Abstract
Non-insulin-dependent diabetes mellitus (NIDDM, type 2 diabetes) is a disorder of glucose homeostasis characterized by hyperglycaemia, peripheral insulin resistance, impaired hepatic glucose metabolism, and diminished glucose-dependent secretion of insulin from pancreatic β-cells1. Glucagon-like-peptide-1(7-37) (GLP-1)2 is an intestinally derived hormone that may be useful for the treatment of NIDDM because it acts in vivo to increase the level of circulating insulin, and thus lower the concentration of blood glucose3,4. This therapeutic effect may result from the ability of GLP-1 to compensate for a defect in the glucose signalling pathway that regulates insulin secretion from β-cells. In support of this concept we report here that GLP-1 confers glucose sensitivity to glucose-resistant β-cells, a phenomenon we term glucose competence. Induction of glucose competence by GLP-1 results from its synergistic interaction with glucose to inhibit metabolically regulated potassium channels that are also targeted for inhibition by sulphonylurea drugs commonly used in the treatment of NIDDM5. Glucose competence allows membrane depolarization, the generation of action potentials, and Ca2+ influx, events that are known to trigger insulin secretion6,7.
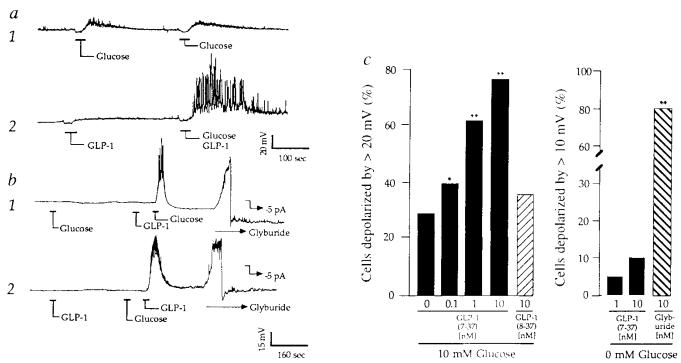
GLP-1 exerts diverse insulinotropic actions on β-cells that include stimulation of cyclic AMP formation8, insulin secretion9, insulin biosynthesis and proinsulin gene expression10. Because all insulinotropic actions of GLP-1 are dependent on simultaneous exposure of β-cells to glucose10,11, GLP-1 may act as a hormonal regulator, or modulator, of the β-cell glucose signalling system. To investigate this possibility, perforated-patch12-14 and cell-attached-patch15 recordings were obtained from solitary β-cells isolated from dispersed rat islets of Langerhans and matained in short-term primary cell culture16. For unknown reasons, these single, isolated β-cells exhibit reduced sensitivity to glucose as measured by glucose-induced insulin secretion17, electrical activity18 or calcium signalling19. When perforated-patch recordings were obtained from such cells under conditions in which the bathing solution contained no glucose, the depolarizing responses to 10 mM glucose were often ⩽10–15 mV in amplitude, and glucose did not always initiate repetitive spiking activity as is frequently observed in intact islets6 (Fig. 1a trace 1). In addition, in the absence of glucose very little change in membrane potential was recorded in response to 10 nM GLP-1 (Fig. 1a left of trace 2). In marked contrast, a 30–35 mV depolarization accompanied by repetitive spiking activity was often observed when glucose and GLP-1 were applied simultaneously (Fig. 1a right of trace 2). This synergistic interaction indicates that GLP-1 acts in a manner analogous to that of a modulatory transmitter. It enhances the reponsiveness of β-cells to glucose, yet is without effect in the absence of glucose.
FIG. 1.
