Abstract
PKCα-activation is a key signaling event governing cell growth, stress-resistance, and drug-resistance. Our recent studies demonstrated that DOX-resistance mediating effects of PKCα require the presence of RLIP76, and their concerted action is sufficient to explain intrinsic DOX-resistance of NSCLC [S.S. Singhal, D. Wickramarachchi, J. Singhal, S. Yadav, Y.C. Awasthi, et al., Determinants of differential doxorubicin sensitivity between SCLC and NSCLC. FEBS Lett. 580 (2006) 2258–2264]. Present studies were carried out to further explore the suggestion from the previous studies that the mitogenic effects of PKCα also require RLIP76. RLIP76−/− MEFs were resistant to PKCα-depletion mediated growth inhibition, as well as to the PKCα-dependent mitogen, phorbol 12-myristate 13-acetate (PMA). Augmenting cellular levels of RLIP76 using purified recombinant RLIP76 increased growth rate in all cells, and restored the sensitivity of RLIP76−/− MEFs to both inhibition through PKCα-depletion and stimulation through PMA. These results show that RLIP76 is a necessary down-stream effector for PKCα-mediated mitogenesis.
Keywords: Protein kinase C, Lung cancer, Doxorubicin, Drug-resistance, RLIP76, siRNA
PKCα is a member of protein kinase family, and is considered one of the ‘classical’ isoforms (α, βI/II, and γ) which bind to and are activated by calcium and diacylglycerol (DAG) resulting in activation of the catalytic domain in response to various stimuli [1,2]. PKCα transmits signals down-stream to pathways regulating cell proliferation, differentiation, cell cycle-control, apoptosis, and cell survival in response to stressors such as the classical chemotherapy drug, doxorubicin (DOX) [1–4]. Cell cycle progression regulation by PKCα (and PKCε) is mediated through activation of cyclin D1 expression through enhanced AP1 binding to the cyclin D1 promoter. However, this mechanism is not uniform, since in some cells, PKCα down-regulates cyclin D and increases p21 and p27, resulting in exit of cells from the cell cycle to G0. Loss of PKCα correlates with induction of apoptosis either through activation of PKCδ, or down-regulation of Bcl [4]. Increased PKCα-mediated augmentation of cell survival is also associated with increased anti-apoptotic protein, Bcl-2, and BclXL. Down-stream targets of PKCα also include Raf-1 kinase which activates the ERK-MAPK cascade involved in stress-defenses and transformation. Interaction of PKC with Rho in the plasma membrane leads to activation of AP-1 through an ERK-MAPK independent pathway [2,4]. Cells with increase PKCα, such as non-small-cell lung carcinoma (NSCLC), exhibit anchorage dependent growth, whereas cells low in PKCα grow in an anchorage independent fashion, like small cell lung carcinoma [4,5]. This difference in morphology is related to the activation of Rho by PKCα and subsequent Rho-dependent cytoskeletal rearrangements [5].
We have recently identified a new target of PKCα, RLIP76 (RALBP1). RLIP76 is a Ral-binding Rho-GAP protein (inhibitor of Rho-signaling) which we have shown to be the predominant cellular mechanism for ATP-dependent effux of glutathione-conjugate (GS-E) and chemotherapy drugs (such as DOX) [6–18]. Although it can function as a drug-resistance transporter, the major physiological role of RLIP76 appears to be the regulation of intracellular levels of lipid–alkenals and alkenal–glutathione conjugates, the formation of which is an early and obligatory event in course of oxidant/radiant stress or signaling [8–10,19] The transport activity of RLIP76 functions to regulate cellular levels of glutathionyl-adducts of lipidoxidation derived reactive oxygen species which are known to exert direct effects in cell proliferation, differentiation, and apoptosis [8–10,19].
