Abstract
The Sodium-dependent Vitamin C Transporter (SVCT2) is responsible for the transport of vitamin C into cells in multiple organs, either from the blood or cerebrospinal fluid. Mice null for SVCT2 (SVCT2(−/−)) do not survive past birth but cause of death has not yet been ascertained. Following mating of SVCT2(+/−) males and SVCT2(+/−) females, fewer SVCT2(−/−) and (+/−) were observed than would be expected according to Mendelian ratios. Vitamin C levels in SVCT2(−/−), (+/−) and (+/+) were genotype-dependent. SVCT2(−/−) fetuses had significantly lower vitamin C levels than littermates in placenta, cortex, and lung but not in liver (the site of vitamin C synthesis). Low vitamin C levels in placenta and cortex were associated with elevations in several different markers of oxidative stress; malondialdehyde, isoketals, F2-isoprostanes, and F4-neuroprostanes. Oxidative stress was not elevated in fetal SVCT2(−/−) lung tissue despite low vitamin C levels. In addition to the expected severe hemorrhage in cortex, we also found hemorrhage in the brain stem, which was accompanied by cell loss. We found evidence of increased apoptosis in SVCT2(−/−) mice and disruption of the basement membrane in fetal brain. Together these data show that vitamin C is critical for maintaining vitamin C levels in fetal and placental tissues and that lack of SVCT2, and resulting low vitamin C levels, results in fetal death, and in SVCT(−/−) that survive the gestation period, in oxidative stress and cell death.
Keywords: Oxidative stress, Ascorbic acid, SVCT2, brain, development
Introduction
Scurvy was once a devastating illness in naval voyagers and those who had little access to fresh fruits and vegetables. Death typically occurs after several months of vitamin C (VC; ascorbate) deprivation in man [1], although after only a month in guinea pigs [2], which like humans are unable to synthesize the vitamin. Pathologic changes in humans and guinea pigs dying of scurvy show marked degeneration of almost every organ except the brain, typified by hemorrhage and putrefaction [1]. The brain and central nervous system appear to be spared relative to other organs, since in severely scorbutic guinea pigs the brain content of VC is almost 25% of normal, whereas it is undetectable in most other organs [2]. Part of the reason for this is that the brain starts out with relatively high VC concentrations of 3–5 mM, and perhaps even higher in certain brain areas and in neurons [3]. Generation of these high intracellular VC concentrations is due to the presence of the Sodium-dependent Vitamin C transporter, type 2 (SVCT2). The cDNA for this transporter was cloned in 1999, along with that for the closely related SVCT1 [4]. The SVCT1 mediates VC absorption in the intestine and reabsorption in the kidney proximal tubule cells. It is also present in liver, lung, and skin [4, 5]. The SVCT2 mediates unidirectional uptake of VC in most other tissues, including brain. In the CNS the SVCT2 is deployed in a novel two-step uptake mechanism, first by transporting the vitamin across the choroid plexus from the blood plasma (30–60 µM) into the CSF (200–300 µM), and then from the CSF and brain interstitium into neurons (2–5 mM) [3]. That the SVCT2 is required for VC to enter the brain has been clearly demonstrated with targeted deletion of the protein in the mouse, despite the ability of this mammal to synthesize its own VC.
Fetuses homozygous for knockout of the SVCT2 generally survive through the completion of gestation, but die at birth without taking a breath [6]. Other than not being expanded and containing little or no VC, the lungs do not show histologic damage and contain normal levels of surfactant. In contrast to the guinea pig dying of scurvy, VC is barely detectable in the brains of the SVCT2(−/−) fetuses. Gross examination showed numerous punctate or coalescing hemorrhages on the convex cortical surface of the brain, which on histological examination penetrated deeply into the cortex. Despite very low VC levels in liver, adrenal, pituitary, pancreas, and muscle, no hemorrhages were observed in these organs and the content of 4-hydroxyproline, a marker of collagen modification, was normal in the skin of the homozygote knockout fetuses. Mice heterozygous for lack of the SVCT2 had decreased VC contents in these various organs, but grew normally and were fertile. Although the immediate cause of death in the homozygous knockout fetuses was proposed as respiratory failure [6], the contribution of VC deficiency in the brain has not been further examined.
VC has been noted to have several functions in brain and in the CNS. Perhaps most frequently considered is its role as an antioxidant to guard against the damage induced by oxidative stress. VC also has multiple non-antioxidant functions in brain [3]. It is a necessary co-factor for dioxygenase enzymes responsible for hydroxylation of neurotransmitters, the transcription factor HIF-1α, and for collagen. For example, SVCT2 knockout mice have been shown to have decreases in catecholamine biosynthesis, although this was not considered the cause of death [7]. Further, VC is necessary for proper myelination of neurons, a process considered to depend on collagen synthesis [8]. Finally, VC levels in cerebrospinal fluid are increased in response to glutamate generated during neurotransmission [9, 10], and VC in turn has been shown to have neuromodulatory effects on synaptic transmission [11, 12]. The combined absence of these diverse functions of VC in brain and neural tissue may account for failure of SVCT2 homozygote fetuses to survive after birth, but more studies of these animals are needed to define the specific mechanisms.
In this work we evaluated several aspects of VC function in SVCT2 knockout fetuses in comparison to their SVCT2(+/−) and SVCT2(+/+) littermates. The major hypothesis in this proposal is that SVCT2(−/−) mice die from neuronal damage due to oxidant stress or hemorrhage, and that brain damage rather than lung abnormalities are responsible for the lack of breathing in SVCT2(−/−) newborns. The results show modest, localized oxidative stress in the brain of SVCT2(−/−) fetuses. In addition, capillary hemorrhage was found to extend to the hindbrain and was associated with cell death. The cause of this hemorrhage was deficient type IV collagen in basement membranes in the brain. We conclude that death in these fetuses or newborns is due to neurologic dysfunction secondary to hemorrhage, especially during to the physical stress of birth.
Methods
SVCT2(+/−) mice
These mice were provided by Dr. Robert Nussbaum. Originally on the 129/SvEvTac background, they were back-crossed more than 10 generations to C57BL/6 mice to place them on this background. Animals were housed in breeding pairs in tub cages in a temperature- and humidity-controlled vivarium. Mice were kept on a 12:12-hour light:dark cycle with lights on at 6 AM. Mice had free access to food and water for the duration of the experiment. Deionized water was supplemented with 0.33 g/L ascorbic acid (Sigma, USA) with 20 μl EDTA to increase stability of VC in solution. This is the standard supplement level that provides adult (non-pregnant) gulo(−/−) mice that cannot synthesize vitamin C with wild-type levels of the vitamin in tissues [13, 14]. All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Biochemistry assays (vitamin C, MDA, F2-isoprostanes, neuroprostanes) were conducted from the offspring of a total of ten litters. Histology and immunohistochemistry data came from litters from a further three dams. Although we did witness a very small number of shriveled placentas not attached to a fetus indicating an aborted fetus, it is not possible to genotype these tissues because they have both maternal and fetal tissue and so these were not recorded. We did not observe any other example of premature births or miscarriages of entire litters.
