Summary
The global obesity epidemic has heightened the need for an improved understanding of how body weight is controlled, and research using mouse models is critical to this effort. In this perspective, we provide a conceptual framework for investigation of feeding behavior in this species, with an emphasis on factors that influence study design, data interpretation, and relevance to feeding behavior in humans. Although we focus on the mouse, the principles presented can be applied to most other animal models. This document represents the current consensus view of investigators from the National Institutes of Health (NIH)-funded Mouse Metabolic Phenotyping Centers (MMPCs).
Introduction
Mouse models have emerged as the tool of choice for basic research into obesity pathogenesis for a variety of reasons. Many key humoral signals (e.g., leptin, ghrelin) and neuronal circuits (e.g., the melanocortin system) involved in energy homeostasis were originally discovered and characterized in mice and subsequently were proven to be critical in humans as well (Farooqi and O’Rahilly, 2008). As in humans, obesity in mice can arise from either monogenic disorders or from complex interactions between genetic background, maternal-fetal environment, learned behaviors and external environmental variables such as diet composition, ambient temperature, threats from predators, and so on. This sensitivity to external variables is a feature shared by many other types of behavior -- what sets food ingestion apart is both the extent of its biological regulation and, since excessive food intake is essential to the pathogenesis of common forms of obesity, the enormous price tag linked to defects in this regulatory process.
These considerations highlight both the importance of food intake studies in mouse models and the potential for such studies to be confounded by variables not anticipated when the study was conceived. The goal of this Perspectives article is to offer guidelines to aid in the design, analysis and interpretation of studies of feeding behavior in mice.
Separating Primary from Secondary Feeding Responses
An important overarching consideration that can usefully inform study design is the context within which a change of food intake is observed. In mice as in humans, body adiposity is determined not by passive accumulation of excess calories as fat, but by active and upward re-regulation of the defended level of body fat stores, a feature that makes obesity an especially challenging biological and therapeutic problem. When investigating a mouse model characterized by increased food consumption, therefore, a key initial question is whether the feeding effect is a direct consequence of the experimental intervention or rather is a secondary, compensatory response. Besides guiding study design, the answer to this question will place limits on the information likely to be obtained from studies aimed at identifying underlying mechanisms.
When hyperphagia occurs in the context of weight maintenance or weight loss, it is likely secondary to either increased energy expenditure or to the loss of energy from the body through other mechanisms (e.g., though glycosuria in uncontrolled diabetes mellitus). In such settings, increased food intake reflects a properly functioning regulatory system, and while mechanistic feeding studies can shed light on how the normal system responds to a defined challenge, the underlying mechanisms are likely to differ from those driving hyperphagia in conditions associated with obesity.
Hyperphagia associated with uncontrolled diabetes is a secondary manifestation of a metabolic disease and, as such, provides a useful paradigm for how to identify and distinguish primary from secondary feeding responses. Evidence supporting the interpretation of “diabetic hyperphagia” as a secondary response includes that it arises in conjunction with progressive loss of adipose mass, is detectable only after body weight and fat content have begun to decrease, and is ameliorated by restoring plasma concentrations of key regulatory hormones (insulin and leptin) from dramatically reduced back to normal values (Havel et al., 1998; Sindelar et al., 1999). Although study of this phenomenon has helped to clarify mechanisms linking a change in hormonal and metabolic milieu to the control of feeding behavior, such insights cannot be relied upon to pertain to other conditions in which food intake is increased (e.g., in response to a genetic perturbation or exposure to a highly palatable diet), and this consideration can inform study design.
Since increased food intake is appropriate when body fat mass is depleted and leptin levels consequently reduced, it follows that apparently “normal” intake (e.g., the absence of hyperphagia) can sometimes be indicative of an altered regulatory system. In mice bearing a targeted mutation that causes a “lean, hypermetabolic” phenotype, for example, comparisons of food intake to genetically normal controls must take into consideration the normal feeding response to reduced body energy stores (Gelling et al., 2008). If mutant mice do not increase their intake relative to that expected in wild-type (WT) controls that have experienced a comparable decrease of body adiposity, this may be indicative of changes in the capacity of the animal to mount an appropriate hyperphagic response. Alternatively, it may be that regulatory responses are intact, but that the threshold for eliciting them has been altered. These possibilities can be distinguished from one another by determining whether the mutant mice are able to mount an appropriate hyperphagic response to a further lowering of body weight (e.g., induced by caloric restriction). If so, the mutation may have “re-set” the defended level of body adiposity at a reduced value. One potential mechanism to explain this outcome would be an increase of leptin sensitivity, such that food is consumed in “normal” amounts (e.g., comparable to WT values) in a setting of low circulating leptin levels. Studies could then be undertaken to test this hypothesis and investigate underlying mechanisms, rather than simply concluding that the mutation did not affect food intake (as tends to occur when food intake is not different between experimental and control groups).
Analysis of Food Intake in the Setting of Excess Weight Gain
When hyperphagia is observed in the context of obesity, a logical inference is that the latter is due, at least in part, to the former, and study design is again informed by this interpretation. For example, the extent to which excessive body weight gain is due to increased food intake can be investigated using a pair-feeding paradigm (see Supplemental Assay Protocols), in which the ability of affected mice to consume food in excess of that eaten by non-obese controls is prevented. Feed efficiency, defined as the ratio of calories consumed divided by body weight gain over a specific time interval, can offer additional insight into how ingested fuel is utilized and as such can aid in the assessment of whether an alteration in energy expenditure or nutrient absorption contributes to a body weight phenotype.
One may also wish to investigate whether the mechanism(s) underlying hyperphagia in obese mice involves 1) a defect in homeostatic control mechanisms that match energy intake to expenditure over long time intervals, 2) an exaggerated response to the rewarding properties of food, 3) an impairment in the ability of meal-related satiation signals to effectively terminate meals once they have begun, or some combination thereof. Distinguishing among these various possibilities requires decisions about which feeding assays will be most informative with respect to underlying mechanism(s) and can thereby guide subsequent studies seeking to identify the brain regions, neurocircuits, and signaling molecules involved.