RLIP76 is activated to increase its GS-E and DOX transporting activity through phosphorylation by PKCα at T297 [16,17]. The greater transporter activity of the phosphorylated form of RLIP76, which predominates in NSCLC, is the primary determinant of intrinsic DOX-resistance of NSCLC [16,17]. Exclusive expression of a mutant RLIP76 which cannot be phosphorylated in NSCLC cells causes reversion of DOX-resistant to a level similar to that seen in the DOX-sensitive SCLC [17]. These findings were confirmed by studies in RLIP76−/− MEFs which lacked any DOX-sensitizing effect of PKCα depletion. During these studies, we also noticed that RLIP76−/− MEFs were also resistant to the anti-proliferative effects of PKCα-depletion by siRNA. These findings suggested that the proliferative signaling by PKCα also requires the presence of RLIP76. In present studies, we have explored this postulate by comparing the effects of PKCα simulation, depletion, and inhibition between RLIP76−/− MEFs as well as SCLC and NSCLC cells. The results of present studies show that RLIP76−/− is a required down-stream effector for PKCα-mediated cell-proliferation. These novel findings have important implications with respect to the role of lipid peroxidation derived reactive-oxygen species, their glutathionylated metabolites, and glutathione-linked metabolism in general in the regulation of cell growth.
Materials and methods
Materials
Sources for reagents for protein purification, tissue culture, and transport studies were the same as described previously [6,15]. Phorbol ester was procured from Sigma Chemical Co., St. Louis, MO. [γ-32P]ATP (3000 Ci/mmol) was purchased from Pharmacia Biotech (Piscataway, NJ). [14C]DOX (specific activity 57 mCi/ mmol) was purchased from Amersham Corporation (Arlington Heights, IL). Source of anti-RLIP76 IgG used in these studies was the same as previously described [6]. RLIP76 siRNA, human PKCα siRNA, and non-specific control siRNA were purchased from Dharmacon (Chicago, IL).
Cell lines and cultures
Human SCLC, H182, H1417, H1618, NSCLC, H226 (squamous cell carcinoma), H2347 (adenocarcinoma), and H358 (bronchio alveolar) cell lines were studied. All cells were cultured at 37 °C in a humidified atmosphere of 5% CO2 in RPMI-1640 medium supplemented with 10% (v/v) heat-inactivated FBS, 1% (v/v) P/S solution, 2 mM l-glutamine, 10 mM Hepes, 1 mM sodium pyruvate, 1.5 g/L sodium bicarbonate, and 4.5 g/L glucose.
Mouse embryonic fibroblast (MEF) cultures
Twelve-week-old C57BL/6 mice born of heterozygous × heterozygous (RLIP76+/− × RLIP76+/−) mating were genotyped by PCR strategy on mouse tail DNA using forward, reverse, and long terminal region (LTR) primers [14]. These mice were commissioned from Lexicon Genetics and were created using Cre-Lox technology as described previously [14]. Embryonic fibroblast lines were prepared from RLIP76+/+, RLIP76+/− , and RLIP76−/− mice on the 13th or 14th day of pregnancy according to the method of Johnson et al. [20] and maintained in RPMI-1640 medium containing 10% (v/v) heat-inactivated FBS, 1% (v/v) P/S solution, 2 mM l-glutamine, 10 mM Hepes, 1 mM sodium pyruvate, 4.5 g/L glucose, and 1.5 g/L sodium bicarbonate and plated in 25-cm2 tissue culture flasks, and incubated at 37 °C in a humidified atmosphere of 5% CO2.
effect of PMA
Cell density during log phase was determined by counting trypan blue excluding cells in a hemocytometer, and 20,000 cells/160 μl medium were plated into each well of 96-well flat-bottomed microtiter plates. The effect of phorbol ester on cell growth was also examined by incubating the cells with 20, 50, and 100 nM PMA for 2 h at 37 °C. Cells were then washed with PBS, followed by 96 h incubation at 37 °C in medium before MTT assay [21]. Levels of RLIP76 protein in cells homogenates were measured by ELISA using anti-RLIP76 IgG as previously described [15].
