Abstract
Context
Guidelines recommend that exercise training be considered for medically stable outpatients with heart failure. Previous studies have not had adequate statistical power to measure the effects of exercise training on clinical outcomes.
Objective
To test the efficacy and safety of exercise training among patients with heart failure.
Design, Setting, and Patients
Multicenter, randomized controlled trial among 2331 medically stable outpatients with heart failure and reduced ejection fraction. Participants in Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) were randomized from April 2003 through February 2007 at 82 centers within the United States, Canada, and France; median follow-up was 30 months.
Interventions
Usual care plus aerobic exercise training, consisting of 36 supervised sessions followed by home-based training, or usual care alone.
Main Outcome Measures
Composite primary end point of all-cause mortality or hospitalization and prespecified secondary end points of all-cause mortality, cardiovascular mortality or cardiovascular hospitalization, and cardiovascular mortality or heart failure hospitalization.
Results
The median age was 59 years, 28% were women, and 37% had New York Heart Association class III or IV symptoms. Etiology was ischemic in 51%. Median left ventricular ejection fraction was 25%. Exercise adherence decreased from a median of 95 minutes per week during months 4 through 6 of follow-up to 74 minutes per week during months 10 through 12. A total of 759 (65%) patients in the exercise group died or were hospitalized, compared with 796 (68%) in the usual care group (hazard ratio [HR], 0.93; 95% confidence interval [CI], 0.84–1.02; P = .13). There were nonsignificant reductions in the exercise training group for mortality (189 [16%] in the exercise group vs 198 [17%] in the usual care group; HR, 0.96; 95% CI, 0.79–1.17; P = .70), cardiovascular mortality or cardiovascular hospitalization (632 [55%] in the exercise group vs 677 [58%] in the usual care group; HR, 0.92; 95% CI, 0.83–1.03; P = .14), and cardiovascular mortality or heart failure hospitalization (344 [30%] in the exercise group vs 393 [34%] in the usual care group; HR, 0.87; 95% CI, 0.75–1.00; P = .06). In prespecified supplementary analyses adjusting for highly prognostic baseline characteristics, the HRs were 0.89 (95% CI, 0.81–0.99; P = .03) for all-cause mortality or hospitalization, 0.91 (95% CI, 0.82–1.01; P = .09) for cardiovascular mortality or cardiovascular hospitalization, and 0.85 (95% CI, 0.74–0.99; P = .03) for cardiovascular mortality or heart failure hospitalization. Other adverse events were similar between the groups.
Conclusions
In the protocol-specified primary analysis, exercise training resulted in nonsignificant reductions in the primary end point of all-cause mortality or hospitalization and in key secondary clinical end points. After adjustment for highly prognostic predictors of the primary end point, exercise training was associated with modest significant reductions for both all-cause mortality or hospitalization and cardiovascular mortality or heart failure hospitalization.
Trial Registration
clinicaltrials.gov Identifier: NCT00047437
Introduction
Heart failure is a major and growing cardiovascular syndrome, and it is the end result of many cardiovascular disorders. An estimated 5 million people in the United States have heart failure, and an additional 500 000 new cases are diagnosed annually.1 Recent data indicate that the prevalence of heart failure in the Medicare population alone exceeds 4 million, with an annual age-adjusted incidence rate of 29 cases per 1000 person-years.2 Although evidence-based drug and device therapies decrease mortality, hospitalizations, and heart failure symptoms and improve quality of life, many patients treated with these regimens often remain burdened by dyspnea and fatigue, diminished exercise tolerance, reduced quality of life, recurrent hospitalizations, and early mortality.2–5
While rest was traditionally recommended for patients with heart failure, over the past 2 decades it has become recognized that physical deconditioning may play a key role in the progression of symptoms and poor outcomes. A number of prior studies have assessed the ability of exercise training to improve functional capacity in patients with heart failure.6–8 Most of these previous studies showed positive effects of exercise training on exercise capacity, quality of life, and biomarkers and observed relatively few complications during training.9 These studies also suggested that exercise training might improve survival and decrease heart failure hospitalizations.6 Two recent meta-analyses suggested improved survival and decreased hospitalizations for heart failure patients undergoing exercise training as compared with a non-exercising control group.10,11
Nonetheless, there remains a safety concern regarding exercise training in heart failure. Although the complication rate for all patients participating in cardiac rehabilitation has been reported to be extremely low, the complication rate for heart failure patients in clinical trials of exercise training has been substantially higher. One potential reason is the 100-fold increased risk for myocardial infarction and 50-fold increased risk of sudden death that exercisers, who are habitually sedentary, experience when initiating exercise training.12
Based on the results of past studies of exercise training, the American College of Cardiology, American Heart Association, European Society of Cardiology, and Canadian Cardiovascular Society have adopted recommendations that physical activity be considered for stable patients with systolic dysfunction.1,13,14 However, previous studies have been relatively small single-center trials, have not been adequately powered to evaluate mortality and morbidity, and have often lacked an adequate control group. The lack of definitive clinical outcome data has hindered the adoption of this potentially promising treatment modality.
Therefore, to examine the issue of exercise safety and effectiveness in a large sample of heart failure patients, Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) was undertaken to determine whether aerobic-type exercise training reduces all-cause mortality or all-cause hospitalization and improves quality of life (the quality-of-life findings are reported in the accompanying manuscript by Flynn et al28) in patients with stable chronic heart failure due to systolic dysfunction. Our primary hypothesis was that in patients with stable heart failure, regular structured exercise training, when added to usual, evidence-based care in accordance with published guidelines, would significantly reduce the incidence of a combined end point of all-cause mortality or all-cause hospitalization.
Methods
Eligibility and Study Design
A complete description of the design of HF-ACTION has been published previously.15 Briefly, HF-ACTION was a multicenter, randomized controlled trial of exercise training vs usual care in patients with left ventricular ejection fraction ≤ 35% and New York Heart Association (NYHA) class II to IV symptoms despite optimal heart failure therapy for at least 6 weeks. Patients were randomized from April 2003 through February 2007 within the United States, Canada, and France. Exclusion criteria included major comorbidities or limitations that could interfere with exercise training, recent (within 6 weeks) or planned (within 6 months) major cardiovascular events or procedures, performance of regular exercise training, or use of devices that limited the ability to achieve target heart rates. The protocol was reviewed and approved by the appropriate institutional review board or ethics committee for each participating center and by the coordinating center institutional review board. All patients provided written voluntary informed consent.
All patients were to undergo baseline cardiopulmonary exercise testing. Test results were reviewed by investigators to identify significant arrhythmias or ischemia that would prevent safe exercise training, to determine appropriate levels of exercise training, and to establish training heart rate ranges. Eligible patients were randomized 1:1 using a permuted block randomization scheme, stratified by clinical center and heart failure etiology (ischemic vs nonischemic). At the baseline clinic visit prior to randomization, demographics, socioeconomic status, past medical history, current medications, physical exam, and the most recent laboratory tests were obtained. Participants reported race and ethnicity at the time of study enrollment using categories defined by the National Institutes of Health. In an analysis to examine the effect of exercise training by subgroup, we used the reported race categories “black or African American” and “white” and combined all others as “other.” All cardiopulmonary exercise tests were sent to the HF-ACTION cardiopulmonary exercise core lab for review.
Exercise Training Protocol
Patients randomized to the exercise training arm first participated in a structured, group-based, supervised exercise program, with a goal of 3 sessions per week for a total of 36 sessions in 3 months. During the supervised training phase, patients performed walking, treadmill, or stationary cycling as their primary training mode. Exercise was initiated at 15 to 30 minutes per session at a heart rate corresponding to 60% of heart rate reserve (maximal heart rate on cardiopulmonary exercise test minus resting heart rate). After 6 sessions, the duration of the exercise was increased to 30 to 35 minutes, and intensity was increased to 70% of heart rate reserve. Details of the exercise training protocol have been reported previously.15 Patients were to begin home-based exercise after completing 18 supervised sessions and were to fully transition to home exercise after 36 supervised sessions. Patients in the exercise training arm were provided home exercise equipment (cycle or treadmill [ICON; Logan, Utah]) and heart rate monitors (Polar USA, Inc, New York, New York). The target training regimen for home exercise was 5 times per week for 40 minutes at a heart rate of 60% to 70% of heart rate reserve. Adherence was evaluated by measuring attendance at the supervised training sessions and by activity logs, telephone and clinic follow-up, and heart rate monitoring data (model A1 or S1, Polar USA Inc) during the home exercise training phase.
Usual Care
Patients in the usual care group were not provided with a formal exercise prescription. All patients, regardless of treatment group, received detailed self-management educational materials, in the form of a booklet, at the time of enrollment, including information on medications, fluid management, symptom exacerbation, sodium intake, and activity level of 30 minutes (as tolerated) of moderate-intensity activity on most days of the week, consistent with the American College of Cardiology/American Heart Association guidelines.1
All patients were asked to return for clinic visits every 3 months for the first 2 years of participation and yearly thereafter for up to 4 years. Cardiopulmonary exercise testing and a 6-minute walk test were performed at the 3-, 12-, and 24-month follow-up visits. The 6-minute walk test was also performed at the 3-year and final visits. Patients made their final visit at the end of the study follow-up period or at 4 years. For patients lost to follow-up, searches of the Social Security Death Index and the National Death Index were performed to assess whether any of these patients had died during the follow-up period.