Synergism of glucose and GLP-1 to depolarize β-cells. a, Trace 1: membrane potential recordings from a β-cell exhibiting weak sensitivity to 10 mM glucose repeatedly applied for 30 s as indicated. Trace 2: recordings from the same cell during application of 10 nM GLP-1 (left) or combined application of 10 mM glucose and 10 nM GLP-1 (right). Initial resting potential (Vm)−62 mV. b, trace 1: a cel1 that initially failed to respond to 10 mM glucose, but which responded to 10 mM glucose after pretreatment with 10 nM GLP-1 (Vm −66 mV). Trace 2: a cell that initially failed to respond to 10 nM GLP-1, but which responded to 10 nM GLP-1 after pretreatment with 10 mM glucose (Vm −60 mV). The initial insensitivity of β-cells to glucose or GLP-1 did not result from their failure to express functional lKATP channels because application of 10 nM glyburide resulted in rapid membrane depolarization (42 ± 11 mV, ±s.e.m., n = 10 cells), as illustrated (b, traces 1,2). b, −5 pA of hyperpolarizing pipette current was applied to repolarize the membrane after application of 10 nM glyburide. Repolarization confirms the integrity of the membrane/pipette seal. c, Cumulative dose–response analysis summarizing the interaction of glucose and GLP-1 to depolarize β-cells. The action of GLP-1(7-37) but not GLP-1(8-37) exhibited dose-dependence under conditions in which the test solution also contained 10 mM glucose (left). In contrast, the action of GLP-1(7-37) but not 10 nM glyburide was abrogated when the test solution contained no added glucose (right). Statistical significance was determined by Student's t-test. Probability values (*P ⩽ 0.05, **P ⩽ 0.005) are expressed relative to control (10 mM glucose and no GLP-1). c, The number of cells tested was 50 for GLP-1(7-37) and 10 mM glucose, 25 for GLP-1(8-37) and 10 mM glucose, 20 for GLP-1(7-37) and 0 mM glucose, and 20 for glyburide and 0 mM glucose. The EC50 value for GLP-1(7-37) was calculated as 1 nM by establishing a cumulative dose–response relationship in which the action of the peptide was assessed over a concentration range of 0.01–100 nM. The EC50 was defined as the concentration of GLP-1(7-37) that depolarized 50% of the cells by ⩾20 mV when the cells were simultaneously challenged with 10 mM glucose. METHODS. Islets were isolated from pancreata of male rats (200–250 g) by collagenase digestion, suspended in culture medium (RPMI 1640 containing 11.1 mM glucose, 10% fetal bovine serum, 100 μg ml−1 streptomycin, 100 U ml−1 penicillin G), and maintained in culture 1–4 days. Single-cell suspensions were prepared by trypsin–EDTA digestion and mechanical dispersion of cultured islets, and cells were plated on Falcon tissue culture dishes coated with concanavalin-A for short-term (⩾16 h) culture. Under these conditions, ⩾90% of the 10–15 μm diameter cells secrete insulin, as determined by the reverse haemolytic plaque assay16, and are therefore considered to be β-cells. The fact that ~80% of our cells responded to glyburide indicates that the primary rat cell cultures are indeed enriched with β-cells. For perforated-patch recordings the pipette solution contained 240 μg ml−1 nystatin and (in mM): 10KCl, 10NaCl, 70K2SO4, 2MgCl2, 10HEPES (pH 7.35, 295 mOsm). In Figs 1-3 the bath solution contained 138 NaCl, 5.6 KCl, 1.2 MgCl2, 2.6 CaCl2, 10 HEPES (pH 7.35, 300 mOsm), and was maintained at 32 °C by a peltier device mounted on the stage of an inverted microscope. All recordings were obtained from solitary cells not organized in clusters, and recordings were initiated within 30 min after transfer of the cells to the glucose-free solution. Pipettes were prepared from borosilicate glass capillary tubes and fire polished to tip resistances of 2–8 MΩ. Measurements were made using an EPC-9 amplifier (bandwidth 10 kHz) interfaced with the Instrutech Data Aquisition and Analysis system (Instrutech Corp., Mineola, NY). The signal was stored on videotape, low-pass filtered (0.5–3.0 kHz, 4-pole Bessel filter, −3 dB attenuation), digitized (100 Hz for recordings of the membrane potential, 5–10 kHz for unitary current recordings), and selected recordings were sampled. Following seal formation, perforation was achieved within 5–10 min, at which time values for the series resistance (Rs), slow capacitance compensation (Cs), and the resting membrane potential were 10–20 MΩ, 6–8 pF and −61 ± 5 mV (n = 50), respectively. No correction was made for liquid junction potentials. Test substances were applied by pressure ejection from ‘puffer’ pipettes. The kinetics of this delivery system were established by measuring the rates of onset (time constant, τ, 1.5 s) and offset (τ, 10 s) of 60 mM KCl-induced depolarizations. The change in membrane potential in response to glucose or GLP-1 was measured as the difference between the resting potential and the plateau potential. GLP-1 was obtained from Scios Nova.
