Abstract
Fluorescent yellow direct repeat (FYDR) mice carry a transgenic reporter for homologous recombination (HR) and have been used to reveal an age dependent increase in HR in the pancreas. An established in vitro model system for accelerated aging of the marrow is the mouse long-term bone marrow culture (LTBMC) system. To determine whether the FYDR system, in which an HR event can lead to a fluorescent cell can be used to study the effects of aging in LTBMCs, we analyzed clonally expanded hematopoietic and marrow stromal cells in FYDR, positive control FYDR-Recombined (FYDR-Rec), and negative control wild-type C57BL/6J (WT) LTBMCs. All groups of cultures demonstrated equivalent parameters of continuous hematopoiesis including generation of multilineage colony forming CFU-GM progenitor cells for over 22 weeks and age associated senescence of hematopoiesis. Results indicate that low expression of the FYDR transgene in bone marrow cells in vivo and in vitro prevents the use of the FYDR mice to study rare combination events in bone marrow. Using an alternative approach for detecting HR, namely the sister chromatid exchange (SCE) assay, we observed a statistically significant increase in the number of SCEs per chromosome in adherent cells subcultured from 20 week compared to 4 week old LTBMCs. These data suggest that adherent marrow stromal cells from LTBMCs become increasingly susceptible to HR events during aging.
Keywords: homologous recombination, long-term marrow culture, FYDR mice
Introduction
Continuous hematopoiesis in murine long-term bone marrow cultures (LTBMC) results from clonal succession of hematopoietic stem cells that reside in the adherent layer and which are in contact with cells of the microenvironment comprised of endothelial, marrow stromal, and macrophage cell lineages (1). Murine continuous bone marrow cultures demonstrate clonal succession of adherent hematopoietic stem cell subsets resulting in release into the non-adherent layer of multilineage and committed hematopoietic progenitor cells (1–4). Depletion of hematopoietic cells by weekly feeding over 20 – 40 plus weeks limits culture longevity and is mouse strain dependent (2–3). The continuous marrow culture system has been shown to reflect several parameters of bone marrow aging, including accumulation of lipid in stromal cells which differentiate into adipocytes (3, 5), and depletion of intracellular antioxidant stores associated with chronic oxidative stress (6, 7). Thus, LTBMC can be used as an in vitro model to study the effects of aging on bone marrow.
The increase in oxidative stress that occurs with age and in LTBMCs (6, 7) can lead to multiple types of DNA damage, including DNA double strand breaks (DSBs). One pathway mammalian cells use to repair DNA DSBs is mitotic homologous recombination (HR). HR uses homologous sequences present on a sister chromatid or homologous chromosome as templates for repair, enabling the repair of DSBs with minimal loss of sequence information (8). Although HR is generally considered to be an error-free DNA repair pathway, recombination between misaligned sequences can result in mutations such as insertions, deletions and translocations, and conditions that stimulate HR increase the risk of acquiring deleterious genetic rearrangements (9). Thus, the frequency of HR is a reflection of both the level of DSBs and the ability of cells to use HR as a repair pathway. For example, acute and chronic oxidative stress has been shown to induce HR events (4–6), and recombinant cells have been shown to accumulate with age in vivo (18).
In terms of the effect of DNA damage on bone marrow cells, while DNA damaging agents, including ionizing irradiation (10–13), phorbol myristate acetate, (14) and alkylating agents (15) have been shown to suppress hematopoiesis, the cell types most susceptible to DNA damage, whether hematopoietic stem cells, bone marrow stromal cells, or both compartments remain unknown. Since LTBMCs recapitulate some characteristics associated with bone marrow aging, here we have used these cultures to study the effects of aging and aging associated oxidative stress on HR in bone marrow cells in vitro.
Using LTBMCs, we explored the effect of aging on the frequency of DNA DSBs in adherent and non-adherent primitive hematopoietic stem cells, by measuring the frequency of HR events. To study HR in bone marrow cells in vivo and in vitro, we set out to use the Fluorescent Yellow Direct Repeat (FYDR) mice in which a HR event at a transgene leads to expression of enhanced yellow fluorescent protein (EYFP) (17). We established continuous bone marrow cultures from FYDR (17), positive control FYDR-Recombined (FYDR-Rec) (16), and negative control WT C57BL/6J female mice. Positive control FYDR-Rec mice carry the full-length EYFP coding sequence under the same promoter and at the same locus as the FYDR mice. Thus, FYDR-Rec mice provide an ideal positive control for FYDR transgene expression (16). To determine if the FYDR system provides a useful tool for analyzing HR in bone marrow cells, we measured the expression of EYFP in positive control FYDR-Rec mice in freshly isolated bone marrow, in the non-adherent hematopoietic cell populations and in colonies formed by harvested and subcultured cells during 40 weeks of continuous marrow culture. Results demonstrate that expression of the FYDR transgene as measured in positive control FYDR-Rec cells is very low. Given the rarity of HR events at the FYDR transgene (17, 18), these data suggest that the FYDR mice cannot be used to study HR in bone marrow cells in vivo or in vitro. In an independent set of experiments, we used an alternative approach to measure HR, namely sister chromatid exchange (SCE) analysis. Interestingly, we found that there is a significant increase in SCEs in adherent cells at 20 weeks, compared to earlier time points, thus showing that cellular susceptibility to HR increases with age in LTBMCs.
Materials and Methods
Animals and Animal Care
FYDR mice homozygous for the transgene (17, 19), FYDR-Rec mice (carrying the full length EYFP sequence) (16), and WT female mice were maintained according to Institutional IACUC protocols and bed standard laboratory chow (Purina). Adult 30 – 33 gram, six to eight week old mice were sacrificed by cervical dislocation, and bone marrow from femur and tibia were flushed into LTBMC flasks according to published methods. The contents of one tibia and one femur (approximately 1 × 107 cells) were established from individual mice.
Long-term bone marrow cultures
Long-term bone marrow cultures (LTBMCS) were established from FYDR-YY, FYDR-REC, and C57BL/6J mice according to published procedures (2, 3). Adult 30 – 33 gram, six to eight week old mice were sacrificed by cervical dislocation, and the contents of the femur and tibia from 4 mice per group were flushed into separate 25 cm. tissue culture flasks containing LTBMC medium using a 10 cc syringe and 21 gauge needle (8 flasks total per group). LTBMC medium consisted of McCoy’s 5A medium (Gibco, Gaithersburg, MD) supplemented with 25% horse serum (Cambrex, Rockland Me), and 10−5 M hydrocortisone sodium hemisuccinate (StemCell Technologies, Vancouver, BC). Cultures were incubated at 33° C in 7% CO2. For maintenance of continuous hematopoiesis, half medium changes were performed weekly. After 4 weeks, the horse serum was replaced with 25% FBS (Gibco, Gaithersburg, MD) containing 10−5 M hydrocortisone sodium hemisuccinate. The cultures were observed weekly for percent confluency, hematopoietic cell production and cobblestone island formation. Cobblestone islands of greater than or equal to 50 cells were scored weekly in each flask. Cultures were maintained until cobblestone islands were not detectable.
Hematopoietic Cell Colony-Forming Assays
Each week the nonadherent cells from each of 8 LTBMC flasks per group were combined. Non adherent cells from the cultures were removed, counted and 5 × 104 cells/dish were plated in triplicate in semi-solid medium consisting of 1% methylcellulose in Iscove’s MDM (StemCell Technologies, Vancouver, BC), 30% Fetal Bovine Serum (FBS), 1% Bovine Serum Albumin (BSA), 10% WEHI-3 conditioned medium (as a source of IL-3), L-glutamine, and 2-mercaptoethanol. CFU-GM colonies of 50 cells or greater were counted on days 7 and 14 after plating.
Establishment of permanent bone marrow stromal cell lines
After cessation of hematopoiesis in LTBMCs, the adherent layer from representative cultures, from each group, was trypsinized and replated into Dulbecco’s medium supplemented with 10% fetal calf serum according to published methods. The cultures were passaged conservatively at 1 to 2, 1 to 5, and 1 to 10 or four weeks, and then passaged at 1 to 100 dilution. Clones were carried out by plating single cells into 96 well Linbro plates using Poissen statistics to derive single cell derived colonies for passage to permanent cell lines containing over 2 × 107, then frozen in liquid nitrogen according to published methods (20).
