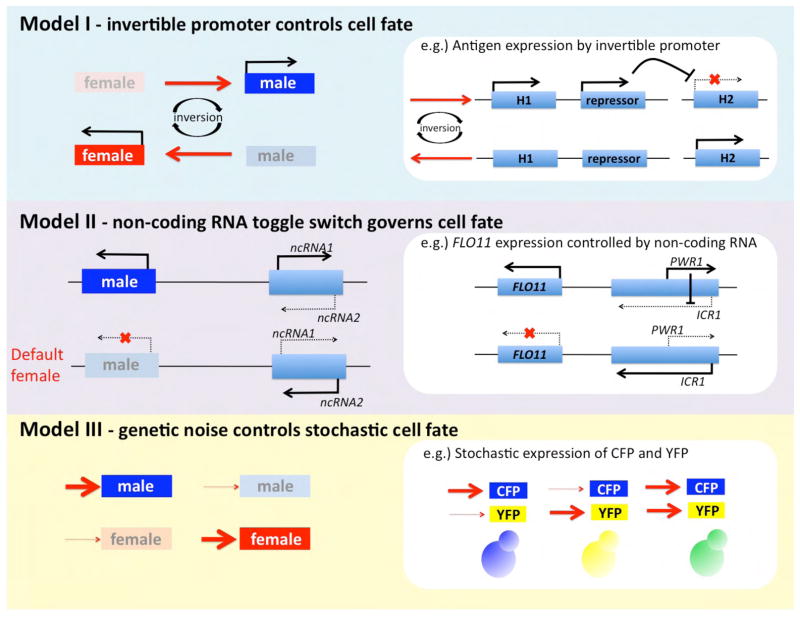
We propose three other speculative models for Plasmodium sex determination involving: 1) inversion of a genetic regulatory element by analogy to flagellar phase variation in Salmonella, 2) competing non-coding RNAs that govern exclusive repression/expression of a cell fate determinant by analogy with the S. cerevisiae FLO11 toggle, and 3) genetic noise/stochastic gene expression (Figure 4). In all of these models, the two gametocyte fates could be uniquely and actively specified, or one could be actively specified and the other a default. For example, in S. cerevisiae the a haploid cell type is the default (cells lacking MAT mate as a), whereas the α cell type is actively determined by the α1 factor. Thus, expression of a single key cell fate determinant could suffice to specify male vs. female gametocytes.
Figure 4.
On how Plasmodium gamete sexual identity may be specified. Three models are presented for how gamete identity might be specified in Plasmodium. These models are speculative and the intent is to suggest ways in which a single clonal isolate of Plasmodium might be capable of producing male or female gametocytes, based on precedents for other regulated switches in bacteria and fungi.
Model 1. Genetic inversion controls cell fate
A paradigmatic example by which clones of a single parental line can adopt two unique cellular fates involves expression of two alternative flagellar antigens in the bacterial pathogen Salmonella (Silverman and Simon, 1980). This system arose as an immune evasion strategy. As the immune system responds to one flagellar antigen, individuals expressing a different flagellar antigen arise in the population and evade immunity. This system involves an ~900 bp invertible DNA locus. In one orientation, it d rives expression of the H2 antigen and a repressor of the unlinked H1 antigen gene. In the opposite orientation, the H2 antigen and H1 repressor are not expressed, and H1 antigen is expressed. The 900 bp region encodes a site-specific recombinase (Hin) that acts on flanking repeats to mediate inversion, and evolved from an ancestral transposon or phage recombination system. A similar molecular switch could underlie the ability of Plasmodium to produce male and female gametocytes based on inversion of a regulatory element that flips between male and female specification (Figure 4). Sequencing genomic DNA from purified male and female gametocytes could identify candidate invertible DNA regions.
Model 2. Competing non-coding RNAs govern cell fate
A novel regulatory element has been defined governing the S. cerevisiae dimorphic transition from yeast to pseudohyphal growth evoked by the Flo11 cell surface adhesin (Bumgarner et al., 2009) (Figure 4). The large FLO11 gene promoter encodes two opposing non-coding RNAs (ncRNA) (ICR1 and PWR1). Expression of one (ICR1) converges on the FLO11 promoter, occluding polymerase to block expression and enforce yeast growth. Expression of the other (PWR1) divergent from FLO11 blocks ICR1 expression and enables FLO11 expression and hyphal growth. An analogous control element could d rive the male-female gametocyte decision in Plasmodium (Figure 4). Analysis of ncRNAs (or small RNAseq) uniquely expressed in male vs. female gametocytes provides an approach to address this model. It is interesting to point out that previous SAGE analysis of Plasmodium falciparum transcripts has identified widespread distribution of both sense and antisense transcribed competing RNA species with an inverse relationship in abundance (Gunasekera et al., 2004). It should also be noted that X chromosome inactivation in mammals is also driven by a non - coding RNA (XIST), with implications for the Plasmodium sex-determination model proposed here.