To provide comparable levels of attention from study personnel in the 2 randomized arms, all patients were to be called every 2 weeks for the first 9 months, monthly until 24 months of follow-up, and quarterly thereafter. During these calls, patients in the exercise training group were asked questions to determine if they were performing the exercise training regimen as prescribed. During these calls, as well as during the supervised training sessions and at follow-up clinic visits, adherence to the exercise training regimen was promoted by study personnel. Patients in the usual care group were asked if they were exercising, but no further information was obtained due to concerns that more detailed questions about exercise would promote exercise behavior among patients in the usual care group. In addition, all patients were asked to complete a physical activity questionnaire at the baseline, 6-month, 12-month, 24-month, 3-year, and final visits. This instrument quantified the amount of moderate or vigorous activity in minutes per week completed during the preceding week.
Primary, Secondary, and Safety Outcomes
The primary end point was a composite of all-cause mortality or all-cause hospitalization. Secondary end points included all-cause mortality, the composite of cardiovascular mortality or cardiovascular hospitalization, and the composite of cardiovascular mortality or heart failure hospitalization. A post hoc analysis was also performed to compare the randomized arms with respect to the composite of cardiovascular mortality, heart failure hospitalization, left ventricular assist device implantation, or heart transplantation. In addition, change from baseline in peak oxygen consumption at 3 months and 1 year, change from baseline in 6-minute-walk distance at 3 months and 1 year, and (as a post hoc analysis) change in NYHA class were evaluated as potential mediators.
Although blinding for patients and investigators was not possible due to the nature of the exercise training intervention, deaths and cardiovascular hospitalizations for each patient were adjudicated by a clinical end point committee blinded to treatment assignment. Once a patient had an adjudicated heart failure hospitalization, no further hospitalizations for that patient were reviewed by the clinical end point committee.
In addition to mortality and hospitalization, other cardiovascular adverse events were captured, including worsening heart failure, myocardial infarction, unstable angina, serious adverse arrhythmia, stroke, and transient ischemic attack. Also captured were hospitalizations for fracture of the hip or pelvis, outpatient fracture repair, implantable cardioverter-defibrillator (ICD) firing events (for subjects with an ICD), all hospitalizations due to an event that occurred during or within 3 hours after exercise, and deaths during or within 3 hours after exercise (or unknown if during or within 3 hours after exercise).
Statistical Analysis
The study was designed to have 90% power to detect an 11% reduction in the 2-year rate of all-cause mortality or all-cause hospitalization for patients randomized to exercise training compared with those randomized to usual care. This estimate was based on assuming an annual primary outcome rate of 30% in the usual care group, treatment nonadherence rates of 30% in the first year of follow-up and 12.5% annually thereafter, an annual crossover rate of 5% from the usual care arm to an active exercise regimen, a planned median follow-up of 2.5 years, and an α level of 0.05.
Statistical analyses were performed by the coordinating center (Duke Clinical Research Institute, Durham, North Carolina) using SAS version 8.2 (SAS Institute, Inc, Cary, North Carolina). Baseline patient characteristics were summarized using medians and interquartile ranges for continuous variables and frequencies and percentages for categorical variables. Statistical comparisons of the treatment arms with respect to clinical outcomes were performed according to the intention-to-treat principle. All statistical tests were 2-tailed. Cumulative event rates were calculated using the Kaplan-Meier method.16 Event (or censoring) times for all patients were measured from the time of randomization (time zero). All information available on the primary and secondary end points was collected until the time of final contact with the patient, including patients who withdrew consent or were lost to follow-up, at which point follow-up was censored. The log-rank test was used to statistically compare the randomized arms with respect to the time until the first occurrence of either component of the primary composite end point and the secondary time-to-event outcomes, adjusting for heart failure etiology.17 Relative risks were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs) and were calculated using the Cox proportional hazards model.18 The Cox model was also used to assess the consistency of the treatment effect by testing for interactions between treatment and prespecified baseline characteristics.
As specified in the HF-ACTION protocol, supplementary analyses of the primary end point of all-cause mortality or hospitalization and the secondary end points of cardiovascular mortality or cardiovascular hospitalization and cardiovascular mortality or heart failure hospitalization were performed, adjusting for baseline characteristics strongly predictive of these clinical outcomes. With time-to-event outcomes, adjustment for strong predictors of the outcome enables the analysis to more specifically compare patients of like risk with like risk and thereby increases the statistical power.19 The baseline predictors of the primary outcome of all-cause mortality or hospitalization were objectively selected using a stepwise variable selection based on treatment-blinded data (ie, omitting treatment group from the variable selection process). To avoid introducing bias, the variables selected were required to have little or no missing data. Only the most highly significant variables (all with P values < .001) identified in this process were used in the covariate-adjusted treatment comparisons. Heart failure etiology (ischemic vs nonischemic), which was a stratification factor in the randomization, was included as a covariate in all time-to-event analyses.
Comparisons of the randomized arms with respect to the change from baseline in physiologic end points were performed using Wilcoxon rank-sum tests. Changes from baseline in NYHA class were compared between treatment groups using ordinal logistic regression.
An independent data and safety monitoring board was appointed by the National Heart, Lung, and Blood Institute to review the interim results of the study and evaluate patient safety and trial feasibility on a semiannual basis. Interim treatment comparisons were monitored by the data and safety monitoring board using 2-sided symmetric O’Brien-Fleming boundaries generated with the Lan-DeMets α-spending function approach to group-sequential testing.20,21 Although the overall significance level was 0.05, a significance level of 0.044 was required at the final analysis due to adjustment for the interim analyses. The data and safety monitoring board allowed the study leadership to have access to the overall primary event rate at some of the DSMB meetings during the study. This rate was higher than projected in the study design. After approximately 2000 patients were enrolled, unconditional power calculations based on the higher overall event rate revealed that the number of events required for 90% statistical power could be achieved with 2300 patients rather than the original goal of 3000. The target sample size was therefore reduced accordingly.
Results
Patient Characteristics
A total of 2331 patients were enrolled at 82 participating centers in the United States, Canada, and France. Baseline characteristics of the patients in each randomized arm are shown in Table 1. The median age of all patients was 59 years; 28% were women, and 40% were racial or ethnic minorities. The median left ventricular ejection fraction was 25%, and 51% of the patients had heart failure with an ischemic etiology. Of patients without an intolerance or contraindication to angiotensin-converting enzyme (ACE) inhibitors or β-blockers, 95% were taking a β-blocker and either an ACE inhibitor or an angiotensin receptor blocker (ARB). Forty-five percent of patients had an implantable cardioverter-defibrillator (ICD) or biventricular pacemaker implanted at the time of enrollment.
Table 1.
Baseline Characteristics
| Characteristics | No. (%) of Patientsa | |
|---|---|---|
| Usual Care (n = 1172) |
Exercise Training (n = 1159) |
|
| Age, median (IQR), y | 59.3 (51.1–68.2) | 59.2 (51.2–67.8) |
| Female sex | 314 (26.8) | 347 (29.9) |
| Hispanic or Latino ethnicity | 48 (4.1) | 40 (3.5) |
| Race | ||
| Black or African American | 372 (31.7) | 377 (32.5) |
| White | 728 (62.1) | 698 (60.2) |
| Other | 56 (4.8) | 65 (5.6) |
| NYHA class | ||
| II | 754 (64.3) | 723 (62.4) |
| III | 409 (34.9) | 422 (36.4) |
| IV | 9 (0.8) | 14 (1.2) |
| Ischemic etiology of heart failure | 599 (51.1) | 598 (51.6) |
| Left ventricular ejection fraction, median (IQR), % | 24.9 (20.0–30.2) | 24.6 (20.0–30.0) |
| Diabetes mellitus | 370 (31.6) | 378 (32.6) |
| Previous myocardial infarction | 499 (42.6) | 480 (41.4) |
| Hypertension | 676 (57.7) | 712 (61.4) |
| Atrial fibrillation or atrial flutter | 241 (20.6) | 247 (21.3) |
| Beck Depression Inventory II score, median (IQR) | 8 (4–15) | 8 (5–15) |
| Systolic blood pressure, median (IQR), mm Hg | 111 (100–126) | 112 (100–126) |
| Diastolic blood pressure, median (IQR), mm Hg | 70 (60–80) | 70 (61–78) |
| Sodium, median (IQR), mEq/Lb | 139 (137–141) | 139 (137–141) |
| Blood urea nitrogen, median (IQR), mg/dLb | 21 (15–28) | 20 (15–28) |
| Serum creatinine, median (IQR), mg/dLb | 1.2 (1.0–1.5) | 1.2 (1.0–1.5) |
| Baseline use of medications and devices | ||
| ACE inhibitor or ARB | 1094 (93.3) | 1105 (95.3) |
| β-Blocker | 1112 (94.9) | 1091 (94.1) |
| Aldosterone receptor antagonist | 528 (45.1) | 523 (45.1) |
| Loop diuretic | 921 (78.6) | 895 (77.2) |
| Digoxin | 547 (46.7) | 499 (43.1) |
| Implantable cardioverter-defibrillator | 448 (38.2) | 490 (42.3) |
| Biventricular pacemaker | 203 (17.3) | 216 (18.6) |
| Functional measures | ||
| Distance of 6-min walk, median (IQR), m | 373.2 (300.0– 432.5) |
365.8 (296.3–436.2) |
| Cardiopulmonary exercise time, median (IQR), min | 9.7 (7.0–12.1) | 9.5 (6.9–12.0) |
| Peak oxygen consumption, median (IQR), mL/kg/min | 14.5 (11.6–17.8) | 14.4 (11.3–17.6) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; IQR, interquartile range; NYHA, New York Heart Association.
Unless otherwise indicated. Percentages may not sum to 100 because of rounding.
Indicates the number of patients/number of patients with nonmissing data for the variable (percentage).
Sodium, blood urea nitrogen, and serum creatinine values are based on standard-of-care laboratory tests measured up to 1 year prior to randomization.