Assay for detection of EYFP fluorescence in cells
Two methods were utilized to quantitate EYFP positive cells. Aliquots of non-adherent cells removed from LTBMCs were scored by fluorescence activated flow analysis according to published methods (14, 17, 18). The sorter gate for yellow color was adjusted to give less than 1 per 4 × 106 false positive cells (autofluorescence) using negative control WT C57BL/6J mouse marrow. Cells were assayed from each culture harvest and mean and standard error calculated for at least four flasks assayed at each time point. A second method for assay of EYFP positive cells was visualization. Using a fluorescence microscope, aliquots of 1 × 105 cells were cytocentrifuged to glass coverslip slides and surveyed visually under a fluorescence objective. The number of cells positive out of 10,000 counted was scored for each of three flasks at each time point.
Assay for Sister Chromatid Exchange by SCE Analysis
To compare the frequency of SCEs in young compared to old culture derived adherent stromal cells, 4, 8, or 20 week LTBM culture derived adherent layers were induced to divide by seeding 2 × 106 cells in T25 flasks in McCoy’s medium supplemented with 25% horse serum and 10−6M Hydrocortisone Sodium Hemissuccinate for the 4 and 8 week cultures, and 25% fetal calf serum and 10−6M Hydrocortisone Sodium Hemissuccinate for the 20 week cultures. After 24 hours, 10 µM bromodeoxyuridine (BrdU) was added for 24–30 hours. Colcemid (0.1 µg/ml) was added for the final 4 hours. Cells were harvested by mitotic shake-off, resuspended, and incubated in hypotonic solution (0.2% potassium chloride, 0.2% sodium chloride, and 10% FBS) for 15 minutes at 37°C and fixed in Carnoy’s solution (25% glacial acetic acid, 75% methanol). Fixed cells were then dropped onto wet slides and dried overnight. To produce differentially-stained chromosomes, a modified fluorescence plus Giemsa technique was used.(24) Slides were stained in Hoechst 33258 (5 µg/ml) in 0.067 M Sorensen’s buffer for 20 minutes, air-dried, mounted in Sorensen’s buffer with a coverslip and exposed to a General Electric 15-watt black light bulb at 65°C for 20 minutes, and air-dried. Slides were then mounted in 20× SSC with a coverslip and incubated at 65°C for 20 minutes, air-dried, and stained in a 5% Giemsa solution in Sorensen’s buffer. At least 26 differentially-stained metaphase spreads per sample were imaged at 400× and SCEs counted. For comparison, a clonal bone marrow stromal cell line derived from the adherent layer of a 34 week old WT LTBMC was analyzed for SCE frequency in exponentially growing stromal cells.
Results
Analysis of FYDR bone marrow in vivo
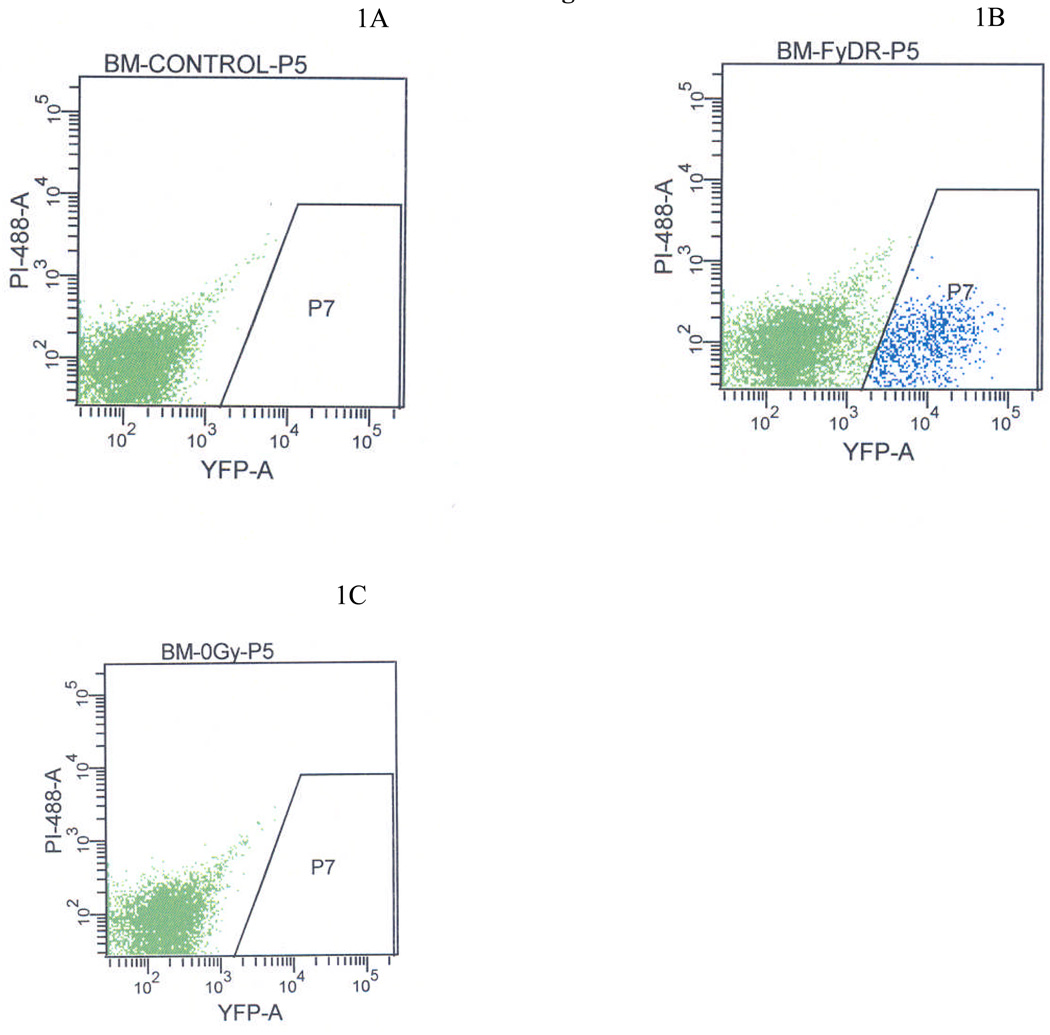
FYDR mice have previously been used to study HR in vivo in pancreas and skin (18, 21, 22) and in vitro in primary fibroblasts (17, 21, 22). To determine if HR could be studied in freshly excised FYDR bone marrow cells, we established parameters for detecting EYFP-positive cells using flow cytometry. By comparing the fluorescence of negative control C57Bl/6J mice (Fig. 1A) and positive control FYDR-Rec mice (Fig. 1B), a region (P7) was established that excluded all negative control cells. We then analyzed 12 million bone marrow cells from C57Bl/6J mice and found that none of the cells fell within the P7 region, indicating a very low level of false positive cells. In contrast, a significant number of fluorescent cells (~5%) were observed in positive control FYDR-Rec bone marrow, in which all cells carry the full-length EYFP coding sequence (Fig. 1B). Analysis of FYDR bone marrow, in which only cells that have under gone an HR event carry full-length EYFP did not yield any detectable fluorescent cells among over 18 million analyzed (Fig. 1C). Given that the frequency of yellow recombinant cells in other tissues of FYDR mice is rare, approximately 1–5 per million [17, 18], at least one million of the FYDR cells analyzed need to have the ability to fluoresce following a recombination event. The positive control FYDR-Rec samples tell us that very few bone marrow cells have the ability to fluoresce (~5%), even though they carry the full length EYFP sequence. Therefore, these studies show that the FYDR mice cannot be used to analyze the frequency of recombinant cells in freshly obtained samples of bone marrow.
Figure 1. Flow analysis of bone marrow sorted from single cell suspensions removed from tibia and femur of FYDR-YY mice.
C57BL/6J (1a); FYDR-Rec (1b); FYDR (1c)
The results are based on sorting of 5 × 107 from the femur and tibia of a single mouse (two femurs, two tibias). Cells on the right side of the P7 window are yellow based on gating according to EYFP yellow color.