Model 3. Stochastic gene expression differences underlie cell fat e
Studies in bacteria and fungi reveal gene expression at the individual cell level and can involve stochastic differences in transcript and protein levels that vary in different cells in a population, a phenomenon called genetic noise (Elowitz et al., 2002; Raser and O’Shea, 2004). In populations of S. cerevisiae cells expressing CFP (blue) and YFP (yellow) from the same gene promoter are observed that are green (blue + yellow, dichromatic), but cells that are only blue or yellow (monochromatic) are also seen (Figure 4). This is not the result of gene loss, but rather attributable to stochastic expression as a result of genetic noise. By analogy, Plasmodium gametocyte fate could be specified by one or two stochastically regulated genes (Figure 4). In the one-gene model, the on state could specify male fate, and the off state female, a possibility consistent with the fact that most known sex-specific developmental mutations are for male. In the two-gene model, expression of one or the other (but not both) by any individual would lead to male or female gametocyte identity. Since a single committed schizont produces only male or female gametocytes, such an event would need to perdure during cell division but then reset to restore totipotency following sex. An approach to address this model could involve RNAseq of purified male and female gametocytes, especially comparing these with parasite strains with developmental defects in male gametocytogenesis.
To summarize, an experimental approach to address these and other models is to purify male and female gametocytes and apply next generation sequencing to DNA (to explore inversion models) and RNA (to identify sex-specific genes or noncoding transcripts). Genetic analysis of existing and newly generated parasite mutants altered in gametocyte production, similar to studies of yeast sterile mutants may also provide another route to define the earliest acting sex determinants.
Conclusions
Studies of sex and its evolution and impact in fungal and parasite pathogens illustrate principles by which genetic diversity is generated and maintained, with implications for both other pathogens and multi-cellular eukaryotes. These findings reveal that rather than losing sex, diverse modifications to sexual reproduction have occurred involving transitions in out - crossing vs. inbreeding, opposite-sex vs. unisex, and meiosis/parameiosis/parasexual modes of reproduction, rendering sexual reproduction cryptic/covert. We propose sex not only admixes pre-existing population genetic diversity, but also serves to generate diversity de novo. In this model, sex is a hypermutagenic state that produces diversity and contributes to evolution. Our understanding of the roles and plasticity of the MAT locus in governing sexual identity in fungi has considerably advanced. By contrast, our understanding of how parasites produce gametocytes/gametes is in its infancy, and nothing is known about how sexes/mating-types are initially established in these ubiquitous microbial pathogens. Comparisons to the fungal MAT/sex loci may provide a propitious conceptual framework to advance fostering laboratory investigations and the development of transmission blocking strategies.
Acknowledgments
I thank Ashley Chi, Maria Cardenas, Eddie Byrnes, Marianna Feretzaki, Cecelia Shertz, Min Ni, Soo Chan Lee, and Ashley Chi for critical reading and comments, Soo Chan Lee for preparing figures, Rachel Mullis for manuscript assistance, the anonymous reviewers for astute insights and suggestions, and the NIH/NIAID for supporting studies on fungal sex and MAT (R01 grants AI39115, AI50113).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akopyants NS, Kimblin N, Secundino N, Patrick R, Peters N, Lawyer P, Dobson DE, Beverley SM, Sacks DL. Demonstration of genetic exchange during cyclical development of Leishmania in the sand fly vector. Science. 2009;324:265–268. doi: 10.1126/science.1169464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano P. Plasmodium falciparum gametocytes: still many secrets of a hidden life. Mol Microbiol. 2007;66:291–302. doi: 10.1111/j.1365-2958.2007.05904.x. [DOI] [PubMed] [Google Scholar]