Follow-up
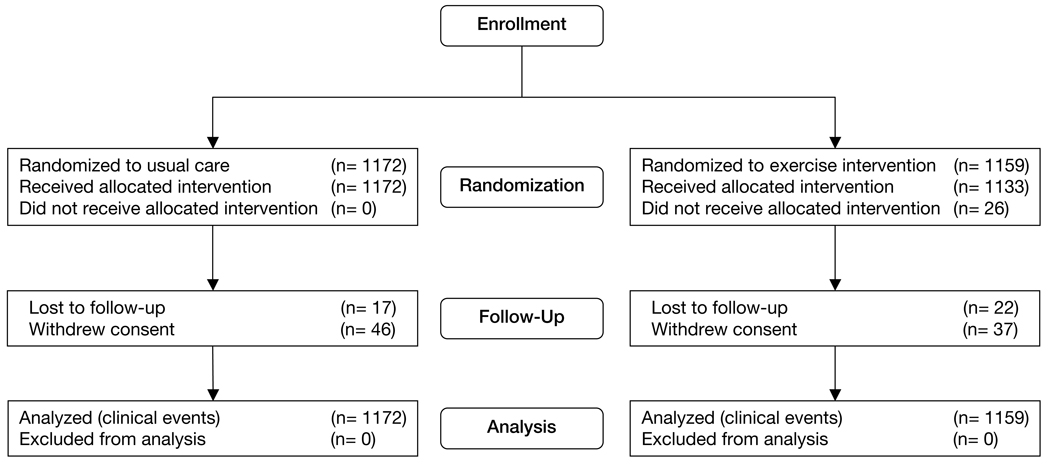
The follow-up period ended March 15, 2008. The median duration of follow-up for the primary end point was 30.1 months (goal of a minimum of 1 year and a maximum of 4 years). Thirty-nine patients (1.7%) were lost to follow-up but had a median follow-up of 14.6 months (Figure 1). Eighty-three patients (4%) withdrew consent at a median time of 6.8 months following randomization. A total of 736 patients completed 36 supervised training sessions with the median time to completion (for 36 sessions) of 3.9 months (interquartile range, 3.4–4.8 months).
Figure 1.
Flow of Participants Through the Trial
At 12 months of follow-up, the number of subjects on an ACE inhibitor and/or an ARB was 93% in the usual care group and 92% in the exercise training group; β-blocker use was 95% in the usual care group and 94% in the exercise training group.
During the first 3 months of follow-up (when patients were still in the supervised training phase of the protocol), patients in the exercise training arm exercised a median of 76 minutes (interquartile range, 39–117) per week. The exercise training goal during this time was 90 minutes per week. The exercise time increased to a median of 95 minutes per week (interquartile range, 26–184) at 4 to 6 months following enrollment and subsequently decreased to a median of 74 minutes per week (interquartile range, 0–180) at 10 to 12 months following enrollment, with a training goal of 120 minutes per week. In the third year of follow-up, patients in the exercise training group were exercising a median of 50 minutes per week (interquartile range, 0–140). At all time points, approximately 30% or more of the patients in the exercise training group exercised at or above the target exercise minutes per week. In these calculations, missing values of minutes per week were conservatively assumed to be no exercise.
Safety of Exercise Training
Overall, the performance of exercise training was well-tolerated and safe. In the exercise group, 37 patients had at least 1 hospitalization due to an event that occurred during or within 3 hours after exercise (Table 2). In the usual care group, 22 patients had such a hospitalization, despite not undergoing a formal exercise program. During the initial 36 sessions of supervised training, the percentages of subjects with an event that caused at least 1 session to be cut short (goal duration not achieved) or the goal training intensity to not be achieved were as follows: 10% for angina, 7% for arrhythmia, 4% for presyncope or syncope, and 2% for hypoglycemia. Only 1 subject had an ICD discharge that caused at least 1 supervised exercise training session to fail to reach the target duration or intensity.
Table 2.
Subjects With Selected Adverse Events
| Adverse Event | Usual Care (N = 1171)a |
Exercise Training (N = 1159) |
|---|---|---|
| Prespecified Cardiovascular Adverse Events | ||
| Worsening heart failure, No. (%) | 340 (29.0) | 303 (26.1) |
| Myocardial infarction, No. (%) | 45 (3.8) | 41 (3.5) |
| Unstable angina, No. (%) | 88 (7.5) | 86 (7.4) |
| Serious adverse arrhythmia, No. (%)b | 164 (14.0) | 167 (14.4) |
| Stroke, No. (%) | 28 (2.4) | 33 (2.8) |
| Transient ischemic attack, No. (%) | 23 (2.0) | 20 (1.7) |
| Any of the above events, No. (%) | 471 (40.0) | 434 (37.4) |
| General Adverse Events | ||
| Hospitalization for fracture of the hip or pelvis, No. (%) | 7 (0.6) | 3 (0.3) |
| Outpatient fracture repair, No. (%) | 20 (1.7) | 13 (1.1) |
| ICD firing, No. fired/No. with ICD (%) | 151/644 (23.0) | 142/641 (22.2) |
| Hospitalization after exercise, No. (%)c | 22 (1.9) | 37 (3.2) |
| Death after (or unknown if after) exercise, No. (%)d | 5 (0.4) | 5 (0.4) |
Abbreviation: ICD, implantable cardioverter-defibrillator.
One subject in the usual care group had no follow-up data forms.
Serious adverse arrhythmias are any of the following: sustained ventricular tachycardia > 30 seconds, ventricular fibrillation, supraventricular tachycardia with rapid ventricular response > 30 seconds, cardiac arrest, or bradycardia (heart rate < 50, symptomatic, and not felt to be related to medication).
Subject had at least 1 hospitalization due to an event that occurred during or within 3 hours after exercise.
Subject died during or within 3 hours after exercise, or unknown if subject died during or within 3 hours after exercise.
Usual Care Crossover
A minority of patients in the usual care group also exercised, based on self-report. For the 8 3-month windows in the first 2 years, 22% to 28% of patients, depending on time point, stated on every telephone call in the 3-month window that they were exercising. As an estimate of the fraction of usual care subjects exercising continuously throughout the trial, 8% of usual care subjects said they were exercising on all telephone calls after the first 3 months. Based on data elicited from the physical activity questionnaire, the median time spent walking at baseline was 40 minutes per week in the usual care group vs 45 minutes per week in the exercise group. At 6 months, the median time walking in the usual care group was 65 minutes per week vs 140 minutes per week in the exercise group. At 12 months, the median time spent walking was 75 minutes per week in the usual care group vs 140 minutes per week in the exercise group. Notably, at the time of randomization, 627 (55%) of the usual care subjects expressed that they were somewhat or very dissatisfied with treatment assignment vs 22 (2%) patients in the exercise group.
Clinical Outcomes
Primary End Point and Its Components
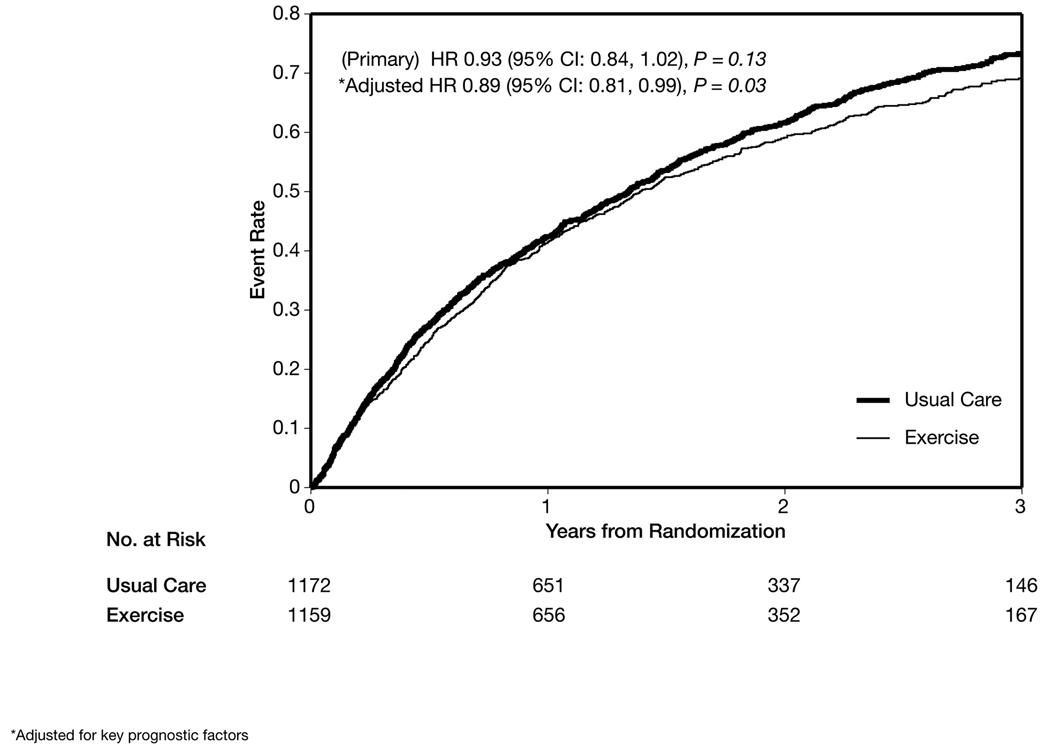
Kaplan-Meier curves depicting the primary end point of death or hospitalization from any cause for each randomized group are shown in Figure 2. During follow-up, 759 patients (65%) in the exercise group and 796 patients (68%) in the usual care group experienced a primary clinical event. The primary analysis (adjusted for heart failure etiology) exercise training resulted in a nonsignificant reduction in the primary end point of all-cause mortality or hospitalization (HR, 0.93; 95% CI, 0.84–1.02; P = .13). The absolute reduction in the primary event rate at 3 years was 4%.
Figure 2.