Effect of FYDR transgene on long term bone marrow cultures
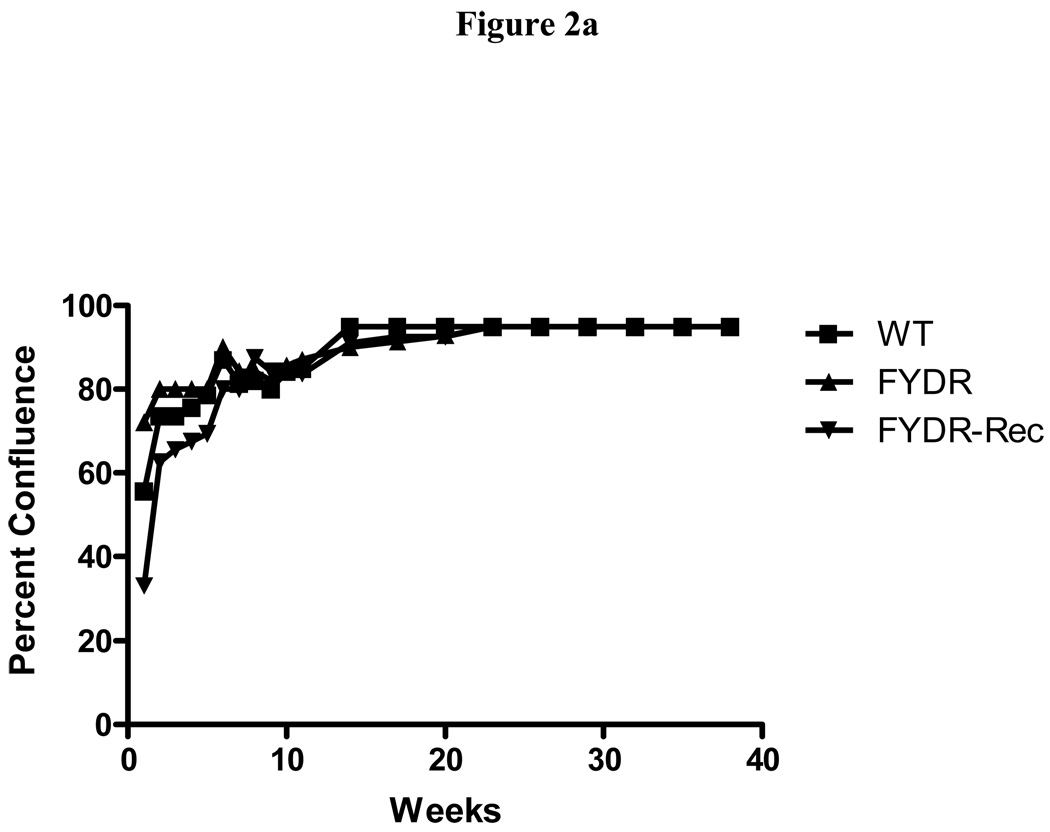
Although the expression levels were low in the freshly obtained FYDR-Rec bone marrow, it remained possible that expression levels of EYFP would be different in cultured cells or in cells differentiated from the initial bone marrow samples. To explore this possibility, LTBMCs were established from groups of four FYDR, FYDR-Rec, and WT mice in two separate experiments. The contents of the femur and tibia from each mouse were flushed into plastic flasks as described in the Materials and Methods. Eight flasks per group of four mice were established for each group. The adherent layer of cultures in all groups reached 100% confluence of the surface area of the flask by 10 weeks. The data is consistent with that from healthy background strain WT mouse derived LTBMCs (2).
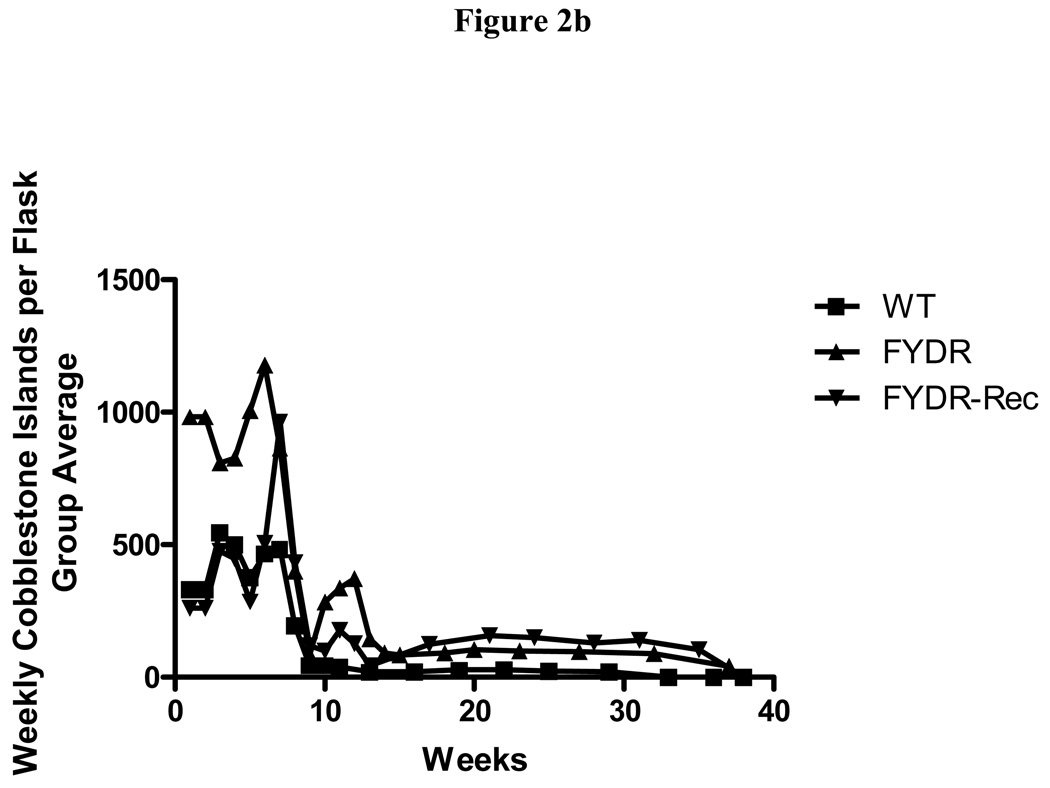
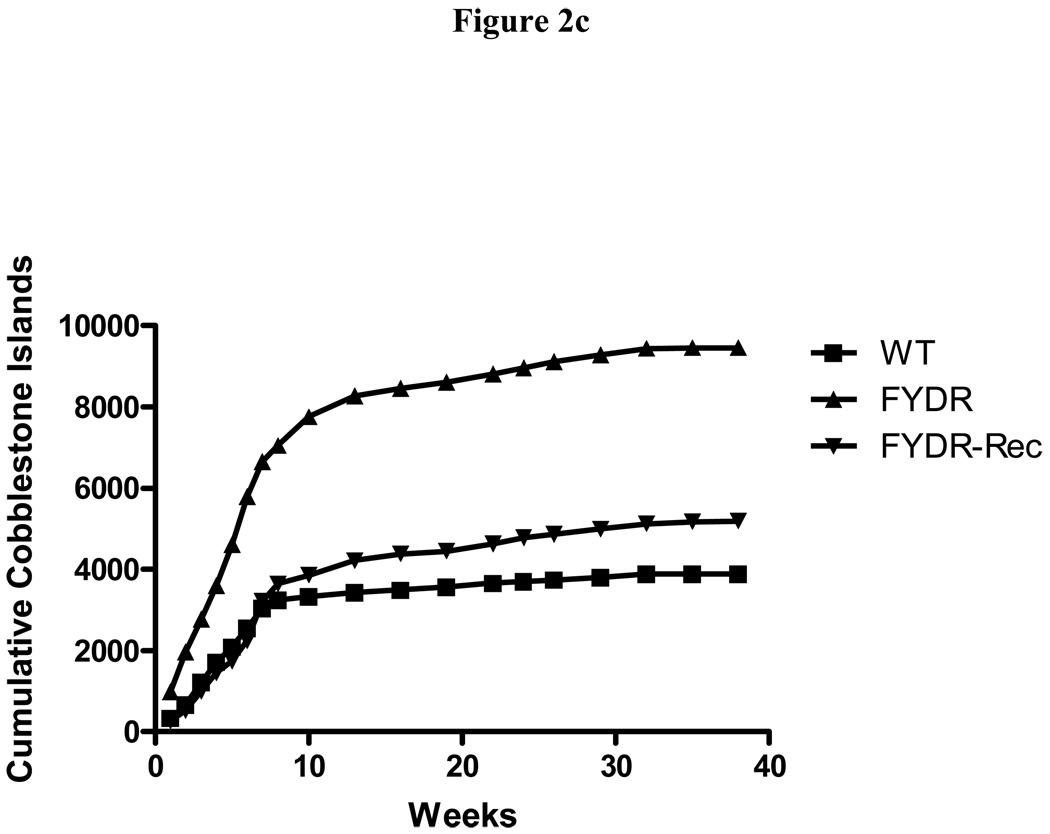
To determine if the FYDR genotype influences the phenotype of LTBMCs, cobblestone islands of adherent hematopoietic cell foci were measured weekly in each flask (Fig. 2b). Cobblestone islands reflected adherent hematopoietic colony forming cells and are an indication of the health and longevity of hematopoiesis long-term bone marrow cultures (2). The fluctuation of cobblestone islands was detected in all groups and is consistent with prior conditions for WT mouse marrow cultures (2). The results established that there was no significant effect of the FYDR, or FYDR-Rec genotype on formation of the adherent layer in LTBMCs. Cumulative cobblestone islands were increased in FYDR compared to FYDR-Rec and WT long-term bone marrow cultures, but the differences between groups were not significant (Fig. 2c).
