Abstract
Protein kinase CK2, a protein serine/threonine kinase, plays a global role in activities related to cell growth, cell death and cell survival. CK2 has a large number of potential substrates localized in diverse locations in the cell including, e.g., NF-κB as an important downstream target of the kinase. In addition to its involvement in cell growth and proliferation it is also a potent suppressor of apoptosis, raising its key importance in cancer cell phenotype. CK2 interacts with diverse pathways which illustrates the breadth of its impact on the cellular machinery of both cell growth and cell death giving it the status of a “master regulator” in the cell. With respect to cancer, CK2 has been found to be dysregulated in all cancers examined demonstrating increased protein expression levels and nuclear localization in cancer cells compared with their normal counterparts. We originally proposed CK2 as a potentially important target for cancer therapy. Given the ubiquitous and essential for cell survival nature of the kinase, an important consideration would be to target it specifically in cancer cells while sparing normal cells. Towards that end, our design of a tenascin based sub-50 nm (i.e., less than 50 nm size) nanocapsule in which an anti-CK2 therapeutic agent can be packaged is highly promising because this formulation can specifically deliver the cargo intracellularly to the cancer cells in vivo. Thus, appropriate strategies to target CK2 especially by molecular approaches may lead to a highly feasible and effective approach to eradication of a given cancer.
Keywords: Protein kinase CK2, casein kinase 2, signaling, cancer, NF-κB, nanocapsule, nanoparticle, tenfibgen, tenascin, antisense, siRNA
1. Introduction
Protein kinase CK2 (adopted acronym for the inappropriate name casein kinase 2 or II, as it may be noted that casein is not a physiological substrate for CK2) has been extensively studied for more than three decades. The protein kinase CK2 complex is a tetramer comprised of catalytic and regulatory subunits such that the two catalytic subunits are linked via two molecules of the beta subunit. The two catalytic subunits α and α′ (∼ 42 and 38 kDa, respectively) and the β subunit (∼ 28 kDa) form complexes such as α2β2, αα′β2, and α′2β2 in varying distribution depending on the cell type. A considerable amount of work has been undertaken to delineate basic biochemistry of the kinase and the details of these studies can be found in several review articles [see, e.g., 1-6]. Much work has also been devoted to the biological functions of CK2 and these types of studies have led to identification of a large number of potential substrates localized in diverse compartments in the cell, just as the kinase itself is found in various locales in the nuclear and cytoplasmic compartments. The kinase was originally found to be elevated in rapidly proliferating cells including cancer cells and over time it has become apparent that CK2 is dysregulated by an increase in protein expression in all cancers examined. It has emerged that CK2 plays a global role in control of cell growth and proliferation, and even more interestingly an equally major role in control of cell death [2,3,7-10]. Since the cancer cell phenotype has the consistently remarkable features of deregulated cell growth (elevation) and cell death (suppressed apoptosis) [e.g., 11,12], the observation that CK2 is elevated in cancer cells provides a key link of the kinase to neoplasia. However, it is now becoming apparent that CK2 may be involved in the pathophysiology of many other disease processes; a detailed elegant discussion of CK2 in diverse diseases was presented in a recent publication [7]. In the present review, we will give a brief overview of the development of our understanding of the biological and pathobiological function of CK2, with a specific focus on its functionality in cancer and consideration of its potential as a key target for cancer therapy. We also consider the feasibility of molecular downregulation in a cancer cell specific manner through delivery of the therapeutic agent in a sub-50 nm tenfibgen nanocapsule.
2. General Features of CK2 Activity
CK2 is among the few protein kinases that can utilize both ATP and GTP for transfer of phosphate groups to serine/threonine residues in the proteins harboring the general consensus sequence S/TXXD/E/Yp/Sp, and it appears that over 300 potential substrates for CK2 may be present in the cell [13]. The question is how CK2 recognizes its substrates in response to diverse signals. An interesting feature of CK2 is that it appears to be constitutively active as its regulation does not follow the general modes of activation commonly observed for protein kinases in the cell. Important insight into the activity of CK2 has been gained by extensive studies on X-ray crystallographic structures of CK2 as has been discussed in detail [see, e.g., 14]. These studies have contributed significantly to the nature of the CK2 structure and aspects of functional activity, although much remains to be learned. These various studies confirmed that the β subunit of CK2 is the linker between the catalytic subunits yielding the α2β2 holoenzyme structure in which the two α subunits do not come into contact with each other. Interestingly, the β subunit harbors a Zn binding motif and it appears that the dimerization of the β subunits requires Zn [14,15]. This dimerization sets the stage for each of the β subunits to bind to a α subunit independently while exhibiting a certain plasticity; the structural details of this interaction have been discussed in detail by Niefind et al. [14]. The various crystallographic studies have also provided some insight into the basis of the ability of CK2 to utilize both ATP and GTP for phosphate transfer as well as the nature of the activation state of the catalytic subunit of the kinase [14]. In this regard, the recent observations that the human CK2α structure can adopt partially inactive conformations, whereas the fully active state of CK2α is pre-formed upon binding of CK2β, are particularly intriguing with respect to the state and regulation of CK2 activity in the cell [14].
3. Aspects of cellular regulation of CK2
The aforementioned structural studies of CK2 highlighted dynamic regions in the kinase that may contribute to functional features of cosubstrate recognition as well as subunit interactions in the cell, and indeed it was proposed that the plasticity of CK2α may reflect a novel mode of regulation of the kinase in the cell [14]. This aspect of CK2 biology has remained an important question over the years. Several modes of intracellular regulation of CK2 function have been postulated. One of the early demonstrations based on studies of androgenic regulation of prostate growth (a physiological normal cell growth and death model) demonstrated dynamic shuttling of CK2 to different intranuclear locations in response to growth stimulus and shuttling out of the nuclear compartment on induction of cell death [e.g., 8,16,17,27]. This view was expanded to suggest that intracellular shuttling of CK2 may represent a general mechanism of its regulation [18]. An important issue that has remained unresolved relates to the nature of CK2 shuttling, i.e., do all the subunits undergo coordinate migration or can the individual subunits migrate independently. Much data indicate that the subunits of CK2 undergo dynamic differential translocation to various intracellular compartments in response to diverse stimuli [3,19]. However, other studies based on a normal cell model suggested the translocation of the holoenzyme rather than individual subunits [20]. The data on individual subunit translocation have generally been derived from use of cancer cell models [19,21,22], and so it is conceivable that the behavior of the kinase in normal vs cancer cells may be distinct. Clearly, further studies need to be undertaken to resolve these issues. To account for the range of CK2 cellular functional activity, it has also been suggested that the interaction of CK2 subunits with diverse intracellular molecules may be a contributory factor [23,24]. It has been further proposed that CK2 controls multiple protein kinases through phosphorylation of a protein kinase targeting molecular chaperone Cdc37 [25]. The fact that CK2 does not follow a single specific pathway of action but rather interacts with diverse pathways illustrates the breadth of its reach in the cellular machinery. These aspects of CK2 function in cell growth accord with its apparently global role in regulation of the growth related activities in the cell [26]. The various models of cellular regulation of CK2 mentioned above have common features and may not necessarily be exclusionary, but clearly these various aspects of CK2 function remain under ongoing consideration.