Sacrifice and tissue collection
Because SVCT2(−/−) pups are not viable and die shortly after the birth [6], we mated SVCT2(+/−) female and male mice to generate SVCT2(−/−) fetuses. Pregnancy was determined by the presence of a vaginal plug. SVCT2(+/−) dams were provided with 0.33 g/L VC in drinking water with 20 µl 0.05 M EDTA. Although SVCT2(+/−) mice can synthesize their own VC, this ensured an adequate supply throughout pregnancy. To obtain fetal tissues, pregnant dams were taken on day 18–19 of gestation, fully anaesthetized using inhaled isoflurane and sacrificed by decapitation. Fetuses (embryonic days (E) 18.5–19.5) were delivered by caesarian section and placed on a Petri dish on ice to induce hypothermia. Fetuses were then quickly decapitated. Liver, whole brain (except cerebellum and brain stem) and lung were removed from each animal and tissues were quickly frozen and stored at −80 °C until needed. In addition to these tissues, the placenta for each fetus was also removed and stored at −80 °C. In a further set of animals, whole brain, including brain stem and top of spinal chord, was removed intact and fixed in 10x formalin for 72 hours before being rinsed and stored in 1x PBS. Brains were then embedded in paraffin and cut and stained as described below. The tail was also removed from each pup, washed in 1x PBS and frozen at −20 °C for 24 hours before extraction of DNA and determination of genotype by PCR. Maternal liver, lung and cortex samples were also removed, quickly frozen and stored at −80 °C until needed for assays. Total numbers of mice used for determination of VC and oxidative stress in tissues were seven SVCT2(−/−) fetuses, nine SVCT2(+/−) fetuses, eight SVCT2(+/+) fetuses, and six SVCT2(+/−) dams. A further six SVCT2(−/−) fetuses, five SVCT2(+/−) fetuses, five SVCT2(+/+) fetuses, and tissue from five SVCT2(+/−) dams were used for lung F2-isoprostane measurements.
VC determination
ASC was measured by HPLC as described [15, 16]. Data are determined per gram tissue (wet weight).
Malondialdehyde (MDA) determination
MDA, a product of lipid peroxidation was measured to detect any differences in oxidative stress among the genotypes. MDA was measured as previously described [17]. Data are determined per gram tissue (wet weight).
F2-isoprostanes, F4-Neuroprostanes
F4-Neuroprostanes and F2-isoprostane levels were measured as described [18]. Data are determined per gram tissue (wet weight).
Histology
Formalin-fixed tissue was paraffin embedded and cut in 5 micron sections on a horizontal plane. Consecutive sections were stained with hematoxylin and eosin (H&E) and thionin. For thionin staining slides were deparaffinized with 2-min. washes in xylene, and ethanol (100%, 100% and 95%, respectively). They were then immersed for 10 min. in distilled water, for 2 min. in thionin, given three washes in distilled water, and finally treated sequentially for 1 min. with 95 % ethanol, 100 % ethanol and xylene. Slides were then coverslipped and allowed to dry.
Immunohistochemistry
A separate group of fetal brains were used for immunohistochemistry studies.
Isoketals
For isoketal staining, brain tissues were fixed in freshly prepared 4% paraformaldehyde-PBS (PFA-PBS) overnight at 4 °C then embedded in paraffin before sectioning. Five-micron sections through the cortex were cut using a cryostat. Sections were treated with 0.3% H2O2 in methanol for 15 min. and then with a Protein block solution (Dako) for 10 min. Sections were incubated with a single chain antibody (D11 ScFv) to isoketals [19] in a humidified chamber for 60 min. at 37 °C. Sections were then incubated with AntiE-tag labeled with horseradish peroxidase (Pharmacia, cat. #27941301) to detect E-tagged ScFv bound to antigens. The enzyme was detected by 3,3'-diaminobenzidine (DAB) using a DAB enhanced liquid substrate system (Sigma). Sections were then counterstained with Mayer’s hematoxylin (Sigma).
TUNEL stain
To examine apoptosis in cortex of fetuses, fetal brain tissue was embedded in O.C.T., then 5-micron cryosections were fixed in 4% PFA-PBS for 20 min at room temperature before being washed in PBS. Fixed brain sections were then treated with 3% citric acid and apoptotic cells were detected by the TUNEL (TdT-mediated dUTP nick end labeling) technique using an in situ cell death detection kit (Roche Applied Science) with the reporter enzyme alkaline phosphatase. Sections were then treated with Fast Red TR/Naphthol AS-NX substrate (Sigma) to detect TUNEL-positive (TUNEL+) cells.
Detection of type IV collagen and laminin in basement membrane in brain
Sections containing the basement membrane were incubated with goat anti-type IV collagen antibodies (SouthernBiotech Associates, Birmingham, AL) and then with rabbit antibodies to human laminin (Abcam Inc. Cambridge, MA). Sections were treated with donkey antibodies to goat and rabbit IgG labeled respectively with Alexa 488 and Alexa 594 (both from Invitrogen Inc, Eugene, OR). Photomicroscopy was performed on an Olympus microscope (BX40) using Olympus camera (DP70).
Statistics
Data were analyzed using SPSS 16.0 for Windows. A univariate ANOVA was conducted for each dependent variable with fetus genotype as the between-groups variable. In cases where samples were also taken from maternal tissue (lung, liver, cortex), the SVCT2(+/−) dams comprised a fourth group. Following significant omnibus ANOVA, follow-up comparisons were conducted using a Bonferroni post hoc test. Genotype distribution within the litters was calculated using a χ2 test against the expected genotype distribution of 1:2:1.
Results
Genotype distribution among the litters
Using data from 9 litters the genotype ratios were 12(−/−): 34(+/−): 31(+/+). A χ2 test against the expected 1:2:1 ratio distribution was significant [χ2=10.428, p<0.01 d.f. = 2]. Given 31 SVCT2(+/+) mice, if Mendelian ratios were followed then we would expect 31 SVCT2(−/−) and 62 SVCT2(+/−) mice. Separate Chi square tests revealed that both of these genotypes were underrepresented in the sample [χ2>11.64, p<0.001].
VC tissue contents
Placenta
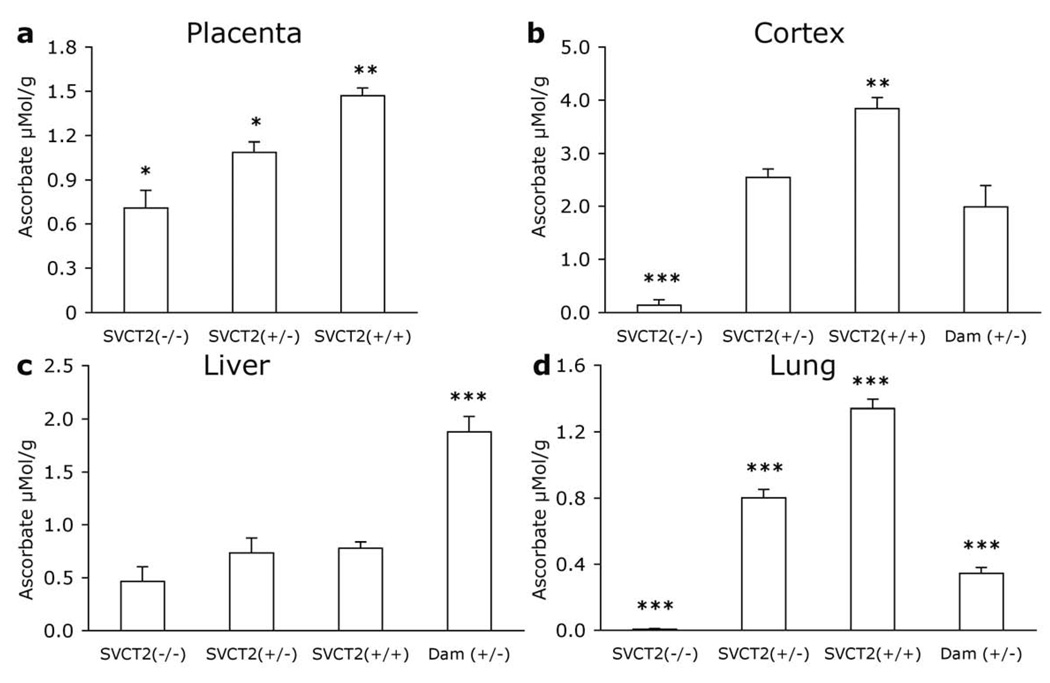
A gene dosage effect was seen in VC levels such that for each additional copy of the SVCT2 a fetus carried VC was increased [F2, 18 = 20.465, p<0.001; Fig. 1a]. SVCT2(−/−) VC levels were lower than both SVCT2(+/−) and SVCT2(+/+) (p<0.05) and SVCT2(+/−) were also lower than SVCT2(+/+) (p<0.01). VC levels were only slightly lower than 1 μmol/g even in SVCT2(−/−) mice due to the combination of fetal and maternal (SVCT2(+/−)) tissue that comprises the placenta.