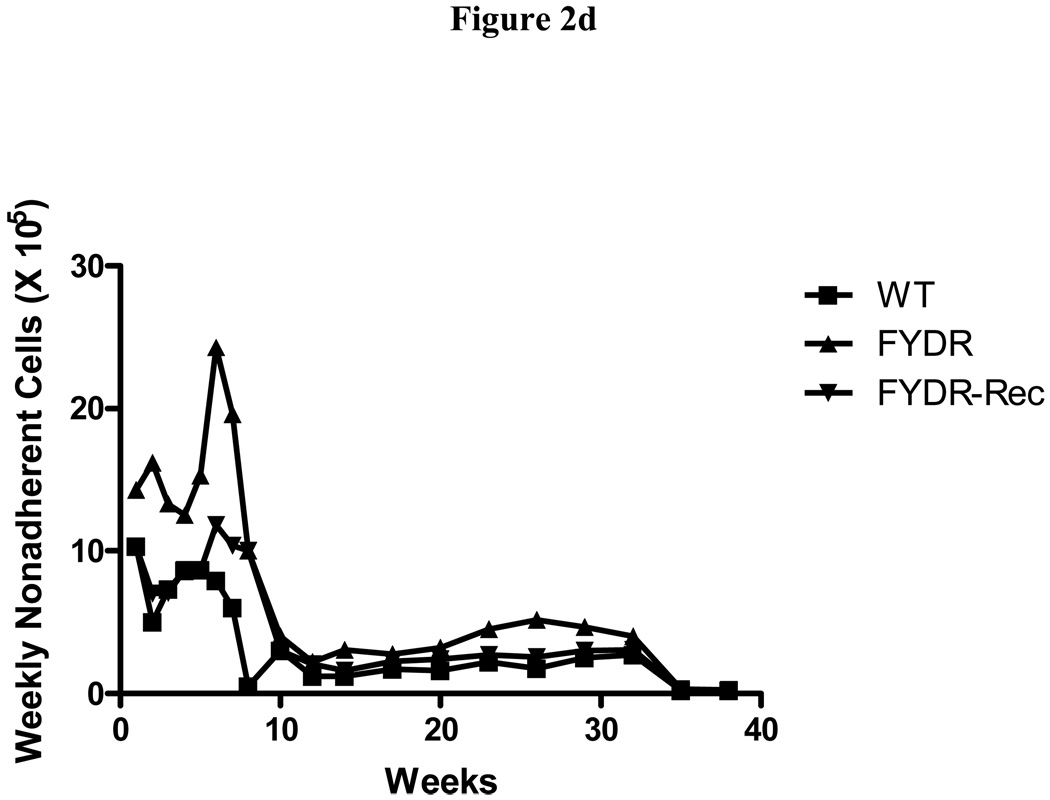
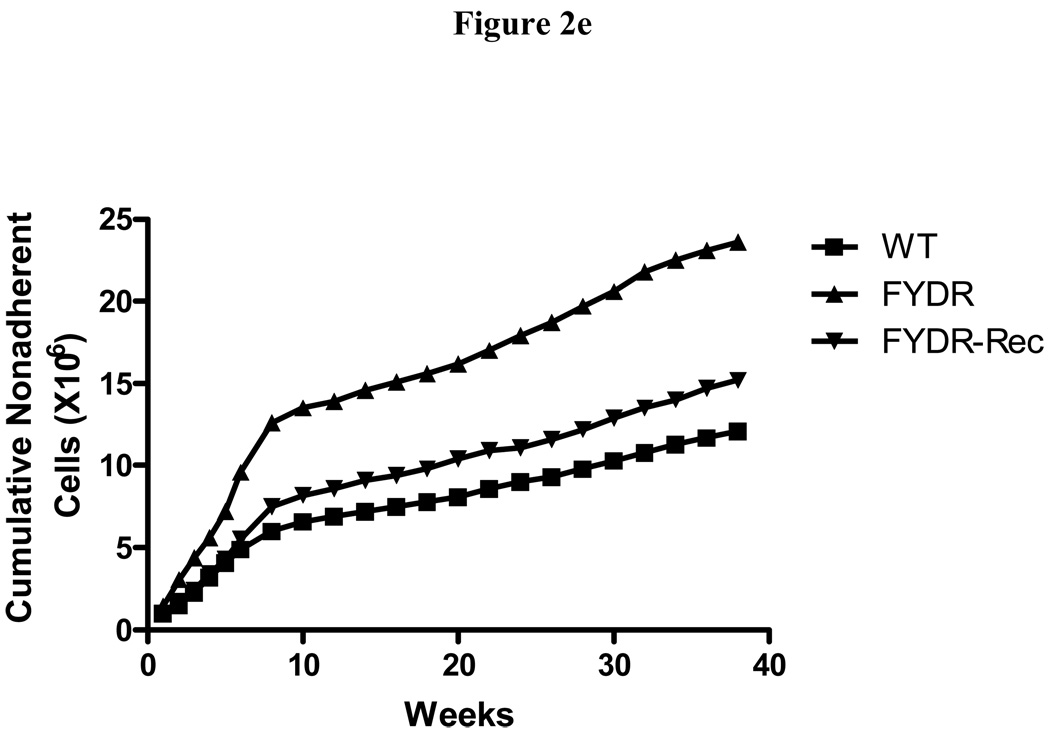
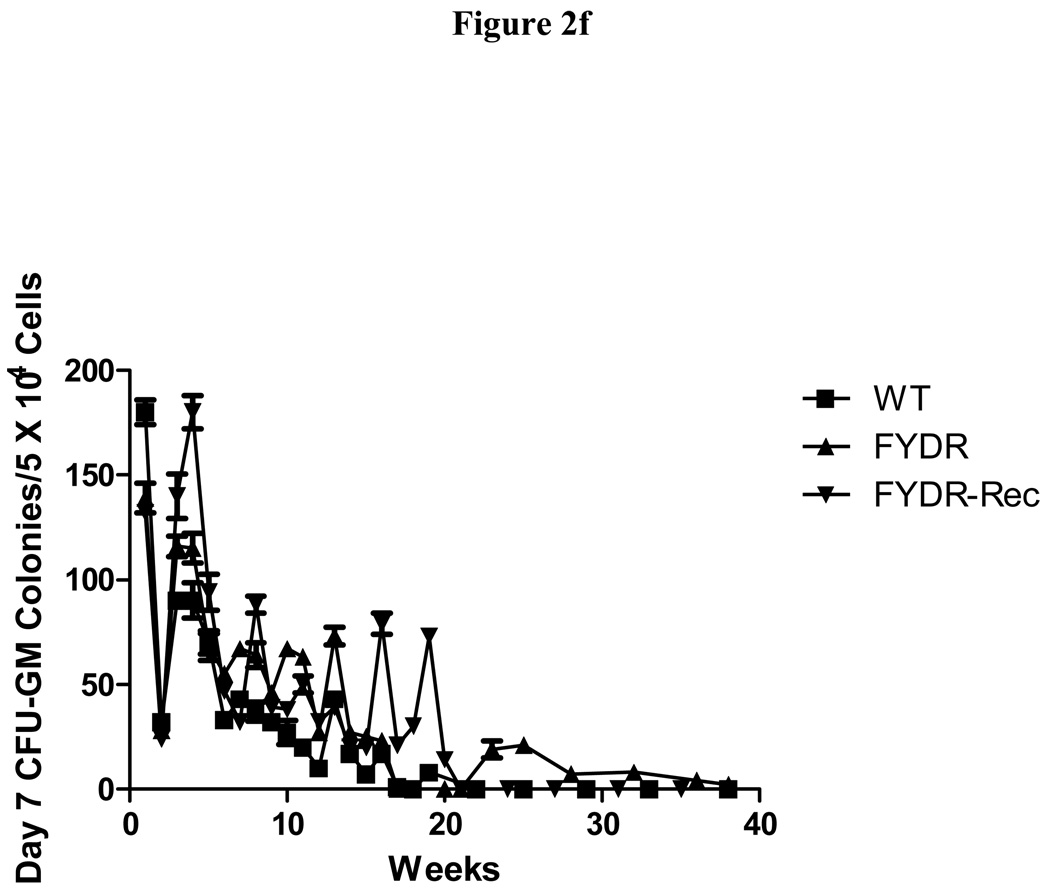
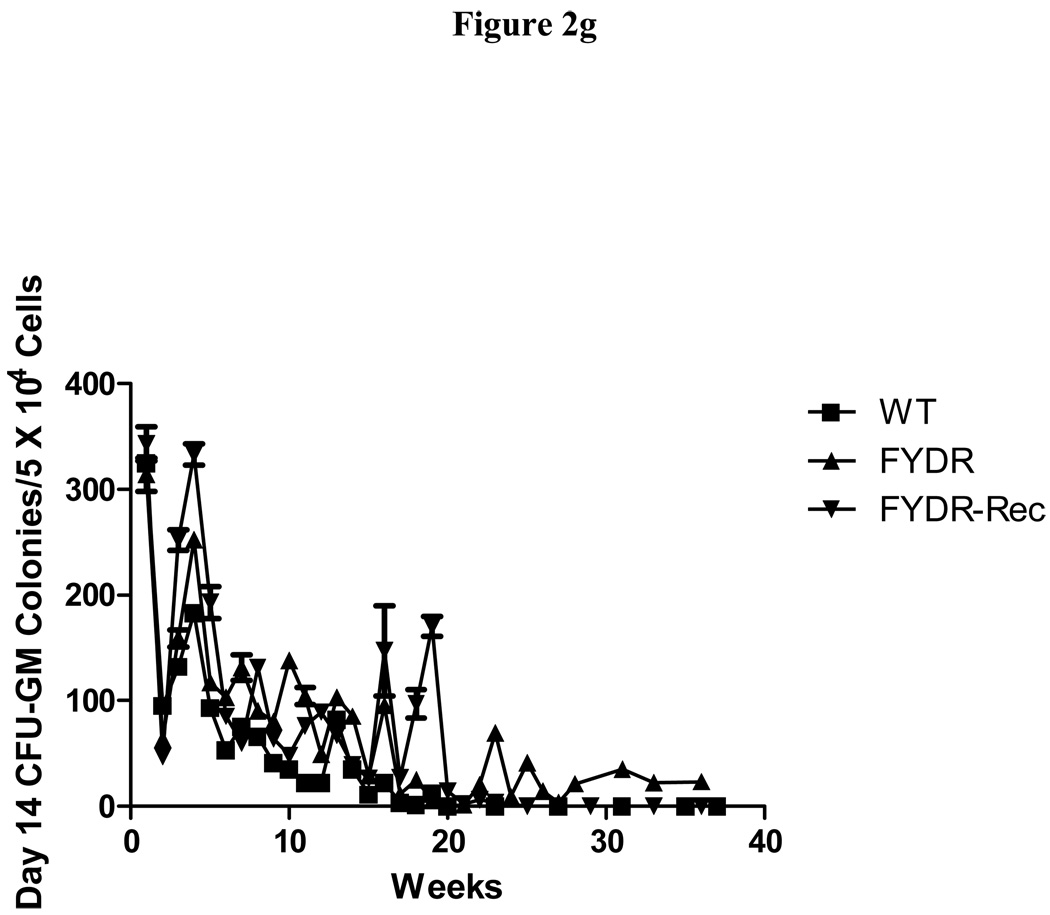
Figure 2. Kinetics of production of cells in colony forming progenitor cells in long-term bone marrow cultures from C57BL/6J, FYDR, and FYDR-Rec mice.
LTBMCs were established from four mice (eight cultures, with a tibia and femur from each mouse inserted into each culture (two per mouse). Nonadherent cells were harvested weekly.
Figure 2a shows percent confluence of the adherent layer in LTBMCs, Figure 2b shows weekly forming of cobblestone islands per flask defined as adherent foci of hematopoietic cells of greater and equal to 50 cells. Figure 2c shows cumulative scoring of cobblestone islands over 40 weeks in LTBMCs for each of the three groups. Figure 2d shows weekly production of nonadherent cells per flask. Each flask was harvested weekly, nonadherent cells counted, and an equal volume of fresh tissue culture being placed in each flask. Figure 2e shows cumulative production of nonadherent cells over 40 weeks in LTBMCs for each of the three culture groups. Figure 2f demonstrates weekly production of nonadherent cells capable of producing greater than 50 cell CFU-GM colonies scored on day 7. The results are presented as number of colony forming cells per 5 × 104 cells in semi-solid auger methylcellulose culture plate. Figure 2g demonstrates scoring of nonadherent cells capable of forming day 14 CFU-GM colonies, consisting of greater than 50 cells per colony.