Results and discussion
effect of RLIP76 loss on cell growth
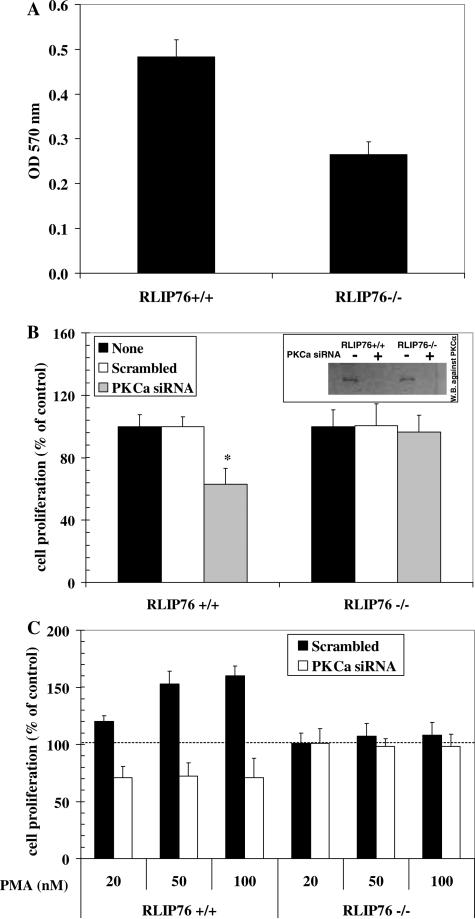
The growth rate of RLIP76+/+ and RLIP76−/− MEFs was compared by inoculating equal number of cells into medium followed by quantitation of cell growth by hemocytometry, cell-counting, MTT as well as colony-forming assays. RLIP76−/− MEFs were significantly slower growing as compared with the wild-type counterpart (Fig. 1A). Data presented are for MTT assays done at 96 h after inoculation of cells into medium, and were consistent with the cell-counting, hemocytometry, and colony-forming assays done in parallel (data not presented). These findings indicated that loss of RLIP76 caused a decrease in basal rate of cell proliferation.
Fig. 1.
effect of RLIP76 loss on PKCα-mediated growth signaling. RLIP76+/+ and RLIP76−/− MEFs was prepared and maintained as described in Materials and methods. Growth rate of the MEFs were measured by performing MTT assays at varying times after inoculation of 1 × 105 cell/ml medium (A). The effect or PKCα-depletion on cell growth was examined in RLIP76+/+ and RLIP76−/− MEFs treated for 3 h with either scrambled or PKCα-siRNA using the Transmessenger Transfection Kit (Qiagen). * means significantly different from RLIP76−/− at p < 0.05 (B). The inset in (B) shows depletion of PKCα using Western-blot analysis with PKCα primary antibodies. The effect of PMA-stimulation was analyzed in cells first treated with PMA at 20, 50, or 100 nM for 2 h at 37 °C, followed by either PKCα-siRNA or scrambled siRNA for 3 h (C). MTT assay was carried out 96 h later. All studies were performed in triplicate with eight replicates for each point.
effect of PKCα-depletion on cell growth in MEFs
For these studies, we used a specific PKCα-siRNA (Dharmacon) and a corresponding scrambled siRNA control. The growth of cells was normalized to controls in the absence of siRNA. The scrambled siRNA caused no effects in the wild-type and RLIP76−/− cells (Fig. 1B). PKCα-depletion was confirmed by Western blot analysis (Fig. 1B, inset). Concomitant with absolute depletion of PKCα protein, cell growth was reduced significantly (p < 0.05) by 36% for the RLIP76+/+ MEFs, whereas growth rate was unaffected by PKCα-depletion in the RLIP76−/− MEFs (Fig. 1B). This observation indicated that PKCα-signaling of cell cycling and proliferation was non-functional in the absence of RLIP76.