Figure 1. VC levels in fetal and maternal tissues.
VC levels were measured in tissue samples from E19.5–20.5 fetuses and dams in (a) placenta, (b) cortex, (c) liver, and (d) lung. Data are expressed as mean + S.E.M. *p<0.05, **p<0.01, ***p<0.001 different from all other groups.
Cortex
Clear differences in VC levels were evident among the groups [F3, 22=38.59, p<0.001; Fig. 1b]. SVCT2(−/−) mice had barely detectable levels of VC that were significantly lower than dams and both other fetal genotypes (p<0.001). SVCT2(+/−) fetuses also had significantly lower levels than their SVCT2(+/+) littermates (p<0.01) but did not differ from SVCT2(+/−) dams. SVCT2(+/+) fetuses had greater VC levels than dams (p<0.001).
Liver
A significant group difference in VC levels arose because SVCT2(+/−) dams had higher VC levels than the fetuses [F3, 24=23.921, p<0.001; Fig. 1c]. VC was higher in the dam than in all three fetus groups (p<0.001). VC levels were similar among the fetuses, with normal levels observed even in SVCT2(−/−) mice.
Lung
As expected, lung tissue in fetal SVCT2(−/−) was completely devoid of ascorbate. Group differences were evident [F3, 25=162.907, p<0.001; Fig. 2d]. SVCT2(−/−) fetus VC lung levels were significantly lower than all other groups (p<0.001). SVCT2(+/−) and (+/+) fetuses had higher VC levels than dams, with a gene-dose-dependent effect between SVCT2(+/−) and SVCT2(+/+). Each group was significantly different from each other group (p<0.001).
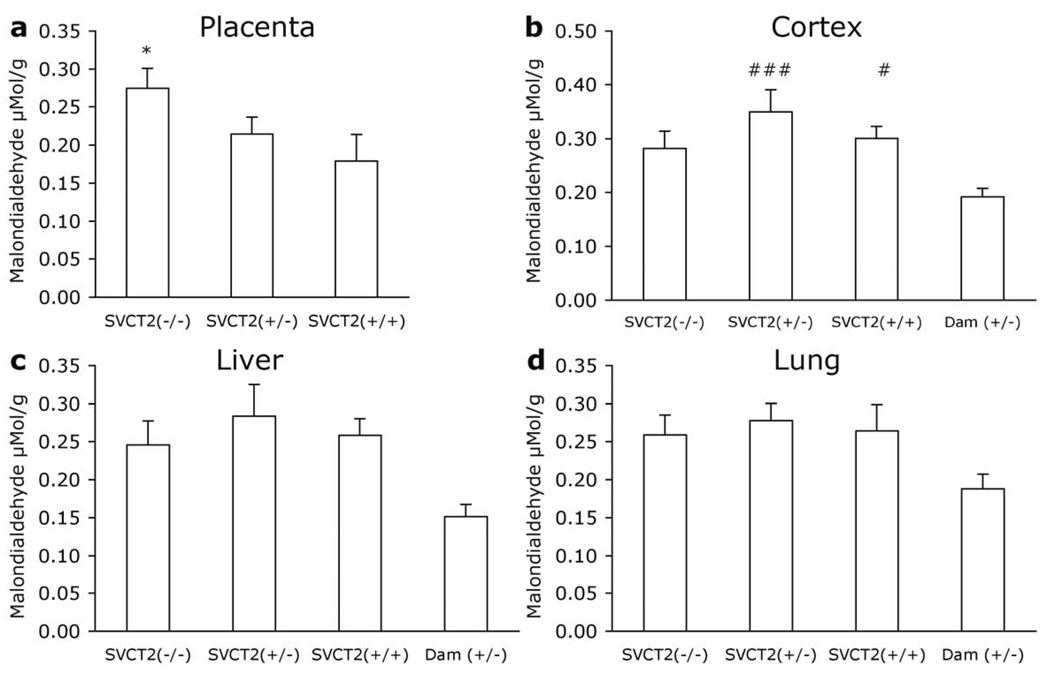
Figure 2. Malondialdehyde in fetal and maternal tissues.
Malondialdehyde (MDA) was measured in tissue samples from E19.5–20.5 fetuses and dams in (a) placenta, (b) cortex, (c) liver, and (d) lung. Data are expressed as mean + S.E.M. *p<0.05 different from all other groups. #p<0.05, ###p<0.001 different from SVCT2(+/−) dam.
Malondialdehyde
Placenta
In the placenta, MDA levels followed an inverse pattern to that seen with VC [F2, 21=9.624, p<0.001; Fig. 2a]. MDA levels were highest in tissue from SVCT2(−/−) fetuses and were significantly greater than in SVCT2(+/−) or SVCT2(+/+) (p<0.05). SVCT2(+/−) and SVCT2(+/+) placental VC levels did not differ.
Cortex
MDA varied among groups in the cortex [F3, 26=6.362, p=0.002; Fig. 2b]. SVCT2(+/−) dams had lower MDA levels than SVCT2(+/−) and SVCT2(+/+) fetuses (p<0.05). Despite varying VC levels, MDA levels did not differ among fetal genotypes.
Liver
Marginally lower MDA levels were also seen in dams than fetuses in the liver but this difference was not significant [F3, 26=2.923, p=0.053; Fig. 2c].
Lung
A similar pattern to the liver MDA was also observed in lung. There were no significant differences among the groups [F3, 25=1.66, p=0.20; Fig. 2d].