4. Suppression of apoptosis by CK2
While it was known for a long time that CK2 plays a role in cell growth and proliferation in normal and cancer cells, the more recent demonstration that CK2 was also a potent suppressor of apoptosis has squarely placed the functionality of the kinase in the cancer cell phenotype since CK2 has been found to be consistently elevated in all cancers studied [7-9]. The original possibility of CK2 as a suppressor of apoptosis was suggested in the studies employing androgenic regulation of the prostate gland where it was shown that loss of androgenic growth stimulus resulted in rapid loss of nuclear associated CK2 and preceded the induction of apoptosis [2,16,17,27]. However, a direct compelling demonstration of the ability of CK2 to suppress apoptosis was shown in an experimental model of drug-induced apoptosis where it was shown that prior overexpression of the α subunit of CK2 (but not the β subunit) resulted in potent suppression of apoptosis [28]. Recent studies from various laboratories have further supported the function of CK2 as a suppressor of apoptosis in diverse experimental models [see, e.g., 10]. It now appears that CK2 may exert a broad impact on the apoptotic machinery by influencing the activity of diverse molecules and pathways involved in the regulation of apoptosis. A few of the examples are the PI3K/Akt pathway [29,30], survivin and other inhibitors of apoptosis proteins (IAPs) [31,32], caspases [33,34], proteins in the Bcl2 pathway, and reactive oxygen species pathways [35-39]. These various observations point to the global impact of CK2 on apoptotic activity in the cell, and further highlight the significance of this function of CK2 in cancer cell biology as discussed subsequently. Finally, in a similar vein, the essential function of CK2 in cell survival is further illustrated by unsuccessful attempts to knockout CK2α and CK2β in yeast and mice [e.g., 40-43]. In mouse embryonic development, disruption of CK2α leads to death mid-gestation with structural defects in heart and neural tube, and disruption of CK2βcauses early lethality at E6.5 [41-43].
5. CK2 and Cancer
The association of CK2 with neoplasia has been known for a long time [e.g., 7-9]. Studies on diverse type of cancers have demonstrated that CK2 is uniformly elevated in all cancers examined. Interestingly, the elevation is noted at the level of protein rather than a significant change at the level of the enzyme message [8]. It is well known that two of the most consistent features of cancer are deregulated proliferation and deregulated apoptotic activity [11,12]. Thus, while CK2 was known to affect proliferation in both normal and cancer cells, the observation that CK2 potently suppressed apoptosis provided a vital link of the kinase to the cancer cell phenotype [10]. In a recent review article we discussed in detail how CK2 function may relate to various key features of cancer cell phenotype [9]. It was recently suggested that a common denominator of diverse cancer cells may be an addiction to CK2 [24]. We propose a further consideration of this appealing concept in view of the following observations. It appears that each tissue has a stable predetermined cellular level of CK2 and this level varies depending on the cell and tissue type. Under normal circumstances, cells resist even a modest upregulation or downregulation of cellular CK2. In the case of cell transformation, it appears that transformed cells acquire a new base level of CK2 and tend to maintain it in a stable manner analogous to that in normal cells, although in this case the cells have a dysregulated level of CK2 compared with that in the original normal cells. This accords with the lack of success in producing stable forced overexpression of CK2 protein in both normal and cancer cells [44,45]. Various observations suggest that a relatively small change in the balance of CK2 expression can have a large impact on cellular homeostasis. This was noted in studies showing that even a modest downregulation of CK2 in the nuclear compartment (chromatin and matrix) leads to induction of widespread cell death by apoptosis. [8,17,46-48]. We have further observed that cancer cells, where the CK2 protein expression is already perturbed, seem to be even more sensitive than normal cells to inhibition of CK2 activity or expression. By the same token, although CK2 by itself is not an oncogene, modest upregulation of CK2 can impart an oncogenic potential to the cells, as observed in experimental animal studies showing the remarkable contributory oncogenicity following increased expression of CK2. For example, overexpression of CK2α in p53 deficient mice or with c-myc or Tal-1 in transgenic mice resulted in a significant increase in the incidence of leukemia and lymphoma in mice [49-52]. Likewise, incorporation of CK2α with MMTV produced a transgenic mouse model of breast cancer with several features resembling the human disease [51]. In each case, modest overexpression of the CK2α transgene was sufficient to evoke enhanced oncogenic potential in the mice.
6. Importance of CK2 as a Target for Cancer Therapy
Given the dual role of CK2 in cell proliferation and cell death, we originally proposed that CK2 could serve as a key target for cancer therapy [17,47,53]. In developing novel avenues of effective cancer therapy, the ultimate goal is to eradicate all tumor cells in the host and achieve a complete cure of the disease. Thus it is important to consider targeting a gene that is uniquely indispensible for cell survival, as otherwise, tumor cells will escape death by recruiting an alternate pathway [e.g., 9,55]. Clearly, CK2 is one such gene. Previous efforts at molecular targeting of protein kinases that were not critical for cell survival might limit their utility for clinical translation to cure the disease [e.g., 54,56,57]. This is not the case for this target because CK2 is essential for cell survival. The importance of CK2 as a target for cancer therapy is derived from the following key considerations. First, CK2 appears to be profoundly responsive to modulations of mitogenic signals from numerous initiating events in cells [2,8,16,48,58,59]. Second, downregulation of CK2 expression affects inflammatory, angiogenic, and drug efflux pathways to the benefit of cancer cell elimination [e.g., 60-63]. Third, dysregulated elevation of CK2 in cancer cells reflects the pathologic status of the tumor [8,65-68]. Fourth, CK2 downregulation impacts not only cell growth and proliferation but also apoptotic activity in cancer cells, making its targeting a two-edged sword [7-10,27,28,46,47,69]. Fifth, CK2 is indispensable for cell survival, and as far as we know there appear to be no redundant pathways to compensate for its downregulation [40-43].