- Alby K, Schaefer D, Bennett RJ. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature. 2009;460:890–893. doi: 10.1038/nature08252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RJ, Johnson AD. Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO J. 2003;22:2505–2515. doi: 10.1093/emboj/cdg235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–514. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- Braks JA, Mair GR, Franke-Fayard B, Janse CJ, Waters AP. A conserved U-rich RNA region implicated in regulation of translation in Plasmodium female gametocytes. Nucleic Acids Res. 2008;36:1176–1186. doi: 10.1093/nar/gkm1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubnick M, Smulian AG. The MAT1 locus of Histoplasma capsulatum is responsive in a mating type-specific manner. Eukaryot Cell. 2007;6:616–621. doi: 10.1128/EC.00020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui T, Lin X, Malik R, Heitman J, Carter D. Isolates of Cryptococcus neoformans from infected animals reveal genetic exchange in unisexual, alpha mating type populations. Eukaryot Cell. 2008;7:1771–1780. doi: 10.1128/EC.00097-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumgarner SL, Dowell RD, Grisafi P, Gifford DK, Fink GR. Toggle involving cis-interfering noncoding RNAs controls variegated gene expression in yeast. Proc Natl Acad Sci U S A. 2009;106:18321–18326. doi: 10.1073/pnas.0909641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler G. Fungal sex and pathogenesis. Clin Microbiol Rev. 2010;23:140–159. doi: 10.1128/CMR.00053-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler G, Rasmussen MD, Lin MF, Santos MA, Sakthikumar S, Munro CA, Rheinbay E, Grabherr M, Forche A, Reedy JL, et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459:657–662. doi: 10.1038/nature08064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes EJ, 3rd, Li W, Lewit Y, Ma H, Voelz K, Ren P, Carter D, Chaturvedi V, Bildfell RJ, May RC, et al. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the Northwest United States. PLoS Pathogens. 2010;6:e1000850. doi: 10.1371/journal.ppat.1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LT, Currie BJ, Krockenberger M, Malik R, Meyer W, Heitman J, Carter D. Clonality and recombination in genetically differentiated subgroups of Cryptococcus gattii. Eukaryot Cell. 2005a;4:1403–1409. doi: 10.1128/EC.4.8.1403-1409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LT, Fraser JA, Nichols CB, Dietrich FS, Carter D, Heitman J. Clinical and environmental isolates of Cryptococcus gattii from Australia that retain sexual fecundity. Eukaryot Cell. 2005b;4:1410–1419. doi: 10.1128/EC.4.8.1410-1419.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D, Saul N, Campbell L, Bui T, Krockenberger M. Sex in Natural Populations of Cryptococcus gattii. In: Heitman J, Kronstad JW, Taylor JW, Casselton LA, editors. Sex in Fungi: Molecular Determination and Evolutionary Implications. Washington, DC: ASM Press; 2007. pp. 477–488. [Google Scholar]
- Cooper A, Tait A, Sweeney L, Tweedie A, Morrison L, Turner CM, MacLeod A. Genetic analysis of the human infective trypanosome Trypanosoma brucei gambiense: chromosomal segregation, crossing over, and the construction of a genetic map. Genome Biol. 2008;9:R103. doi: 10.1186/gb-2008-9-6-r103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Adam RD, Worobey M, Sterling CR. Population genetics provides evidence for recombination in Giardia. Curr Biol. 2007;17:1984–1988. doi: 10.1016/j.cub.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Daniels KJ, Srikantha T, Lockhart SR, Pujol C, Soll DR. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J. 2006;25:2240–2252. doi: 10.1038/sj.emboj.7601099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva EK, Gehrke AR, Olszewski K, Leon I, Chahal JS, Bulyk ML, Llinas M. Specific DNA-binding by apicomplexan AP2 transcription factors. Proc Natl Acad Sci U S A. 2008;105:8393–8398. doi: 10.1073/pnas.0801993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener AC, Fink GR. DLH1 is a functional Candida albicans homologue of the meiosis-specific gene DMC1. Genetics. 1996;143:769–776. doi: 10.1093/genetics/143.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MW, Thompson J, Gardiner DL, Trenholme KR. Sex in Plasmodium: a sign of commitment. Trends Parasitol. 2008;24:168–175. doi: 10.1016/j.pt.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Dumitru R, Navarathna DH, Semighini CP, Elowsky CG, Dumitru RV, Dignard D, Whiteway M, Atkin AL, Nickerson KW. In vivo and in vitro anaerobic mating in Candida albicans. Eukaryot Cell. 2007;6:465–472. doi: 10.1128/EC.00316-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- Escandon P, Sanchez A, Martinez M, Meyer W, Castaneda E. Molecular epidemiology of clinical and environmental isolates of the Cryptococcus neoformans species complex reveals a high genetic diversity and the presence of the molecular type VGII mating type a in Colombia. FEMS Yeast Res. 2006;6:625–635. doi: 10.1111/j.1567-1364.