F2-isoprostanes
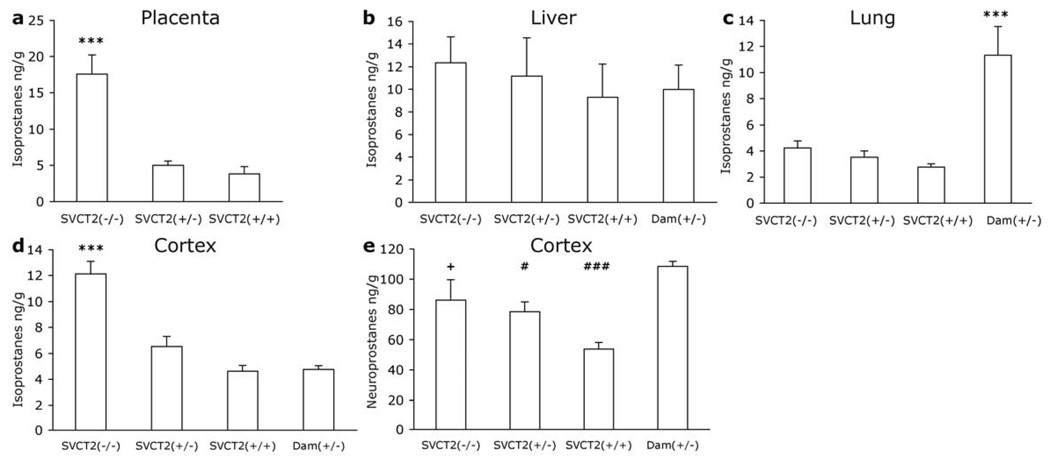
F2-isoprostanes were measured in placenta, liver, and lung. In placenta F2-isoprostanes were elevated in SVCT2(−/−) fetuses [F2, 12 = 21.441, p<0.001; Fig. 3a] compared to both SVCT2(+/−) and SVCT2(+/+) littermates (p<0.001). In the liver, there were no group differences in F2-isoprostane levels (Fig. 3b). In the lung F2-isoprostane levels in SVCT2(−/−) fetuses were 150% of those in SVCT2(+/+) littermates, and SVCT2(+/−) fetuses had an intermediate value, however, this trend was not significant (p>0.05; Fig. 3c). When fetal lung F2-isoprostanes were analyzed with maternal lung, there was a significant effect of group [F3, 21 = 12.05, p>0.001), because F2-isoprostanes were higher in lung tissue from the oxygen-exposed dam than in any of her offspring. F2-isoprostane levels were also measured in the cortex in 2 to 5 mice per group. There were significant differences among the groups [F3, 13 =20.689, p<0.001; Fig. 3d]. F2-isoprostanes were higher in cortex of SVCT2(−/−) mice than in littermates and in SVCT2(+/−) dam (p<0.001). SVCT2(+/−) and SVCT2(+/+) littermates and SVCT2(+/−) dams did not differ.
Figure 3. F2-isoprostanes and F4-neuroprostanes in fetal and maternal tissues.
F2-isoprostanes were measured in tissue samples from E19.5–20.5 fetuses and dams in (a) placenta, (b) liver, (c) lung, and (d) cortex. (e) F4-neuroprostanes were measured in cortex only. Data are expressed as mean + S.E.M. ***p<0.001 different from all other groups. #p<0.05, ###p<0.001 different from SVCT2(+/−) dam. +p<0.05 different from SVCT2(+/+).
F4-Neuroprostanes
F4-Neuroprostanes were analyzed in 5 to 9 mice per group. There was a significant group effect on neuroprostanes [F3, 24 = 9.906, p<0.001; Fig. 3e]. SVCT2(+/+) neuroprostane levels were significantly lower than SVCT2(−/−) littermates (p<0.05) and than SVCT2(+/−) dams (p<0.001) but did not differ significantly from SVCT2(+/−) littermates (p=0.057). Neuroprostanes in the dam were also greater than in SVCT2(+/−) fetuses (p<0.05).
Histology
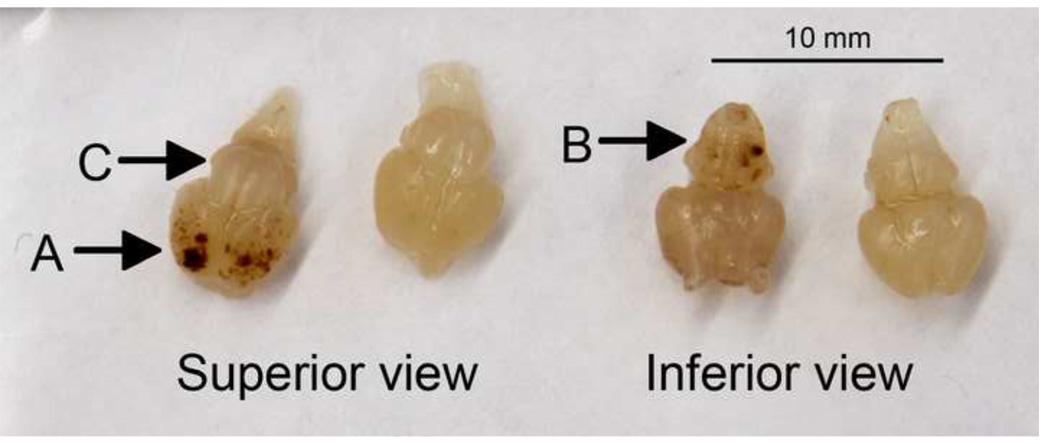
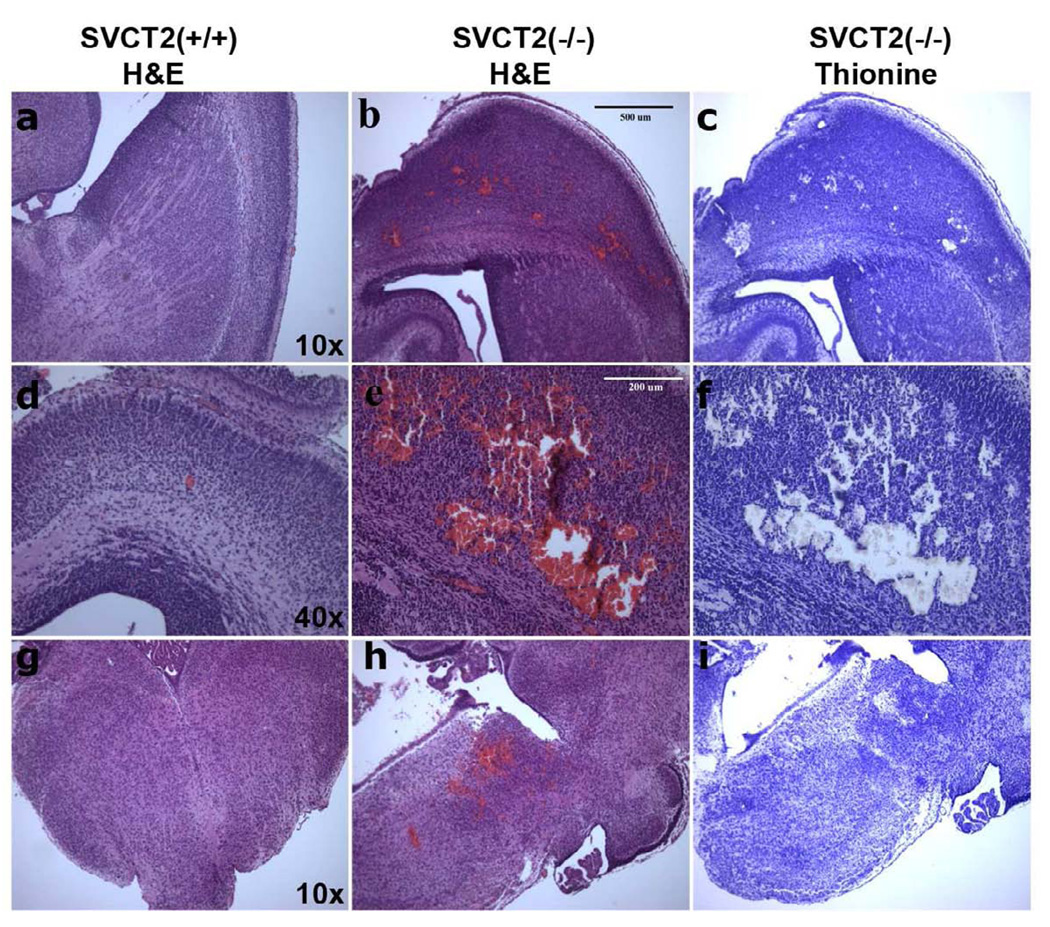
In Sotirou, et al [6], hemorrhage was reported in the cortex. In accordance with this earlier report, cortical hemorrhages in SVCT2(−/−) mice were immediately discernable upon examination of whole brain as it was removed. Hemorrhages were also observed on the underside of the SVCT2(−/−) brains in the brain stem area (Fig. 4). The cerebellum, on the other hand, was free from hemorrhage. H&E and thionine stains of consecutive brain sections revealed the extent of hemorrhage in SVCT2(−/−) mice. Large areas of hemorrhage (Fig. 5b, e, h) in cortex and brain stem of SVCT2(−/−) mice were empty of neuronal cells (Fig. 5 c, f, i).