Following our proposal, considerable interest in using CK2 as a target for cancer therapy has now emerged. The approaches being proposed are to use small molecule chemical inhibitors of CK2 [70-76], a peptide inhibitor to block CK2 phosphorylation sites in CK2 substrates [77], and molecular downregulation of CK2 using antisense or siRNA [7,9,17,46,47,53,78,80]. Targeting CK2 for cancer therapy raises the issue of its ubiquitous and essential for cell survival nature as being a potential problem for host toxicity. However, it appears that normal cells exhibit relative resistance to induction of apoptosis in response to agents such as antisense CK2α ODN or inhibitors of CK2 relative to cancer cells [46,69]. Despite this, it is important that approaches are devised that will optimally achieve the downregulation of CK2 only in cancer while sparing normal cells. The use of small molecule inhibitors [e.g., 71] or peptides to block CK2 phosphorylation sites [77] in vivo is not based on protected or targeted delivery of these agents in vivo, and relies on the pharmacologic window; however, their future success remains to be determined with regard to the potential of toxicity to normal cells and also the issue of tumor cell drug resistance, which may contribute to the problem of efficacy of these agents in vivo.
Our focus has been on the utilization of molecular downregulation of CK2 with attempts to do so specifically in cancer cells while sparing the normal cells in vivo. This approach, if successful, has the clear advantage that it is likely to be highly effective for cell death, and to overcome issues such as drug resistance in tumor cells. Starting with antisense CK2α ODN to downregulate CK2 in cell culture and in prostate cancer (PCa) and head and neck squamous cell carcinoma (HNSCC) xenografts [17,46,69], we originally demonstrated that potent tumor cell death is achieved in these experimental models. More recently, we have now devised novel antisense and siRNA constructs that downregulate both α and α′ subunits of CK2, thus ensuring more complete downregulation of CK2 in vivo [78,80].
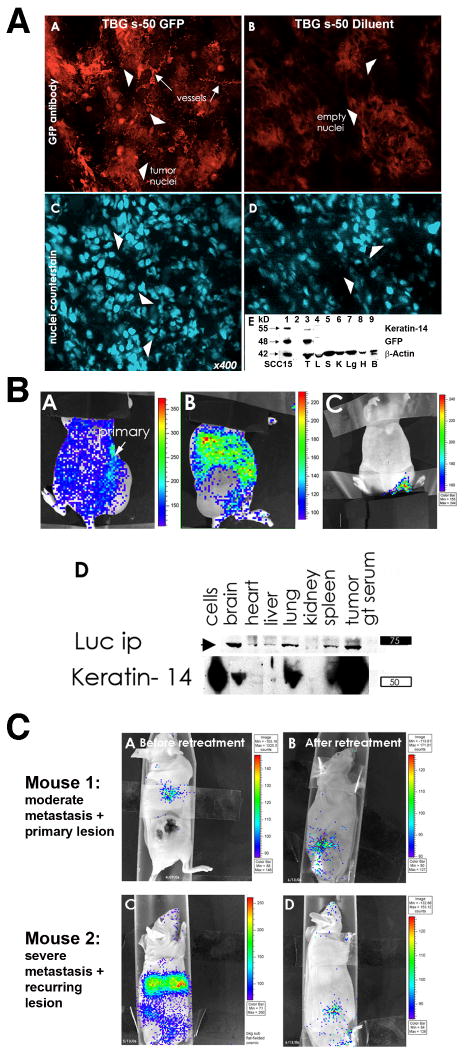
To achieve the goal of specific molecular downregulation of the targeted signal in tumors, we have developed a novel sub-50 (s-50) nm (i.e., less than 50 nm size) tenfibgen nanocapsule to deliver the CK2 targeting agent specifically to primary and distant tumors in vivo. Tenfibgen (TBG), the nanocapsule material, is a subdomain (fibrinogen binding fragment) of tenascin C. The commonly employed experimental methods for nucleic acid delivery have included the use of viral vectors and non-viral vectors such as cationic lipid complexes, polycation complexes, macromolecular conjugates and liposomes. However, none of these have yielded satisfactory cancer cell specific targeting and have not addressed other critical issues essential for satisfactory drug delivery. The novel s-50 nm nanoencapsulation process to date displays many attributes of a potential clinically applicable in vivo delivery system [47,53,79,80]. The resulting product is an ultra small neutrally charged particle, with a protective shell of the targeting ligand having a non-ordered surface stabilized by crystallization. For tumor targeting, a protein ligand, tenfibgen (TBG), enables tumor specific accumulation due to the increased expression of tenascin receptors specifically on the tumor cells, with negligible uptake observed by the reticuloendothelial system (RES) or other organs. The s-50 nanocapsule is also suitable for magnetic resonance imaging (MRI) with demonstrated ability to overcome compartmental boundaries in vivo. Furthermore, this system meets the key biological objective challenges for nanomedicine as stipulated by the FDA, NIH and NIST [81]. Our s-50 TBG nanocapsule containing antisense or siRNA directed against CK2 protects the nucleic acid in a tumor cell-specific protein ligand shell during circulation and releases the cargo within the cell following entry via the caveolar pathway, thereby bypassing the endosomal trap. Thus, this nanocapsule technology holds enormous promise for the successful molecular targeting of CK2 specifically in tumor cells. Additionally, our data suggest that the s-50 TBG nanocapsule has the ability to target metastases (Fig. 1), including bone metastases (Unger, G., Ahmed, K., et al., unpublished data), which considerably raises its potential for advanced cancer therapy.
Fig. 1. TBG s-50 nanocapsule tumor-homing and biodistribution in xenograft mice following systemic administration.
A. TBG s-50 nanocapsules target tumor cells and tumor-derived microvessels following systemic distribution: Panels A-D - Tumor cryosections from mice bearing SCC-15 tongue carcinoma xenografts were immunodetected for nuclear GFP signal 3 weeks following i.p. administration at 0.2 mg/kg of TBG s-50 nanocapsules bearing nuclear-localized GFP plasmid (Panel A) or diluent (Panel B). Positioning of cell nuclei in tumors is depicted by bisbenzamide counterstain (Panels C,D). GFP immunosignal was located in the tumor-derived microvasculature (arrows, Panel A) and clusters of tumor cells (arrowheads, Panel A). Note the lack of nuclear GFP staining in the tumor sections from the TBG s-50 diluent injected mouse. Panel E - Immunoblot analysis of tumor and tissue lysates from mice treated with TBG s-50 nuclear GFP or diluent nanocapsules confirms microscopy by another method (only results for GFP treated mice shown). Lysates were subjected to electrophoresis on a 4-12% gradient gel and immunodetected for keratin-14 to identify tumor cells (Panel E-top line), GFP fusion protein (Panel E, middle line), and β-actin as a loading control (Panel E, lower line). The lanes contain the following samples: 1) GFP transfected SCC-15 cell line as positive control, 2) MW marker, 3) tumor, 4) liver, 5) spleen, 6) kidney, 7) lung, 8) heart and 9) brain.