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- Forche A, Alby K, Schaefer D, Johnson AD, Berman J, Bennett RJ. The parasexual cycle in Candida albicans provides an alternative pathway to meiosis for the formation of recombinant strains. PLoS Biol. 2008;6:e110. doi: 10.1371/journal.pbio.0060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser JA, Diezmann S, Subaran RL, Allen A, Lengeler KB, Dietrich FS, Heitman J. Convergent evolution of chromosomal sex-determining regions in the animal and fungal kingdoms. PLoS Biol. 2004;2:e384. doi: 10.1371/journal.pbio.0020384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser JA, Giles SS, Wenink EC, Geunes-Boyer SG, Wright JR, Diezmann S, Allen A, Stajich JE, Dietrich FS, Perfect JR, et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature. 2005;437:1360–1364. doi: 10.1038/nature04220. [DOI] [PubMed] [Google Scholar]
- Fraser JA, Subaran RL, Nichols CB, Heitman J. Recapitulation of the sexual cycle of the primary fungal pathogen Cryptococcus neoformans variety gattii: implications for an outbreak on Vancouver Island. Eukaryot Cell. 2003;2:1036–1045. doi: 10.1128/EC.2.5.1036-1045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya T, Mu J, Hayton K, Liu A, Duan J, Nkrumah L, Joy DA, Fidock DA, Fujioka H, Vaidya AB, et al. Disruption of a Plasmodium falciparum gene linked to male sexual development causes early arrest in gametocytogenesis. Proc Natl Acad Sci U S A. 2005;102:16813–16818. doi: 10.1073/pnas.0501858102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galagan JE, Calvo SE, Cuomo C, Ma LJ, Wortman JR, Batzoglou S, Lee SI, Basturkmen M, Spevak CC, Clutterbuck J, et al. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature. 2005;438:1105–1115. doi: 10.1038/nature04341. [DOI] [PubMed] [Google Scholar]
- Gaunt MW, Yeo M, Frame IA, Stothard JR, Carrasco HJ, Taylor MC, Mena SS, Veazey P, Miles GA, Acosta N, et al. Mechanism of genetic exchange in American trypanosomes. Nature. 2003;421:936–939. doi: 10.1038/nature01438. [DOI] [PubMed] [Google Scholar]
- Gibson W, Peacock L, Ferris V, Williams K, Bailey M. Analysis of a cross between green and red fluorescent trypanosomes. Biochem Soc Trans. 2006;34:557–559. doi: 10.1042/BST0340557. [DOI] [PubMed] [Google Scholar]
- Gibson W, Peacock L, Ferris V, Williams K, Bailey M. The use of yellow fluorescent hybrids to indicate mating in Trypanosoma brucei. Parasit Vectors. 2008;1:4–19. doi: 10.1186/1756-3305-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles SS, Dagenais TR, Botts MR, Keller NP, Hull CM. Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect Immun. 2009;77:3491–3500. doi: 10.1128/IAI.00334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse V, Krappmann S. The asexual pathogen Aspergillus fumigatus expresses functional determinants of Aspergillus nidulans sexual development. Eukaryot Cell. 2008;7:1724–1732. doi: 10.1128/EC.00157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekera AM, Patankar S, Schug J, Eisen G, Kissinger J, Roos D, Wirth DF. Widespread distribution of antisense transcripts in the Plasmodium falciparum genome. Mol Biochem Parasitol. 2004;136:35–42. doi: 10.1016/j.molbiopara.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Heitman J. Sexual reproduction and the evolution of microbial pathogens. Curr Biol. 2006;16:R711–725. doi: 10.1016/j.cub.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Hirai M, Arai M, Mori T, Miyagishima SY, Kawai S, Kita K, Kuroiwa T, Terenius O, Matsuoka H. Male fertility of malaria parasites is determined by GCS1, a plant-type reproduction factor. Curr Biol. 2008;18:607–613. doi: 10.1016/j.cub.2008.03.045. [DOI] [PubMed] [Google Scholar]
- Hiremath SS, Chowdhary A, Kowshik T, Randhawa HS, Sun S, Xu J. Long-distance dispersal and recombination in environmental populations of Cryptococcus neoformans var. grubii from India. Microbiology. 2008;154:1513–1524. doi: 10.1099/mic.0.2007/015594-0. [DOI] [PubMed] [Google Scholar]
- Huang G, Srikantha T, Sahni N, Yi S, Soll DR. CO(2) regulates white-to-opaque switching in Candida albicans. Curr Biol. 2009;19:330–334. doi: 10.1016/j.cub.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Wang H, Chou S, Nie X, Chen J, Liu H. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc Natl Acad Sci U S A. 2006;103:12813–12818. doi: 10.1073/pnas.0605270103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull CM, Davidson RC, Heitman J. Cell identity and sexual development in Cryptococcus neoformans are controlled by the mating-type-specific homeodomain protein Sxi1α. Genes Dev. 2002;16:3046–3060. doi: 10.1101/gad.1041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull CM, Johnson AD. Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science. 1999;285:1271–1275. doi: 10.1126/science.285.5431.1271. [DOI] [PubMed] [Google Scholar]