Figure 4. Hemorrhage in fetal brains.
Photographs of brains from SVCT2(−/−) (left-hand example of each pair) and SVCT2(+/+) fetuses from above (superior) and below (inferior), showing severe hemorrhage in cortex (A) and brain stem (B), and lack of hemorrhage in the cerebellum (C) in SVCT2(−/−) mice.
Figure 5. Histology of hemorrhage and cell loss in SVCT2(−/−) and SVCT2(+/+) fetal brains.
Paraffin-embedded E18.5–19.5 brains were sliced at 5 microns on a horizontal plane and mounted on slides. Consecutive slides were stained with H&E (a, b, d, e, g, h) and thionine (c, f, i). Magnification at 4X showed no hemorrhage in SVCT2(+/+) cortex (a) and extensive hemorrhage in SVCT(−/−) cortex (b), with accompanying cell loss in SVCT(−/−) cortex (c). Cortex at 10x magnification shows the severity of hemorrhage SVCT2(−/−) mice (e–f) compared to lack of hemorrhage in SVCT2(+/+) littermates (d). Brain stem at 4x magnification shows that hemorrhage is also found in brain stem of SVCT2(−/−) mice (h–i) but not in the same region of SVCT2(+/+) mice (g).
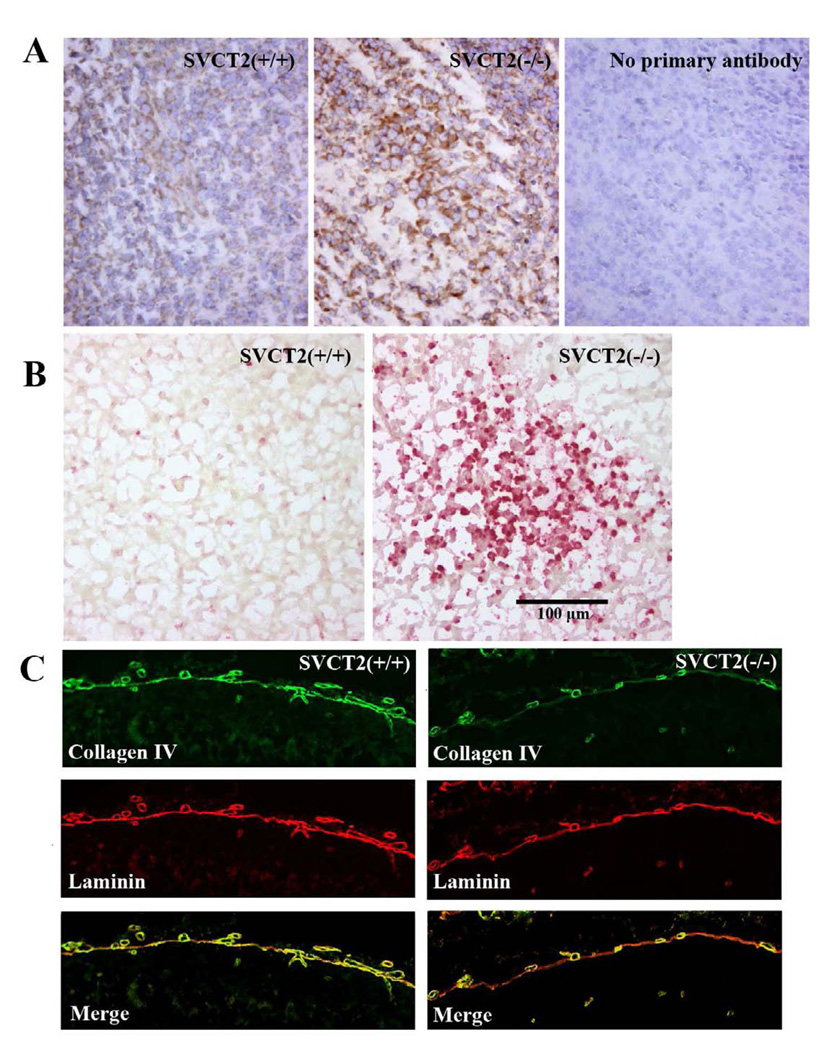
The finding of elevated oxidative stress in SVCT2(−/−) mice was confirmed by immunostaining for isoketals with the single chain variable antibody D11. Greater isoketal staining was observed in the brains of SVCT2(−/−) fetuses compared to wild-type littermates (Fig. 6a). Staining of additional sections with TUNEL stain showed there was also increased accumulation of apoptotic cells in SVCT2(−/−) mice compared to wild-types (Fig. 6b). Both stains were observed throughout the cortical tissue in discrete areas, and were often congruent.
Figure 6. Isoketal staining, apoptosis and type IV collagen in late-stage SVCT2(−/−) and SVCT2(+/+) fetuses.
(A) Isoketal immunostaining was conducted with D11 ScFv antibody in mouse cortex and counterstained with hematoxylin and eosin. Greater isoketal staining was observed in SVCT2(−/−) embryos (center panel), compared to SVCT2(+/+) fetuses (left panel). The right panel shows no primary antibody (20x magnification). (B) Localized accumulations of TUNEL-positive cells were observed in cortex of SVCT2(−/−) fetuses compared to age-matched wild-type fetuses (20x magnification). (C) Double-immunofluorescent detection of collagen IV (green) and laminin (red) was conducted in brain of SVCT2(+/+) and SVCT2(−/−) fetuses. Note collagen IV is co-localized with laminin in the basement membranes of wild type (left panel) but not SVCT2(−/−) vascular vessels (right panel) (20x magnification).
To evaluate the impact of low brain VC levels on collagen synthesis in the brains of SVCT2(−/−) fetuses, immunostains of the basement membrane for type IV collagen and laminin were compared. As can be seen in Fig. 6c, type IV collagen staining in the basement membrane was decreased in SVCT2(−/−) fetuses compared to SVCT2(+/+) fetuses. On the other hand, staining of laminin was similar in the membranes of the two genotypes. This is best demonstrated in the merged images, which show incomplete staining of the membrane for type IV collagen compared to laminin in the SVCT2(−/−) fetuses. Staining of the parietal endodermal cells was not decreased in SVCT2(−/−) fetuses, suggesting that type IV collagen was being synthesized, but not exported.
Discussion
Contrary to previous report [6], we found fewer than expected SVCT2(−/−) and SVCT2(+/−) mice in litters delivered by caesarian section, indicating that a smaller proportion of these fetuses survive until birth. This result suggests that lack of SVCT2, and therefore lack of VC in several major organs, contributes to fetal death. In both the present and the earlier study [6] SVCT2(+/−) dams were supplemented with VC in the drinking water, however the amounts given were varied. In the present study mice received 0.33 g/L, whereas Sotirou et al. provided 2.5 g/L. It is possible that the additional VC availability to the dam increased survival of the SVCT2(−/−) fetuses in utero. The mechanism by which this would have occurred is unclear, however, because neither placental tissue, nor SVCT2-dependent tissue in the fetus (including brain, lung and liver) would have been able to benefit from this additional VC.
