Abstract
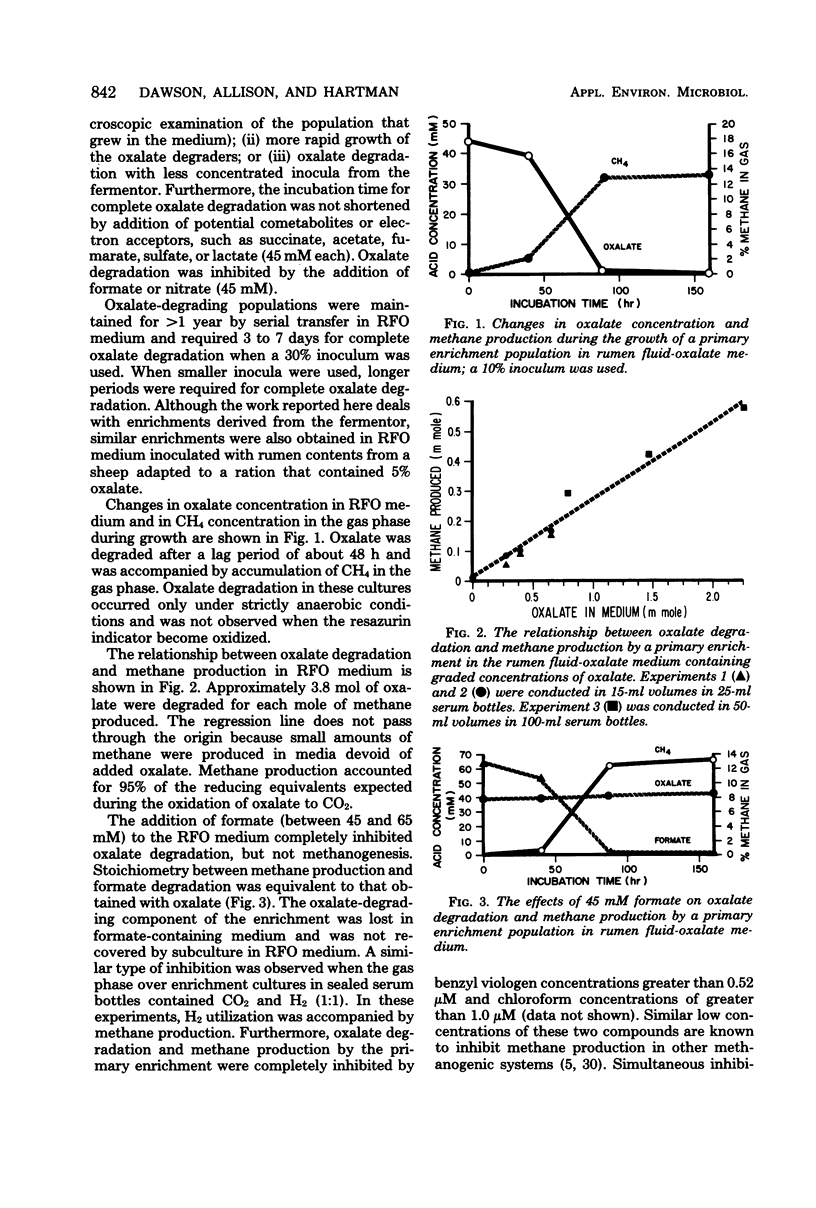
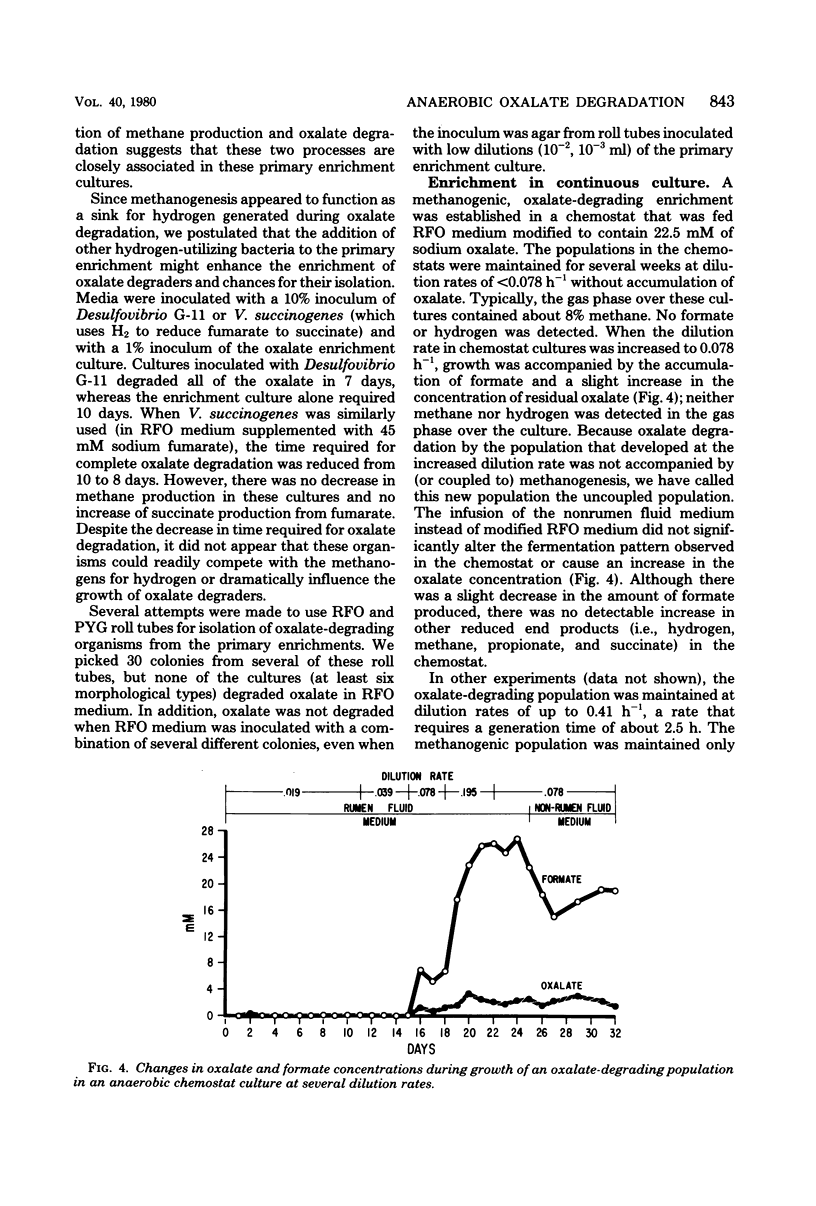
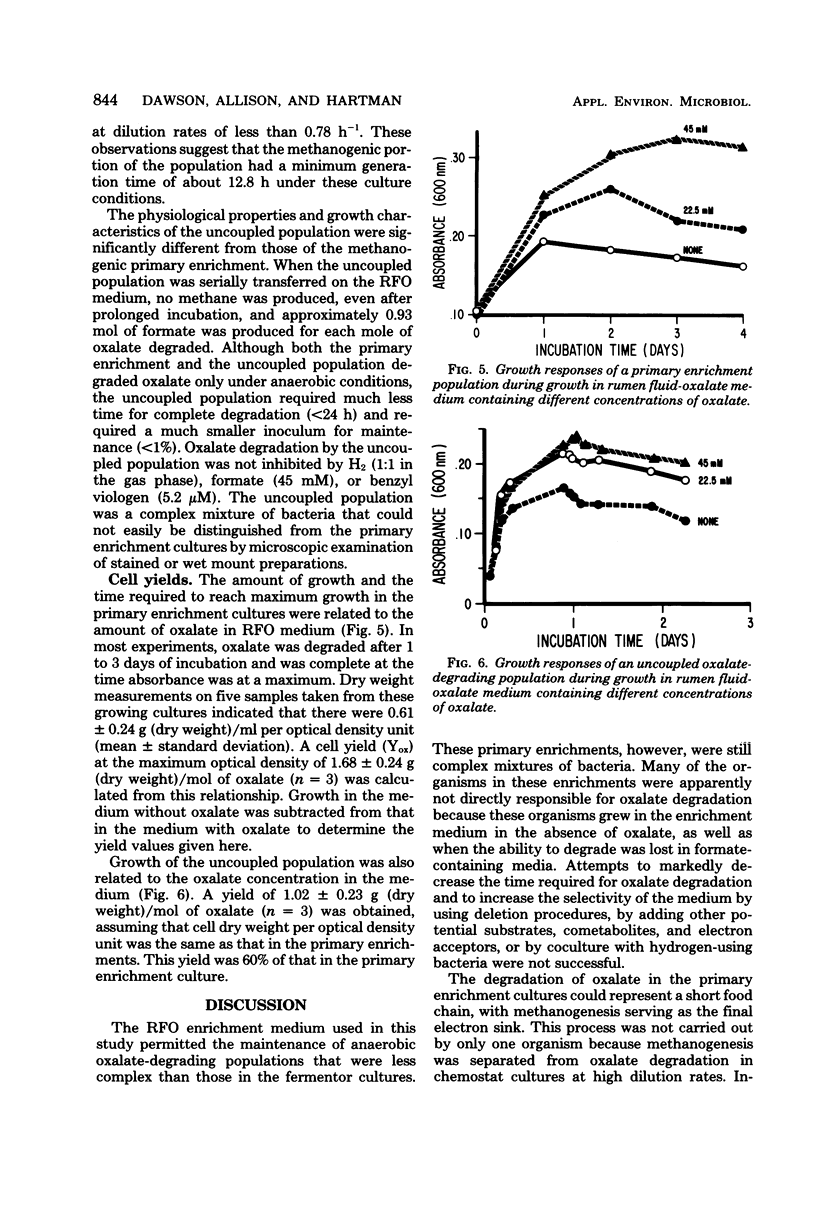
Enrichment cultures of rumen bacteria degraded oxalate within 3 to 7 days in a medium containing 10% rumen fluid and an initial level of 45 mM sodium oxalate. This capability was maintained in serially transferred cultures. One mole of methane was produced per 3.8 mol of oxalate degraded. Molecular hydrogen and formate inhibited oxalate degradation but not methanogenesis; benzyl viologen and chloroform inhibited both oxalate degradation and methanogenesis. Attempts to isolate oxalate-degrading bacteria from these cultures were not successful. Oxalate degradation was uncoupled from methane production when enrichments were grown in continuous culture at dilution rates greater than or equal to 0.078 h-1. Growth of the uncoupled population (lacking methanogens) in batch culture was accompanied by degradation of 45 mM oxalate within 24 h and production of 0.93 mol of formate per mol of oxalate degraded. Oxalate degradation by the uncoupled population was not inhibited by molecular hydrogen or formate. Cell yields (grams [dry weight]) per mole of oxalate degraded by the primary enrichment and the uncoupled populations were 1.7 and 1.0, respectively.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Kumeno F. In vitro simulation of rumen fermentation: apparatus and effects of dilution rate and continuous dialysis on fermentation and protozoal population. J Anim Sci. 1973 May;36(5):941–948. doi: 10.2527/jas1973.365941x. [DOI] [PubMed] [Google Scholar]