The interaction of GLP-1 and glucose is remarkable in that the timing of the application of the two substances need not be simultaneous. Glucose-insensitive β-cells were rendered glucose-competent (capable of responding to glucose) by pretreatment with GLP-1 (Fig. 1b trace 1). Conversely, GLP-1-insensitive cells were rendered GLP-1-sensitive by prior application of glucose (Fig. 1b trace 2). These ‘priming’ effects associated with GLP-1 or glucose pretreatment may reflect either the prolonged action of cytosolic second messengers and/or slow dissociation of GLP-1 from its receptor.
Synergism between glucose and GLP-1 was dose-dependent (effector concentration for half maximum response, EC50, 1 nM), and exhibited pharmacological specificity because deletion of a single amino acid at the amino terminus to yield GLP-1(8-37)21 abrogated activity (Fig. 1c, left). For these reasons, the action of GLP-1 is mediated by a receptor with pharmacological properties similar to that which mediates GLP-1-induced stimulation of insulin secretion from insulinoma cell lines10,22,23. That the action of GLP-1 is in fact a receptor-mediated process is supported by a recent report of the complementary DNA cloning of a β-cell GLP-1 receptor that binds GLP-1 (but not glucagon) with high affinity and which stimulates cAMP production24.
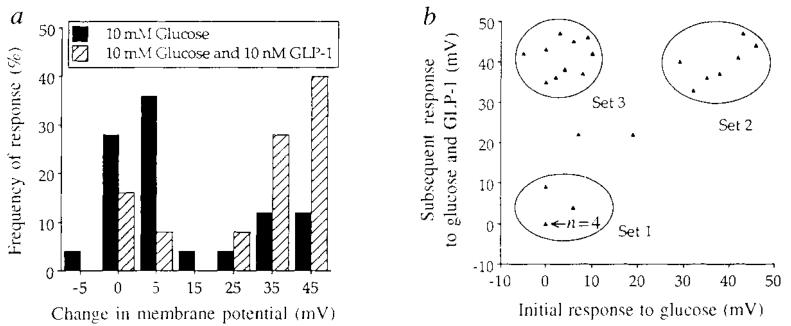
The induction of glucose competence by GLP-1 was specific for a distinct subpopulation of β-cells (Fig. 2a, b). When initially challenged with 10 mM glucose for 30 s, most cells exhibited either no response or a small (⩽15 mV) depolarizing response (Fig. 2a, cross-hatched bars). The glucose-sensitivity of individual β-cells therefore is diminished in comparison to β-cells of whole, intact islets. In contrast, when glucose-insensitive β-cells were subsequently challenged with a combined application of glucose and GLP-1, most cells were depolarized by ⩾30 mV (Fig. 2a, cross-hatched bars). Scatter plot analysis revealed that the interaction between GLP-1 and glucose segregated among three distinct sets of observations (Fig. 2b, sets 1–3). Cells included in set 2 comprised 28% of all cells tested and were fully responsive to glucose (that is, constitutively glucose-competent). In contrast, cells included in sets 1 and 3 exhibited very little response when initially challenged with glucose, and these comprised 64% of all cells tested. When the cells that showed a poor initial response to glucose were subsequently challenged with glucose and GLP-1, introduction of glucose competence was observed in 40% of all cells tested, and it was this subpopulation of β-cells that comprised set 3. These findings are consistent with previous reports of micro-heterogeneity among β-cells16,17,19.
FIG. 2.
A frequency-of-response histogram (a) and scatter plot (b) analysis summarizing the interaction of glucose and GLP-1 to depolarize β-cells. When initially challenged with 10 mM glucose the majority of cells exhibited a ⩽15mV depolarizing response, as indicated by either the fully shaded histogram bars in a, or the position of the triangles relative to the x-axis in b (where one triangle equals one cell except for the n = 4 cells triangle). When subsequently challenged with a combined application of 10 mM glucose and 10 nM GLP-1, the distribution of the histogram plot was shifted to the right (a, cross-hatched bars) and a subpopulation of β-cells labelled set 3 was rendered glucose competent (b, as indicated by the position of the triangles relative to the y-axis). In contrast, cells that comprised set 2 exhibited a dose-dependent depolarizing response to glucose over a concentration range of ~7–20 mM glucose and were constitutively glucose competent. Each illustration depicts results obtained from the same 25 cells. Test substances were applied for 30 s at 10-min intervals, and only cells exhibiting a resting membrane potential of at least −55 mV were included in the data analysis. More prolonged (3–5 min) application of 10 or 20 mM glucose (without GLP-1) to glucose-insensitive β-cells that comprised sets 1 and 3 did not significantly increase the magnitude of their depolarizing response. Such glucose resistance may reflect metabolic dysfunctions resulting from disaggregation of the islets and the loss of cell-to-cell contacts, or alternatively the loss of paracrine/endocrine influences that regulate glucose responsiveness.