- Hull CM, Raisner RM, Johnson AD. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science. 2000;289:307–310. doi: 10.1126/science.289.5477.307. [DOI] [PubMed] [Google Scholar]
- Idnurm A, Walton FJ, Floyd A, Heitman J. Identification of the sex genes in an early diverged fungus. Nature. 2008;451:193–196. doi: 10.1038/nature06453. [DOI] [PubMed] [Google Scholar]
- Keeling P. Five questions about microsporidia. PLoS Pathog. 2009;5:e1000489. doi: 10.1371/journal.ppat.1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SM, Franke-Fayard B, Mair GR, Lasonder E, Janse CJ, Mann M, Waters AP. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell. 2005;121:675–687. doi: 10.1016/j.cell.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Kidd SE, Chow Y, Mak S, Bach PJ, Chen H, Hingston AO, Kronstad JW, Bartlett KH. Characterization of environmental sources of the human and animal pathogen Cryptococcus gattii in British Columbia, Canada, and the Pacific Northwest of the United States. Appl Environ Microbiol. 2007;73:1433–1443. doi: 10.1128/AEM.01330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, Macdougall L, Boekhout T, Kwon-Chung KJ, Meyer W. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada) Proc Natl Acad Sci U S A. 2004;101:17258–17263. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Liang WJ, Chaloupka J, Ruoff C, Schroppel K, Naglik JR, Eckert SE, Mogensen EG, Haynes K, Tuite MF, et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol. 2005;15:2021–2026. doi: 10.1016/j.cub.2005.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung KJ. Emmonsiella capsulata: perfect state of Histoplasma capsulatum. Science. 1972;177:368–369. doi: 10.1126/science.177.4046.368. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung KJ. A new species of Filobasidiella, the sexual state of Cryptococcus neoformans B and C serotypes. Mycologia. 1976b;68:943–946. [PubMed] [Google Scholar]
- Kwon-Chung KJ, Edman JC, Wickes BL. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect Immun. 1992;60:602–605. doi: 10.1128/iai.60.2.602-605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung KJ, Sugui JA. Sexual reproduction in Aspergillus species of medical or economical importance: why so fastidious? Trends Microbiol. 2009;17:481–487. doi: 10.1016/j.tim.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke SA, Lockhart SR, Daniels KJ, Soll DR. Skin facilitates Candida albicans mating. Infect Immun. 2003;71:4970–4976. doi: 10.1128/IAI.71.9.4970-4976.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Corradi N, Byrnes EJ, 3rd, Torres-Martinez S, Dietrich FS, Keeling PJ, Heitman J. Microsporidia evolved from ancestral sexual fungi. Curr Biol. 2008;18:1675–1679. doi: 10.1016/j.cub.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Ni M, Li W, Shertz C, Heitman J. The evolution of sex: a perspective from the fungal kingdom. Microbiol Mol Biol Rev. 2010;74:298–340. doi: 10.1128/MMBR.00005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler KB, Fox DS, Fraser JA, Allen A, Forrester K, Dietrich FS, Heitman J. Mating-type locus of Cryptococcus neoformans: a step in the evolution of sex chromosomes. Eukaryot Cell. 2002;1:704–718. doi: 10.1128/EC.1.5.704-718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Metin B, White TC, Heitman J. Organization and evolutionary trajectory of the mating type (MAT) locus in dermatophyte and dimorphic fungal pathogens. Eukaryot Cell. 2010;9:46–58. doi: 10.1128/EC.00259-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X. Cryptococcus neoformans: morphogenesis, infection, and evolution. Infect Genet Evol. 2009;9:401–416. doi: 10.1016/j.meegid.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Lin X, Heitman J. Mechanisms of homothallism in fungi--transitions between heterothallism and homothallism. In: Heitman J, Kronstad JW, Taylor JW, Casselton LA, editors. Sex in Fungi: Molecular Determination and Evolutionary Implications. Washington, DC: ASM Press; 2007. pp. 35–58. [Google Scholar]
- Lin X, Hull CM, Heitman J. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature. 2005;434:1017–1021. doi: 10.1038/nature03448. [DOI] [PubMed] [Google Scholar]