effect of PKCα-stimulation on growth of MEFs
The phorbol ester PMA (phorbol-12-myristate-13-acetate) caused a dose-dependent and saturable stimulation of cell growth in the RLIP76+/+ MEFs, whereas no growth stimulation was apparent in the RLIP76−/− MEFs (p < 0.001) (Fig. 1C). For the RLIP76+/+ MEFs, depletion of cells by PKCα resulted in a reduction of cell growth by ~35%; more importantly, the effect of PMA was completely abrogated, confirming that PKCα is necessary for the proliferative response elicited by PMA. Remarkably, neither the scrambled siRNA nor PKCα-siRNA pre-treatment affected the growth of RLIP76−/− MEFs, either in the absence or presence of PMA. These results strongly indicated that proliferative signaling through PKCα stimulation requires the presence of RLIP76.
effect of RLIP76 supplementation on proliferation signaling by PKCα
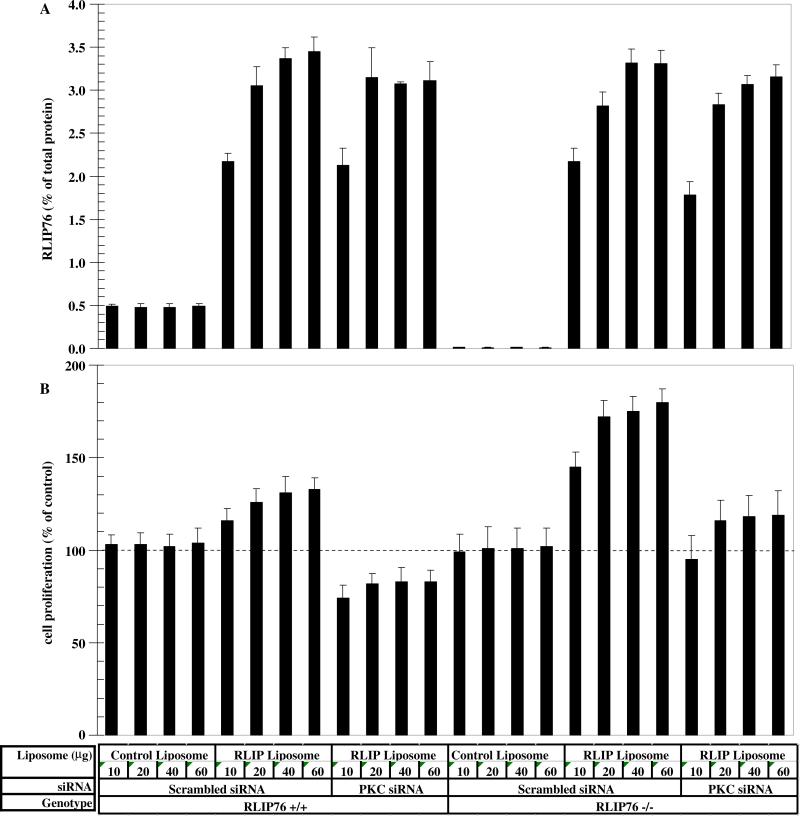
If RLIP76 were the key ingredient missing in RLIP76−/− MEFs that resulted in loss of PKCα-mediated proliferation signaling, replacement of RLIP76 should overcome this defect. To address this question, we measured cell-proliferation rate and cellular RLIP76 protein between RLIP76+/+ and RLIP76−/− MEFs in which RLIP76 level was augmented by addition of purified recombinant human RLIP76 through liposomal delivery, as previously used [6,15]. Measurement of total RLIP76 protein in crude cell homogenate revealed absence of detectable protein in the RLIP76−/− MEFs as expected (Fig. 2A). Total RLIP76 protein normalized to total crude protein showed that RLIP76 represented ~0.5% of total protein in these MEFs, an amount in close agreement with that reported previously for other human cells [15]. Addition of increasing volumes of control liposomes (not containing purified RLIP76) caused no change in RLIP76 level, but addition of increasing amounts of RLIP76-liposomes caused an apparently saturable 4 to 7-fold increase in cellular RLIP76 protein in the RLIP76+/+ MEFs. The quantity of RLIP76 protein in RLIP76−/− MEFs treated with RLIP76 liposomes was comparable, though slightly lower than the corresponding values obtained upon augmenting RLIP76 in RLIP76+/+ MEFs (Fig. 2A). Scrambled PKCα-siRNA had no significant effect on the uptake of RLIP76 from liposomes in either the RLIP76+/+ or RLIP76−/− MEFs. These results confirmed previous studies showing absence of RLIP76 in RLIP76−/− MEFs, and the ability to augment cellular RLIP76 using liposomal delivery.