When using increased body weight as a framework within which to investigate mechanisms of hyperphagia, it is important to appreciate that “not all instances of weight gain are equal.” Elevated food intake and body weight occur not only in obese animals, but in the context of accelerated linear growth as well. To distinguish between these two, body composition and body length should be assessed before studies to investigate underlying mechanisms are undertaken. If accelerated linear growth, rather than obesity, is the cause of increased body weight gain, hyperphagia in this setting is more likely to be an appropriate, adaptive response than the primary mechanism driving the phenotype (in contrast to what is observed in many forms of obesity).
A related question that often arises is whether measurements of food intake should be normalized to body weight, as is often done with measures of energy expenditure. We offer two arguments in opposition to this practice for the analysis of food intake data. The first is that differences in body weight can reflect differences in lean body mass, fat mass, or both; consequently, normalized intake values must be interpreted differently when comparing obese and lean animals. Second, normalizing by simple division makes assumptions about the nature of the relationship between intake and body weight that have yet to be validated. As one example, normalizing food intake in this way presumes that intake is regulated as a function of changes in body weight, whereas the reverse is commonly the case. As one extreme example, intake normalized to body weight can lead to the conclusion that leptin-deficient lepob/lepob mice are either hypophagic or hyperphagic relative to WT controls, depending on the age at which measurements are made, even if absolute intake is unchanging. This is because when the mice are young, the relative increase of intake exceeds the weight increase; as weight continues to increase with age, however, it eventually exceeds the increase of intake, relative to controls. Yet comparisons of absolute (i.e., non-normalized) intake reveal lepob/lepob mice to be hyperphagic relative to controls at any age. Including body weight and/or composition data in the figure legend when food intake is reported may help to minimize misinterpretation of non-normalized food intake.
When group differences in body weight reflect differences of age, gender or linear growth, meaningful comparisons of intake tend to be confounded regardless of whether or how intake data are normalized. As was recently reported for the analysis of energy expenditure data in mice (Kaiyala et al., 2010), multiple regression may permit insight into whether an experimental intervention affects intake after adjusting for differences of other variables, but such an approach awaits validation in a large cohort of mice. Until such an analysis has been undertaken, we suggest that quantitative comparisons of food intake can be inherently misleading and should therefore be avoided under certain circumstances (e.g., when an experimental intervention is undertaken in groups of old vs. young or male vs. female mice).
Limitations
A key issue that should be considered when planning experiments is that feeding assays can lack the sensitivity needed to identify differences in energy intake that although subtle may nonetheless affect body fat mass over time. Even the most sensitive assay tools cannot be relied upon to detect group mean differences of intake below 10% on a day-to-day basis in mouse models, yet such differences, if they persist, can substantially influence body weight and fat content. As reviewed in Supplementary Assay Protocols, the ability to detect small differences of daily intake can be increased by comparing cumulative values measured over long time intervals, rather than relying solely on daily measures. Of course, many other limitations to the use of mouse models to study feeding behavior exist and are highlighted throughout the discussion below.
Basic Considerations when Designing a Study
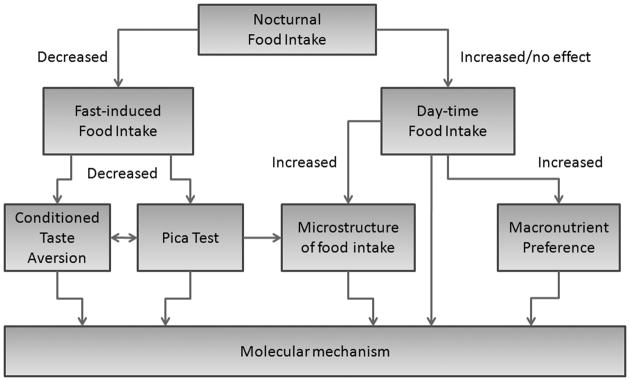
Because behavioral and metabolic responses in mice are highly sensitive to genetic and environmental factors, even subtle aspects of an experimental paradigm can influence feeding and other pertinent measures (Bailey et al., 2006; Champy et al., 2004; Champy et al., 2008; Crabbe et al., 1999; Mandillo et al., 2008) (Figure S1). Some of these factors are discussed below.
Choice of Strain
The phenotype resulting from even quite subtle genetic, dietary or pharmacological manipulations can differ widely depending on the background strain. Currently, the C57BL/6J background strain is by far the most commonly used for energy homeostasis studies in mice, as this was the first strain to have its genome completely sequenced (Gregory et al., 2002) and, in contrast to other commonly used strains, is relatively susceptible to diet-induced obesity (DIO; for review see (Champy et al., 2008; Collins et al., 2004). Even variation within sub-strains such as C57BL/6J vs. C57BL/6N (Bryant et al., 2008; Roth et al., 2002; Yang et al., 2003) can affect experimental outcomes. This phenomenon is well described in the Jackson Laboratory publication “Genetic background: understanding its importance in mouse-based biomedical research” (http://jaxmice.jax.org/manual/index.html). As one example, the severity of many aspects of the phenotype of lepob/lepob mice, including diabetes (Coleman, 1978; Coleman and Hummel, 1973; Haluzik et al., 2004; Qiu et al., 2001), fertility (Ewart-Toland et al., 1999; Qiu et al., 2001) and adiposity (Qiu et al., 2001), varies substantially across different genetic backgrounds. Similarly, predisposition to DIO and associated metabolic impairments among genetically normal mice also varies greatly with background strain. To summarize, variability in key endpoints (e.g., food intake, body composition, weight gain, etc.) is increased by inclusion of more than one background strain in a given experiment and this, in turn, can confound the ability to attribute a change in experimental endpoints to a specific intervention (e.g., administration of a drug or targeted gene knockout). For this reason, studies involving measures of food intake are usually performed in mice bred onto a pure background strain, most often C57BL/6J.