Nonadherent cells are continuously released from the adherent population, and these nonadherent cells can be collected and analyzed. We found that the number of nonadherent cell produced weekly per flask was similar between groups and began to decrease by around week 15 for all three groups (Fig. 2d). Cumulative production of nonadherent cells showed a similar production in all three cell lines (Fig. 2e).
LTBMCs are capable of producing CFU-GM colonies. We measured the ability of the FYDR, positive control FYDR-Rec and negative control C57Cl/6JWT cultures to give rise to CFU-GM colonies starting 7 days (Fig. 2f) and 14 days (Fig. 2g) after initial plating. These numbers fluctuated week to week over the course of 20 weeks with sporadic production of colony forming cells after 20 weeks. The results are similar to those previously published for C57BL/6J mice (2). These data reflect movement from the adherent to the nonadherent phase of the cultures of hematopoietic colony forming cells. Weekly production of cells capable of producing the more primitive day 14 CFU-GM colony forming cells was not significantly different than the production from WT and FYDR-Rec cultures. Using two-way ANOVA test with week and culture type being the independent variables, the F-test for culture type effect indicated that the three culture types produced the same number of colony forming cells. With the post hoc Tukey’s multiple comparisons, FYDR and FYDR-Rec cultures did not produce a significantly different number of colony forming cells compared to each other, or WT cultures. From these studies, it is clear that the FYDR and the FYDR-Rec genotypes do not interfere with the ability of LTBMCs to give rise to CFU-GM colonies.