Fig. 2.
Effect of RLIP76 augmentation on PKCα-depletion. RLIP76 protein levels were measured by specific ELISA [15] and normalized to total protein in crude cell homogenate measured by Bradford's assay (A). The normalized values, showing RLIP76 protein as a percentage of total protein, are presented for RLIP76+/+ and RLIP76−/− MEFs treated with RLIP76-proteoliposomes (10, 20, 40 or 60 μg purified RLIP76 protein/ml). Control liposomes contained an equal amount of heat-inactivated purified RLIP76. After 24 h incubation with liposomes, cells were treated with PKCα-siRNA or scrambled control. MTT assay was used for measuring cell growth (B). Results presented are average and SD of triplicate determinations with eight replicates each.
Cell growth measurements, normalized to untreated control cells of the corresponding genotype, showed that augmentation of RLIP76 caused increased cell proliferation above control, increasing to ~130% of control in RLIP76+/+ MEFs augmented to contain seven times the normal amount of RLIP76. The growth inhibitory effect of PKCα-depletion in these cells was partially, but not completely, antagonized by augmentation of RLIP76 (Fig. 2B). In contrast to the RLIP76+/+ MEFs, the RLIP76−/− MEFs had a much greater increase in cell proliferation upon addition of RLIP76. Indeed, the growth-rate of these cells exceeded the wild-type (Fig. 2B). In the RLIP76 supplemented RLIP76−/− cells, sensitivity to inhibition by PKCα-depletion was restored. These findings provided strong evidence for the assertion that cell-proliferative signaling involves that activity of RLIP76 and PKCα, and that in the absence of RLIP76, PKCα-mediated mitogenesis is absent.
Correlation of cell-growth findings in NSCLC and SCLC
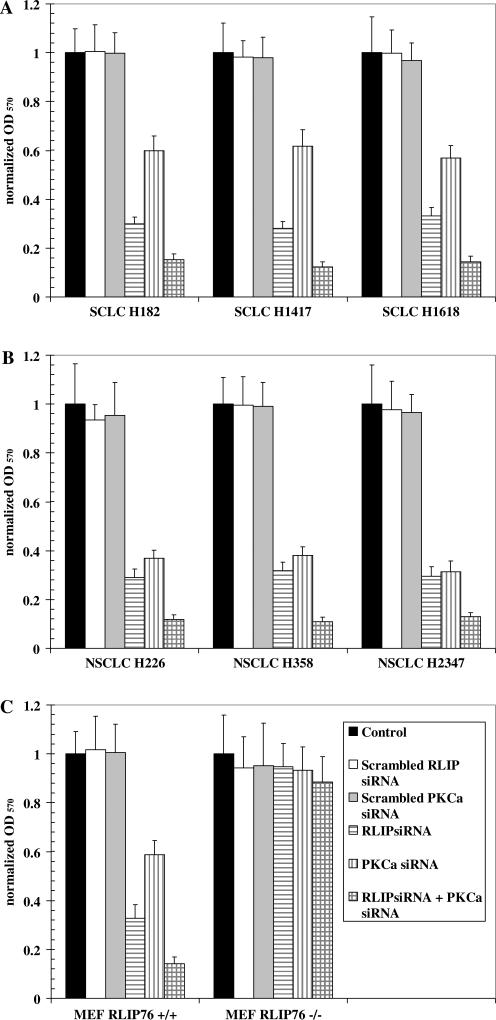
SCLC and NSCLC represent a good model system for studying differential effects of PKCα and RLIP76, since the latter has much higher PKCα activity as compared with the former, and the specific activity of RLIP76 in NSCLC is twice that in SCLC [15–17]. For these studies, cell growth was compared between 3 SCLC and 3 NSCLC cell lines in the presence or absence of specific depletion of PKCα or RLIP76 by siRNA (Fig. 3). Scrambled siRNAs had no effect on growth. RLIP76-depletion caused a consistent ~70% decrease in surviving cells in both SCLC and NSCLC. We have previously shown that RLIP76-depletion causes apoptosis in both cell types [15,18]. Depletion of PKCα by siRNA also reduced cell growth, though the effect was more prominent in NSCLC as compared with SCLC. Combined depletion of PKCα and RLIP76 had an additive effect, reducing cell growth by >85% in both SCLC and NSCLC (Fig. 3). These findings showed that specific depletion of PKCα preferentially affects NSCLC as expected, since these cells have greater PKCα activity [16,17].