In placenta, cortex and lung, SVCT2 genotype predictably determined VC levels. SVCT2 is present in placenta [20, 21] and is the likely source of VC transport. There was approximately 50% decrease of VC in placenta from SVCT2(−/−) mice, where half of the placental tissue derives from the SVCT2(+/−) dam. Some VC may also have been due to transport by SVCT1 which has also been detected in placenta, although it is not necessary for VC transport [22]. Following this reasoning, it is likely that the placenta from an SVCT2(+/+) fetus derived from an SVCT2(+/+) dam would have even higher VC than levels reported here, since this normal situation will have maximal VC uptake into both fetal- and maternal-derived cells. An inverse relationship exists between VC content and the levels of both MDA and F2-isoprostanes in the placenta. Both measures of oxidative stress were increased in SVCT2(−/−) placenta notwithstanding the modest decrease in overall placental VC levels supporting the contention that VC is a vital antioxidant for the fetal side of the placenta and that its entry into the fetal side is controlled by the SVCT2. Loss of only one allele of the SVCT2, resulted in approximately a 25 % decrease in VC and did not increase lipid peroxidation. Nonetheless, if combined with other oxidative stressors such as smoking, the resulting increased oxidative stress in the placenta may put the fetus at risk as it may lead to calcification of villae and inadequate nutrient transfer [23]. The direct relationship between SVCT2 genotype and placental VC content demonstrated in this study strongly suggests that in vivo placental VC uptake requires the SVCT2 and thus does not involve dehydroascorbate as has previously been suggested [24, 25].
VC levels in the lung were almost undetectable in SVCT2(−/−) fetuses and there were clear differences between each group. Thus, the role of SVCT1 in VC uptake in lung must be minimal despite its reported presence in lung tissues [4, 5]. Nevertheless, fetal lung tissue was not under additional oxidative stress, with normal MDA and F2-isoprostane levels. Although MDA levels were the same in fetal lungs compared to lungs from oxygen-exposed dams, F2-isoprostanes in the lungs of dams were between 2.5x and 4x that seen in fetuses. The lack of a clear oxidant injury in SVCT2(−/−) fetal lung tissue supports the contention that lung damage is not the cause of respiratory failure and thus death in these mice. Nevertheless, there are further areas to be investigated in determining the cause of death of these mice. Airway Surface liquid (ASL) is a critical layer protecting epithelial cells in the respiratory tract, where SVCT2 is expressed, from the external environment. VC is normally present in the ASL, although lower levels are found in diseases with adverse respiratory symptoms. VC has recently been shown to regulate cystic fibrosis trans-membrane conductance regulator (CFTR) chloride channels that determine fluid secretion [26]. Thus in the SVCT2(−/−) fetuses, it is possible that failure of these channels to open leads to ASL without sufficient water, and that ‘sticky’ lungs are therefore unable to open at birth.
VC levels were doubled in the liver of SVCT2(+/−) dams (~1.8 umol/g) relative to the levels typically observed in non-pregnant SVCT2(+/−) and SVCT2(+/+) mice (~0.8–1 μmol/g, unpublished results). A likely reason for this difference is that VC synthesis was increased in the SVCT2(+/−) dams in order to supply the growing fetuses. A similar increase in plasma VC was also observed during pregnancy in non-supplemented SVCT1(−/−) mice, with a slightly greater increase when these dams were supplemented with 0.33 g/L VC in drinking water [22]. Thus in the present study the high liver VC level in dams may be attributable to both increased synthesis and the VC supplementation, and this phenomena should be investigated further. In humans, where synthesis is not possible, VC intake must be greatly increased to supply these additional requirements. Mice synthesize VC in liver starting on about E16 [27] and this would also apply to SVCT2(−/−) fetuses. It was therefore not surprising that VC levels were similar in the fetal liver in all genotypes. The presence of SVCT1 in liver [4] may also have helped prevent VC depletion in SVCT2(−/−) mice. These data differ from the findings of Sotirou, et al. [6] who reported significantly decreased VC in fetal liver tissues in mice of approximately the same age (E18.5). The higher values in the SVCT2(+/+) and (+/−) fetuses in [6] may reflect a combination of VC-synthesis and also uptake via SVCT2 of some of the additional VC available from the high-supplemented dam. Sotirou, et al. [6] found that serum VC in fetuses was genotype–dependent, despite the fact that synthesis was presumably equivalent in all offspring. It is possible that placing the SVCT2(−/−) mice on the C57BL/6 background modified liver VC levels, but otherwise we have no explanation for the differences between the two studies. Normal VC levels in fetal livers in the present study fits with our finding that levels of oxidative stress did not vary among the genotypes. In addition to a genotype-dependent effect on VC levels in brain, spleen and muscle in adult, non-supplemented SVCT2(+/−) versus (+/+) mice, Kuo et al. [28] also observed gender differences. Female mice had greater VC levels in SVCT1-dependent tissues (kidney, liver) and plasma, and excreted less VC in urine. Gulonolactone oxidase activity did not differ among the groups indicating no gender or genotype effect on VC-synthesis. In the present study we did not record the gender of the fetuses but otherwise the fetal data agree with Kuo et al.’s results in adult mice: liver VC did not vary between SVCT2(+/−) and (+/+) genotypes but we did observe genotype differences in cortex, lung and placenta. Thus it seems that the effects of SVCT2 transporter level are maintained in SVCT2(+/−) animals from the late fetal stage throughout adulthood.
VC levels in the cortex were close to zero in SVCT2(−/−) fetuses, confirming previous findings [6]. Such low levels demonstrate that transfer of dehydroascorbate via GLUT transporters across the blood brain barrier is inadequate to supply VC to brain cells. The highest cortical VC levels were observed in SVCT2(+/+) fetuses. Higher VC levels during development and in the first few postnatal days have previously been reported [29, 30]. Differences between the SVCT2(+/+) fetus and the SVCT2(+/−) dam could be attributable solely to the extra copy of the SVCT2 carried by the fetus, or it could reflect the normal elevation in the fetus that could not be matched by the SVCT2(+/−) littermates. Previous data from our laboratory suggest there is little difference between adult SVCT2(+/+) and SVCT2(+/−) mice, but the difference seen here may be attributable to both reasons. MDA was greater in the cortex of the fetuses compared to dams, although there were no differences among the genotypes. In contrast, both F2-isoprostanes and F4-neuroprostanes, which are more specific markers of lipid peroxidation [31], were significantly greater in SVCT2(−/−) fetuses than in SVCT2(+/+) littermates. However, the elevations observed were modest and isoketal staining showed that they were restricted to certain regions not obviously associated with hemorrhage. This would also account for the finding of spotty distribution of apoptosis, as measured by Tunel staining. Although these areas of lipid peroxidation and cortical cell death may not be large enough to generate a dramatic lipid peroxidation signal or cause death in the mouse, they nonetheless show that VC provides vital protection against lipid peroxidation in the developing fetal brain. In this experiment TUNEL and isoketal staining were not performed on serial sections throughout the brain but were identified in a number of different sections of cortical tissue. In future, a more thorough examination should explore localization, and co-localization of these phenomena, along with hemorrhage, and other markers of damage such as inflammatory response. Such a study may help to elucidate how the different factors interrelate.