One drawback of using C57BL/6J mice is that they produce relatively small litters and thus are not optimal for the production of transgenic or knock-out animals. The Animal Models of Diabetic Complications Consortium (AMDCC) has detailed phenotypic information for numerous mouse strains that manifest different diabetic complications, including cardiovascular dysfunction, nephropathy, neuropathy, retinopathy and uropathy, in addition to hyperglycemia and metabolic impairments, thereby enabling researchers to select the animal model that best mimics the human condition they are investigating. Numerous online resources (Table 1) are available to aid in the selection of an appropriate mouse strain for a specific study, including phenotypic and genetic databases.
Table 1.
Resources to help select a mouse strain
| Title | Link |
|---|---|
| Phenotype Resources | |
| Mouse Phenome Database | www.jax.org/phenome |
| Europhenome Mouse Phenotyping Resource | www.europhenome.org |
| The Animal Models of Diabetic Complications Consortium (AMDCC) | www.amdcc.org |
| Eumorphia | www.eumorphia.org |
| Centre for Modeling Human Disease | www.cmhd.ca |
| Genetics/Genomics Resources | |
| Mouse Genome Informatics Database | www.informatics.jax.org |
| Mouse SNP Database | Mousesnp.roche.com |
| Priorities for Mouse Functional Genomic Research Across Europe (PRIME) | www.prime-eu.org |
| SOP/Protocol Resources | |
| European Mouse Phenotyping Resource of Standardised Screens (EMPRESS) | www.empress.har.mrc.ac.uk |
| Centre for Modeling Human Disease | www.cmhd.ca |
| Mouse/Embryonic Stem Cell Repositories | |
| Riken Bioresource Center | www.brc.riken.jp/lab/animal/en |
| Mutant Mouse Regional Resource Center | www.mmrrc.org |
| The European Mouse Mutant Archive (EMMA) | www.emmanet.org |
| Mousebook (Medical Research Council) | www.mousebook.org |
| North American Conditional Mouse Mutagenesis Project (NorCOMM) | www.norcomm.org |
| European Conditional Mouse Mutagenesis program (EuCOMM) | www.eucomm.org |
| Knock Out Mouse Project (KOMP) | www.komp.org |
Beyond these considerations, overreliance on the C57BL/6J strain may yield a skewed understanding of systems governing feeding behavior and energy homeostasis, considering that humans are not an inbred population. By focusing on one or another inbred mouse strain to interpret an anomaly of feeding behavior, we may therefore limit our understanding of human physiology, especially for studies seeking to clarify gene-by-environment interactions that predispose to obesity. Rather than systematically excluding the study of mice with mixed genetic backgrounds, the argument can be made that such mice offer an important opportunity to identify gene variants that affect energy balance (see Table 1) (Su et al., 2008).
A number of ongoing gene-targeting projects (Table 1) such as the European Conditional Mouse Mutagenesis (EUCOMM) program and the KnockOut Mouse Project (KOMP) use the C57BL/6N line as a background strain, a movement that may lead to a switch in the predominant mouse strain used for studies on energy homeostasis. Despite being closely related, there are numerous genetic polymorphisms that distinguish C57BL/6N (National Institutes of Health; NIH) and C57BL6/J (Jackson Laboratory) substrains (Bryant et al., 2008) and yield phenotypic differences. Of particular relevance here is that in contrast to the C57BL6/J line, the C57BL6/N strain does not carry the nicotinamide nucleotide transhydrogenase (Nnt) deletion that contributes to impaired glucose homeostasis in C57BL6/J mice (Freeman et al., 2006; Huang et al., 2006; Toye et al., 2005).
Genetic Background in Transgenic and Knockout Mice
When creating a novel transgenic or knockout mouse strain, initial characterization should be performed on animals bred from heterozygous × heterozygous pairings. In addition to ensuring that the WT controls have exactly the same genetic background, this approach also controls for differences in in utero environment and early-life experience. Many investigators choose to backcross strains of mixed genetic background to ensure that their mice remain on a pure congenic background; alternatively they may opt to change the genetic background of their mice to a strain that is more commonly used for their disease model. The important point is that having genetically-modified mice on a pure genetic background simplifies the interpretation of results and comparison with published data, despite inherent limitations noted above.
Although ≥10 generations of successive backcrossing to an inbred strain is generally accepted as being needed to produce a congenic line, this number is somewhat arbitrary and the relative contribution of the original strain depends upon the breeders selected. Some companies offer “speed congenic” services, which can accelerate and improve the accuracy of the backcrossing process. This service is based on selecting those breeders that display the highest percentage of genetic similarity to the target strain, thus reducing the number of generations required before the mice become statistically identical at all genetic loci to the targeted congenic strain, except for the modified locus and genes linked to it (for review see (Wakeland et al., 1997; Wong, 2002)).
Once a genetically modified strain is bred onto a congenic background, mice should be out-crossed to a congenic mouse from the parent strain every 8–10 generations to reduce genetic drift and the consequent generation of a new sub-strain within a colony. Although not immune to genetic drift, most commercial vendors employ rigorous quality control for each strain, and it is recommended that commercially sourced mice, as opposed to “WT congenics” from your own colony, be used for out-crossing. Yet despite the best efforts of commercial vendors to maintain consistency, available evidence suggests that phenotypic and behavioral differences exist among mice of the same strain that are purchased from different vendors (Bryant et al., 2008) or even from different facilities operated by the same vendor. Thus, when mice of the same strain are purchased from two different vendors, one cannot assume that they are genetically or phenotypically identical. We recommend maintaining consistency in both vendor and vendor location when purchasing animals for a series of related studies.