B. TBG s-50 nanocapsules deliver nucleic acid cargo specifically to tumor metastases. Panels A-D - A mouse with a large UM-11b laryngeal carcinoma xenograft flank tumor was injected i.p. with TBG s-50 nanocapsules containing 12.5 μg of luciferase plasmid and imaged using the Xenogen method 5 days later to allow for development of luciferase gene expression (Panels A,B). A control mouse, first injected with TBG s-50 nanocapsules bearing trehalose, was injected after 5 days with luciferin substrate and imaged together with the luciferase mouse for background control and comparison (Panel C). Luciferase signal was apparent in the viable rim of the cytolytic tumor mass (Panel A) and throughout the lung and abdominal region (Panel B). Large peritoneal metastases were encountered upon necropsy, but not assayed further. Panel D - Tissues from the metastases-burdened mouse were immunoprecipitated for luciferase, and the immunoprecipitates were analyzed by immunoblot along with lysate to correlate luciferase expression with the presence of keratin-14, a marker for head neck cancer. Substantial keratin-14 signal was co-present with luciferase protein signal in brain, lung, spleen and tumor. The lane labeled “cells” represents UM-11b cells used for xenograft injection.
C. Existing metastases are cleared by TBG s-50 anti-CK2 nanocapsules in mice at ng/kg dosing. Panels A,C - Mice with SCC-15 tongue carcinoma xenografts were assayed for tumor burden 6 months following docetaxel/cisplatin treatment by the Xenogen method following i.p. administration of 12.5 μg of TBG s-50 luciferase plasmid. Panels B,D - Mice were administered additional tumor-targeted TBG s-50 luciferase plasmid nanocapsules and reimaged 4-6 weeks later following continual TBG s-50 anti-CK2 oligonucleotide nanocapsule treatment. The AB panel series was imaged after 6 weeks of 100 ng/kg q2d i.p. dosing together with topical 3 μg/ml TBG s-50 anti-CK2 oligonucleotide nanocapsule treatment for the last 2 weeks. The CD panel series was reimaged following 4 weeks of 100 ng/kg q2d i.p. treatment with TBG s-50 anti-CK2 oligonucleotide nanocapsules. Following reimaging, mice were treated with surgery (mouse in Panel B) or not (mouse in Panel D) and combination low-dose metronomic chemotherapy (20 μg/kg cisplatin, 1/1000 of murine MTD) with increasing doses of TBG s-50 anti-CK2 oligonucleotide nanocapsules before cessation of treatment. Mice were euthanized six months later at two years of age with no evidence of recurrence by imaging analysis.
How does CK2 downregulation achieve therapeutic goals in cancer? Given that CK2 impacts over 300 potential substrates in the cell and a wide range of pathways that pertain to cell growth and apoptosis [e.g., 13], it is likely that its downregulation would have a vast reach on activities that regulate cell function. Consistent with this, NF-κB is among the various pathways that have received considerable attention pertaining to a link with CK2 signaling. Here we consider the response of this pathway to molecular downregulation of CK2. The following discussion supplements the elegant consideration of NF-κB in development and cancer in reference to CK2 involvement [82]. NF-κB is known to have a broad role in regulation of many genes involved in diverse processes, among which are those relating to cell growth and proliferation, cell death, inflammation, migration, and angiogenesis [e.g., 82-86]. Aberrant activation of NF-κB has been documented in several cancers including mammary gland, prostate, and head and neck cancer [e.g., 83-86]. The activation of NF-κB in response to upstream signals is achieved by release of the inhibitory complex with IκBs, whose phosphorylation by various kinases including CK2 results in its degradation. Upon release, NF-κB (e.g., p65/p50) is translocated to the nucleus where it binds to regulatory sites of a variety of genes [82-84].
Studies on the activation of NF-κB in mammary gland [82,87,88] and head and neck cancer [89] demonstrated the involvement of CK2 in this process. Further, it is of note that CK2 is also involved in the phosphorylation of p65 directly thereby influencing its activity [90]. Interestingly, activation of p65 and a related gene cluster is also linked with repression of tumor suppressor TP53 mRNA and protein expression in a subset of head and neck squamous cell carcinomas retaining wt TP53 genotype (HNSCC) [91,92]. Involvement of CK2 in modulating the activity of TP53 has also been noted [93-96], thus providing a possible link in these various pathways. Further recent investigations along these lines undertaken in HNSCC have demonstrated differential responses of the NF-κB and TP53 pathways upon modulation of individual subunits of CK2 [80]. Knockdown of individual subunits of CK2 demonstrated a differential decrease of gene expression of not only NF-κB but also cell survival (BCL-XL) and cell cycle progression (CCND1) genes, whereas an increase of TP53 family genes known to promote growth arrest and apoptosis (p53 and Tap63) was observed. Knockdown of CK2α demonstrated a significant decrease in ITGA3 and ITGB4, while knockdown of CK2α′ resulted in decrease of ITGA6. Interestingly, the angiogenic factor VEGF was significantly reduced by downregulation of both α and α′ subunits of CK2 [80]. The involvement of CK2 in the process of angiogenesis has also been documented previously [60,61]. Likewise, altered expression of certain integrin genes (ITG) involved in HNSCC adhesion and migration has been previously reported [97,98]. Based on the observations that CK2 influences the expression of integrin genes in HNSCC, a further analysis of the effects of downregulation of CK2 subunits α and β on wound healing showed a marked inhibitory effect [80]; these observations have provided novel information on the important role of CK2 in cell migration.