Detection of EYFP positive cells within nonadherent cultures and CFU-GM colonies
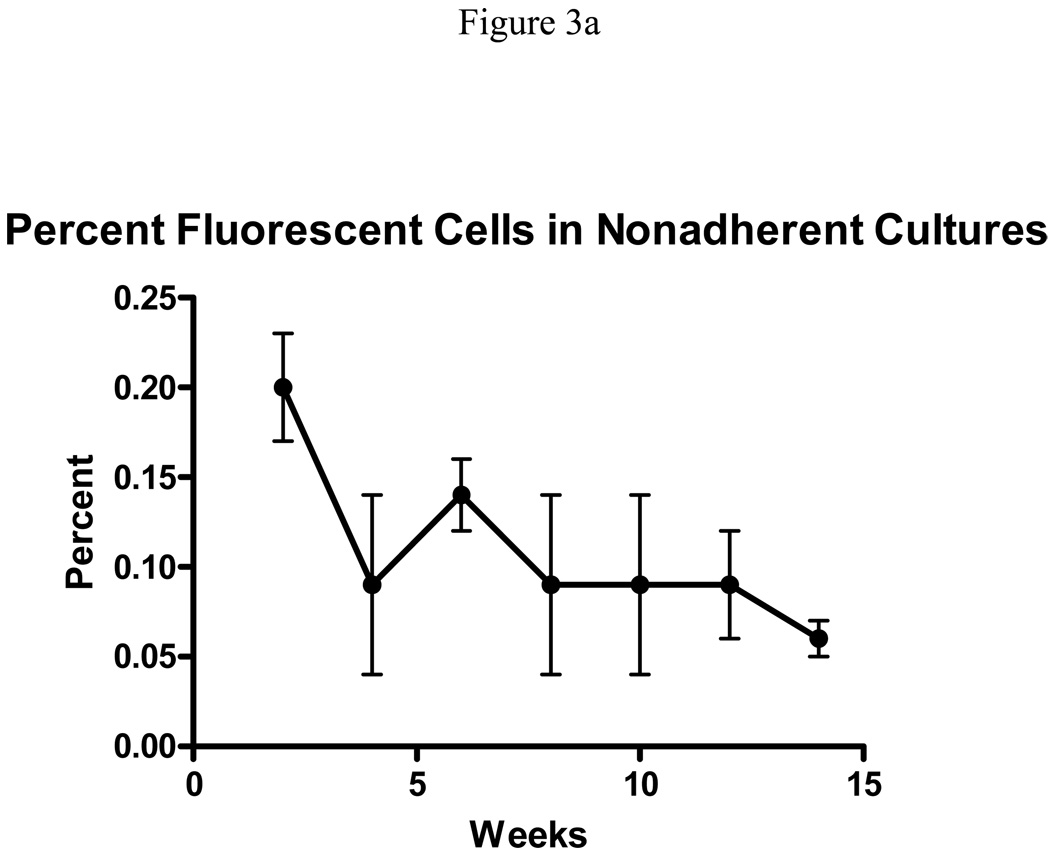
The above results establish that FYDR- mouse derived LTBMCs were as healthy as those derived from positive control FYDR-Rec and negative control WT mice. To explore the possibility that these cultures could be used to study HR using the FYDR system, we first assessed expression of EYFP in the positive control FYDR-Rec non-adherent cells. Since positive control FYDR-Rec mice are an ideal control for expression of the EYFP coding sequence in FYDR mice, analysis of the percentage of fluorescent cells in FYDR-Rec cultures reveals the likelihood that a recombinant cell in FYDR cultures will actually fluoresce. We therefore measured the percentage of EYFP positive cells among non-adherent cells harvested from FYDR-Rec LTBMC over 14 weeks. We found that initially, only approximately 0.2% of positive control cells actually yield a detectable fluorescent signal (Fig. 3a) and that over time, this percentage decreases. Given this low percentage among the positive control samples, we did not anticipate that we would detect any recombinant cells among the FYDR cultures. Assuming a frequency that is similar to what has been observed previously in other tissues (~1–5 per million) (17, 18), we would need to analyze over 500 million cells per sample to detect just a few recombinant cells. Nevertheless, parallel studies of the FYDR cultures were performed. Although it appeared that there were detectable recombinant cells (data not shown), given the low EYFP expression capacity, the number of cells that are necessary for robust data make the study of HR using the FYDR detection system in non-adherent bone marrow cultures infeasible.
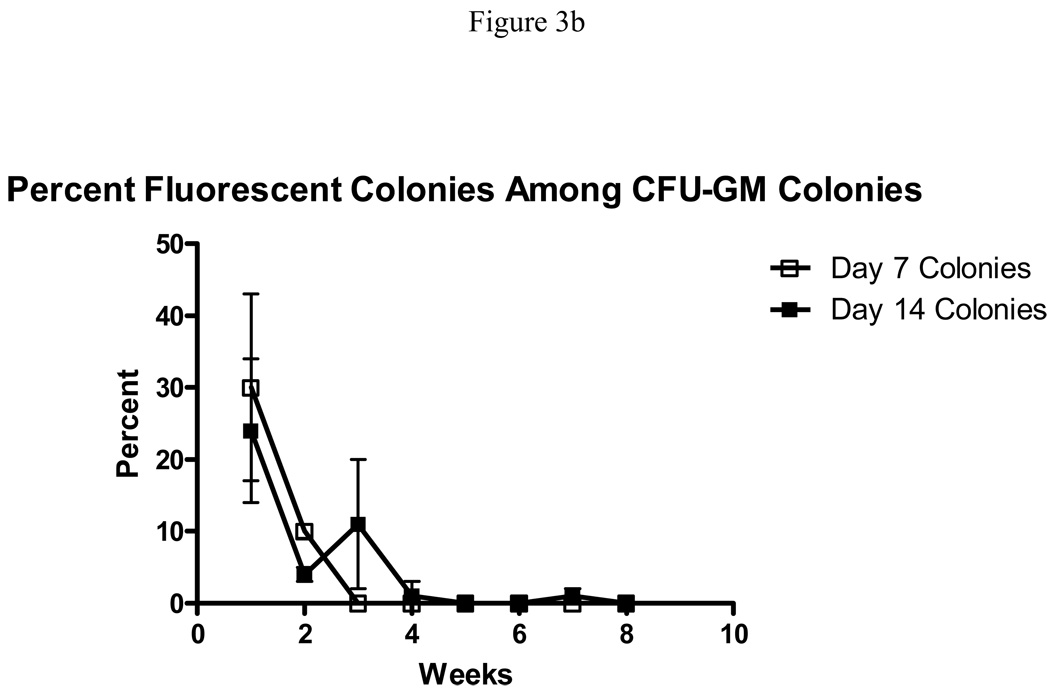
Figure 3.
Expression of the FYDR transgene in positive control FYDR-Rec bone marrow cells. (A) Percentage of fluorescent cells in non-adherent FYDR-Rec cultures as measured by flow cytometry. Error bars represent 1 standard deviation. (B) Percentage of colonies containing detectable EYFP positive cells as measured by visual inspection under a fluorescent microscope in day 7 and day 14 CFU-GM colonies.
In addition to non-adherent cells, we also scored the number of day 7 and day 14 CFU-GM colonies from positive control FYDR-Rec cultures which contained yellow cells detectable via visual examination under a fluorescent microscope. Initially, a significant percentage of both day 7 and day 14 CFU-GM colonies from FYDR-Rec bone marrow cultures contain yellow cells, 30% and 24%, respectively (Fig. 3b). However, the percentage of colonies with yellow cells decreases with time until, by week 5, virtually no colonies contain yellow cells (Fig. 3b). We also examined the fraction of day 7 and day 14 CFU-GM colonies containing at least one yellow cell from FYDR bone marrow cultures, in which EYFP positive cells occur only after a HR event. Of the more than 4700 colonies analyzed, only one FYDR colony contained a yellow cell. Taken together, these data suggest that the FYDR HR detection system is not suitable for studying HR in either non-adherent cells or CFU-GM colonies.
Age-Dependent increase in SCEs detectable in LTBMC adherent stromal cells
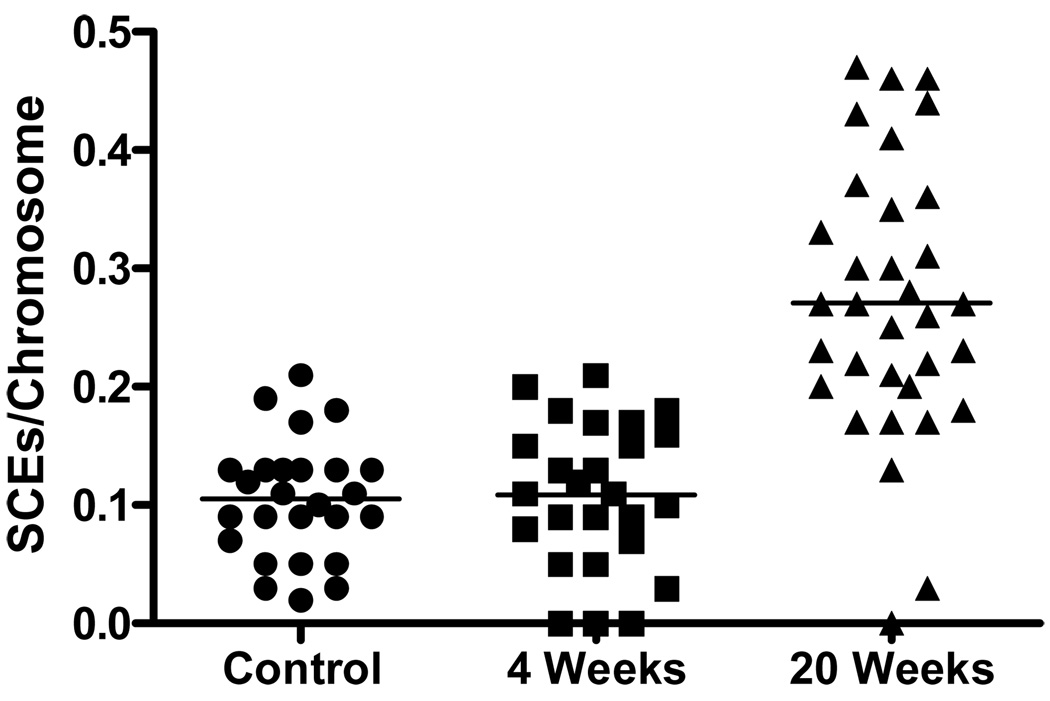
Recombination events using the FYDR system are detected at a transgene located at one specific locus on chromosome #1 (17). However, HR events are not limited to this transgene, but rather occur throughout the genome. In order to detect genome-wide HR events, we measured sister chromatid exchanges (SCEs). For SCE analysis, differentially stained sister chromatids reveal HR events that have occurred within the previous two cell divisions. HR events can potentially arise due to an age dependent increase in oxidative stress over time in cells in the adherent layer, which were in plateau phase during the culture duration. We measured SCEs in adherent cells removed from LTBMCs at 4, 8, or 20 weeks, subcultured for 48 hours, and labeled with bromodeoxyuridine (BrdU) as described in Materials and Methods. Controls for this experiment included a clonal bone marrow stromal cell line derived from a WT LTBMC. In addition, we also used 32D cl 3 hematopoietic progenitor cells (22) as another control cell line for non-adherent cells (data not shown). This clonal bone marrow stromal cell line controlled for subculture in prolonged growth phase and showed an average SCE frequency of 0.11 SCEs per chromosome (Fig. 4, Table 1). An independent culture of hematopoietic cell line 32D cl 3 (22) showed a frequency of 0.07 SCEs per chromosome (data not shown). We next compared adherent cells harvested from 4 or 8 week old (young) and twenty week old (old) LTBMCs for SCEs, and at least 26 metaphase spreads were scored per condition. The results demonstrate a significant increase in SCEs in the adherent cells removed from 20 week compared to 4 week old LTBMCs. (Fig. 4, Table 1). Therefore, we conclude that changes associated with aging of LTBMCs lead to a significant increase in susceptibility to HR events.