Genetic vs. Diet-Induced Obese (DIO) Mice
The most commonly used mouse models of obesity are either genetic (e.g., induced by a natural or experimentally-initiated mutation) or diet-induced, and the model selected depends on the questions being asked. For example, generating DIO mice, as described in detail in Supplemental Assay Protocols, is time-consuming and can be costly (due to extended periods of housing), but has the advantage of having greater relevance to common forms of human obesity than most monogenic obesity syndromes. This assertion is based on the fact that as in most obese humans, the DIO phenotype arises from combined effects of a polygenic susceptibility and exposure to palatable, calorically dense diets, especially when the diet is high in fat and/or refined carbohydrates. Further, the DIO model allows comparisons between obese and lean mice that are genetically identical, thus eliminating a major source of experimental variability. DIO C57BL/6 mice are available in limited numbers from commercial vendors including The Jackson Laboratory and Taconic, but vendors typically cannot provide information about the rate of weight gain in individual mice.
The most widely used mouse models of monogenic obesity are lepob/lepob, lepdb/lepdb and AY. Because each has been studied for more than 50 years, considerable phenotypic data are available in the literature for each, and they along with many other mouse models of genetic obesity are commercially available. In addition, several non-profit organizations have repositories of mouse stocks as well as embryonic stem (ES) cell lines of previously generated strains that have interesting phenotypes but are not in sufficient demand to be stocked by commercial vendors. Some of these are listed in Table 1.
Age and sex
Male mice are often preferred over females because many key determinants of energy balance are affected by hormonal variation associated with the estrous cycle (for review see (Asarian, 2006; Fernandez-Fernandez et al., 2006; Hill et al., 2008)). Additionally, like humans, body composition and body fat distribution differ between male and female mice, with mature females having a higher percentage of body fat, but relatively less fat deposited within the abdomen, than males. Consequently, experimental groups of mixed sexes are not generally recommended for studies of energy balance and male and female animals should be studied separately, as discussed earlier. Similarly, age matching is critical, especially when investigating mice that are not fully mature.
Stress and habituation
Mice are easily stressed, and stress per se influences all aspects of energy homeostasis including food intake, energy expenditure, locomotor activity and body composition. Further, whereas some stressors decrease food intake, others have the opposite effect (Adam and Epel, 2007; Tamashiro et al., 2007a; Tamashiro et al., 2007b; Ulrich-Lai and Herman, 2009). In addition to designed experimental stressors such as restraint, forced-swim or foot shock, stress in mice can also be induced inadvertently by routine handling, noise or social isolation, or by placement in a clean home cage, a metabolic cage or other experimental apparatus.
The degree of stress experienced by animals can vary with both the number of investigators involved in an experiment and their skill and experience working with mice (Mandillo et al., 2008). Stress can therefore be minimized by assigning responsibility for animal handling to a single, experienced individual throughout a study, a key issue when designing study protocols. Even subtle factors such as a change in the perfume of an investigator can be a source of stress. Many relevant study endpoints in addition to food intake are also sensitive to stress, including circulating insulin, glucose and corticosterone levels, and it is important to consider whether reported outcomes involving these measures are reflective of stress rather than, or in addition to, the intended experimental manipulation. Practical issues related to experimental stress and its reduction are detailed in Supplemental Assay Protocols.
Energy Intake and Considerations in Assay Selection
Meal Patterns in Mice
When housed under a standard 12-h light/12-h dark cycle, mice consume the majority of their food during the dark, with short bouts of feeding during the light. Water consumption is strongly linked to food intake and declines dramatically in fasted or food-restricted animals. Typical 24-h food intake for a 7–9 week old male mouse fed standard chow is 10–12 Kcal/g body weight (mean across 13 strains; Jackson Laboratory, Mouse Phenome Database) with approximately 70% consumed during the dark. Obese mice are often hyperphagic, and can eat significantly more than this, although sustained consumption of more than twice control intake is rare. Practical guidelines for the selection of a feeding paradigm are provided in Supplementary Assay Protocols.
To minimize the impact of variables that can affect food intake (Figure S1), most studies of feeding behavior are conducted in mice that are individually housed, matched for age, sex, and background strain, and have unrestricted access to their diet. Even with these controls in place, variables such as room temperature, humidity and noise can have a major influence on feeding behavior (Fregly et al., 1957). Specifically, because the thermoneutral temperature for a mouse is 29–32°C, mice kept at standard vivarium temperatures such as 22°C must expend extra energy to keep warm, and this in turn triggers adaptive increases of food intake. It is noteworthy that advertised vivarium temperatures are not always accurate or constant. The effect of even small fluctuations in ambient temperature on energy homeostasis is illustrated in mice that lack uncoupling protein-1 (UCP1), a protein that is expressed in the mitochondria of brown adipose tissue (BAT). These mice have a heightened susceptibility to cold, do not develop obesity under standard housing conditions (Enerback et al., 1997), but do when housed at thermoneutrality (Feldmann et al., 2009). Other parameters such as food preference and gut flora can also be affected by experimental pharmacological and/or genetic manipulations and can affect study outcomes.
Questions to Consider When Studying the Feeding Effects of a Drug Intervention
Does the Compound Alter Normal Feeding Behavior?
To determine if a candidate compound affects short-term food intake, measures of nocturnal feeding are often the first to be undertaken, especially if the intervention is hypothesized to reduce intake, since mice eat their largest meals soon after “lights off.” Dark-cycle readings can be taken under red-light illumination to avoid disturbing the animals, and intake should be monitored at regular intervals throughout the dark-period to uncover acute effects on satiation or satiety. For example, a compound that induces satiation may reduce the size of an initial meal but no others, whereas a compound that increases satiety may prolong the inter-meal interval. However, neither of these effects may be detectable if the only measure obtained is 24-h food consumption, since mice compensate for early reductions of energy intake by eating more later on. As one example, exogenous administration of the gut peptide cholecystokinin (CCK) potently reduces intake for 30–60 min, followed by a subsequent, compensatory increase of intake (Moran and Kinzig, 2004). When investigating such short-acting compounds, intake should be assessed every 30 min for the first 2 or 3 h. When applying procedures that act over longer intervals (e.g., exogenous leptin), hourly or even daily assessments can be used. Assessing these parameters is greatly facilitated by use of automated feeding systems that require no investigator intervention.