Fig. 3.
effects of RLIP76 or PKCα depletion on inhibition of growth of lung cancer cells or RLIP76 MEFs. Cells were treated as shown in the figure for 3 h. Transmessenger transfection reagent was used for transfection of all siRNA species. Cells were then allowed to grow for 48 h at 37 °C with 5% CO2 in fresh medium. MTT assay was performed 96 h later. The combinations of treatments are shown in the figure.
Significance
The fundamental nature of these findings with respect to the nature of PKCα-mediated signaling is evident from the cell-growth studies showing that RLIP76 is a required effector for signaling cell-proliferation. The mechanism for the proliferative effects of PKCα-mediated stimulation of RLIP76 transport activity is likely rooted in the physiological function of RLIP76 as a transporter of GS-E of membrane-lipid derived alkenals (such as 4-hydroxynonenal, 4HNE). 4HNE is a pro-apoptotic and anti-proliferative lipid alkenal formed during oxidative signaling and stress. It is metabolized primarily to a GS-E, which are removed from cells by RLIP76 [22] and other GS-E transporters such as ABCC1 and ABCG2 [23]. Removal of this conjugate is essential to maintain continued metabolism of 4HNE to 4HNE-SG, because the latter is a potent product-inhibitor or glutathione-transferases which catalyze the conjugation. Thus, a mechanism such as RLIP76 which directly regulates 4HNE-SG levels in cells indirectly also regulates the level of free 4HNE, which would tend to accumulate if 4HNE-SG removal was inhibited with resultant inhibition of GST. Inhibition of RLIP76 would thus be predicted to increase intracellular pro-apoptotic/growth inhibitory alkenals, and conversely an increased level of activity or RLIP76 should lower this. Apoptosis triggered by RLIP76 inhibition or depletion [15,18] alone, as well as increased cellular proliferation caused by augmenting cellular RLIP76 [15] or by depleting 4HNE levels through increased expression of the 4HNE metabolizing GST isozymes, hGSTA4-4 [24], strongly supports this model. Studies of alkenal levels in RLIP76-transfected cells as well as in RLIP76−/− animal tissues confirm this model [14]. The crucial role of RLIP76 as a regulator of cell proliferation and resistance, and the central role of the RLIP76-PKCα interaction in the differential drug-resistance of NSCLC suggest a new paradigm for understanding growth signaling in cancer.
Acknowledgments
This work was supported in part by NIH Grants CA 77495, CA 104661 (S.A.), and ES 012171 (Y.C.A.), and Cancer Research Foundation of North Texas (S.S. and S.Y.).