Time to All-Cause Death or All-Cause Hospitalization
Four baseline characteristics (duration of the cardiopulmonary exercise test, left ventricular ejection fraction, Beck Depression Inventory II score, and history of atrial fibrillation or flutter) were identified as highly prognostic of the primary end point of all-cause mortality or hospitalization, independent of treatment assignment. After adjusting for these covariates and heart failure etiology, exercise training was found to reduce the incidence of all-cause mortality or all-cause hospitalization (the primary end point) by 11% (HR, 0.89; 95% CI, 0.81–0.99; P = .03) (Figure 2).
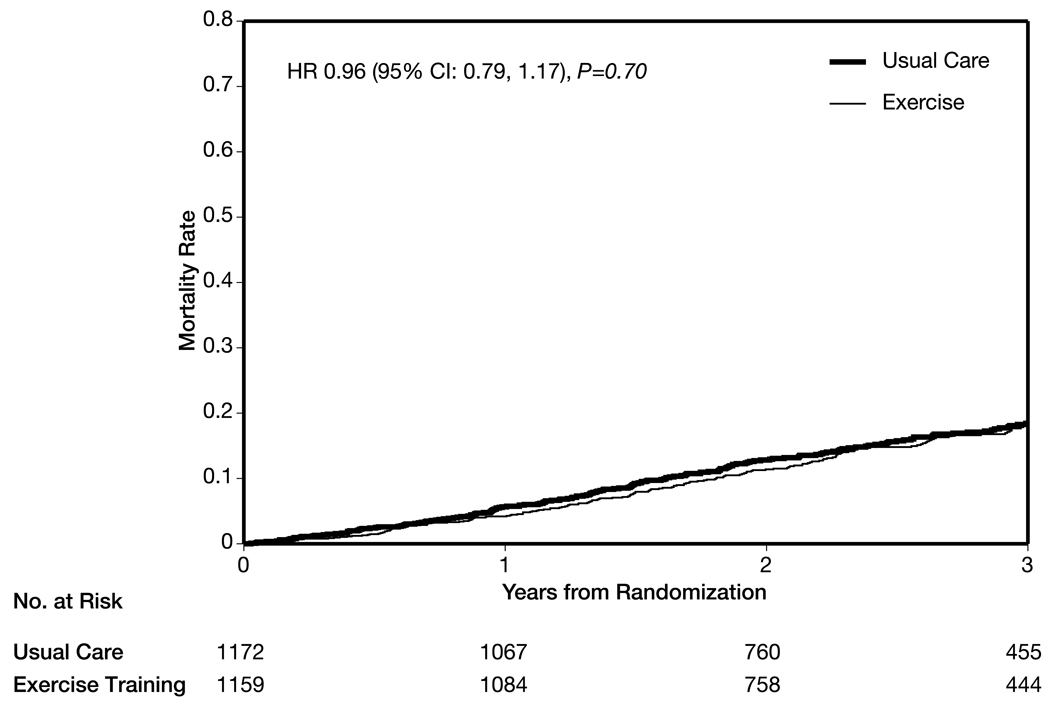
There was no significant difference in the number of deaths (189 [16%] in the exercise group vs 198 [17%] in the usual care group (HR, 0.96; 95% CI, 0.79–1.17; P = .70) (Table 3, Figure 3). At least 1 hospitalization was experienced in 729 patients (63%) in the exercise group vs 760 (65%) in the usual care group.
Table 3.
Clinical Events
| Event | Usual Care (N = 1171)a |
Exercise Training (N = 1159) |
Hazard Ratio (95% CI) |
P Value |
|---|---|---|---|---|
| All-cause mortality or all-cause hospitalization (primary end point), No. (%) |
796 (68) | 759 (65) | 0.93 (0.84–1.02) | .13 |
| Cardiovascular mortality or cardiovascular hospitalization, No. (%) |
677 (58) | 632 (55) | 0.92 (0.83–1.03) | .14 |
| Cardiovascular mortality or heart failure hospitalization, No. (%) |
393 (34) | 344 (30) | 0.87 (0.75–1.00) | .06 |
| Cardiovascular mortality or heart failure hospitalization or cardiac transplantation or left ventricular assist device, No. (%) |
403 (34) | 353 (30) | 0.87 (0.75–1.00) | .06 |
| All-cause mortality, all-cause hospitalization, emergency department visit, or urgent clinic visit for heart failure exacerbation, No. (%) |
906 (77) | 885 (76) | 0.99 (0.90–1.08) | .79 |
| All-cause mortality, No. (%) | 198 (17) | 189 (16) | 0.96 (0.79–1.17) | .70 |
| Cardiovascular mortality, No. (%) | 143 (12) | 131 (11) | 0.92 (0.74–1.15) | .47 |
Abbreviation: CI, confidence interval.
One subject in the usual care group had no follow-up data forms.
Figure 3.
Time to All-Cause Mortality
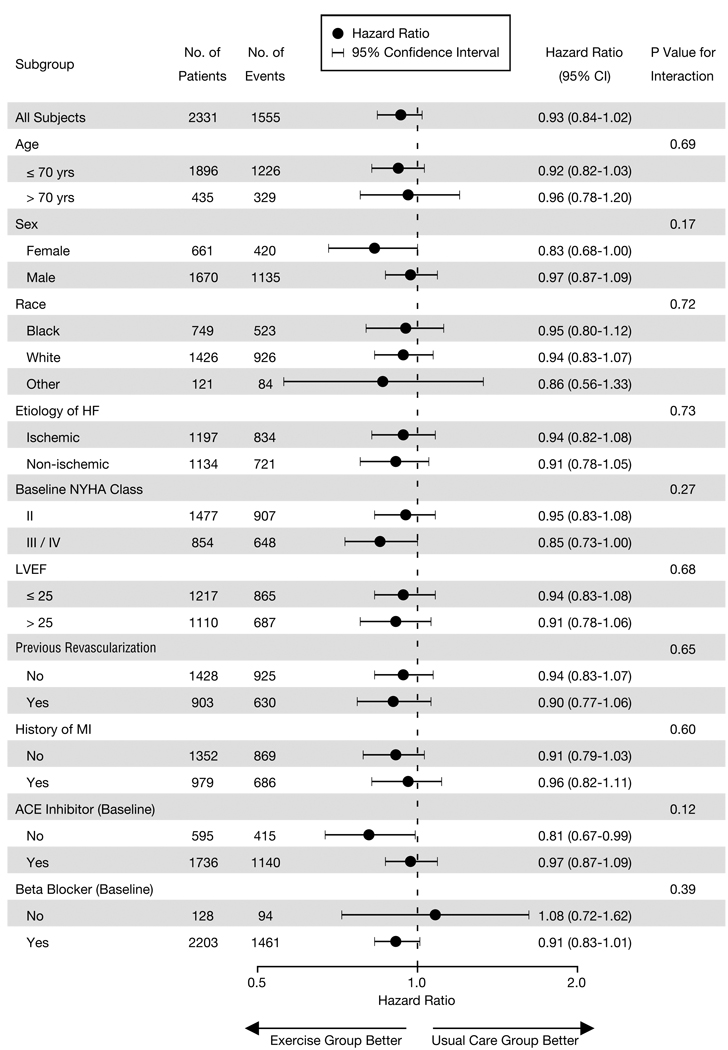
The HRs and CIs for the comparison of treatment arms within selected subgroups are shown in Figure 4. As reflected by the interaction P values, there was no significant interaction of exercise training with any of the factors defining these subgroups; that is, the overall study result was consistent among the various subgroups.
Figure 4.