To begin to define the signalling system by which GLP-1 acts, the actions of membrane-permeant cAMP analogues were tested for their effects on the induction of glucose competence. As summarized in Table 1, in β-cells that initially exhibited a weak depolarizing response (<15 mV) to 10 mM glucose, the application of 10 μM Rp-cAMPS, an antagonist of endogenous cAMP signalling pathways, inhibited the synergistic depolarizing interaction between glucose and GLP-1. In contrast, 10 μM of the cAMP agonist Sp-cAMPS potentiated the depolarizing response to glucose even in the absence of GLP-1. These findings suggest that cAMP-mediated signalling systems are necessary for the induction of glucose competence by GLP-1. Previous studies have implicated cAMP-mediated signalling systems in the regulation of the β-cell membrane potential25,26, possibly by controlling the activity of ATP-sensitive potassium channels (IkATP) and/or voltage-sensitive calcium channels14,27-29.
TABLE 1.
Effects of membrane-permeant analogues of cAMP on the interaction of glucose and GLP-1(7-37) to depolarize rat pancreatic β-cells
| Treatment | Number of cells tested |
mV Change in membrane potential (±s.e.m.) |
|---|---|---|
| 10 mM glucose | 10 | 12 ± 5 |
| 10 mM glucose | 5 | 35 ± 10* |
| 10 nM GLP-1(7-37) | ||
| 10 mM glucose | 5 | 8 ± 6 |
| 10 μM Rp-cAMPS | ||
| 10 mM glucose | 5 | 10 ± 7 |
| 10 nM GLP-1(7-37) | ||
| 10 μM Rp-cAMPS | ||
| 10 mM glucose | 5 | 40 ± 12* |
| 10 μM Sp-cAMPS |
Sp- and Rp-cAMPS were obtained from BioLog Life Science Institute, La Jolla, CA and prepared as 1 mM stock solutions in distilled water. The analogues were then diluted to a final concentration of 10 μM in the standard extracellular recording solution described in Fig. 1. Relatively glucose-insensitive β-cells were initially identified by testing for their inability to generate a substantial depolarizing response to 10 mM glucose applied for 30 s. After allowing 5 min recovery, such cells were subsequently challenged with a 30 s application of test solutions containing the indicated concentrations of glucose, GLP-1(7-37), and Sp-cAMPS. For those experiments examining the action of Rp-cAMPS the cells were pretreated in 10 μM Rp-cAMPS for 30 min at 37 °C. The cells were then challenged with the indicated test solutions containing glucose, GLP-1(7-37), and Rp-cAMPS. Statistical significance was evaluated by Student's t-test.
A value that is significantly different (P ⩽ 0.05) from control (10 mM glucose alone).
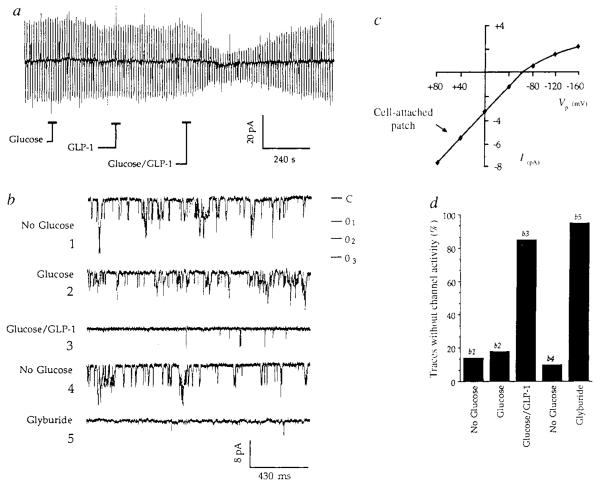
To begin to define the biophysical basis for induction of glucose competence by GLP-1, attention was focused on β-cells that exhibited a ⩽10mV depolarizing response when initially challenged with glucose. Voltage clamp analysis was done in the perforated patch configuration and the effects of glucose and GLP-1 on the resting membrane conductance were monitored under conditions in which the bath solution contained no glucose. Brief application of 10 mM glucose or 10 nM GLP-1 was without significant effect on membrane conductance, whereas the conductance was reversibly inhibited by combined application of both test substances (Fig. 3a). A very similar inhibitory action was also recorded during application of 10 nM glyburide (a sulphonylurea that blocks IKATP with high specificity6,20) to these same cells (data not shown). These findings suggest that glucose and GLP-1 synergize to depolarize β-cells by closing sulphonylurea-sensitive potassium channels (IKATP channels) that are open at or near the resting potential.