Footnotes
Abbreviations: RLIP76 (RALBP1), Ral-interacting protein; DOX, doxorubicin; SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer; GSH, glutathione; GS-E, glutathione-electrophile conjugate; DNP-SG, dinitrophenyl S-glutathione; MRP, multi-drug-resistance associated protein; PMA, phorbol ester (phorbol 12-myristate 13-acetate); PKC, protein-kinase-C.
References
- 1.Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal. 1998;10:529–542. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 2.Nakashima S. Protein kinase C alpha (PKC alpha): regulation and biological function. J. Biochem. 2002;132:669–675. doi: 10.1093/oxfordjournals.jbchem.a003272. [DOI] [PubMed] [Google Scholar]
- 3.Lahn M, Paterson BM, Sundell K, Ma D. The role of protein kinase C-alpha (PKC-α) in malignancies of the gastrointestinal tract. Eur. J. Cancer. 2004;40:10–20. doi: 10.1016/j.ejca.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Michie AM, Nakagawa R. The link between PKCα regulation and cellular transformation. Immunol. Lett. 2005;96:155–162. doi: 10.1016/j.imlet.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Holinstat M, Mehta D, Kozasa T, Minshall RD, Malik AB. Protein kinase Cα-induced p115RhoGEF phosphorylation signals endothelial cytoskeletal rearrangement. J. Biol. Chem. 2003;278:28793–28798. doi: 10.1074/jbc.M303900200. [DOI] [PubMed] [Google Scholar]
- 6.Awasthi S, Cheng J, Singhal SS, Saini MK, Pandya U, Pikula S, Pikula J, Singh SV, Zimniak P, Awasthi YC. Novel function of human RLIP76: ATP-dependent transport of glutathione conjugates and doxorubicin. Biochemistry. 2000;39:9327–9334. doi: 10.1021/bi992964c. [DOI] [PubMed] [Google Scholar]
- 7.Awasthi S, Cheng J, Singhal SS, Sharma R, Pandya U, Zimniak P, Awasthi YC. Functional reassembly of xenobiotic transport from the N-terminal and C-terminal domains of RLIP76 and identification of ATP binding sequences. Biochemistry. 2001;40:4159–4168. doi: 10.1021/bi002182f. [DOI] [PubMed] [Google Scholar]
- 8.Cheng J, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, Singh SV, Zimniak P, Awasthi S, Awasthi YC. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J. Biol. Chem. 2001;276:41213–41223. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]
- 9.Awasthi S, Sharma R, Singhal SS, Zimniak P, Awasthi YC. RLIP76, a novel transporter catalyzing ATP-dependent efflux of xenobiotics. Drug Metab. Disp. 2002;30:1300–1310. doi: 10.1124/dmd.30.12.1300. [DOI] [PubMed] [Google Scholar]
- 10.Awasthi S, Singhal SS, Sharma R, Zimniak P, Awasthi YC. Transport of glutathione-conjugates and chemotherapeutic drugs by RLIP76 (RALBP1): a novel link between G-protein and tyrosine kinase signaling and drug resistance. Int. J. Cancer. 2003;106:635–646. doi: 10.1002/ijc.11260. [DOI] [PubMed] [Google Scholar]
- 11.Sharma R, Singhal SS, Wickramarachchi D, Awasthi YC, Awasthi S. RLIP76 (RALBP1) mediated transport of leukotriene C4 (LTC4) in cancer cells: implications in drug-resistance. Int. J. Cancer. 2004;112:934–942. doi: 10.1002/ijc.20516. [DOI] [PubMed] [Google Scholar]
- 12.Yadav S, Singhal SS, Singhal J, Wickramarachchi D, Knutson E, Albrecht TB, Awasthi YC, Awasthi S. Identification of membrane anchoring domains of RLIP76 using deletion mutants analyses. Biochemistry. 2004;43:16243–16253. doi: 10.1021/bi0482811. [DOI] [PubMed] [Google Scholar]