In the present study we report brain VC levels of 2.9 – 4.0 µmol/g in SVCT2(+/−) and (+/+) mice. These are comparable to fetal rat brain levels of 637–712 mg/g (approximately 3.6–4 µmol/g) at a similar gestational age [29] and also to adult SVCT2(+/−) and (+/+) brain levels 2.6 – 4.2 nmol/mg (µmol/g) [28], and other studies that use adult wild-type mice (2–3.8 µmol/g) [13, 32]. In the liver, we report SVCT2(+/−) and (+/+) levels of approximately 0.9 µmol/g and up to 2.0 µmol/g in the dam which are within the range of other reports in adult SVCT2(+/−) and (+/+) mice [28]. Wild-type mouse liver is typically found to be between 0.8 and 1.0 µmol/g [13, 32]. Overall our data are within normal range compared to other data, although fetal brain was a little lower than might be expected given that fetal brain tissues are typically much higher in VC than adult brain. Unfortunately, due to idiosyncratic measurement reporting in [6], we are unable to directly compare our data to the other report of fetal SVCT2 mice. Some differences may be accounted for by use of alternative methods of measurement but more importantly, all fetuses, even SVCT2(+/+) had lower SVCT2, and therefore transfer of VC, across the placenta due to SVCT2(+/−) genotype of dam.
The cause of cortical and brainstem hemorrhage in the SVCT(−/−) fetuses is most likely due to failure to supply enough VC for synthesis of vascular type IV collagen for the basement membranes of brain microvessels. In support of this notion, our results show markedly decreased type IV collagen in fetal basement membrane in comparison to laminin. On the other hand, parietal endodermal cells responsible for synthesis of the type IV collagen in the basement membrane stain strongly for type IV collagen in SVCT2(−/−) fetuses, reflecting the expected cellular retention of under-hydroxylated type IV procollagen as well as a defect in collagen secretion. That defective type IV collagen deposition causes capillary hemorrhage in SVCT2(−/−) fetuses is also supported by a similar punctate pattern of hemorrhage and decrease in basement membrane type IV collagen seen in mice carrying a mutation of type IVa1 collagen, which is a cause of cortical damage and reabsorption in the human syndrome of porencephaly [33]. Why hemorrhage is restricted to the brain and is not observed in other organs is unclear, but is likely due to a severe deficiency of VC in brain vascular endothelial cells relative to endothelial cells in other organs. This in turn is due to the fact that brain capillary endothelial cells from normal mice do not express the SVCT2 [34, 35], while it is prominently expressed in endothelial cells from other organs [36, 37]. The supply of VC for type IV collagen synthesis in brain vascular endothelial cells might thus be limited, so that a deficiency of the SVCT2 in supporting pericytes and in the sub-endothelial region causes frank deficiency in the endothelial cells leading to decreased hydroxylation and extrusion of type IV collagen.
Regarding the cause of death in the SVCT2 fetuses, we conclude that it is likely due to loss of neurons and supporting cells in crucial brain areas due to hemorrhage and oxidative stress. Sotiriou, et al. [6] noted hemorrhage in the cortex of SVCT2(−/−) mice, and we found it also in the brainstem region. Damage to the brain stem, which is responsible for regulation of many autonomic functions, including respiration, is a very likely candidate for cause of death in these mice. In support of this notion, we previously showed that sequential depletion of both vitamins E and C in guinea pigs caused an ascending paralysis and death [38]. The cause of the neurologic deficits proved to be neuronal degeneration and fiber loss in the pons and upper spinal cord [39]. Another factor to consider regarding the death of SVCT(−/−) fetuses is that it occurred largely, although not entirely, at parturition. The trauma of birth would be expected to increase hemorrhage from fragile brain capillaries [33] and acutely impair the respiratory drive and other brain functions.
There may be clinical relevance in these data that support the need for VC during gestation. It is typically assumed that most western diets are nutritionally replete. However, sub-clinical VC deficiency has been identified in a number of populations including pregnant and lactating women [40–42]. Extrapolation from the data presented here suggests that fetuses of women who do not ingest sufficient vitamin C during pregnancy could be at risk for elevated oxidative stress in the brain as well as in placenta. It is not yet known whether or how this may impact fetal development. We have previously reported deficits in sensorimotor deficits in gulo(−/−) mice that are unable to synthesize their own VC [13]. We hypothesize that damage to the brain occurs during pregnancy or lactation and that both decreased supply from gulo(−/−) dams and inability to synthesize VC in the gulo(−/−) fetuses were the cause.
In conclusion, the present results show that VC and the SVCT2 are crucial to prevent capillary hemorrhage and death in mice. The role of VC during pregnancy is not limited to maintaining strong membranes in the uterus and preventing preterm delivery. Extrapolations from these data to humans indicated the vital importance of ensuring that pregnant women are adequately supplemented with VC to prevent developmental abnormalities, including death of the fetus, which may be brought about by low VC and elevated oxidative stress.
Acknowledgements
This work was supported by NIH grant NS057674-03 to J.M.M. and NIH grant DK59637 to the Lipid, Lipoprotein and Atherosclerosis Core of the Vanderbilt Mouse Metabolic Phenotype Centers. The authors would like to thank Lewis F Pennock Jr for the photography of fetal brains.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.Lind J. A Treatise on the scurvy in three parts: containing an inquiry into the nature, causes, and cures of that disease: together with a critical and chronological view of what has been published on the subject. Birmingham, Ala: Classics of Medicine Library; 1980. [Google Scholar]
- 2.Hughes RE, Hurley RJ, Jones PR. The retention of ascorbic acid by guinea-pig tissues. Br J Nutr. 1971;26:433–438. doi: 10.1079/bjn19710048. [DOI] [PubMed] [Google Scholar]
- 3.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 5.Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids. 2007 doi: 10.1007/s00726-007-0555-7. [DOI] [PubMed] [Google Scholar]
- 6.Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, Hoogstraten-Miller S, Miller GF, Kwon O, Levine M, Guttentag SH, Nussbaum RL. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med. 2002;8:514–517. doi: 10.1038/0502-514. [DOI] [PubMed] [Google Scholar]
- 7.Bornstein SR, Yoshida-Hiroi M, Sotiriou S, Levine M, Hartwig HG, Nussbaum RL, Eisenhofer G. Impaired adrenal catecholamine system function in mice with deficiency of the ascorbic acid transporter (SVCT2) Faseb J. 2003;17:1928–1930. doi: 10.1096/fj.02-1167fje. [DOI] [PubMed] [Google Scholar]
- 8.Eldridge CF, Bunge MB, Bunge RP, Wood PM. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol. 1987;105:1023–1034. doi: 10.1083/jcb.105.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cammack J, Ghasemzadeh B, Adams RN. The pharmacological profile of glutamate-evoked ascorbic acid efflux measured by in vivo electrochemistry. Brain Res. 1991;565:17–22. doi: 10.1016/0006-8993(91)91731-f. [DOI] [PubMed] [Google Scholar]