FIG. 3.
Glucose and GLP-1 synergize to decrease the resting membrane conductance and inhibit the activity of single lKATP channels, a, The membrane conductance was monitored by perforated-patch recording in the voltage clamp mode under conditions in which the bath solution contained no added glucose. Application for 30 s of either 10 mM glucose or 10 nM GLP-1 was without significant effect on the magnitude of evoked current responses to ±10 mV voltage steps from a holding potential of −80 mV, whereas the evoked currents were reversibly inhibited by simultaneous application of 10 mM glucose and 10 nM GLP-1. The conductance decreased 80% from 2.0 nS to 0.4 nS and no shift in the holding current was observed. Outward currents are indicated by upward deflections, b, Cell-attached patch recordings of unitary inward currents measured when the bath solution contained no added glucose (traces 1 and 4), 20 mM glucose (trace 2), 20 mM glucose and 10 nM GLP-1 (trace 3), or 10 nM glyburide (trace 5), each applied for 30 s. Inward currents are indicated by downward deflections from a closed level (C) to three superimposed levels of openings (01–3). Filter 1 kHz, sample rate 5kHz. c, The l–V relationship for unitary currents recorded in the cell-attached patch configuration. The slope of the l–V relationship decreased at very negative pipette potentials (inward rectification), reversed direction when the pipette potential (Vp) was more negative than −70 mV, and the single channel conductance inferred from the slope of the linear portion of the l–V relationship indicated a value of 60 pS, as expected for lKATP6,20 d, Histogram analysis summarizing the actions of glucose, GLP-1, and glyburide, as illustrated in b, traces 1–5, to inhibit lKATP. The effects of these test substances were assessed by determining the frequency of occurrence of 500-ms oscilloscope traces that exhibited no channel activity (blanks). Fifty traces were recorded before, during the peak effect, and after recovery from each test substance.
METHODS, a, For measurements of the resting membrane conductance the command potential was shifted by ±10 mV for 1.5 s at a frequency of 0.1 Hz. The conductance was monitored while simultaneously compensating for the series resistance by 80%. Glucose and GLP-1 were applied at 4-min intervals to avoid priming effects that are observed at shorter intervals, b, For cell-attached-patch recordings the pipette solution contained (in mM): 140 KCI, 5CaCI2, 5MgCI2, 10 HEPES (pH adjusted to 7.35 with KOH, 305 mOsm) and Vp was +50 mV. Test substances were applied at 10-min intervals, and the current traces illustrated are representative of the peak effects observed. In b, trace 2, the relatively small effect of 20 mM glucose on channel activity was accompanied by a decreased unitary current amplitude, suggestive of a decreased driving force for K+, possibly due to a depolarizing action of glucose at the whole-cell level. The decreased unitary current amplitude observed in trace 2 may therefore suggest an action of glucose to depolarize β-cells, not simply by inhibiting lKATP channels, but also by inducing an uncharacterized conductance change occurring in the membrane outside the patch.