Subgroup Analysis of the Primary End Point
Secondary End Points
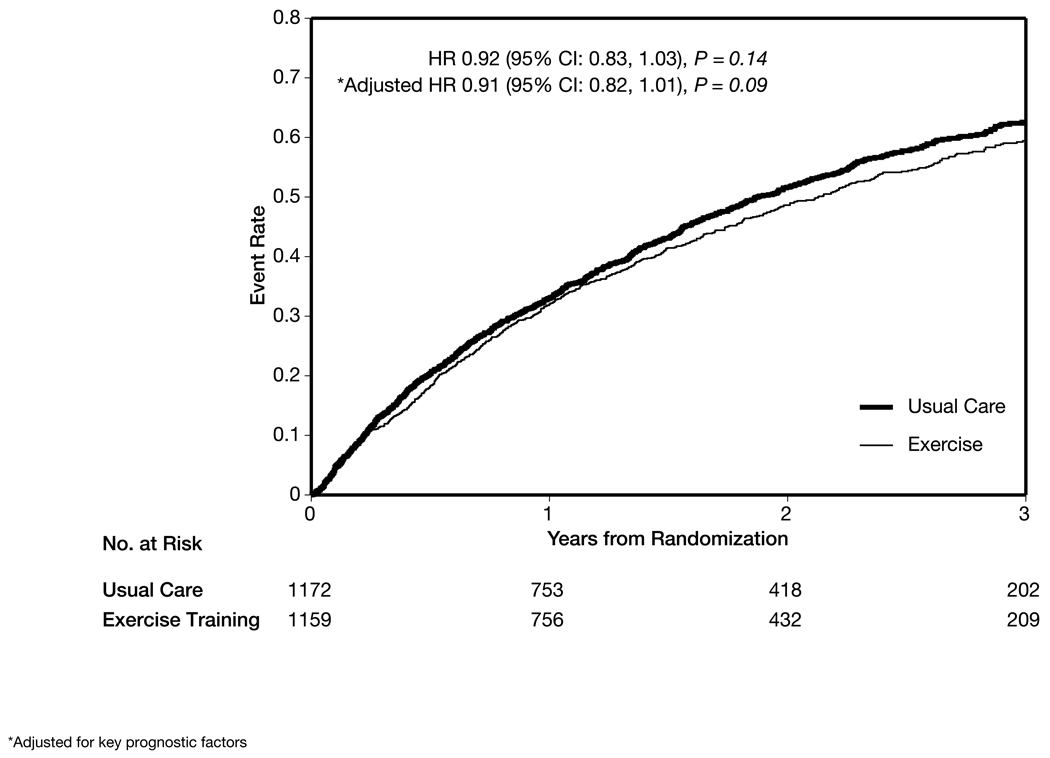
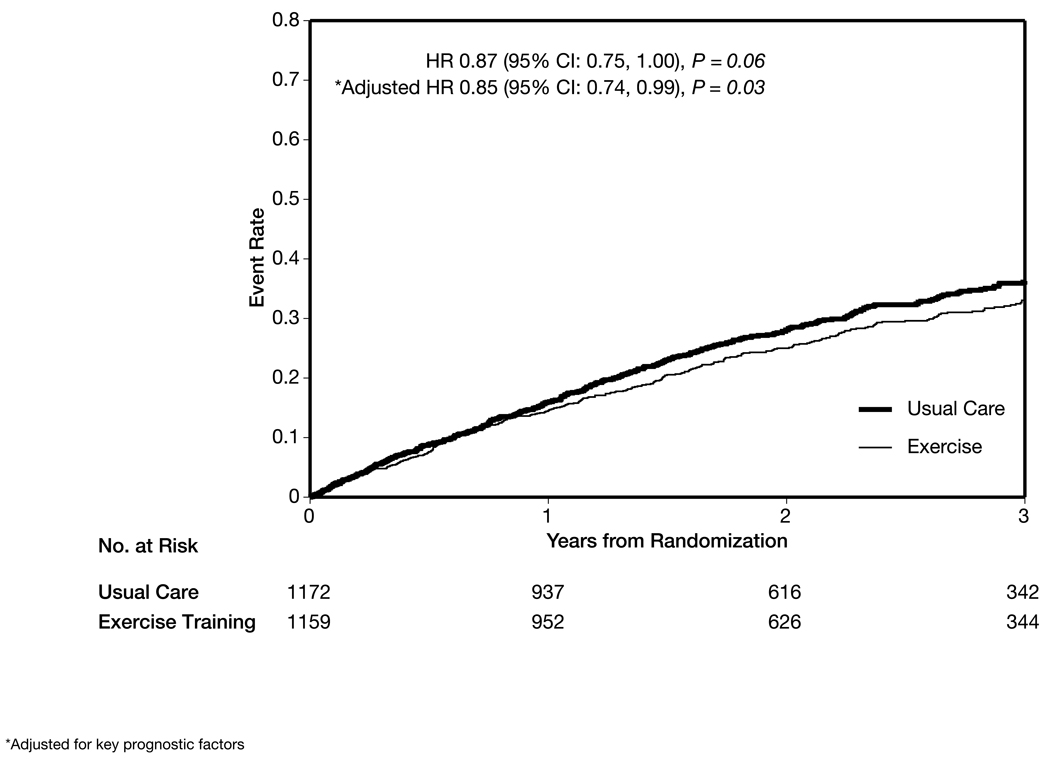
Exercise training had a nonsignificant modest reduction in the combined end point of cardiovascular mortality or cardiovascular hospitalization in the main analysis (632 [55%] in the exercise group vs 677 [58%] in the usual care group; HR, 0.92; 95% CI, 0.83–1.03; P = .14) and after adjustment for prognostic factors (HR, 0.91; 95% CI, 0.82–1.01; P = .09) (Figure 5). There was a nonsignificant reduction in cardiovascular mortality or heart failure hospitalization (344 [30%] in the exercise group vs 393 [34%] in the usual care group; HR, 0.87; 95% CI, 0.75–1.00; P = .06), which was statistically significant after adjustment for prognostic factors (HR, 0.85; 95% CI, 0.74–0.99; P = .03) (Figure 6).
Figure 5.
Time to Cardiovascular Mortality or Cardiovascular Hospitalization
Figure 6.
Time to Cardiovascular Mortality or Heart Failure Hospitalization
Exercise Training Effects
The changes from baseline in peak oxygen consumption and 6-minute walk distance at 3 months and 1 year are presented in Table 4). Compared to usual care patients at 3 months of follow-up, patients in the exercise training arm had a greater improvement in 6-minute walk distance (median, 20 vs 5 meters; P < .001), exercise duration on cardiopulmonary exercise test (1.5 vs 0.3 minutes; P < .001), and peak VO2 (0.6 vs 0.2 mL/min/kg; P < .001). The number of patients who had a 50-meter or greater improvement in 6-minute walk at 3 months was 166 (19%) in the usual care group and 273 (28%) in the exercise training arm. The number of patients with a 1 mL/min/kg or greater improvement in peak VO2 was 297 (33%) in the usual care group and 423 (44%) in the exercise training arm. The median percent improvement of 4% in peak VO2 in the exercise training group fell short of the 10% improvement targeted in the protocol that is customarily used as a clinically relevant improvement. At 12 months, the differences in cardiopulmonary exercise test results remained but there was no significant difference in 6-minute walk distance. The analyses presented are complete cases only and do not take into account missing data (33% at 12 months).
Table 4.
Change in 6-Minute Walk Test and Cardiopulmonary Exercise Test Resultsa
| Median (IQR) | |||
|---|---|---|---|
| Test | Usual Care | Exercise Training | P Value |
| Baseline to 3 monthsa | |||
| Six-minute walk distance, m (n = 1835) | 5 (−28, 37) | 20 (−15, 57) | < .001 |
| Cardiopulmonary exercise duration, min (n = 1914) | 0.3 (−0.6, 1.4) | 1.5 (0.3, 3.0) | < .001 |
| Peak VO2, mL/kg//min (n = 1870) | 0.2 (−1.2, 1.4) | 0.6 (−0.7, 2.3) | < .001 |
| Baseline to 12 monthsa | |||
| Six-minute walk distance, m (n = 1444) | 12 (−30, 55) | 13 (−28, 61) | .26 |
| Cardiopulmonary exercise duration, min (n = 1476) | 0.2 (−1.0, 1.7) | 1.5 (0.0, 3.2) | < .001 |
| Peak VO2, mL/kg//min (n = 1442) | 0.1 (−1.5, 1.8) | 0.7 (−1.0, 2.5) | < .001 |
Abbreviation: IQR, interquartile range.
Complete case analysis. Expected 2284 at 3 months.
Complete case analysis. Expected 2159 at 12 months.
Post Hoc Analyses
For the post hoc end point of cardiovascular mortality, heart failure hospitalization, heart transplantation, or left ventricular assist device implantation, the reduction in HR was 13% with exercise training (353 [30%] in the exercise group vs 403 [34%] in the usual care group; HR, 0.87; 95% CI, 0.75–1.00; P = .06). A post hoc analysis of NYHA class showed a difference between the groups, with 30% of the exercise cohort improving by 1 class or more vs 25% of the usual care cohort (ordinal regression P = .03).
Comment
HF-ACTION is the largest multicenter, randomized controlled trial of exercise training in heart failure to date. The size and duration of this trial are sufficient to examine for the first time the effect of exercise training on the combined primary end point of all-cause death or all-cause hospitalization in patients with left ventricular systolic dysfunction. In this cohort of patients with reduced left ventricular function, NYHA class II to IV symptoms, and treated with optimal, guideline-based background heart failure therapy, exercise training was safe, but provided a nonsignificant reduction in the risk for the primary end point of all-cause mortality or all-cause hospitalization and key secondary clinical end points. However, the reduction in risk for the primary end point and for the risk of cardiovascular mortality or heart failure hospitalization was significant after adjusting for highly prognostic predictors of the primary end point.
It is important to recognize that the main or primary analysis for the study that adjusted only for heart failure etiology did not result in a significant reduction in the primary end point or secondary end points. The change from a nonsignificant to a significant result after adjustment for strongly predictive factors is unusual in large clinical trials, but can occur when the treatment differences are close to significance.19 The overall interpretation of the results, then, is that this structured exercise training intervention had at best a modest effect on clinical end points in a large cohort of subjects. The changes in cardiopulmonary exercise testing parameters and 6-minute walk distance at 3 months were consistent with the finding of a modest benefit in reducing clinical events.
The ability to achieve a 13% risk reduction for the end point of cardiovascular mortality or heart failure hospitalization is important, given the exceptional use of evidence-based therapies among the patients in this study at baseline and throughout the trial. HF-ACTION arguably represents the largest trial to date with nearly uniform adherence to guideline-based therapy. In this study, 95% of patients without a contraindication or intolerance to β-blockers or ACE inhibitors received optimal heart failure therapy, defined as a β-blocker and either an ACE inhibitor or an ARB. In addition, 45% of the patients were treated with an ICD or biventricular pacemaker prior to randomization. The magnitude of effect of exercise training on the combined end point of cardiovascular mortality or heart failure hospitalization was similar to that observed with candesartan in the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM; HR, 0.84; 95% CI, 0.77–0.91) and valsartan in the Valsartan Heart Failure Trial (Val-HeFT; HR, 0.87; 95% CI, 0.77–0.97).22,23 In CHARM and Val-HeFT, only 55% and 35% of patients, respectively, received β-blocker therapy, and an ICD was reported in only 2.4% to 2.6% of patients enrolled in CHARM.22,23
A major challenge of HF-ACTION was to design and implement an exercise training protocol in patients with heart failure that could be translated into clinical practice. We based the study design on the traditional 36-session cardiac rehabilitation model, followed by regular home exercise. Unlike the study by Belardinelli et al,6 evaluating a strategy of only supervised training was not feasible, and it is unlikely that such a strategy would be adopted in practice. As expected in an unblinded study of a behavioral intervention, the HF-ACTION investigators had to deal with issues of crossover, adherence, and site variation. In fact, based on a survey of the patients following randomization, 55% of usual care patients were not satisfied with the arm to which they were randomly assigned, and many continued some level of physical activity.