Fasting-induced feeding can also be used for investigating anorexigenic compounds. In a typical paradigm, mice are fasted for a fixed period such as 4–6 h or longer (e.g., overnight), and the return of food is preceded by administration of the test compound or other procedure. In normal mice, fasting triggers a hyperphagic response during the re-feeding period (relative to free-feeding mice) that lasts several hours and can aid in the detection of reduced intake; as body energy stores are replenished, intake returns to normal, and detection of milder forms of anorexia may become problematic.
The important point is that the best time of day for measuring food intake depends upon the question being asked. If a treatment is hypothesized to reduce short-term intake, the test will be more sensitive if intake at baseline is relatively high. Conducting the test in the dark or withholding food prior to the test increases the baseline value and may therefore simplify detection of modest feeding effects. Conversely, if a treatment is hypothesized to increase acute intake (e.g., administering the hormone ghrelin), it can be advantageous to have a lower baseline value. This can be accomplished by a shorter period of deprivation and/or by conducting the test during the light cycle when mice eat less. A final consideration is that due to their relatively high metabolic rate, a 12–16 h fast is a major stressor and longer periods of fasting are not recommended because of their potent effects on feeding behavior. In general, water should be freely available at all times when feeding is assessed.
Does the Compound Reduce Food Intake through a Homeostatic Mechanism? – Anorexia vs. Aversion
In addition to interventions that impinge upon neurocircuits governing homeostatic food intake control, food intake in mice can also be reduced by stress (noted above), sickness or drug toxicity. Once a test compound is shown to reduce food intake, therefore additional studies may be warranted to determine whether a non-homeostatic mechanism underlies the effect. The terminology used here is necessarily explicit, and the terms “physiological” and “non-physiological,” and “specific” and “nonspecific,” are avoided since reductions of food intake caused by sickness-like behavior in mice may be induced via the same receptors or neuronal circuitry that induce satiation. As an example, CCK is secreted during every meal, and while administration of small doses of exogenous CCK reduce food intake without aversive effects (Gibbs and Smith, 1977), higher doses are associated with aversive or sickness-like behavior (Deutsch and Hardy, 1977; Perez and Sclafani, 1991). Thus, satiation and sickness-induced reductions of food intake may exist along a continuum with illness/nausea representing one extreme. Although rodents lack the emetic reflex (i.e., they cannot vomit), “sickness-like” behavior can manifest itself as a spiky coat or hunched posture, altered breathing rate, labored movements, reduced activity and/or subdued behavior. Two well-characterized behavioral assays, conditioned taste aversion (CTA) and pica (a disorder of feeding behavior characterized by increased consumption or chewing of non-food materials), are commonly employed to assess whether a reduction in food intake is a manifestation of malaise. For a detailed description of how to perform CTA and pica tests and the relative merits of these tools for assessing sickness-like behavior in rodents, see the Supplemental Assay Protocols section and (Andrews and Horn, 2006).
Does the Compound Affect Energy Balance Independently of Food Intake?
Pair-feeding is a technique in which the amount of food provided to a control group of mice is matched to that consumed by the experimental group, so as to determine the extent to which the effect of a treatment on body weight or body composition occurred independently of changes of energy intake (Dubuc et al., 1984; Levin et al., 1996). If body weight is reduced to a greater extent in treated mice than in controls fed the exact same amount of food, this outcome is suggestive of a change in metabolic rate elicited by the treatment, which could subsequently be verified by indirect calorimetry. Because pair-fed mice consume the same test diet as experimental mice, but in lesser amounts, factors such as macronutrient content and palatability are also controlled. Additional details and limitations regarding pair-feeding are provided in Supplemental Assay Protocols.
In summary, decisions about when and how food intake is to be measured should be made only after considering a variety of parameters including diet and time of day, how often to take readings, and whether the animals should be fed or fasted prior to food presentation. A sample decision-making chart for assessing the effect of a compound/intervention on feeding behavior is shown in Figure 1. In addition, assessment of potential illness should be considered for novel interventions that reduce intake, and pair-fed controls can be used to differentiate reduced feeding from other causes of weight loss.
Figure 1.
A sample decision making chart for assessment of the effect of a drug/compound on energy intake
Questions to Consider When Analyzing Feeding Behavior in Genetically-Modified Mice
Is Food Intake Normal in My Model?
If a genetically modified mouse weighs more or less than controls, a reasonable first step in characterizing the phenotype is to assess food intake as described above. When increased food intake is observed in the context of obesity, the investigative approach can focus on whether the underlying mechanism involves a defect in homeostatic control, in the perception of satiating or rewarding properties of food, or in other mechanisms. When increased food intake is observed in the context of reduced body weight or fat mass (e.g., in mice with a lean, hypermetabolic phenotype), the mechanisms driving intake may be compensatory in nature and in fact reflect a properly operating regulatory system.
A simple test to determine whether a homeostatic regulation of food intake is intact is to measure fasting-induced refeeding. In this assay, animals are fasted during a period of high baseline consumption (usually overnight) and then re-fed, and their intake measured until body weight returns to pre-fasted values (usually, within 2–3 d). Animals normally consume large meals during this re-feeding period to correct for their negative energy balance, and failure to re-feed normally may indicate a defect in this homeostatic response. Alternatively, excessive re-feeding hyperphagia may be indicative of altered satiation pathways or other changes affecting the control of feeding behavior.