To characterize the channels inhibited by glucose and GLP-1, cell-attached patch recordings were obtained under conditions that allow analysis of inwardly-directed IKATP currents. Baseline activity of single IKATP channels exhibited a mean unitary current amplitude of 6.0 pA (pipette potential +50 mV) when the bath solution contained no added glucose (Fig. 3b trace 1), and the single channel conductance inferred from the unitary current amplitude as a function of voltage (I–V) relationship was 60 pS (Fig. 3c), as expected for IKATP6,7. In 6 of 10 cells tested, application of 10–20 mM glucose had very little effect on channel activity (Fig. 3b trace 2), whereas a decrease in channel activity similar to that previously reported6,30 was recorded from 4 cells (data not shown). When the initially glucose-insensitive cells were challenged with a combined application of 20 mM glucose and 10 nM GLP-1, the channel activity was decreased in two cells and was nearly eliminated in four cells (Fig. 3b trace 3). In addition, the unitary current amplitude decreased to 4.2 pA, as expected if glucose/GLP-1-induced whole-cell depolarization resulted in a decreased driving force for K+. Channel activity recovered (Fig. 3b trace 4) and was then blocked by glyburide (Fig. 3b trace 5), indicating that these currents are in fact attributable to IKATP. This experiment is summarized in Fig. 3d which illustrates the maximal inhibitory effects of glucose, GLP-1, and glyburide on IKATP activity, expressed as the percentage of 500 ms oscilloscope traces (n = 50 traces per treatment) that exhibited no channel activity (blanks). Because IKATP channels within the patch were inhibited by application of glucose and GLP-1 to the membrane outside the patch, a cytosolic signalling mechanism is implicated. Whether cAMP-dependent protein phosphorylation is responsible for the inhibition of IKATP will require further analysis given that conflicting reports exist concerning how phosphorylation influences the activity of these channels30-32.
The inhibitory action of glucose on IKATP, as observed in the intact islet, is proposed to result from a glucose signalling system whereby uptake of glucose is mediated by a facilitative glucose transporter, intracellular glucose is converted to glucose-6-phosphate by glucokinase, and aerobic glycolysis generates ATP that is required to inhibit channel function6,7. In contrast, the best evidence available to date indicates that GLP-1 exerts its effects on β-cells by stimulating the production of cAMP8,21,24. The action of GLP-1 to induce glucose competence is therefore suggestive of crosstalk between the cAMP and glucose signalling systems. The consequences of crosstalk are likely to be manifest not only at the level of IKATP modulation, but possibly also at more proximal components (glucose transporter, glucokinase) or distal components (voltage-sensitive calcium channels, vesicular insulin secretion) in the glucose signalling system.
These findings indicate that GLP-1, a circulating hormone, synergizes with glucose to inhibit IKATP, thereby allowing β-cells to depolarize and generate action potentials, events that are necessary prerequisites to glucose-stimulated insulin secretion. Induction of glucose competence by GLP-1 is a receptor-mediated process that is distinct from the more direct inhibitory action of sulphonylureas on IKATP, and provides a straightforward explanation for the ability of GLP-1 to stimulate insulin secretion from the pancreas. Furthermore, as is the case for its insulinotropic action in vivo, the inhibition of IKATP by GLP-1 exhibits an absolute requirement for glucose. It is for these reasons that GLP-1 may offer distinct therapeutic advantages over sulphonylureas when it is used for treatment of NIDDM. Administration of GLP-1 by intravenous infusion produces a rise in circulating insulin accompanied by a lowering of blood glucose, a process that is self-terminating3, as expected because the insulinotropic actions of GLP-1 are glucose-dependent. The likelihood for development of hypoglycaemia during treatment with GLP-1 is therefore reduced, and an extra margin of safety is provided that sulphonylureas or insulin do not offer.