Studies by Brown et al. [80] on the effects of various subunits of CK2 on induction of cell death also demonstrated that downregulation of α but not α′ or β subunit was most prominent in inducing cell death in cultured HNSCC cells, analogous to previous observations in prostate cancer cells [17]. Analysis of cell cycle under these various conditions suggested similar increases in cells arrested in G0/G1 in each case, but specific decreases in S phase by knockdown of CK2α and CK2α′ while that of CK2β resulting in a decrease in cells in G2/M phase [80]. The therapeutic implications of these observations are highlighted by observations that modest downregulation of CK2α in cells sensitizes them to agents such as TRAIL or etoposide [35], and analogous observations by Brown et al. [80] have shown that downregulation of CK2 also sensitizes HNSCC to cisplatin. Further, studies were undertaken to examine the in vivo effects of downregulation of CK2 by employing anti-CK2α/α′ ODN which target both the CK2α and CK2α′ subunits in the s-50 nm TBG nanocapsule formulation delivered systemically to a xenograft model of HNSCC in mice. The results demonstrated potent induction of apoptosis in tumor cells in vivo associated with downregulation of CK2α and CK2α′ expression, a decrease in total NF-κB p65, and decreased p65 serine 536 and serine 529 phosphorylation. Additionally, other genes related to growth and survival such as Cyclin D1, BCL-XL, and BCL2 demonstrated decreased expression while TP53 and p63 were increased. Previous studies have investigated the interaction of CK2 with p53 [e.g., 92-95]; however, it may be noted also that p53 is not required for induction of apoptosis on downregulation of CK2 [17,99]. The involvement of the downstream pathways relating to NF-κB and TP53 that respond to downregulation of CK2 in vivo is illustrated in Fig. 2. Together with previous observations on the therapeutic effectiveness of knocking down CK2α [9,17,46,47,53], our recent studies [78,80] further highlight the importance of targeting both CK2α and CK2α′ as a novel and potentially key strategy for targeted cancer therapy.
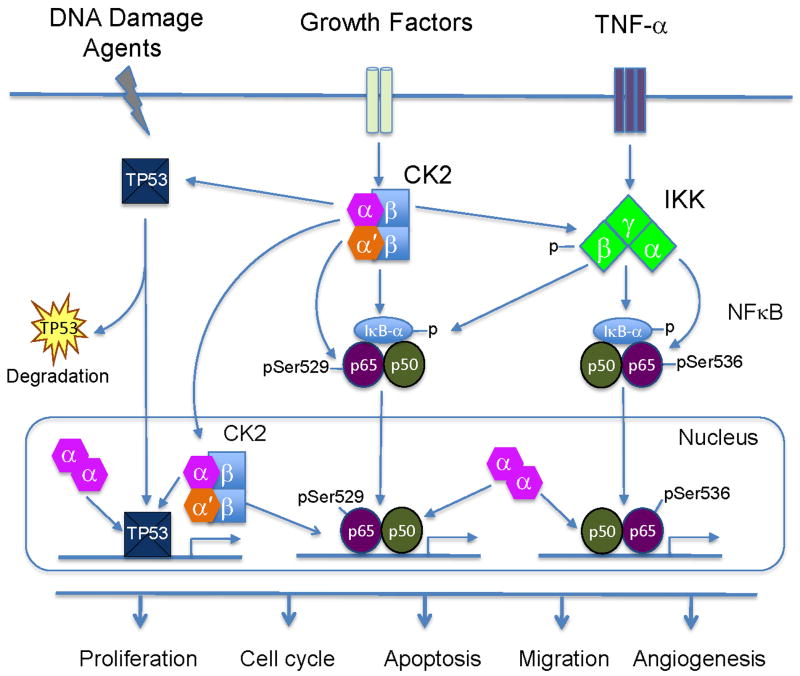
Fig. 2. Proposed model for CK2 modulation of NF-κB and TP53 pathways.
Increased external signaling events, such as from TNF-α and other growth factors, bind and activate cell surface receptors, which induce aberrant CK2 and NF-κB activation. CK2 modulates IKKβ and IκBα phosphorylation and degradation, as well as promotes IKK mediated phosphorylation of NF-κB p65 at serine536. In addition, CK2 is able to directly phosphorylate p65 at serine529. At present, it is not clear if these phosphorylations occur on the same or different molecules. Activated NF-κB subunits translocate into the nucleus, bind to the NF-κB target gene promoters, and modulate transcriptional activity. In addition, CK2 is able to regulate TP53 and family members through protein phosphorylation, degradation, and modulation of expression. Abundant CK2α subunits are present in the nucleus of tumor cells and co-regulate NF-κB and TP53 family proteins and target gene expression. Thus, CK2 subunits regulate broad cellular functions affecting proliferation, cell cycle, apoptosis, migration and angiogenesis, which promote tumorigenesis of head and neck or other cancers. TNF-α: Tumor necrosis factor-α; NF-κB: nuclear factor κB; IKK: Inhibitor kappaB kinase; IκBα: Inhibitor κBα
7. Concluding remarks
The availability of the s-50 nm TBG nanocapsule to deliver the therapeutic agent (such as antisense, siRNA, or chemical inhibitors for CK2) has important implications as it provides for the first time a means to target cancer cells while sparing normal cells in vivo, and thus obviating issues relating to potential host toxicity as a result of CK2 downregulation in general or from the nanocapsule, which includes a normal tissue protein. Given that downregulation of CK2 causes death in diverse types of cancer cells, we postulate that this therapeutic modality could find application in cancers other than prostate cancer and squamous cell carcinoma of head and neck that we have studied. Thus, we propose that the molecular downregulation of CK2 achieved by its targeting via the s-50 TBG nanocapsule in a tumor cell specific manner has the potential of successful application to therapy of diverse types of cancers.
Acknowledgments
The original work in the authors laboratories is supported by Department of Veterans Affairs Research Funds and National Cancer Institute (NIH) grant UO1-CA15062 (KA), NIH grant RO1-DK067436 (BK), DOD Contract W81XWH-05-C-0084 (GU), and ZIA-DC-00016 (CVW, ZC).