- Lin X, Litvintseva AP, Nielsen K, Patel S, Floyd A, Mitchell TG, Heitman J. αADα hybrids of Cryptococcus neoformans: evidence of same-sex mating in nature and hybrid fitness. PLoS Genet. 2007;3:1975–1990. doi: 10.1371/journal.pgen.0030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Patel S, Litvintseva AP, Floyd A, Mitchell TG, Heitman J. Diploids in the Cryptococcus neoformans serotype A population homozygous for the alpha mating type originate via unisexual mating. PLoS Pathog. 2009;5:e1000283. doi: 10.1371/journal.ppat.1000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvintseva AP, Marra RE, Nielsen K, Heitman J, Vilgalys R, Mitchell TG. Evidence of sexual recombination among Cryptococcus neoformans serotype A isolates in sub-Saharan Africa. Eukaryot Cell. 2003;2:1162–1168. doi: 10.1128/EC.2.6.1162-1168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tewari R, Ning J, Blagborough AM, Garbom S, Pei J, Grishin NV, Steele RE, Sinden RE, Snell WJ, et al. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev. 2008;22:1051–1068. doi: 10.1101/gad.1656508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn MS, Lewis MD, Acosta N, Yeo M, Carrasco HJ, Segovia M, Vargas J, Torrico F, Miles MA, Gaunt MW. Trypanosoma cruzi IIc: phylogenetic and phylogeographic insights from sequence and microsatellite analysis and potential impact on emergent Chagas disease. PLoS Negl Trop Dis. 2009;3:e510. doi: 10.1371/journal.pntd.0000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Hagen F, Stekel DJ, Johnston SA, Sionov E, Falk R, Polacheck I, Boekhout T, May RC. The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc Natl Acad Sci U S A. 2009a;106:12980–12985. doi: 10.1073/pnas.0902963106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LJ, Ibrahim AS, Skory C, Grabherr MG, Burger G, Butler M, Elias M, Idnurm A, Lang BF, Sone T, et al. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 2009b;5:e1000549. doi: 10.1371/journal.pgen.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLα strains. Science. 2000;289:310–313. doi: 10.1126/science.289.5477.310. [DOI] [PubMed] [Google Scholar]
- Mair GR, Braks JA, Garver LS, Wiegant JC, Hall N, Dirks RW, Khan SM, Dimopoulos G, Janse CJ, Waters AP. Regulation of sexual development of Plasmodium by translational repression. Science. 2006;313:667–669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel MA, Barker BM, Kroken S, Rounsley SD, Orbach MJ. Genomic and population analyses of the mating type loci in Coccidioides species reveal evidence for sexual reproduction and gene acquisition. Eukaryot Cell. 2007;6:1189–1199. doi: 10.1128/EC.00117-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland CM, Chang YC, Varma A, Kwon-Chung KJ. Uniqueness of the mating system in Cryptococcus neoformans. Trends Microbiol. 2004;12:208–212. doi: 10.1016/j.tim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- McDonough ES, Lewis AL. Blastomyces dermatitidis: production of the sexual stage. Science. 1967;156:528–529. doi: 10.1126/science.156.3774.528. [DOI] [PubMed] [Google Scholar]
- Metin B, Findley K, Heitman J. The mating type locus (MAT) and sexual reproduction of Cryptococcus heveanensis: insights into the evolution of sex and sex-determining chromosomal regions in fungi. PLoS Genetics. 2010;6:e10000961. doi: 10.1371/journal.pgen.1000961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MG, Johnson AD. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell. 2002;110:293–302. doi: 10.1016/s0092-8674(02)00837-1. [DOI] [PubMed] [Google Scholar]
- Morgan JA, Vredenburg VT, Rachowicz LJ, Knapp RA, Stice MJ, Tunstall T, Bingham RE, Parker JM, Longcore JE, Moritz C, et al. Population genetics of the frog-killing fungus Batrachochytrium dendrobatidis. Proc Natl Acad Sci U S A. 2007;104:13845–13850. doi: 10.1073/pnas.0701838104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison LJ, Tweedie A, Black A, Pinchbeck GL, Christley RM, Schoenefeld A, Hertz-Fowler C, MacLeod A, Turner CM, Tait A. Discovery of mating in the major African livestock pathogen Trypanosoma congolense. PLoS ONE. 2009;4:e5564. doi: 10.1371/journal.pone.0005564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow CA, Fraser JA. Sexual reproduction and dimorphism in the pathogenic basidiomycetes. FEMS Yeast Res. 2009;9:161–177. doi: 10.1111/j.1567-1364.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- Mu J, Myers RA, Jiang H, Liu S, Ricklefs S, Waisberg M, Chotivanich K, Wilairatana P, Krudsood S, White NJ, et al. Plasmodium falciparum genome-wide scans for positive selection, recombination hot spots and resistance to antimalarial drugs. Nat Genet. 2010;42:268–271. doi: 10.1038/ng.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K, Cox GM, Litvintseva AP, Mylonakis E, Malliaris SD, Benjamin DK, Jr, Giles SS, Mitchell TG, Casadevall A, Perfect JR, et al. Cryptococcus neoformans α strains preferentially disseminate to the central nervous system during coinfection. Infect Immun. 