Another assay that can be used to investigate whether normal homeostatic feeding is intact is to examine the response to a high-fat diet (HFD; see Supplemental Assay Protocols). Normal mice become hyperphagic within 48 h of exposure to a HFD and begin to gain body weight beyond that of control values within the first 1–2 wks, and caloric intake often returns toward normal levels after this initial period of hyperphagia (when making such measurements, it is important to monitor calorie intake, rather than food consumption in grams, since the high-fat and control low-fat diets typically differ in energy density). Failure to exhibit the gradual normalization of energy intake as body weight and fat mass increase on a HFD may indicate an alteration in homeostatic control of food intake, as occurs in mice with impaired melanocortin signaling (Butler et al., 2001).
Is the Body Weight/Composition Phenotype Dependent on Altered Feeding Behavior?
A simple means of establishing whether reduced food intake is responsible for a phenotype of lowered body weight is to use pair-feeding as described above. If a genetically altered mouse is both hyperphagic and obese, restricting the intake of a cohort of these animals to match that consumed by WT controls sheds light on the extent to which the obesity is due to excessive intake, while keeping in mind precautions described in Supplemental Assay Protocols. If the results of this type of pair-feeding study indicate that differences of caloric intake per se cannot fully account for observed changes of body weight, a useful next step is measure energy expenditure by indirect calorimetry.
The Mouse as a Model of Human Feeding Behavior
Ultimately, information gained from analysis of feeding behavior in mice is most useful if it sheds light on human physiology and/or pathophysiology, and many observations support this view. As noted earlier, monogenic causes of human obesity have been identified principally on the basis of mouse models (Farooqi and O’Rahilly, 2008). Of therapeutic relevance is that many drug targets under investigation for the treatment of human obesity were originally identified and characterized in mouse models. Conversely, genome-wide association studies have identified variation in genes previously characterized as participating in the central nervous system control of food intake in mice (e.g., Sh2b1) as being associated with human obesity (Ren et al., 2007; Willer et al., 2009). Lastly, the ease with which modern genetic and pharmacological tools are applied to mouse models supports their application to the study of human obesity pathogenesis and treatment.
This being said, there are many obvious and important differences in feeding behavior between mice and humans. Laboratory mice are nocturnal and consume most of their food in frequent, small meals throughout the dark. By comparison, humans tend to consume most of their calories in three or four meals during the light. The extent to which this pattern of human feeding behavior has evolved due to social constraints is an open question – the observation that rodents are readily trained to receive their food in discrete meals in meal-entrainment studies suggests that this aspect of feeding behavior can be strongly influenced by learned variables.
Another pertinent issue is eating for physiological versus psychological need. Humans are strongly predisposed to alter patterns of food consumption in response to a wide range of emotional states, with one consequence being that altered feeding patterns are common features of psychiatric illness. Perhaps even more obvious is the volitional aspect of feeding behavior – deliberately choosing to eat or not – which may be a uniquely human quality. As such, it can be argued that feeding behavior in humans is uniquely complex and that mouse models are useful primarily to study physiological aspects of feeding behavior after psychological considerations have been stripped away.
Even this assertion can be challenged, however, since the determinants of energy balance in mice differ substantially from those in humans. Mice have a much higher surface area to volume ratio than humans, requiring them to expend a greater proportion of their daily energy budget to maintain core temperature. Consequently, changes of ambient temperature have a greater impact on energy demands in rodents, and this in turn can strongly affect feeding behavior, as discussed earlier. Another difference is that rodents lack an emetic reflex, which complicates the study of aversion and drug toxicology.
In a recent provocative perspectives article, Martin and colleagues argue that due to their generally sedentary lives in a nutrient-rich environment many of the “control” laboratory rodents used in research studies are in fact metabolically morbid which may skew data interpretation (Martin et al., 2010). They state that by human standards most laboratory rodents are in relatively poor health and that a second control group of animals that have access to increased exercise and/or more limited food should be included to in many studies to represent the “healthy” human population.
Conclusions
This document represents the opinions of the investigators from the various NIH funded MMPCs. It is a working document and will be updated from time to time to incorporate the knowledge we acquire through the performance of studies with WT, knockout and transgenic animals. For alternative points of view and for coverage of relevant aspects of mouse phenotyping not covered in this article we also refer readers to the EUMORPHIA (http://www.eumorphia.org), a European consortium of 18 research institutes across 8 countries developing and validating standard operating protocols (SOPs), as well as the following books (Fox et al., 2006; Hedrich, 2004; Hrabe De Angelis et al., 2006)