- Allison M. J., Littledike E. T., James L. F. Changes in ruminal oxalate degradation rates associated with adaptation to oxalate ingestion. J Anim Sci. 1977 Nov;45(5):1173–1179. doi: 10.2527/jas1977.4551173x. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber H. H., Gallimore E. J. The metabolism of oxalic acid in the animal body. Biochem J. 1940 Feb;34(2):144–148. doi: 10.1042/bj0340144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T. Inhibition of rumen methanogenesis by methane analogues. J Bacteriol. 1967 Jul;94(1):171–175. doi: 10.1128/jb.94.1.171-175.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson K. A., Allison M. J., Hartman P. A. Isolation and some characteristics of anaerobic oxalate-degrading bacteria from the rumen. Appl Environ Microbiol. 1980 Oct;40(4):833–839. doi: 10.1128/aem.40.4.833-839.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A., Grubb J. A. Basal medium for the selective enumeration of rumen bacteria utilizing specific energy sources. Appl Environ Microbiol. 1976 Nov;32(5):703–710. doi: 10.1128/aem.32.5.703-710.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson H. R., Hinds F. C., Bryant M. P., Owens F. N. Efficiency of energy utilization by mixed rumen bacteria in continuous culture. J Dairy Sci. 1975 Nov;58(11):1645–1659. doi: 10.3168/jds.S0022-0302(75)84763-1. [DOI] [PubMed] [Google Scholar]
- James L. F., Butcher J. E. Halogeton poisoning of sheep: effect of high level oxalate intake. J Anim Sci. 1972 Dec;35(6):1233–1238. doi: 10.2527/jas1972.3561233x. [DOI] [PubMed] [Google Scholar]