2005b;73:4922–4933. doi: 10.1128/IAI.73.8.4922-4933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K, De Obaldia AL, Heitman J. Cryptococcus neoformans mates on pigeon guano: implications for the realized ecological niche and globalization. Eukaryot Cell. 2007;6:949–959. doi: 10.1128/EC.00097-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K, Marra RE, Hagen F, Boekhout T, Mitchell TG, Cox GM, Heitman J. Interaction between genetic background and the mating-type locus in Cryptococcus neoformans virulence potential. Genetics. 2005a;171:975–983. doi: 10.1534/genetics.105.045039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Gorman CM, Fuller HT, Dyer PS. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature. 2009;457:471–474. doi: 10.1038/nature07528. [DOI] [PubMed] [Google Scholar]
- Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, Chretien F, Heitman J, Dromer F, Nielsen K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathogens. 2010;6:e1000953. doi: 10.1371/journal.ppat.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti M, Rydholm C, Schwier EU, Anderson MJ, Szakacs G, Lutzoni F, Debeaupuis JP, Latge JP, Denning DW, Dyer PS. Evidence for sexuality in the opportunistic fungal pathogen Aspergillus fumigatus. Curr Biol. 2005;15:1242–1248. doi: 10.1016/j.cub.2005.05.045. [DOI] [PubMed] [Google Scholar]
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- Peacock L, Ferris V, Bailey M, Gibson W. Dynamics of infection and competition between two strains of Trypanosoma brucei brucei in the tsetse fly observed using fluorescent markers. Kinetoplastid Biol Dis. 2007;6:4–14. doi: 10.1186/1475-9292-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock L, Ferris V, Bailey M, Gibson W. Intraclonal mating occurs during tsetse transmission of Trypanosoma brucei. Parasit Vectors. 2009;2:43–55. doi: 10.1186/1756-3305-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke SS, Zufall RA. Rapid diversification of mating systems in ciliates. Biological Journal of the Linnean Society. 2009;98:187–197. [Google Scholar]
- Poxleitner MK, Carpenter ML, Mancuso JJ, Wang CJ, Dawson SC, Cande WZ. Evidence for karyogamy and exchange of genetic material in the binucleate intestinal parasite Giardia intestinalis. Science. 2008;319:1530–1533. doi: 10.1126/science.1153752. [DOI] [PubMed] [Google Scholar]
- Pyrzak W, Miller KY, Miller BL. Mating type protein Mat1–2 from asexual Aspergillus fumigatus drives sexual reproduction in fertile Aspergillus nidulans. Eukaryot Cell. 2008;7:1029–1040. doi: 10.1128/EC.00380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh MA, Malik SB, Logsdon JM., Jr A phylogenomic inventory of meiotic genes; evidence for sex in Giardia and an early eukaryotic origin of meiosis. Curr Biol. 2005;15:185–191. doi: 10.1016/j.cub.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Ramirez-Zavala B, Reuss O, Park YN, Ohlsen K, Morschhauser J. Environmental induction of white-opaque switching in Candida albicans. PLoS Pathog. 2008;4:e1000089. doi: 10.1371/journal.ppat.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raser JM, O’Shea EK. Control of stochasticity in eukaryotic gene expression. Science. 2004;304:1811–1814. doi: 10.1126/science.1098641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedy JL, Floyd AM, Heitman J. Mechanistic plasticity of sexual reproduction and meiosis in the Candida pathogenic species complex. Curr Biol. 2009;19:891–899. doi: 10.1016/j.cub.2009.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni N, Yi S, Daniels KJ, Srikantha T, Pujol C, Soll DR. Genes selectively up-regulated by pheromone in white cells are involved in biofilm formation in Candida albicans. PLoS Pathog. 2009;5:e1000601. doi: 10.1371/journal.ppat.1000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul N, Krockenberger M, Carter D. Evidence of recombination in mixed-mating-type and alpha-only populations of Cryptococcus gattii sourced from single eucalyptus tree hollows. Eukaryot Cell. 2008;7:727–734. doi: 10.1128/EC.00020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoustra SE, Debets AJ, Slakhorst M, Hoekstra RF. Mitotic recombination accelerates adaptation in the fungus Aspergillus nidulans. PLoS Genet. 2007;3:e68. doi: 10.1371/journal.pgen.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurko AM, Logsdon JM., Jr Using a meiosis detection toolkit to investigate ancient asexual “scandals” and the evolution of sex. Bioessays. 2008;30:579–589. doi: 10.1002/bies.20764. [DOI] [PubMed] [Google Scholar]