It is not easy for participants in an exercise training program, particularly in patients such as in this study who have chronic symptomatic heart failure and multiple comorbid conditions, to continue training during long-term follow-up. Although the study invested substantial effort and resources into optimizing adherence, we understand that lack of compliance is likely due to many factors, including a limitation of the disease state and concomitant comorbid conditions, diminishing motivation, or other factors, some of which are not easily modifiable. The level of adherence achieved in HF-ACTION likely approaches the maximal amount one could achieve in a broad population of heart failure patients, given the extensive attention provided by study personnel to promoting compliance, the provision of exercise equipment and heart rate monitors and other adherence optimization efforts. By implementing these additional strategies, we were able to improve, in a significantly larger and broadly representative cohort of heart failure patients, to a median of 1.8 supervised exercise training sessions per week from the 1.7 sessions per week seen in the study by McKelvie et al,9 which used a similar design of initial supervised training followed by home-based training. At 3 months, patients in the training arm did have changes in exercise parameters (median 4% increase in peak VO2), which were less than those seen in both the Belardinelli et al6 (18% increase in peak VO2) and McKelvie et al9 (10% increase in peak VO2) studies. All 3 studies had patients engaged in supervised training during the early phases. The differences between studies may be due to adherence during the early stages or differences in baseline characteristics, including β-blocker use. The observed 1-minute difference between groups in exercise time is similar to the changes in exercise time observed in the early ACE inhibitor trials.24–26
The lack of change in exercise testing parameters at 12 months may have been due to an insufficient training stimulus in most patients. A potential cause for the blunted training effect was the very high utilization of β-blockers, which have been shown to limit peak VO2 changes in healthy subjects. The improvement at 12 months seen in the usual care group that reduced the differences between arms of the study was potentially due to crossover to exercise training in the usual care patients. Differences in health status between those who returned for the 12-month cardiopulmonary exercise test and those who did not may have also played a role. Finally, there is significant variability in peak VO2 measurements, particularly when obtained from multiple centers.27
The results of HF-ACTION should be interpreted in the context of the following potential limitations. The patients enrolled in this trial were relatively young compared to the general heart failure population and did not have heart failure with preserved left ventricular function (or diastolic heart failure). The only measure of exercise for patients in the usual care arm was the Physical Activity Questionnaire, which provided a snapshot of activity over the prior 7 days. Blinding of patients and research personnel was not possible. Over 50% of the patients randomized to the usual care arm were either somewhat or very dissatisfied with their treatment assignment; a number of these patients likely crossed over and initiated training. Despite the extensive efforts of the study, adherence to the exercise regimen and crossovers to exercise in the usual care group may have diminished the study’s ability to detect a significant effect of exercise training on the primary outcome. The lack of blinding of may have also caused differential attention to patients by study personnel. However, the investigators attempted to control for the inherent differences in the amount of contact with caregivers by regular telephone contact and follow-up of the patients in both arms of the trial. Due to the fact that not all patients underwent cardiopulmonary exercise testing at 3-month and 12-month follow-up visits, changes in peak VO2 should be interpreted with caution. The level of missing home exercise data makes exercise group adherence difficult to quantify. Some safety end points were measured only in the exercise arm and thus have no within-trial comparator group.
Conclusion
Regular exercise training in patients with systolic heart failure was safe. Based on the main analysis adjusted for heart failure etiology only, exercise training produced a nonsignificant reduction in the primary end point (all-cause mortality or all-cause hospitalization) and key secondary clinical end points. In protocol-specified supplementary analyses adjusted for prognostic factors, the treatment effect was statistically significant for the primary end point and for the secondary end point of cardiovascular mortality or heart failure hospitalization. These findings are consistent with the 33 previous trials and the meta-analyses showing improved outcomes. Based on the safety of exercise training and the modest reduction in clinical events in addition to the modest increases in health-related quality of life (reported in the accompanying manuscript by Flynn et al28), the HF-ACTION results support a prescribed exercise training program for patients with reduced left ventricular function and heart failure symptoms in addition to evidence-based therapy.
Acknowledgments
Funding/Support: HF-ACTION was funded by grants 5U01HL063747 (Duke University, C. O’Connor, coordinating center), 5U01HL066461 (Duke University, K. Schulman, economics and quality of life), 5U01HL068973 (Boston Medical Center, W. Colucci), 5U01HL066501 (Case Western Reserve University, I. Piña), 5U01HL066482 (Emory University, A. Smith), 5U01HL064250 (Henry Ford Hospital, S. Keteyian), 5U01HL066494 (Ohio State University, W. Abraham), 5U01HL064257 (Oregon Health & Science University, R. Hershberger), 5U01HL066497 (University of Alabama at Birmingham, V. Bittner), 5U01HL068980 (University of California, Los Angeles, G. Fonarow), 5U01HL064265 (University of Colorado Health Sciences Center, E. Wolfel), 5U01HL066491 (Wake Forest University, D. Kitzman), and 5U01HL064264 (Washington University in St. Louis, G. Ewald) from the National Heart, Lung, and Blood Institute.
Role of the Sponsor: The National Heart, Lung, and Blood Institute had a role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; and in the preparation, review, and approval of the manuscript.
HF-ACTION Investigators
Executive Committee: Christopher M. O’Connor, MD; Kerry L. Lee, PhD; Stephen J. Ellis, PhD; David S. Rendall, BA, PA-C; Duke University, Durham, North Carolina; David J. Whellan, MD, Thomas Jefferson University, Philadelphia, Pennsylvania; Ileana L. Piña, MD, Case Western Reserve University, Cleveland, Ohio; Steven J. Keteyian, PhD, Henry Ford Hospital, Detroit, Michigan; Lawton S. Cooper, MD, MPH; Robin Boineau, MD; Lawrence J. Fine, MD, DrPH; Jerome L. Fleg, MD; Eric S. Leifer, PhD; National Heart, Lung, and Blood Institute, Bethesda, Maryland; Virginia Erickson, RN, PhD, University of California, Los Angeles; Jonathan G. Howlett, MD, Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia; Nancy Houston Miller, RN, BSN, Stanford University, Stanford, California; Debra Isaac, MD, Foothills Hospital, Calgary, Alberta; Robert McKelvie, MD, Hamilton Health Sciences, Ontario; Faiez Zannad, MD, PhD, Université Henri Poincaré, Nancy, France.
Coordinating Center (Duke Clinical Research Institute): Sharon Boozer, BA; Patty Connolly; Anthony Doll, BA; Stephen J. Ellis, PhD; Camille Frazier, MD, MHS; Deb Mark; Michelle McClanahan-Crowder, AA; Marcia Meyer, RN, BSN; Brenda S. Mickley, BS; David S. Rendall, BA, PA-C; Molly Rich; Hoss Rostami, BSMSE; Sharon Settles, MS; Kathy Spinella, BBA; Jessica Staib, PhD; Omar Thompson, BA; Duke University, Durham, North Carolina.
Steering Committee (U01 Grant Sites): William Abraham, MD; Danuta Biniakiewicz, PhD; Joann Homan, RN; Ohio State University, Columbus; Vera Bittner, MD, MSPH; Meredith Fitz-Gerald, RN, BSN; University of Alabama at Birmingham; Gregory Ewald, MD; Heidi Craddock, RN; Jean Flanagan, RN, MSN; Washington University in St. Louis, Missouri; Gregg Fonarow, MD; Virginia Erickson, RN, PhD; University of California, Los Angeles; Wilson Colucci, MD; Nancy Z. Lim, RN; Elena Tokareva, PhD; Boston Medical Center, Massachusetts; Rami Alharethi, MD; Ray Hershberger, MD; Deirdre Nauman; Oregon Health & Science University, Portland; Steven J. Keteyian, PhD; Matthew Saval, MS; Henry Ford Hospital, Detroit, Michigan; Dalane W. Kitzman, MD; Brittney L. Fray, MS; Brian Moore, MS; Wake Forest University, Winston-Salem, North Carolina; Ileana L. Piña, MD; Marianne Vest, MA, BSN; Case Western Reserve University, Cleveland, Ohio; Andrew Smith, MD; Gail Snell, RN, BSN, CCRC; Emory University, Atlanta, Georgia; Eugene Wolfel, MD; Mona Cantu, RN, NP; University of Colorado Hospital, Aurora.