ACKNOWLEDGEMENTS
We thank R. Kramer for critical reading of the manuscript, and C. P. Miller for advice regarding preparation of islet cell cultures. This work was supported by a USPHS grant J.F.H. is an Investigator with the Howard Hughes Medical Institution
References
- 1.Unger RH, Foster DW. In: Williams Textbook of Endocrinology. Wilson JD, Foster DW, editors. Saunders; Philadelphia: 1992. pp. 1255–1333. [Google Scholar]
- 2.Mojsov S, et al. J. biol. Chem. 1986;261:11880–11889. [PubMed] [Google Scholar]
- 3.Nathan DM, Schreiber E, Fogel H, Mojsov S, Habener JF. Diabetes Care. 1992;15:270–276. doi: 10.2337/diacare.15.2.270. [DOI] [PubMed] [Google Scholar]
- 4.Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. New Eng. J. Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- 5.Gerich JE. New Engl. J. Med. 1989;321:1231–1245. doi: 10.1056/NEJM198911023211805. [DOI] [PubMed] [Google Scholar]
- 6.Ashcroft FM, Rorsman P. Prog. Biophys. molec. Biol. 1991;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 7.Rajan AS, et al. Diabetes Care. 1990;13:340–363. doi: 10.2337/diacare.13.3.340. [DOI] [PubMed] [Google Scholar]
- 8.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Proc. natn. Acad. Sci. U.S.A. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mojsov S, Weir GC, Habener JF. J. clin. Invest. 1987;79:616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fehmann HC, Habener JF. Endocrinology. 1992;130:159–166. doi: 10.1210/endo.130.1.1309325. [DOI] [PubMed] [Google Scholar]
- 11.Weir GC, Mojsov S, Hendrick GK, Habener JF. Diabetes. 1989;38:338–342. doi: 10.2337/diab.38.3.338. [DOI] [PubMed] [Google Scholar]
- 12.Horn R, Marty A. J. gen. Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falke LC, Gillis KD, Pressel DM, Misler S. FEBS Lett. 1989;251:167–172. doi: 10.1016/0014-5793(89)81448-6. [DOI] [PubMed] [Google Scholar]
- 14.Smith PA, Ashcroft FM, Rorsman P. FEBS Lett. 1990;261:187–190. doi: 10.1016/0014-5793(90)80667-8. [DOI] [PubMed] [Google Scholar]
- 15.Hamill OP, Marty A, Neher E, Sakman B, Sigworth F. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 16.Hiriart M, Matteson DR. J. gen. Physiol. 1988;91:617–639. doi: 10.1085/jgp.91.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pipeleers D, In't Veld P, Maes E, Van De Winkel M. Proc. natn. Acad. Sci. U.S.A. 1982;79:7322–7325. doi: 10.1073/pnas.79.23.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherman A, Rinzel J, Keizer J. Biophys. J. 1988;54:411–425. doi: 10.1016/S0006-3495(88)82975-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Baimbridge KG, Brown JC. Endocrinology. 1992;131:146–152. doi: 10.1210/endo.131.1.1611994. [DOI] [PubMed] [Google Scholar]
- 20.Ashcroft FM. A. Rev. Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- 21.Blackmore PF, Mojsov S, Exton JH, Habener JF. FEBS Lett. 1991;283:7–10. doi: 10.1016/0014-5793(91)80541-a. [DOI] [PubMed] [Google Scholar]
- 22.Goke R, Conlon JM. J. Endocrinol. 1988;116:357–362. doi: 10.1677/joe.0.1160357. [DOI] [PubMed] [Google Scholar]
- 23.Fehmann HC, Habener JF. Endocrinology. 1991;128:2880–2888. doi: 10.1210/endo-128-6-2880. [DOI] [PubMed] [Google Scholar]
- 24.Thorens B. Proc. natn. Acad. Sci. U.S.A. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henquin JC, Meissner HP. Endocrinology. 1984;115:1125–1134. doi: 10.1210/endo-115-3-1125. [DOI] [PubMed] [Google Scholar]
- 26.Eddlestone GT, Oldham SB, Lipson LG, Premdas FH, Beigelman PM. Am. J. Physiol. 1985;248:C145–C153. doi: 10.1152/ajpcell.1985.248.1.C145. [DOI] [PubMed] [Google Scholar]
- 27.Smith PA, Rorsman P, Ashcroft FM. Nature. 1989;342:550–553. doi: 10.1038/342550a0. [DOI] [PubMed] [Google Scholar]
- 28.Prenkti M, Glennon MC, Geschwind JF, Matschinsky FM, Corkey BE. FEBS Lett. 1987;220:103–107. doi: 10.1016/0014-5793(87)80884-0. [DOI] [PubMed] [Google Scholar]
- 29.Rajan AS, Hill RS, Boyd AE. Diabetes. 1989;38:874–880. doi: 10.2337/diab.38.7.874. [DOI] [PubMed] [Google Scholar]
- 30.Ribalet B, Ciani S, Edelstone GT. J. gen. Physiol. 1989;94:693–717. doi: 10.1085/jgp.94.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichols CG, Lederer WJ. J. gen. Physiol. 1991;97:1095–1098. doi: 10.1085/jgp.97.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, Giebisch G. Proc. natn. Acad. Sci. U.S.A. 1991;88:9722–9725. doi: 10.1073/pnas.88.21.9722. [DOI] [PMC free article] [PubMed] [Google Scholar]