- James L. F., Street J. C., Butcher J. E. In vitro degradation of oxalate and of cellulose by rumen ingesta from sheep fed Halogeton glomeratus. J Anim Sci. 1967 Nov;26(6):1438–1444. doi: 10.2527/jas1967.2661438x. [DOI] [PubMed] [Google Scholar]
- Kafkewitz D. Improved growth media for Vibrio succinogenes. Appl Microbiol. 1975 Jan;29(1):121–122. doi: 10.1128/am.29.1.121-122.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton A. M., Wolfe R. S. Adenosine triphosphate pools in Methanobacterium. J Bacteriol. 1970 Apr;102(1):43–51. doi: 10.1128/jb.102.1.43-51.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Muirhead P. A. Quantitative method for the gas chromatographic analysis of short-chain monocarboxylic and dicarboxylic acids in fermentation media. Appl Microbiol. 1975 Mar;29(3):374–381. doi: 10.1128/am.29.3.374-381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley E. K., Schmidt-Nielsen K. Oxalate metabolism in the pack rat, sand rat, hamster and white rat. J Nutr. 1967 Apr;91(4):496–502. doi: 10.1093/jn/91.4.496. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward G., Harbers L. H., Blaha J. J. Calcium-containing crystals in alfalfa: their fate in cattle. J Dairy Sci. 1979 May;62(5):715–722. doi: 10.3168/jds.S0022-0302(79)83314-7. [DOI] [PubMed] [Google Scholar]
- Wolin E. A., Wolfe R. S., Wolin M. J. Viologen dye inhibition of methane formation by Methanobacillus omelianskii. J Bacteriol. 1964 May;87(5):993–998. doi: 10.1128/jb.87.5.993-998.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin M. J. Metabolic interactions among intestinal microorganisms. Am J Clin Nutr. 1974 Nov;27(11):1320–1328. doi: 10.1093/ajcn/27.11.1320. [DOI] [PubMed] [Google Scholar]