Supplementary Material
Acknowledgments
Authors thank members of the MMPC for their helpful discussion and careful reading of the manuscript. The MMPC is funded by NIH grant U24 DK059637.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton Neurosci. 2006;125:100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L. Membrane estrogen receptors and energy homeostasis. J Neurosci. 2006;26:11255–11256. doi: 10.1523/JNEUROSCI.3717-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KR, Rustay NR, Crawley JN. Behavioral phenotyping of transgenic and knockout mice: practical concerns and potential pitfalls. ILAR J. 2006;47:124–131. doi: 10.1093/ilar.47.2.124. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, McRoberts JA. Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J Neurogenet. 2008;22:315–331. doi: 10.1080/01677060802357388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4:605–611. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- Champy MF, Selloum M, Piard L, Zeitler V, Caradec C, Chambon P, Auwerx J. Mouse functional genomics requires standardization of mouse handling and housing conditions. Mamm Genome. 2004;15:768–783. doi: 10.1007/s00335-004-2393-1. [DOI] [PubMed] [Google Scholar]
- Champy MF, Selloum M, Zeitler V, Caradec C, Jung B, Rousseau S, Pouilly L, Sorg T, Auwerx J. Genetic background determines metabolic phenotypes in the mouse. Mamm Genome. 2008;19:318–331. doi: 10.1007/s00335-008-9107-z. [DOI] [PubMed] [Google Scholar]
- Coleman DL. Obese and diabetes: Two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Coleman DL, Hummel KP. The influence of genetic background on the expression of the obese (Ob) gene in the mouse. Diabetologia. 1973;9:287–293. doi: 10.1007/BF01221856. [DOI] [PubMed] [Google Scholar]
- Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81:243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Deutsch JA, Hardy WT. Cholecystokinin produces bait shyness in rats. Nature. 1977;266:196. doi: 10.1038/266196a0. [DOI] [PubMed] [Google Scholar]
- Dubuc PU, Cahn PJ, Willis P. The effects of exercise and food restriction on obesity and diabetes in young ob/ob mice. Int J Obes. 1984;8:271–278. [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- Ewart-Toland A, Mounzih K, Qiu J, Chehab FF. Effect of the genetic background on the reproduction of leptin-deficient obese mice. Endocrinology. 1999;140:732–738. doi: 10.1210/endo.140.2.6470. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, O’Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab. 2008;4:569–577. doi: 10.1038/ncpendmet0966. [DOI] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez R, Martini AC, Navarro VM, Castellano JM, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Novel signals for the integration of energy balance and reproduction. Mol Cell Endocrinol. 2006;254–255:127–132. doi: 10.1016/j.mce.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Fox JG, Barthold S, Davisson M, Newcomer CE, Quimby FW, Smith A, editors. History, Genetics and Wild-Mice. 1–4. Academic Press; 2006. The Mouse in Biomedical Research. [Google Scholar]
- Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes. 2006;55:2153–2156. doi: 10.2337/db06-0358. [DOI] [PubMed] [Google Scholar]
- Fregly MJ, Marshall NB, Mayer J. Effect of changes in ambient temperature on spontaneous activity, food intake and body weight of goldthioglucose-obese and nonobese mice. Am J Physiol. 1957;188:435–438. doi: 10.1152/ajplegacy.1957.188.3.435. [DOI] [PubMed] [Google Scholar]
- Gelling RW, Yan W, Al-Noori S, Pardini A, Morton GJ, Ogimoto K, Schwartz MW, Dempsey PJ. Deficiency of TNFalpha converting enzyme (TACE/ADAM17) causes a lean, hypermetabolic phenotype in mice. Endocrinology. 2008;149:6053–6064. doi: 10.1210/en.2008-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Smith GP. Cholecystokinin and satiety in rats and rhesus monkeys. Am J Clin Nutr. 1977;30:758–761. doi: 10.1093/ajcn/30.5.758. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Sekhon M, Schein J, Zhao S, Osoegawa K, Scott CE, Evans RS, Burridge PW, Cox TV, Fox CA, Hutton RD, Mullenger IR, Phillips KJ, Smith J, Stalker J, Threadgold GJ, Birney E, Wylie K, Chinwalla A, Wallis J, Hillier L, Carter J, Gaige T, Jaeger S, Kremitzki C, Layman D, Maas J, McGrane R, Mead K, Walker R, Jones S, Smith M, Asano J, Bosdet I, Chan S, Chittaranjan S, Chiu R, Fjell C, Fuhrmann D, Girn N, Gray C, Guin R, Hsiao L, Krzywinski M, Kutsche R, Lee SS, Mathewson C, McLeavy C, Messervier S, Ness S, Pandoh P, Prabhu AL, Saeedi P, Smailus D, Spence L, Stott J, Taylor S, Terpstra W, Tsai M, Vardy J, Wye N, Yang G, Shatsman S, Ayodeji B, Geer K, Tsegaye G, Shvartsbeyn A, Gebregeorgis E, Krol M, Russell D, Overton L, Malek JA, Holmes M, Heaney M, Shetty J, Feldblyum T, Nierman WC, Catanese JJ, Hubbard T, Waterston RH, Rogers J, de Jong PJ, Fraser CM, Marra M, McPherson JD, Bentley DR. A physical map of the mouse genome. Nature. 2002;418:743–750. doi: 10.1038/nature00957. [DOI] [PubMed] [Google Scholar]
- Haluzik M, Colombo C, Gavrilova O, Chua S, Wolf N, Chen M, Stannard B, Dietz KR, Le Roith D, Reitman ML. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology. 2004;145:3258–3264. doi: 10.1210/en.2004-0219. [DOI] [PubMed] [Google Scholar]
- Havel PJ, Uriu-Hare JY, Liu T, Stanhope KL, Stern JS, Keen CL, Ahren B. Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats: reversal by insulin. Am J Physiol. 1998;274:R1482–1491. doi: 10.1152/ajpregu.1998.274.5.R1482. [DOI] [PubMed] [Google Scholar]
- Hedrich H, editor. The Laboratory Mouse. Elsevier Limited; 2004. [Google Scholar]
- Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294:E827–832. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabe De Angelis M, Chambon P, Brown S, editors. Standards in Mouse Model Phenotyping. Wiley-VCH; 2006. [Google Scholar]