- 13.Yadav S, Zajac E, Singhal SS, Singhal J, Drake K, Awasthi YC, Awasthi S. POB1 over-expression inhibits RLIP76 mediated transport of glutathione-conjugates, drugs and promotes apoptosis. Biochem. Biophys. Res. Commun. 2005;328:1003–1009. doi: 10.1016/j.bbrc.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 14.Awasthi S, Singhal SS, Yadav S, Singhal J, Drake K, Nadkar A, Zajac E, Wickramarachchi D, Rowe N, Yacoub A, Boor PJ, Dwivedi S, Dent P, Jarman WE, John B, Awasthi YC. RLIP76 is a major determinant of radiation sensitivity. Cancer Res. 2005;65:6022–6028. doi: 10.1158/0008-5472.CAN-05-0968. [DOI] [PubMed] [Google Scholar]
- 15.Singhal SS, Yadav S, Singhal J, Zajac E, Awasthi YC, Awasthi S. Depletion of RLIP76 sensitizes lung cancer cells to doxorubicin. Biochem. Pharmacol. 2005;70:481–488. doi: 10.1016/j.bcp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Singhal SS, Yadav S, Singhal J, Drake K, Awasthi YC, Awasthi1 S. The role of PKCa and RLIP76 in transport-mediated doxorubicin-resistance in lung cancer. FEBS Lett. 2005;579:4635–4641. doi: 10.1016/j.febslet.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 17.Singhal SS, Wickramarachchi D, Singhal J, Yadav S, Awasthi YC, Awasthi S. Determinants of differential doxorubicin sensitivity between SCLC and NSCLC. FEBS Lett. 2006;580:2258–2264. doi: 10.1016/j.febslet.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Singhal SS, Awasthi YC, Awasthi S. Regression of melanoma in a murine model by RLIP76 depletion. Cancer Res. 2006;66:2354–2360. doi: 10.1158/0008-5472.CAN-05-3534. [DOI] [PubMed] [Google Scholar]
- 19.Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, Srivastava SK. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): Role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J. Biol. Chem. 2006;281:17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- 20.Johnson DR, Finch RA, Lin Z-P, Zeiss CJ, Sartorelli AC. The pharmacological phenotype of combined multidrug-resistance mdr1a/1b- and mrp1-deficient mice. Cancer Res. 2001;61:1469–1476. [PubMed] [Google Scholar]
- 21.Awasthi S, Singhal SS, He N-G, Chaubey M, Zimniak P, Srivastava SK, Singh SV, Awasthi YC. Modulation of doxorubicin cytotoxicity by ethacrynic acid. Int. J. Cancer. 1996;68:333–339. doi: 10.1002/(SICI)1097-0215(19961104)68:3<333::AID-IJC11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Sharma R, Singhal SS, Cheng J, Yang Y, Sharma A, Zimniak P, Awasthi S, Awasthi YC. RLIP76 is the major ATP-dependent transporter of glutathione-conjugates and doxorubicin in human erythrocytes. Arch. Biochem. Biophys. 2001;391:171–179. doi: 10.1006/abbi.2001.2395. [DOI] [PubMed] [Google Scholar]
- 23.Borst P, Evers R, Kool M, Wijnholds J. The multidrug resistance protein family. Biochim Biophys Acta. 1999;1461:347–357. doi: 10.1016/s0005-2736(99)00167-4. [DOI] [PubMed] [Google Scholar]
- 24.Cheng J, Yang Y, Singh SP, Singhal SS, Awasthi S, Singh SV, Zimniak P, Awasthi YC. Two distinct 4-hydroxynonenal metabolizing glutathione S-transferase isozymes are differentially expressed in human tissues. Biochem. Biophys. Res. Commun. 2001;282:1268–1274. doi: 10.1006/bbrc.2001.4707. [DOI] [PubMed] [Google Scholar]