References
- 1.Allende JE, Allende CC. Protein kinase CK2 - an enzyme with multiple substrates and a puzzling regulation. FASEB J. 1995;9:313–323. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed K. Nuclear matrix and protein kinase CK2 signaling. Crit Rev Eukaryot Gene Expr. 1999;9:329–336. doi: 10.1615/critreveukargeneexpr.v9.i3-4.170. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed K, Gerber DA, Cochet C. Joining the cell survival squad: An emerging role for protein kinase CK2. Trends in Cell Biology. 2002;12:226–230. doi: 10.1016/s0962-8924(02)02279-1. [DOI] [PubMed] [Google Scholar]
- 4.Pinna LA. Protein kinase CK2: A challenge to canons. J Cell Sci. 2002;115:3873–3878. doi: 10.1242/jcs.00074. [DOI] [PubMed] [Google Scholar]
- 5.Litchfield DW. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pyerin W, Ackermann K. The genes encoding human protein kinase CK2 and their functional links. Progr Nucl Acid Res Mol Biol. 2003;74:239–273. doi: 10.1016/s0079-6603(03)01015-8. [DOI] [PubMed] [Google Scholar]
- 7.Guerra B, Issinger OG. Protein kinase CK2 in human disease. Curr Medicinal Chem. 2008;15:1870–1886. doi: 10.2174/092986708785132933. [DOI] [PubMed] [Google Scholar]
- 8.Tawfic S, Yu S, Wang H, Faust R, Davis A, Ahmed K. Protein kinase CK2 signal in neoplasia. Histol Histopathol. 2001;16:573–582. doi: 10.14670/HH-16.573. [DOI] [PubMed] [Google Scholar]
- 9.Trembley JH, Wang G, Unger G, Slaton J, Ahmed K. CK2: A key player in cancer biology. Cell Mol Life Sci. 2009;66:1858–1867. doi: 10.1007/s00018-009-9154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad KA, Wang G, Unger G, Slaton J, Ahmed K. Protein kinase CK2 – A key suppressor of apoptosis. Adv Enzyme Regul. 2008;48:179–187. doi: 10.1016/j.advenzreg.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 12.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 13.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 14.Niefind K, Raaf J, Issinger OG. Protein kinase CK2: From structure to insights. Cell Mol Life Sci. 2009;66:1800–1816. doi: 10.1007/s00018-009-9149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chantalat L, Leroy D, Filhol O, Nueda A, Benitez MJ, Chambaz EM, Cochet C, Dideberg O. Crystal structure of the human protein kinase CK2 regulatory subunit reveals its zinc finger-mediated dimerization. EMBO J. 1999;18:2930–2940. doi: 10.1093/emboj/18.11.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu S, Wang H, Davis A, Ahmed K. Consequences of CK2 signaling to the nuclear matrix. Mol Cell Biochem. 2001;227:67–71. [PubMed] [Google Scholar]
- 17.Wang H, Davis A, Yu S, Ahmed K. Response of cancer cells to molecular interruption of the CK2 signal. Mol Cell Biochem. 2001;227:167–174. [PubMed] [Google Scholar]
- 18.Faust M, Montenarh M. Subcellular localization of protein kinase CK2 – A key to its function? Cell & Tissue Res. 2000;301:329–340. doi: 10.1007/s004410000256. [DOI] [PubMed] [Google Scholar]
- 19.Filhol O, Cochet C. Cellular functions of protein kinase CK2: a dynamic affair. Cell Mol Life Sci. 2009;66:1830–1839. doi: 10.1007/s00018-009-9151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvi M, Sarno S, Marin O, Meggio F, Itarte E, Pinna LA. Discrimination between the activity of protein kinase CK2 holoenzyme and its catalytic subunits. FEBS Lett. 2006;580:3948–3952. doi: 10.1016/j.febslet.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 21.Guerra B, Siemer S, Boldyreff B, Issinger OG. Protein kinase CK2: evidence for a protein kinase CK2β subunit fraction, devoid of the catalytic CK2α subunit in mouse brain and testicles. FEBS Lett. 1999;462:353–357. doi: 10.1016/s0014-5793(99)01553-7. [DOI] [PubMed] [Google Scholar]
- 22.Martel V, Filhol O, Nueda A, Cochet C. Dynamic localization/association of protein kinase CK2 subunits in living cells: a role in its cellular regulation? Ann NY Acad Sci. 2002;973:272–277. doi: 10.1111/j.1749-6632.2002.tb04648.x. [DOI] [PubMed] [Google Scholar]
- 23.Olsten MEK, Weber JE, Litchfield DW. CK2 interacting proteins: emerging paradigms for CK2 regulation? Mol Cell Biochem. 2005;274:115–124. doi: 10.1007/s11010-005-3072-6. [DOI] [PubMed] [Google Scholar]
- 24.Ruzzene M, Pinna LA. Addiction to protein kinase CK2: A common denominator of diverse cancer cells. Biochim Biophys Acta. 2010;1804:499–504. doi: 10.1016/j.bbapap.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Miyata Y. CK2: The kinase controlling the Hsp90 chaperone machinery. Cell Mol Life Sci. 2009;66:1840–1849. doi: 10.1007/s00018-009-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barz T, Ackerman K, Dubois G, Eils R, Pyerin W. Genome-wide expression screens indicate a global role for protein kinase CK2 in chromatin remodeling. J Cell Sci. 2003;116:1563–1577. doi: 10.1242/jcs.00352. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed K, Yenice S, Davis A, Goueli SA. Association of casein kinase 2 (CK-2) with nuclear chromatin in relation to androgenic regulation of rat prostate. Proc Natl Acad Sci USA. 1993;90:4426–4430. doi: 10.1073/pnas.90.10.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo C, Yu S, Davis AT, Green JE, Ahmed K. A potential role of nuclear matrix-associated protein kinase CK2 in protection against drug-induced apoptosis in cancer cells. J Biol Chem. 2001;276:5992–5999. doi: 10.1074/jbc.M004862200. [DOI] [PubMed] [Google Scholar]
- 29.Di Maira G, Salvi M, Arrigoni G, Marin O, Sarno S, Brustolon F, Pinna LA, Ruzzene M. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Different. 2005;12:668–677. doi: 10.1038/sj.cdd.4401604. [DOI] [PubMed] [Google Scholar]
- 30.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 31.Tapia JC, Torres VA, Rodriguez DA, Leyton L, Quest AF. Casein kinase 2 (CK2) increases survivin expression via enhanced beta-catenin-T cell factor/lymphoid enhancer binding factor-dependent transcription. Proc Natl Acad Sci USA. 2006;103:15079–15084. doi: 10.1073/pnas.0606845103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang G, Ahmad KA, Ahmed K. Impact of protein kinase CK2 on inhibitor of apoptosis proteins (IAPs) in prostate cancer cells. Mol Cell Biochem. 2008;316:91–97. doi: 10.1007/s11010-008-9810-9. [DOI] [PubMed] [Google Scholar]