- Huang TT, Naeemuddin M, Elchuri S, Yamaguchi M, Kozy HM, Carlson EJ, Epstein CJ. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum Mol Genet. 2006;15:1187–1194. doi: 10.1093/hmg/ddl034. [DOI] [PubMed] [Google Scholar]
- Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of Body Fat Mass as a Major Determinant of Metabolic Rate in Mice. Diabetes. 2010 doi: 10.2337/db09-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin N, Nelson C, Gurney A, Vandlen R, de Sauvage F. Decreased food intake does not completely account for adiposity reduction after ob protein infusion. Proc Natl Acad Sci U S A. 1996;93:1726–1730. doi: 10.1073/pnas.93.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandillo S, Tucci V, Holter SM, Meziane H, Banchaabouchi MA, Kallnik M, Lad HV, Nolan PM, Ouagazzal AM, Coghill EL, Gale K, Golini E, Jacquot S, Krezel W, Parker A, Riet F, Schneider I, Marazziti D, Auwerx J, Brown SD, Chambon P, Rosenthal N, Tocchini-Valentini G, Wurst W. Reliability, robustness, and reproducibility in mouse behavioral phenotyping: a cross-laboratory study. Physiol Genomics. 2008;34:243–255. doi: 10.1152/physiolgenomics.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci U S A. 2010;107:6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TH, Kinzig KP. Gastrointestinal satiety signals II. Cholecystokinin. Am J Physiol Gastrointest Liver Physiol. 2004;286:G183–188. doi: 10.1152/ajpgi.00434.2003. [DOI] [PubMed] [Google Scholar]
- Perez C, Sclafani A. Cholecystokinin conditions flavor preferences in rats. Am J Physiol. 1991;260:R179–185. doi: 10.1152/ajpregu.1991.260.1.R179. [DOI] [PubMed] [Google Scholar]
- Qiu J, Ogus S, Mounzih K, Ewart-Toland A, Chehab FF. Leptin-deficient mice backcrossed to the BALB/cJ genetic background have reduced adiposity, enhanced fertility, normal body temperature, and severe diabetes. Endocrinology. 2001;142:3421–3425. doi: 10.1210/endo.142.8.8323. [DOI] [PubMed] [Google Scholar]
- Ren D, Zhou Y, Morris D, Li M, Li Z, Rui L. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J Clin Invest. 2007;117:397–406. doi: 10.1172/JCI29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DM, Swaney JS, Dalton ND, Gilpin EA, Ross J., Jr Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol. 2002;282:H2134–2140. doi: 10.1152/ajpheart.00845.2001. [DOI] [PubMed] [Google Scholar]
- Sindelar DK, Havel PJ, Seeley RJ, Wilkinson CW, Woods SC, Schwartz MW. Low plasma leptin levels contribute to diabetic hyperphagia in rats. Diabetes. 1999;48:1275–1280. doi: 10.2337/diabetes.48.6.1275. [DOI] [PubMed] [Google Scholar]
- Su Z, Korstanje R, Tsaih SW, Paigen B. Candidate genes for obesity revealed from a C57BL/6J x 129S1/SvImJ intercross. Int J Obes (Lond) 2008;32:1180–1189. doi: 10.1038/ijo.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Hegeman MA, Nguyen MM, Melhorn SJ, Ma LY, Woods SC, Sakai RR. Dynamic body weight and body composition changes in response to subordination stress. Physiol Behav. 2007a;91:440–448. doi: 10.1016/j.physbeh.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Ostrander MM, Gardner SR, Ma LY, Woods SC, Sakai RR. Social stress and recovery: implications for body weight and body composition. Am J Physiol Regul Integr Comp Physiol. 2007b;293:R1864–1874. doi: 10.1152/ajpregu.00371.2007. [DOI] [PubMed] [Google Scholar]
- Toye AA, Lippiat JD, Proks P, Shimomura K, Bentley L, Hugill A, Mijat V, Goldsworthy M, Moir L, Haynes A, Quarterman J, Freeman HC, Ashcroft FM, Cox RD. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia. 2005;48:675–686. doi: 10.1007/s00125-005-1680-z. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeland E, Morel L, Achey K, Yui M, Longmate J. Speed congenics: a classic technique in the fast lane (relatively speaking) Immunol Today. 1997;18:472–477. doi: 10.1016/s0167-5699(97)01126-2. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, Soranzo N, Tanaka T, Timpson NJ, Almgren P, Bennett A, Bergman RN, Bingham SA, Bonnycastle LL, Brown M, Burtt NP, Chines P, Coin L, Collins FS, Connell JM, Cooper C, Smith GD, Dennison EM, Deodhar P, Elliott P, Erdos MR, Estrada K, Evans DM, Gianniny L, Gieger C, Gillson CJ, Guiducci C, Hackett R, Hadley D, Hall AS, Havulinna AS, Hebebrand J, Hofman A, Isomaa B, Jacobs KB, Johnson T, Jousilahti P, Jovanovic Z, Khaw KT, Kraft P, Kuokkanen M, Kuusisto J, Laitinen J, Lakatta EG, Luan J, Luben RN, Mangino M, McArdle WL, Meitinger T, Mulas A, Munroe PB, Narisu N, Ness AR, Northstone K, O’Rahilly S, Purmann C, Rees MG, Ridderstrale M, Ring SM, Rivadeneira F, Ruokonen A, Sandhu MS, Saramies J, Scott LJ, Scuteri A, Silander K, Sims MA, Song K, Stephens J, Stevens S, Stringham HM, Tung YC, Valle TT, Van Duijn CM, Vimaleswaran KS, Vollenweider P, Waeber G, Wallace C, Watanabe RM, Waterworth DM, Watkins N, Witteman JC, Zeggini E, Zhai G, Zillikens MC, Altshuler D, Caulfield MJ, Chanock SJ, Farooqi IS, Ferrucci L, Guralnik JM, Hattersley AT, Hu FB, Jarvelin MR, Laakso M, Mooser V, Ong KK, Ouwehand WH, Salomaa V, Samani NJ, Spector TD, Tuomi T, Tuomilehto J, Uda M, Uitterlinden AG, Wareham NJ, Deloukas P, Frayling TM, Groop LC, Hayes RB, Hunter DJ, Mohlke KL, Peltonen L, Schlessinger D, Strachan DP, Wichmann HE, McCarthy MI, Boehnke M, Barroso I, Abecasis GR, Hirschhorn JN. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GT. Speed congenics: applications for transgenic and knock-out mouse strains. Neuropeptides. 2002;36:230–236. doi: 10.1054/npep.2002.0905. [DOI] [PubMed] [Google Scholar]
- Yang Y, Beyer BJ, Otto JF, O’Brien TP, Letts VA, White HS, Frankel WN. Spontaneous deletion of epilepsy gene orthologs in a mutant mouse with a low electroconvulsive threshold. Hum Mol Genet. 2003;12:975–984. doi: 10.1093/hmg/ddg118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.