- Schurko AM, Neiman M, Logsdon JM., Jr Signs of sex: what we know and how we know it. Trends Ecol Evol. 2009;24:208–217. doi: 10.1016/j.tree.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Selmecki A, Forche A, Berman J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science. 2006;313:367–370. doi: 10.1126/science.1128242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RK, Bennett RJ. Fungal meiosis and parasexual reproduction--lessons from pathogenic yeast. Curr Opin Microbiol. 2009;12:599–607. doi: 10.1016/j.mib.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M, Simon M. Phase variation: genetic analysis of switching mutants. Cell. 1980;19:845–854. doi: 10.1016/0092-8674(80)90075-6. [DOI] [PubMed] [Google Scholar]
- Silvestrini F, Alano P, Williams JL. Commitment to the production of male and female gametocytes in the human malaria parasite Plasmodium falciparum. Parasitology. 2000;121:465–471. doi: 10.1017/s0031182099006691. [DOI] [PubMed] [Google Scholar]
- Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll DR. “White-opaque transition”: a second high-frequency switching system in Candida albicans. J Bacteriol. 1987;169:189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TG, Lourenco P, Carter R, Walliker D, Ranford-Cartwright LC. Commitment to sexual differentiation in the human malaria parasite, Plasmodium falciparum. Parasitology. 2000;121:127–133. doi: 10.1017/s0031182099006265. [DOI] [PubMed] [Google Scholar]
- Stanton BC, Giles SS, Staudt MW, Kruzel EK, Hull CM. Allelic exchange of pheromones and their receptors reprograms sexual identity in Cryptococcus neoformans. PLoS Genet. 2010;6:e1000860. doi: 10.1371/journal.pgen.1000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibayrenc M, Ayala FJ. The clonal theory of parasitic protozoa: 12 years on. Trends Parasitol. 2002;18:405–410. doi: 10.1016/s1471-4922(02)02357-7. [DOI] [PubMed] [Google Scholar]
- Torres EM, Sokolsky T, Tucker CM, Chan LY, Boselli M, Dunham MJ, Amon A. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science. 2007;317:916–924. doi: 10.1126/science.1142210. [DOI] [PubMed] [Google Scholar]
- Torres I, Garcia AM, Hernandez O, Gonzalez A, McEwen JG, Restrepo A, Arango M. Presence and expression of the mating type locus in Paracoccidioides brasiliensis isolates. Fungal Genet Biol. 2010;47:373–380. doi: 10.1016/j.fgb.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Tsaousis AD, Kunji ER, Goldberg AV, Lucocq JM, Hirt RP, Embley TM. A novel route for ATP acquisition by the remnant mitochondria of Encephalitozoon cuniculi. Nature. 2008;453:553–556. doi: 10.1038/nature06903. [DOI] [PubMed] [Google Scholar]
- Tzung KW, Williams RM, Scherer S, Federspiel N, Jones T, Hansen N, Bivolarevic V, Huizar L, Komp C, Surzycki R, et al. Genomic evidence for a complete sexual cycle in Candida albicans. Proc Natl Acad Sci U S A. 2001;98:3249–3253. doi: 10.1073/pnas.061628798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya AB, Muratova O, Guinet F, Keister D, Wellems TE, Kaslow DC. A genetic locus on Plasmodium falciparum chromosome 12 linked to a defect in mosquito-infectivity and male gametogenesis. Mol Biochem Parasitol. 1995;69:65–71. doi: 10.1016/0166-6851(94)00199-w. [DOI] [PubMed] [Google Scholar]
- Velagapudi R, Hsueh YP, Geunes-Boyer S, Wright JR, Heitman J. Spores as infectious propagules of Cryptococcus neoformans. Infect Immun. 2009;77:4345–4355. doi: 10.1128/IAI.00542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Pujol C, Lockhart SR, Soll DR. Chromosome loss followed by duplication is the major mechanism of spontaneous mating-type locus homozygosis in Candida albicans. Genetics. 2005;169:1311–1327. doi: 10.1534/genetics.104.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Saunders CW, Hu P, Grant RA, Boekhout T, Kuramae EE, Kronstad JW, Deangelis YM, Reeder NL, Johnstone KR, et al. Dandruff-associated Malassezia genomes reveal convergent and divergent virulence traits shared with plant and human fungal pathogens. Proc Natl Acad Sci U S A. 2007;104:18730–18735. doi: 10.1073/pnas.0706756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C, Tada Y, Dong X, Heitman J. The human fungal pathogen Cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell Host Microbe. 2007;1:263–273. doi: 10.1016/j.chom.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Yi S, Sahni N, Daniels KJ, Pujol C, Srikantha T, Soll DR. The same receptor, G protein, and mitogen-activated protein kinase pathway activate different downstream regulators in the alternative white and opaque pheromone responses of Candida albicans. Mol Biol Cell. 2008;19:957–970. doi: 10.1091/mbc.E07-07-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordan RE, Galgoczy DJ, Johnson AD. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc Natl Acad Sci U S A. 2006;103:12807–12812. doi: 10.1073/pnas.0605138103. [DOI] [PMC free article] [PubMed] [Google Scholar]