Steering Committee (Non–U01 Grant Sites): Kirkwood Adams, MD; Jana Glotzer, RN, MSN, ACNP; Valerie Johnson, RN; Kate Schumacher; UNC Hospitals, Chapel Hill, North Carolina; Gordon Blackburn, PhD; Carrie Geither, RN; Susan Moore, RN, BSN; Cleveland Clinic Foundation, Cleveland, Ohio; A. Bleakley Chandler, Jr, MD; Shanda Browning Vaughn, RN; Paula J. Easler, RN, CCRC; Debbie Williams; University Hospital, Augusta, Georgia; Julius M. Gardin, MD; Kelly Dimick, RN; Sharon K. Sklar, LPN; Sherri Teller, RN, CCRP; St. John Hospital and Medical Center, Detroit, Michigan; Jalal K. Ghali, MD; Karen Hale-Stenson, RN; LSU Health Sciences Center, Shreveport, Louisiana; Mihai Gheorghiade, MD; Theresa Strzelczyk, APN, CNS; Northwestern University Medical Center, Chicago, Illinois; Maryl R. Johnson, MD; Cassondra Vander Ark, RN, MS, CCRC; University of Wisconsin-Madison; Lee R. Goldberg, MD, MPH; Andrew Kao, MD; Jennifer Dekerlegand, MPT, PhD; Hospital of the University of Pennsylvania, Philadelphia; William E. Kraus, MD; Johanna Johnson, MS; Brian D. Duscha, MS; Duke University Medical Center, Durham, North Carolina; Mandeep R. Mehra, MD; Hector Ventura, MD; Bobbett Harris, RN; Ochsner Clinic Foundation, New Orleans, Louisiana; Monica Colvin-Adams, MD; Kathy Duderstadt, BSN; Karen Meyer, RN, BSN; Melissa Steger, RN, CCRC; University of Minnesota Medical Center, Fairview; Barry Cabuay, MD; Ron M. Oren, MD; Page Scovel, RN, BSN, CCRC; University of Iowa Hospitals and Clinic, Iowa City; Andrew Kao, MD; Tracey Stevens, MD; Karen Haffey, RN, BSN, CCRC; Christy Mandacina; Ann Stewart, RN, BSN; Mid America Heart Institute, Kansas City, Missouri; Ann M. Swank, PhD, CSCS; John Manire, MS; University of Louisville, Kentucky; Paul D. Thompson, MD; Ludmila Cosio-Lima, PhD; Marie Lagasse, MS; Hartford Hospital, Connecticut; Tehmina Naz, MD; Lynne Wagoner, MD; Susan K. Roll, RN, BSN; University of Cincinnati, Ohio; Frank G. Yanowitz, MD; Johnny Walker; Adam Mueller; LDS Hospital, Salt Lake City, Utah; Peter McCullough, MD; Cathy Coleman, RN, BSN, CCRC; Kimberly A. Dorrell; Tamika Washington; William Beaumont Hospital, Royal Oak, Michigan; Eileen Handberg, PhD; James A. Hill, MD; Jacqueline Bakos, RN; Alice Boyette; Pamela Smith; Cynthia Williams, RN, BSN, MS; University of Florida, Gainesville; Dalynn Badenhop, PhD; Susan Schroeder; Kelly Walter; Medical University of Ohio, Toledo; Peter Kokkinos, PhD; Elisse Collins; Lauren Korsak, MS; Washington DC VA Medical Center; Eric Eichhorn, MD; Allison Leonard, RN, BSN; Tina Worley, RN, BSN; Medical City Dallas Hospital, Texas; Gerald Fletcher, MD; Phil Peasley, RN; Pam Oldano, RN; Mayo Clinic Jacksonville, Florida; John Kostis, MD; Nora M. Cosgrove, RN, BS; University of Medicine and Dentistry of New Jersey, New Brunswick; Udho Thadani, MD; Lisa Rogan, RN, BSN; Michelle Thresher, RN, BSN; John Turner, RN; University of Oklahoma Health Sciences Center, Oklahoma City; Denise Barnard, MD; Denise Herman, MD; Annette Contasti, RN; Marcy Sagerian, RN; University of California, San Diego Medical Center; Elizabeth Ofili, MD; Anekwe Onwuanyi, MD; Sunday Nkemdiche, MD; Morehouse School of Medicine, Atlanta, Georgia; Howard Eisen, MD; James Fitzpatrick, MD; Joyce Wald, DO; Jennie Wong, RN, CCRP; Temple University Hospital, Philadelphia, Pennsylvania; Myrvin Ellestad, MD; Leslie Kern, RN, PhD; Long Beach Memorial Medical Center, California; Ezra A. Amsterdam, MD; Mary J. Burns, RN; UC Davis Medical Center, Sacramento, California; Savitri Fedson, MD; Ravi K. Garg, MD; Peggy Bennett, RN; Linda Bond, RN, MSN; University of Chicago Hospitals, Illinois; Leway Chen, MD, MPH; Janice Schrack, RN, BSN; Strong Memorial Hospital, Rochester, New York; Douglas Pearce, MD; Linda Bond, MSN, RN; Greg Palevo; Margarett Serfass, RN; Saint Thomas Hospital, Nashville, Tennessee; Daniel Forman, MD; Maria M. Lopez; Yemi Talabi-Oates; April Williams; Brigham and Women’s Hospital and Boston VA Medical Center, Boston, Massachusetts; David Truitte, MD; Cindy Baumann, RN, CCRN; Lynchburg General Hospital, Virginia; Jenny Adams, PhD; Anne Lawrence, RN; Baylor Hamilton Heart and Vascular Hospital, Dallas, Texas; Dennis McNamara, MD; Emily Gruendler, RN, BSN; Virginia Schneider; University of Pittsburgh Medical Center, Pennsylvania; Steven Hutchins, MD; Alyce Hartwick, RN; Heart Clinic Arkansas, Little Rock; Paul Campbell, MD; Michele Esposito; Northeast Medical Center, Concord, North Carolina; Carol Buchter, MD; Rebecca A. Letterer, RN, BSN; University of Washington Medical Center, Seattle; Robert Taylor, MD; Cheri Wells, RN, MSN; University of New Mexico Health Sciences Center, Albuquerque; Bruce Johnson, PhD; Beth Kaping, RN; Susan Leathes, RN; Mayo Clinic, Rochester, Minnesota; Joseph O’Bryan, MD; Lynn Langley, RN; Southwest Florida Heart Group, Fort Myers; Edward T. Hastings, MD; Cassandra Clancy, RN, CCRC; St. Luke’s Medical Center, Milwaukee, Wisconsin; Neil Agruss, MD; Christine Lawless, MD; Robin Fortman, MS, APN/CNP, CCRC; Central DuPage Hospital, Winfield, Illinois; Timothy R. McConnell, PhD; Deb Wantz, MSN, RN, CCNS, CCRC; Geisinger Medical Center, Danville, Pennsylvania; Mary N. Walsh, MD; Regina Margiotti, CMS, CCRC; The Care Group, Indianapolis, Indiana; Stuart Russell, MD; Elizabeth Heck, RN, BSN; Johns Hopkins Hospital, Baltimore, Maryland; Justine Lachmann, MD; Diane Lippman, RN; Jeannette McLaughlin, RN; St. Francis Hospital, Roslyn, New York; Joel Landzberg, MD; Susan Mathus, RN, BSN; Hackensack University Medical Center, New Jersey; David W. Cullinane, MD; Wyatt Voyles, MD; Dione Lenz, RN; Scott Kaczkowski. BS, CCRC; Medical Center of the Rockies Foundation, Loveland, Colorado; Jack L. David, MD; Eve Gillespie, MD, PhD; Pat Keane-Richmond, RN, CCRC; Glacier View Cardiology, Kalispell, Montana; Steven K. Krueger, MD; Lori Heiss, RN, BS; BryanLGH Heart Institute, Lincoln, Nebraska; Stephen Gottlieb, MD; Nancy Greenberg, RN, BSN, MS; University of Maryland School of Medicine, Baltimore; Neil Gordon, MD; Emily Parks, BS; Melanie Willoughby, RN, BSN, CCRN; St. Joseph’s/Candler Hospital, Savannah, Georgia; Marvin W. Kronenberg, MD; Jennie Glenn, RN; Carol Madison, RN; Vanderbilt University Medical Center, Nashville, Tennessee; Malcolm Arnold, MD; Julie K. Smith, RN; London Health Sciences Centre, Ontario; Eduardo Azevedo, MD; Glen Drobot, MD; Estrellita Estrella-Holder, RN, BN, MSA, CCN(C); St. Boniface General Hospital, Winnipeg, Manitoba; Jonathan Howlett, MD; Darlene Cooley-Warnell; Sheila Yarn, RN; Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia; Debra Isaac, MD; Jane Grant, RN; Kim Lyzun; Foothills Hospital, Calgary, Alberta; Marie-Helene LeBlanc, MD; Rachel Vienneau, RN, BSc; Hôpital Laval, Sainte-Foy, Quebec; Robert S. McKelvie, MD; Linda Beare; Jill Hancock, LRN; Hamilton Health Sciences, Ontario; Gordon Moe, MD; Delores Golob, RN, BA; St. Michael’s Hospital, Toronto, Ontario; Kenneth Melvin, MD; Anne Cymet, RN; Judith Renton, RN; Toronto General Hospital, Ontario; Anil Nigam, MD; Julie LaLonge; Montreal Heart Institute, Quebec; Karim Djaballah, MD; Hôpital Brabois, Vandoeuvre-lès-Nancy, France; Patrick Aebehard, MD; Centre Cardiologique du Nord, Saint-Denis, France; Marie Christine Iliou, MD; Hôpital Broussais, Paris, France; Remi Sabatier, MD; Annette Belin, MD; Centre Hospitalier Universitaire de Caen, France; Alain Cohen-Solal, MD; Hôpital Beaujon, Clichy, France; Luc Hittinger, MD, Hôpital Henri Mondor, Créteil, France.
Data and Safety Monitoring Board: Bertram Pitt, MD (chair), University of Michigan, Ann Arbor; Philip A. Ades, MD, University of Vermont, South Burlington; Lotfy L. Basta, MD, San Francisco, California; Victor Froelicher MD, Stanford University and Palo Alto VA Medical Center, California; Mary Elizabeth Hamel, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts; Barry M. Massie, MD, San Francisco VA Hospital, California; Lemuel Moyé, MD, PhD, University of Texas Health Science Center at Houston; Lynda H. Powell, PhD, Rush University, Chicago, Illinois.
Cardiopulmonary Exercise Core Lab: William E. Kraus, MD (director); Johanna Johnson, MS; Lucy Piner, MS; Duke University, Durham, North Carolina; Daniel Bensimhon, MD (codirector), LeBauer Cardiovascular Research Foundation, Greensboro, North Carolina; Stuart Russell, MD, Johns Hopkins University, Baltimore, Maryland.