- 10.Miele M, Boutelle MG, Fillenz M. The physiologically induced release of ascorbate in rat brain is dependent on impulse traffic, calcium influx and glutamate uptake. Neuroscience. 1994;62:87–91. doi: 10.1016/0306-4522(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 11.Grunewald RA. Ascorbic acid in the brain. Brain Res Brain Res Rev. 1993;18:123–133. doi: 10.1016/0165-0173(93)90010-w. [DOI] [PubMed] [Google Scholar]
- 12.Rebec GV, Pierce RC. A vitamin as neuromodulator: ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog Neurobiol. 1994;43:537–565. doi: 10.1016/0301-0082(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 13.Harrison FE, Yu SS, Van Den Bossche KL, Li L, May JM, McDonald MP. Elevated oxidative stress and sensorimotor deficits but normal cognition in mice that cannot synthesize ascorbic acid. J Neurochem. 2008;106:1198–1208. doi: 10.1111/j.1471-4159.2008.05469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci U S A. 2000;97:841–846. doi: 10.1073/pnas.97.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pachla LA, Kissinger PT. Analysis of ascorbic acid by liquid chromatography with amperometric detection. Methods Enzymol. 1979;62:15–24. doi: 10.1016/0076-6879(79)62183-3. [DOI] [PubMed] [Google Scholar]
- 16.May JM, Qu ZC, Mendiratta S. Protection and recycling of alpha-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys. 1998;349:281–289. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- 17.Harrison FE, May JM, McDonald MP. Vitamin C deficiency increases basal exploratory activity but decreases scopolamine-induced activity in APP/PSEN1 transgenic mice. Pharmacol Biochem Behav. 2009 doi: 10.1016/j.pbb.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts LJ, 2nd, Montine TJ, Markesbery WR, Tapper AR, Hardy P, Chemtob S, Dettbarn WD, Morrow JD. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273:13605–13612. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- 19.Davies SS, Amarnath V, Roberts LJ., 2nd Isoketals: highly reactive gamma-ketoaldehydes formed from the H2-isoprostane pathway. Chem Phys Lipids. 2004;128:85–99. doi: 10.1016/j.chemphyslip.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Rajan DP, Huang W, Dutta B, Devoe LD, Leibach FH, Ganapathy V, Prasad PD. Human placental sodium-dependent vitamin C transporter (SVCT2): molecular cloning and transport function. Biochem Biophys Res Commun. 1999;262:762–768. doi: 10.1006/bbrc.1999.1272. [DOI] [PubMed] [Google Scholar]
- 21.Biondi C, Pavan B, Dalpiaz A, Medici S, Lunghi L, Vesce F. Expression and characterization of vitamin C transporter in the human trophoblast cell line HTR-8/SVneo: effect of steroids, flavonoids and NSAIDs. Mol Hum Reprod. 2007;13:77–83. doi: 10.1093/molehr/gal092. [DOI] [PubMed] [Google Scholar]
- 22.Corpe CP, Tu H, Eck P, Wang J, Faulhaber-Walter R, Schnermann J, Margolis S, Padayatty S, Sun H, Wang Y, Nussbaum RL, Espey MG, Levine M. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J Clin Invest. 2010 doi: 10.1172/JCI39191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klesges LM, Murray DM, Brown JE, Cliver SP, Goldenberg RL. Relations of cigarette smoking and dietary antioxidants with placental calcification. Am J Epidemiol. 1998;147:127–135. doi: 10.1093/oxfordjournals.aje.a009424. [DOI] [PubMed] [Google Scholar]
- 24.Choi JL, Rose RC. Transport and metabolism of ascorbic acid in human placenta. Am J Physiol. 1989;257:C110–C113. doi: 10.1152/ajpcell.1989.257.1.C110. [DOI] [PubMed] [Google Scholar]
- 25.Rybakowski C, Mohar B, Wohlers S, Leichtweiss HP, Schroder H. The transport of vitamin C in the isolated human near-term placenta. Eur J Obstet Gynecol Reprod Biol. 1995;62:107–114. doi: 10.1016/0301-2115(95)02117-p. [DOI] [PubMed] [Google Scholar]
- 26.Fischer H, Schwarzer C, Illek B. Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci U S A. 2004;101:3691–3696. doi: 10.1073/pnas.0308393100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kratzing CC, Kelly JD. Ascorbic acid synthesis by the mammalian fetus. Int J Vitam Nutr Res. 1986;56:101–103. [PubMed] [Google Scholar]
- 28.Kuo SM, MacLean ME, McCormick K, Wilson JX. Gender and sodium-ascorbate transporter isoforms determine ascorbate concentrations in mice. J Nutr. 2004;134:2216–2221. doi: 10.1093/jn/134.9.2216. [DOI] [PubMed] [Google Scholar]
- 29.Kratzing CC, Kelly JD, Kratzing JE. Ascorbic acid in fetal rat brain. J Neurochem. 1985;44:1623–1624. doi: 10.1111/j.1471-4159.1985.tb08804.x. [DOI] [PubMed] [Google Scholar]
- 30.Terpstra M, Tkac I, Rao R, Gruetter R. Quantification of vitamin C in the rat brain in vivo using short echo-time 1H MRS. Magn Reson Med. 2006;55:979–983. doi: 10.1002/mrm.20854. [DOI] [PubMed] [Google Scholar]
- 31.Roberts LJ, 2nd, Fessel JP. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Chem Phys Lipids. 2004;128:173–186. doi: 10.1016/j.chemphyslip.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Harrison FE, Hosseini AH, Dawes SM, Weaver S, May JM. Ascorbic acid attenuates scopolamine-induced spatial learning deficits in the water maze. Behav Brain Res. 2009;205:550–558. doi: 10.1016/j.bbr.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, Aguglia U, van der Knaap MS, Heutink P, John SW. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–1171. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- 34.Garcia Mde L, Salazar K, Millan C, Rodriguez F, Montecinos H, Caprile T, Silva C, Cortes C, Reinicke K, Vera JC, Aguayo LG, Olate J, Molina B, Nualart F. Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia. 2005;50:32–47. doi: 10.1002/glia.20133. [DOI] [PubMed] [Google Scholar]
- 35.Qiao H, May JM. Development of ascorbate transporters in brain cortical capillary endothelial cells in culture. Brain Res. 2008;1208:79–86. doi: 10.1016/j.brainres.2008.02.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiao H, Li L, Qu ZC, May JM. Cobalt-induced oxidant stress in cultured endothelial cells: prevention by ascorbate in relation to HIF-1alpha. Biofactors. 2009;35:306–313. doi: 10.1002/biof.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steiling H, Longet K, Moodycliffe A, Mansourian R, Bertschy E, Smola H, Mauch C, Williamson G. Sodium-dependent vitamin C transporter isoforms in skin: Distribution, kinetics, and effect of UVB-induced oxidative stress. Free Radic Biol Med. 2007;43:752–762. doi: 10.1016/j.freeradbiomed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Hill KE, Montine TJ, Motley AK, Li X, May JM, Burk RF. Combined deficiency of vitamins E and C causes paralysis and death in guinea pigs. Am J Clin Nutr. 2003;77:1484–1488. doi: 10.1093/ajcn/77.6.1484. [DOI] [PubMed] [Google Scholar]
- 39.Burk RF, Christensen JM, Maguire MJ, Austin LM, Whetsell WO, Jr, May JM, Hill KE, Ebner FF. A combined deficiency of vitamins E and C causes severe central nervous system damage in guinea pigs. J Nutr. 2006;136:1576–1581. doi: 10.1093/jn/136.6.1576. [DOI] [PubMed] [Google Scholar]
- 40.Johnston CS, Thompson LL. Vitamin C status of an outpatient population. J Am Coll Nutr. 1998;17:366–370. doi: 10.1080/07315724.1998.10718777. [DOI] [PubMed] [Google Scholar]
- 41.Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci U S A. 2001;98:9842–9846. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salmenpera L. Vitamin C nutrition during prolonged lactation: optimal in infants while marginal in some mothers. Am J Clin Nutr. 1984;40:1050–1056. doi: 10.1093/ajcn/40.5.1050. [DOI] [PubMed] [Google Scholar]