Figure 4. Sister chromatid exchange (SCE) increase in bone marrow stromal cells removed from 4 week or 20 week old C57BL/6J LTBMCs.
Cells were removed from the adherent layer of representative long-term bone marrow culture flasks by scraping with a rubber policeman, transferred to liquid culture, labeled with BrdU, and carried for two cell divisions as described in the methods. The results are for scoring 26 metaphases per time point for sister SCEs. Results compare 4 week and 20 week old LTBMCs. Control is a clonal C57BL/6J mouse LTBMC derived stromal cell line carried in logarithmic/exponential phase growth. Median SCEs per chromosome are indicated by horizontal bars. Twenty week samples showed a statistically significant increase compared to 4 weeks. (Mann-Whitney test: p < 0.0001)
Table I.
Culture Duration Dependent Increased Sister Chromatid Exchange in Adherent Bone Marrow Stromal Cells from Long-Term Marrow Cultures
| Week | Number of Observations |
SCE/Chromosome (mean ± SE) |
|---|---|---|
| 4 | 26 | 0.11 ± 0.01 |
| 8 | 26 | 0.11 ± 0.01 |
| 20 | 33 | 0.27 ± 0.02 |
P value (two sided two sample t-test): Difference between 4 and 8 wks. (p = 0.81) Difference between 4 and 20 wks. (p < 0.0001)
Discussion
The FYDR mice carry two copies of the coding sequence for EYFP arranged in tandem, each copy carrying different deletions of essential coding sequence that prevent EYFP expression. A HR event between the two coding sequences can restore full-length EYFP coding sequence, resulting in yellow fluorescence (17). Previous studies have revealed that FYDR mice can be used to study HR in vivo in skin and pancreas (18, 21, 22) and in vitro in cultured primary fibroblasts (17, 21). In addition, in the pancreas of FYDR mice, a direct age related increase in the number of EYFP positive cells occurring as a result of both de novo recombination events and clonal expansion of previously existing recombinant cells was demonstrated (17, 25).
In the present studies, we sought to determine whether the FYDR transgene affected the health of non-adherent hematopoietic cells or adherent stromal cells of LTBMCs. We obtained LTBMCs from FYDR, positive control FYDR-Rec and negative control WT mice. In each of several parameters of hematopoiesis in LTBMCs, including cumulative production of cobblestone islands (reflecting adherent hematopoietic colonies), non-adherent hematopoietic cell production, and colony forming non-adherent hematopoietic cell production (cells which form day 7 and day 14 multilineage CFU-GM colonies containing granulocyte/macrophage cells), the FYDR transgene did not negatively affect the health of marrow cultures. Thus, the FYDR genotype was not associated with a detectable diminution of hematopoiesis in continuous bone marrow culture (2).
To determine if the FYDR transgene can be used to study the effects of bone marrow aging on HR, HR events that occur at the FYDR transgene must be detectable, meaning the recombined full-length EYFP coding sequence must be expressed and translated into the EYFP protein. Although the FYDR transgene was designed using a promoter that had previously been shown to be ubiquitously expressed in all tissues (26), we and others have found that expression of EGFP and EYFP is highly variegated in vivo (16, 26). Indeed, histological analysis of positive control FYDR-Rec mice shows significant variation in expression levels among tissue types and even among the same cell type within a tissue (16). Previous studies using FYDR mice indicated that HR events are very rare (~1–5 recombinant cells per million) (17, 18). Thus, in order to detect recombinant cells, the number of cells expressing the FYDR substrate (EYFP expression) must be high. We determined the expression level of the FYDR transgene in bone marrow cells from positive control FYDR-Rec mice, in which all cells carry the full-length EYFP coding sequence (16). Only ~5% of freshly excised bone marrow cells from positive control FYDR-Rec mice expressed EYFP, a level too low to be feasible for the detection of rare HR events in FYDR mice in vivo, due to limitations in the number of cells that can be obtained per mouse.
The expression of the FYDR transcript has been shown to vary in vivo and in vitro (16, 18, 21). Thus, we also analyzed the expression of the FYDR transgene in bone marrow cultures in vitro. Non-adherent cells removed from positive control FYDR-Rec LTBMCs show that less than 0.2% of cells are EYFP positive. In addition, while day 7 and day 14 CFU-GEMM positive control FYDR-Rec colonies initially showed that 25–30% of colonies were EYFP positive, the percentage of EYFP positive colonies decreased to zero with time. These data establish that bone marrow cells from FYDR-Rec mice do not express EYFP a high level, indicating significant differences in EYFP expression among bone marrow, skin, and pancreas (16, 18, 21). The decrease in EYFP expression over time in culture may reflect a decrease in EYFP mRNA transcription, a decrease in EYFP mRNA translation or a change in any factor that may affect EYFP protein stability.
With the low expression of the FYDR substrate in positive control FYDR-Rec bone marrow and the observed loss of yellow color over time in FYDR-Rec LTBMCs, the chance of detecting a significant age-dependent change in EYFP positive cells in FYDR LTBMC would have required sorting of over 5 × 108 harvested non-adherent cells, which is impractical for the LTBMC system. If 100% of the FYDR-Rec cells had been yellow, we may have been able to detect changes in the frequency of recombination in FYDR LTBMCs. However, given the low percentage of EYFP expressing cells, the FYDR system is not amenable to studying HR in bone marrow. In contrast, we observed a statistically significant increase in SCEs in the aged subcultured adherent cells from LTBMCs. The results indicate that aging of nondividing plateau phase adherent cells in the stromal microenvironment of LTBMCs (27) is associated with an increase in SCEs in cells removed and allowed to undergo two cell divisions. These results are consistent with oxidative stress that is cumulative over time in LTBMC adherent cells held in a nondividing state, and is associated with their decreased capacity to support hematopoiesis (2). Early cessation of hematopoiesis has been reported in LTBMCs derived from mice that contain deficient levels of the antioxidant protein MnSOD (21–22). Furthermore, enhanced hematopoiesis has been observed in LTBMCs to which is added the antioxidant enzyme catalase (7). Thus, the increase in SCEs in the adherent cells of the marrow environment may be due to an increase in DSBs caused by the oxidative stress of aging. Taken together, the present studies indicate that while the FYDR system cannot be used to study HR in LTBMCs, while SCE analysis is a potentially valuable method for measuring the effects of aging on DNA DSB-induced and HR in LTBMCs.
The distributions of the data for number of SCEs/chromosome at 4, 8, and 20 weeks were examined with the Anderson-Darling normality test using stromal cells form C57BL6/JNHsd LTBMCs of each age. The p-values for these three tests were all greater than 0.25, indicating that the data can be regarded as normally distributed and hence the two sample t-test can be used to compare between different weeks. At least 30 metaphase spreads were scored from stromal cells prepared as described in the methods.