- 33.McDonnell MA, Abedin MJ, Melendez M, Platikanova TN, Ahmed K, Kelekar A. Phosphorylation of murine Caspase-9 by the protein kinase CK2 regulates its cleavage by Caspase-8. J Biol Chem. 2008;283:20149–20158. doi: 10.1074/jbc.M802846200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin S, Lee Y, Kin W, Ko H, Choi H, Kim K. Caspase-2 primes cancer cells for TRAIL-mediated apoptosis by processing procaspase-8. EMBO J. 2005;24:3532–3542. doi: 10.1038/sj.emboj.7600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G, Ahmad KA, Ahmed K. Role of CK2 in regulation of TRAIL induced apoptosis in prostate cancer cells. Canc Res. 2006;66:2242–2249. doi: 10.1158/0008-5472.CAN-05-2772. [DOI] [PubMed] [Google Scholar]
- 36.Ravi R, Bedi A. Sensitization of tumor cells to Apo2 ligand/TRAIL-induced apoptosis by inhibition of casein kinase II. Canc Res. 2002;62:4180–4185. [PubMed] [Google Scholar]
- 37.Izeradjene K, Douglas L, Delaney A, Houghton JA. Casein kinase II (CK2) enhances death-inducing signaling complex (DISC) activity in TRAIL-induced apoptosis in human colon carcinoma cell lines. Oncogene. 2005;24:2050–2058. doi: 10.1038/sj.onc.1208397. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad KA, Wang G, Ahmed K. Intracellular Hydrogen Peroxide Production is an Upstream Event in Apoptosis Induced by Downregulation of CK2 in Prostate Cancer Cells. Mol Canc Res. 2006;4:331–338. doi: 10.1158/1541-7786.MCR-06-0073. [DOI] [PubMed] [Google Scholar]
- 39.Hanif IM, Ahmad KA, Ahmed K, Pervaiz S. Involvement of reactive oxygen species in apoptosis induced by pharmacological inhibition of protein kinase CK2. Ann NY Acad Sci. 2009;1171:591–599. doi: 10.1111/j.1749-6632.2009.04916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padmanabha R, Chen-Wu JLP, Hanna DE, Glover CVC. Isolation, sequencing, and disruption of the yeast CKA2 gene: Casein kinase II is essential for viability in S. cerevisiae. Mol Cell Biol. 1990;10:4089–4099. doi: 10.1128/mcb.10.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchou T, Nernet M, Blond O, Jensen HH, Pointu H, Olsen BB, Cochet C, Issinger OG, Boldyreff B. Disruption of the regulatory β subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol Cell Biol. 2003;23:908–915. doi: 10.1128/MCB.23.3.908-915.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lou DY, Dominguez I, Toselli P, Landesman-Bollag E, O'Brien C, Seldin DC. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol Cell Biol. 2008;28:131–139. doi: 10.1128/MCB.01119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seldin DC, Lou DY, Toselli P, Landesman-Bollag E, Dominguez I. Gene targeting of CK2 catalytic subunits. Mol Cell Biochem. 2008;316:141–147. doi: 10.1007/s11010-008-9811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Yu S, Davis AT, Ahmed K. Cell cycle dependent regulation of protein kinase CK2 signaling to the nuclear matrix. J Cell Biochem. 2003;88:812–822. doi: 10.1002/jcb.10438. [DOI] [PubMed] [Google Scholar]
- 45.Yu S, Davis AT, Guo C, Green JE, Ahmed K. Differential targeting of protein kinase CK2 to the nuclear matrix upon transient overexpression of its subunits. J Cell Biochem. 1999;74:127–134. [PubMed] [Google Scholar]
- 46.Slaton JW, Sloper DT, Unger G, Davis A, Ahmed K. Induction of apoptosis by antisense CK2 in human prostate cancer xenograft model. Mol Canc Res. 2004;2:712–721. [PubMed] [Google Scholar]
- 47.Ahmad KA, Wang G, Slaton JW, Unger G, Ahmed K. Targeting CK2 for cancer therapy. Anticancer Drugs. 2005;16:1037–1043. doi: 10.1097/00001813-200511000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed K, Davis AT, Wang H, Faust RA, Yu S, Tawfic S. Significance of protein kinase CK2 nuclear signaling in neoplasia. J Cell Biochem Supp. 2000;35:130–135. doi: 10.1002/1097-4644(2000)79:35+<130::aid-jcb1136>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 49.Kelliher MA, Seldin DC, Leder P. Tal-1 induces T cell acute lymphoblastic leukemia accelerated by casein kinase IIα. EMBO J. 1996;15:5160–5166. [PMC free article] [PubMed] [Google Scholar]
- 50.Landesman-Bollag E, Channavajhala PL, Cardiff RD, Seldin DC. p53 deficiency and misexpression of protein kinase CK2 α collaborate in the development of thymic lymphomas in mice. Oncogene. 1998;16:2965–2974. doi: 10.1038/sj.onc.1201854. [DOI] [PubMed] [Google Scholar]
- 51.Landesman-Bollag E, Song DH, Romieu-Mourez R, Sussman DJ, Cardiff RD, Sonenshein GE, Seldin DC. Protein kinase CK2: Signaling and tumorigenesis in the mammary gland. Mol Cell Biochem. 2001;227:153–165. [PubMed] [Google Scholar]
- 52.Channavajhala P, Seldin DC. Functional interaction of protein kinase CK2 and c-Myc in lymphomagenesis. Oncogene. 2002;21:5280–5288. doi: 10.1038/sj.onc.1205640. [DOI] [PubMed] [Google Scholar]
- 53.Unger GM, Davis AT, Slaton JW, Ahmed K. Protein kinase CK2 as regulator of cell survival: Implications for cancer therapy. Curr Canc Drug Targets. 2004;4:77–84. doi: 10.2174/1568009043481687. [DOI] [PubMed] [Google Scholar]
- 54.Gleave ME, Monia B. Antisense therapy for cancer. Nat Rev Cancer. 2005;5:468–479. doi: 10.1038/nrc1631. [DOI] [PubMed] [Google Scholar]
- 55.Wang G, Ahmad KA, Unger G, Slaton JW, Ahmed K. CK2 signaling in androgen-dependent and –independent prostate cancer. J Cell Biochem. 2006;99:382–391. doi: 10.1002/jcb.20847. [DOI] [PubMed] [Google Scholar]
- 56.Cho-Chung YS. Protein kinase A-directed antisense restrains cancer growth: Sequence-specific inhibition of gene expression. Antisense Nucleic acid Drug Dev. 1996;6:237–244. doi: 10.1089/oli.1.1996.6.237. [DOI] [PubMed] [Google Scholar]