Echo Core Lab: Julius M. Gardin, MD (director); Renee L. Bess, BS, RDCS, RVT; Gerald I. Cohen, MD; St. John Hospital and Medical Center, Detroit, Michigan.
Heart Rate Training Core Lab: Steven J. Keteyian, PhD (director); Jonathan K. Ehrman, PhD (associate director); Clinton A. Brawner, MS (coordinator); Henry Ford Hospital, Detroit, Michigan.
Adherence Core Lab: Nancy Houston Miller, RN, BSN (codirector), Stanford University, Stanford, California; James A. Blumenthal, PhD (codirector); Krista Barbour, PhD; Duke University, Durham, North Carolina; Tanya M. Spruill, PhD, Columbia University, New York City, New York; Bess Marcus, PhD, Brown Medical School and Miriam Hospital, Providence, Rhode Island; Jim Raczynski, MD, University of Arkansas for Medical Sciences, Little Rock.
Biomarker and DNA Core Lab: Kirkwood Adams, MD, University of North Carolina at Chapel Hill; Mark Donahue, MD; Mike Felker, MD; Duke University, Durham, North Carolina.
Economics and Quality of Life Group: Kevin A. Schulman, MD; Kevin P. Weinfurt, PhD; Kathryn E. Flynn, PhD; Shelby Reed, PhD; Ann Burnette, BS; Linda Davidson-Ray, MA; Joëlle Y. Friedman, MPA; Yanhong Li, MS; Li Lin, MS; Betsy O’Neal, BA;, Duke University, Durham, North Carolina.
Clinical End Point Committee: Michael Zile, MD (chair), Ralph H. Johnson; VA Medical Center and Medical University of South Carolina, Charleston; Daniel R. Bensimhon, MD, LeBauer Cardiovascular Research Foundation, Greensboro, North Carolina; Vera Bittner, MD, MSPH, University of Alabama at Birmingham; Robin Boineau, MD, National Heart, Lung, and Blood Institute, Bethesda, Maryland; Mark E. Dunlap, MD, Case Western Reserve University, Cleveland, Ohio; William E. Kraus, MD; Christopher M. O’Connor; Duke University, Durham, North Carolina; Gordon Moe, MSC, MD, FRCP(C), St. Michael’s Hospital, Toronto, Ontario; John Wertheimer, MD, Thomas Jefferson University and Drexel University Medical Residents at Abington Memorial Hospital, Philadelphia, Pennsylvania; David J. Whellan, MD, Jefferson Medical College, Philadelphia, Pennsylvania.
Footnotes
Author Contributions: Dr O’Connor had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: O’Connor, Whellan, Lee, Keteyian, Cooper, Kraus, Kitzman, Blumenthal, Zannad, Piña. Acquisition of data: O’Connor, Whellan, Lee, Keteyian, Kraus, Kitzman, Rendall, McKelvie, Zannad, Piña. Analysis and interpretation of data: O’Connor, Whellan, Lee, Keteyian, Cooper, Ellis, Leifer, Kraus, Kitzman, Blumenthal, Houston Miller, Fleg, Schulman, Zannad. Drafting of the manuscript: O’Connor, Whellan, Lee, Keteyian, Kraus, Kitzman, Piña. Critical revision of the manuscript for important intellectual content: O’Connor, Whellan, Lee, Keteyian, Cooper, Ellis, Leifer, Kraus, Kitzman, Blumenthal, Rendall, Houston Miller, Fleg, Schulman, McKelvie, Zannad, Piña. Statistical analysis: Lee, Ellis, Leifer. Obtained funding: O’Connor, Whellan, Lee, Keteyian, Kitzman. Administrative, technical, or material support: O’Connor, Whellan, Lee, Keteyian, Cooper, Kitzman, Blumenthal, Rendall, Fleg, Schulman, Zannad, Piña. Study supervision: O’Connor, Whellan, Keteyian, Cooper, Houston Miller, Schulman, McKelvie, Zannad.
Financial Disclosures: Dr O’Connor reports receiving grants or funding, personal income for consulting, and honoraria from GE Medical, Roche, and the National Institutes of Health. Dr Whellan reports receiving grants or funding from GE Medical and the National Institutes of Health. Dr Keteyian reports receiving honoraria for lectures to scientific, educational, and community groups; and receiving royalties from books published by Human Kinetics and McGraw-Hill. Dr Ellis reports receiving grants from GE Medical. Dr Kraus reports receiving grants or funding from the National Institutes of Health. Dr Blumenthal reports receiving grants or funding from the National Institutes of Health. Mr Rendall reports receiving grants from GE Medical. Ms Houston Miller reports receiving personal income for consulting from Triage Wireless; and receiving honoraria from Pfizer, CV Therapeutics, and AstraZeneca. Dr Schulman reports receiving research support from Actelion Pharmaceuticals, Allergan, Amgen, Astellas Pharma, Bristol-Myers Squibb, The Duke Endowment, Genentech, Inspire Pharmaceuticals, Johnson & Johnson, Kureha Corporation, LifeMasters Supported SelfCare, Medtronic, Merck & Co, Nabi Biopharmaceuticals, National Patient Advocate Foundation, North Carolina Biotechnology Center, NovaCardia, Novartis, OSI Eyetech, Pfizer, Sanofi-Aventis, Scios, Tengion, Theravance, Thomson Healthcare, and Vertex Pharmaceuticals; receiving personal income for consulting from McKinsey & Company and the National Pharmaceutical Council; having equity in Alnylam Pharmaceuticals; having equity in and serving on the board of directors of Cancer Consultants; and having equity in and serving on the executive board of Faculty Connection LLC. Dr Schulman has made available online a detailed listing of financial disclosures (http://www.dcri.duke.edu/research/coi.jsp). Dr Piña reports receiving grants or funding from the National Institutes of Health; receiving personal income for consulting from the Food and Drug Administration; and receiving honoraria from AstraZeneca, Innovia, Merck, Novartis, Sanofi-Aventis, and Solvay.
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Previous Presentation: Presented as a late-breaking clinical trial at the American Heart Association Scientific Sessions; November 11, 2008; New Orleans, La.
Additional Contributions: We thank Wendy Gattis Stough, PharmD, Duke University, for editorial contributions; Damon M. Seils, MA, Duke University, for assistance with manuscript preparation; and Karen E. Joynt, MD, Brigham and Women’s Hospital, for technical assistance. Dr Gattis Stough received non-trial funding from the study coordinating center. Mr Seils did not receive compensation for his assistance apart from his employment at the study coordinating center. Dr Joynt did not receive compensation for her assistance.
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med. 2008;168(4):418–424. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 3.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 4.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 5.Konstam MA, Gheorghiade M, Burnett JC, Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 6.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99(9):1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 7.Coats AJ, Adamopoulos S, Radaelli A, et al. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992;85(6):2119–2131. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- 8.Hambrecht R, Niebauer J, Fiehn E, et al. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol. 1995;25(6):1239–1249. doi: 10.1016/0735-1097(94)00568-B. [DOI] [PubMed] [Google Scholar]
- 9.McKelvie RS. Exercise training in patients with heart failure: clinical outcomes, safety, and indications. Heart Fail Rev. 2008;13(1):3–11. doi: 10.1007/s10741-007-9052-z. [DOI] [PubMed] [Google Scholar]
- 10.Piepoli MF, Davos C, Francis DP, Coats AJ. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328(7433):189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116(10):693–706. doi: 10.1016/j.amjmed.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Siscovick DS, Weiss NS, Fletcher RH, Lasky T. The incidence of primary cardiac arrest during vigorous exercise. N Engl J Med. 1984;311(14):874–877. doi: 10.1056/NEJM198410043111402. [DOI] [PubMed] [Google Scholar]
- 13.Arnold JM, Liu P, Demers C, et al. Canadian Cardiovascular Society consensus conference recommendations on heart failure 2006: diagnosis and management. Can J Cardiol. 2006;22(1):23–45. doi: 10.1016/s0828-282x(06)70237-9. Erratum in: Can J Cardiol. 2006;22(3):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29(19):2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 15.Whellan DJ, O’Connor CM, Lee KL, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153(2):201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Kalbfleisch J, Prentice R. the statistical analysis of failure time data. 2nd ed. New York, NY: John Wiley & Sons, Inc; 2002. [Google Scholar]
- 18.Cox D. Regression models and life-tables (with discussion) J Royal Statist Soc B. 1972;34:187–220. [Google Scholar]
- 19.Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21(19):2917–2930. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
- 20.Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70(3):659–663. [Google Scholar]
- 21.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–556. [PubMed] [Google Scholar]
- 22.Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362(9386):759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 23.Cohn JN, Tognoni G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 24.A placebo-controlled trial of captopril in refractory chronic congestive heart failure. J Am Coll Cardiol. 1983;2(4):755–763. doi: 10.1016/s0735-1097(83)80316-7. [DOI] [PubMed] [Google Scholar]
- 25.Comparative effects of therapy with captopril and digoxin in patients with mild to moderate heart failure. The Captopril-Digoxin Multicenter Research Group. JAMA. 1988;259(4):539–544. [PubMed] [Google Scholar]
- 26.Narang R, Swedberg K, Cleland JG. What is the ideal study design for evaluation of treatment for heart failure? Insights from trials assessing the effect of ACE inhibitors on exercise capacity. Eur Heart J. 1996;17(1):120–134. doi: 10.1093/oxfordjournals.eurheartj.a014670. [DOI] [PubMed] [Google Scholar]
- 27.Bensimhon DR, Leifer ES, Ellis SJ, et al. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of exercise traiNing) Am J Cardiol. 2008;102(6):712–717. doi: 10.1016/j.amjcard.2008.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]