The number of SCEs/chromosome at Week 8 and Week 20 were compared with Week 4. It can be seen that the number of SCEs/chromosome at Week 20 was significantly higher than the value at Week 4 (p<0.0001). The value at Week 8 was not significantly different from the value at Week 4 (p=0.81).
Acknowledgments
Supported by NIH Grants: RO1-CA083876 and U19-A168021. Additional support was received from the Department of Energy Grant FG01-04ER04-21. D.M.W.-B. was supported by the NIEHS Grant T32ES007020. We also thank the MIT Center for Environmental Health Sciences (NIEHS P30 ES001209-26A1).
References
- 1.Mauch P, Greenberger JS, Botnick LE, Hannon EC, Hellman S. Evidence for structured variation in self-renewal capacity within long-term bone marrow cultures. PNAS, USA. 1980;77:2927–2930. doi: 10.1073/pnas.77.5.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakakeeny MA, Greenberger JS. Granulopoiesis longevity in continuous bone marrow cultures and factor dependent cell line generation: Significant variation among 28 inbred mouse strains and outbred stocks. J Nat Canc Inst. 1982;68:305–317. [PubMed] [Google Scholar]
- 3.Greenberger JS. Sensitivity of corticosteroid-dependent, insulin-resistant lipogenesis in marrow preadipocytes of mutation diabetic-obese mice. Nature. 1978;275:752–754. doi: 10.1038/275752a0. [DOI] [PubMed] [Google Scholar]
- 4.Greenberger JS, Newburger PE, Lipton JM, Moloney WC, Sakakeeney MA, Jackson PL. Viral and cellular requirements for Friend virus granulocytic leukemogenesis in long-term marrow cultures. J Nat Canc Inst. 1980;64:867–878. [PubMed] [Google Scholar]
- 5.Epperly MW, Cao S, Goff J, et al. Increased longevity of hematopoiesis in continuous bone marrow cultures and adipocytogenesis in marrow stromal cells derived from SMAD3−/− mice. Exp Hematol. 2005;33:353–362. doi: 10.1016/j.exphem.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Epperly MW, Cao S, Zhang X, et al. Increased longevity of hematopoiesis in continuous bone marrow cultures derived from mtNOS−/− homozygous recombinant negative mice correlates with increased radioresistance of hematopoietic and bone marrow stromal cells. Exp Hematol. 2007;35:137–145. doi: 10.1016/j.exphem.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Gupta R, Karpatkin S, Basch RS. Hematopoiesis and stem cell renewal in long-term bone marrow cultures containing catalase. Blood. 2006;107:1837–1846. doi: 10.1182/blood-2005-03-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair (Amst) 2007;6:923–935. doi: 10.1016/j.dnarep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Reliene R, Bishop AJ, Schiestl RH. Involvement of homologous recombination in carcinogenesis. Adv Genet. 2007;58:67–87. doi: 10.1016/S0065-2660(06)58003-4. [DOI] [PubMed] [Google Scholar]
- 10.Greenberger JS, Otten JA, Eckner RJ, Tennant RW. In vitro quantitation of lethal and leukemogenic effects of gamma irradiation on stromal and hematopoietic stem cells in continuous mouse bone marrow culture. Int J Rad Oncol Biol Phys. 1982;8:1155–1165. doi: 10.1016/0360-3016(82)90063-3. [DOI] [PubMed] [Google Scholar]
- 11.Greenberger JS, Hoffman N, Lieberman M, Botnick LE, Sakakeeny MA, Eckner RJ. Pool size of pluripotential hematopoietic stem cells increased in continuous bone marrow culture by Friend spleen focus forming virus. J N C I. 1983;70:323–331. [PubMed] [Google Scholar]
- 12.Greenberger JS, Klassen V, Kase K, Sakakeeny MA. Effects of low-dose-rate irradiation on plateau phase bone marrow stromal cells in vitro: Demonstration of a new form of nonlethal physiologic alteration of support of hematopoietic stem cells. Int J Rad Oncol Biol Phys. 1984;10:1027–1037. doi: 10.1016/0360-3016(84)90174-3. [DOI] [PubMed] [Google Scholar]
- 13.Naparstek E, Donnelly T, Kase K, Greenberger JS. Biologic effects of in vitro x-irradiation of murine long-term bone marrow cultures on the production of granulocyte-macrophage colony stimulating factors. Exp Hematol. 1985;13:701–708. [PubMed] [Google Scholar]
- 14.Greenberger JS, Newburger PE, Sakakeeny MA. Phorbol myristate acetate stimulates macrophage proliferation and differentiation and alters granulopoiesis and leukemogenesis in long-term bone marrow cultures. Blood. 1980;56:368–379. [PubMed] [Google Scholar]
- 15.Greenberger JS, Palaszynski EW, Pierce JH, et al. Biologic effects of prolonged L-Phenylalanine mustard treatment of murine long-term bone marrow cultures and IL-3 dependent hematopoietic progenitor cell lines. J Nat Canc Inst. 1985;74:247–262. [PubMed] [Google Scholar]
- 16.Wiktor-Brown DM, Hendricks CA, Olipitz W, Rogers AB, Engelward BP. Applications of fluorescence for detecting rare sequence rearrangements in vivo. Cell Cycle. 2006;5:2715–2719. doi: 10.4161/cc.5.23.3527. [DOI] [PubMed] [Google Scholar]
- 17.Hendricks CA, Almeida KH, Stitt MS, et al. Spontaneous mitotic homologous recombination at an enhanced yellow fluorescent protein (EYFP) cDNA direct repeat in transgenic mice. Proc Natl Acad Sci USA. 2003;100:6325–6330. doi: 10.1073/pnas.1232231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiktor-Brown DM, Hendricks CA, Olipitz W, Engelward BP. Age-dependent accumulation of recombinant cells in the mouse pancreas revealed by in situ fluorescence imaging. Proc Natl Acad Sci USA. 2006;103:11862–11867. doi: 10.1073/pnas.0604943103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendricks CA, Almedia KH, Stitt MS, et al. Spontaneous mitotic homologous recombination at an enhanced yellow fluorescent protein (EYFP) cDNA direct repeat in transgenic mice. Proc Natl Acad Sci USA. 2003;100(11):6325–6330. doi: 10.1073/pnas.1232231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberger JS, Anderson J, Berry LA, Epperly MW, Cronkite EP, Boggs SS. Effects of irradiation of CBA/Ca mice on hematopoietic stem cells and stromal cells in long-term bone marrow cultures. Leukemia. 1996;10(3):514–527. [PubMed] [Google Scholar]
- 21.Wiktor-Brown DM, Olipitz W, Hendricks CA, Rugo RE, Engelward BP. Tissue-specific differences in the accumulation of sequence rearrangements with age. DNA Repair (Amst) 2008;7:694–703. doi: 10.1016/j.dnarep.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovalchuk O, Hendricks CA, Cassie S, Engelward AJ, Engelward BP. In vivo recombination after chronic damage exposure falls to below spontaneous levels in "recombomice". Mol Cancer Res. 2004;2:567–573. [PubMed] [Google Scholar]
- 23.Epperly MW, Bernarding M, Gretton J, Jefferson M, Nie S, Greenberger JS. Overexpression of the transgene for manganese superoxide dismutase (MnSOD) in 32D cl 3 cells prevents apoptosis induction by TNF-α, IL-3 withdrawal and ionizing irradiation. Exp Hematol. 2003;31(6):465–474. doi: 10.1016/s0301-472x(03)00041-9. [DOI] [PubMed] [Google Scholar]
- 24.Perry P, Wolff S. New giemsa method for the differential staining of sister chromatids. Nature. 1974;251:156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- 25.Wiktor-Brown DM, Kwon HS, Nam YS, So PT, Engelward BP. Integrated one-and two-photon imaging platform reveals clonal expansion as a major driver of mutation load. Proc Natl Acad Sci USA. 2008;105:10314–10319. doi: 10.1073/pnas.0804346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. 'Green mice' as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 27.Biankin SA, Collector MI, Biankin AV, et al. A histological survey of green fluorescent protein expression in 'green' mice: implications for stem cell research. Pathology. 2007;39:247–251. doi: 10.1080/00313020701230807. [DOI] [PubMed] [Google Scholar]
- 28.Lechpammer S, Epperly MW, Zhou S, Nie S, Glowacki J, Greenberger JS. Antioxidant pool regulated adipocyte differentiation Sod2−/− bone marrow stromal cells. Exp Hematol. 2005;33:1201–1208. doi: 10.1016/j.exphem.2005.06.026. [DOI] [PubMed] [Google Scholar]