- 57.Cho YS, Kim MK, Tan L, Srivastava R, Agrawal S, Cho-Chung YS. Protein kinase A RIα antisense inhibition of PC3M prostate cancer cell growth: Bcl-2 hyperphosphorylation, Bax up-regulation, and Bad-hyperphosphorylation. Clin Canc Res. 2002;8:607–614. [PubMed] [Google Scholar]
- 58.Ahmed K. Significance of the casein kinase system in cell growth and proliferation with emphasis on studies of the androgenic regulation of the prostate. Cell Mol Biol Res. 1994;40:1–11. [PubMed] [Google Scholar]
- 59.Ji H, Wang J, Nika H, Hawke D, Keezer S, Ge Q, Fang B, Fang X, Fang D, Litchfield DW, Aldape K, Lu Z. EGF-induced ERK activation promotes CK2-mediated disassociation of α-catenin from β-catenin and transactivation of β-catenin. Mol Cell. 2009;36:547–559. doi: 10.1016/j.molcel.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kramerov AA, Saghizadeh M, Pan H, Kabosova A, Montenarh M, Ahmed K, Penn JS, Chan CK, Hinton DR, Grant MB, Ljubimov AV. Expression of protein kinase CK2 in astroglial cells of normal and neovascularized retina. Amer J Path. 2006;168:1722–1736. doi: 10.2353/ajpath.2006.050533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kramerov AA, Saghizadeh M, Caballero S, Shaw LC, Li Calzi S, Bretner M, Montenarh M, Pinna LA, Grant MB, Ljubimov AV. Inhibition of protein kinase CK2 suppresses angiogenesis and hematopoietic stem cell recruitment to retinal neovascularization sites. Mol Cell Biochem. 2008;316:177–186. doi: 10.1007/s11010-008-9831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh NN, Ramji DP. Protein kinase CK2, an important regulator of the inflammatory response? J Mol Med. 2008;86:887–897. doi: 10.1007/s00109-008-0352-0. [DOI] [PubMed] [Google Scholar]
- 63.di Maira G, Brustolon F, Bertacchini J, Tosoni K, Marmiroli S, Pinna LA, Ruzzene M. Pharmacological inhibition of protein kinase CK2 reverts the multidrug resistance phenotype of a CEM cell line characterized by high CK2 level. Oncogene. 2007;26:6915–6926. doi: 10.1038/sj.onc.1210495. [DOI] [PubMed] [Google Scholar]
- 64.Faust RA, Gapany M, Tristani P, Davis A, Adams GL, Ahmed K. Elevated protein kinase CK2 activity in chromatin of head and neck tumors: Association with malignant transformation. Cancer Letts. 1996;101:31–35. doi: 10.1016/0304-3835(96)04110-9. [DOI] [PubMed] [Google Scholar]
- 65.Gapany M, Faust RA, Tawfic S, Davis A, Adams GL, Ahmed K. Association of elevated CK2 activity with aggressive behavior of squamous cell carcinoma of the head and neck. Mol Med. 1995;1:659–666. [PMC free article] [PubMed] [Google Scholar]
- 66.Faust RA, Niehans GA, Gapany M, Hoistad D, Knapp D, Cherwitz D, Davis A, Adams GL, Ahmed K. Subcellular immunolocalization of protein kinase CK2 in normal and carcinoma cells. Int J Biochem Cell Biol. 1999;31:941–949. doi: 10.1016/s1357-2725(99)00050-3. [DOI] [PubMed] [Google Scholar]
- 67.Laramas M, Pasquier D, Filhol O, Ringeisen F, Desxotes JL, Cochet C. Nuclear localization of protein kinase CK2 catalytic subunit (CK2α) is associated with poor prognostic factors in human prostate cancer. Eur J Cancer. 2007;43:928–934. doi: 10.1016/j.ejca.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 68.O-Charoenrat P, Rusch V, Talbot SG, Sarkaria I, Viale A, Socci N, Ngai I, Rap P, Singh B. Casein kinase II alpha subunit and C1-inhibitor are independent predictors of outcome in patients with squamous cell carcinoma of the lung. Clin Cancer Res. 2004;10:5792–5803. doi: 10.1158/1078-0432.CCR-03-0317. [DOI] [PubMed] [Google Scholar]
- 69.Wang G, Unger GM, Ahmad KA, Slaton JW, Ahmed K. Downregulation of CK2 induces apoptosis in cancer cells: A potential approach to cancer therapy. Mol Cell Biochem. 2005;274:77–84. doi: 10.1007/s11010-005-3077-1. [DOI] [PubMed] [Google Scholar]
- 70.Pinna LA, Allende JE. Protein kinase CK2: An ugly duckling in the kinome pond. Cell Molec Life Sci. 2009;66:1795–1799. doi: 10.1007/s00018-009-9148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderes KL, Siddiqui-Jain A, Streiner N, Chua P, Pierre F, Omori M, Darjarnia L, Stansfield R, Phung J, Bliesath J, Ho C, Drygin D, O'Brien S, Rice W. Discovery and characterization of CX-4945 a selective orally bioavailable small molecule inhibitor of protein kinase CK2: Phase 1 initiated [abstract]. AACR; 2009; Proceedings of the 100th Annual Meeting of the American Association for Cancer Research; 2009 Apr 18-22; Denver, CO. Philadelphia (PA). 2009. Abstract nr 4660. [Google Scholar]
- 72.Battistutta R. Structural bases of protein kinase CK2 inhibition. Cell Molec Life Sci. 2009;66:1868–1889. doi: 10.1007/s00018-009-9155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mishra S, Pertz V, Zhang B, Kaur P, Shimada H, Groffen J, Kazimierczuk Z, Pinna LA, Heisterkamp N. Treatment of P190 Bcr/Abl lymphoblastic leukemia cells with inhibitors of the serine/threonine kinase CK2. Leukemia. 2007;21:178–180. doi: 10.1038/sj.leu.2404460. [DOI] [PubMed] [Google Scholar]
- 74.Zień P, Bretner M, Zastapilo K, Szyszka R, Shugar D. Selectivity of 4,5,6,7-tetrabromobenzimidazole as an ATP-competitive potent inhibitor of protein kinase CK2 from various sources. Biochem Biophys Res Commun. 2003;306:129–133. doi: 10.1016/s0006-291x(03)00928-8. [DOI] [PubMed] [Google Scholar]
- 75.Prudent R, Cochet C. New Protein Kinase CK2 inhibitors: Jumping out of the catalytic box. Chemistry and Biology. 2009;16:112–120. doi: 10.1016/j.chembiol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 76.Sandholt IS, Olsen BB, Guerra B, Issinger OG. Resorufin: a lead for a new protein kinase CK2 inhibitor. Anticancer Drugs. 2009;20:238–248. doi: 10.1097/CAD.0b013e328326472e. [DOI] [PubMed] [Google Scholar]
- 77.Solares AM, Santana A, Baldrón I, Valenzuela C, González CA, Díaz A, Castillo D, Ramos T, Gómez R, Alonso DF, Herrera L, Sigman H, Perea SE, Acevedo BE, López-Saura P. Safety and preliminary efficacy data of a novel casein kinase 2 (CK2) peptide inhibitor administered intralesionally at four dose levels in patients with cervical malignancies. BMC Cancer. 2009;9146 doi: 10.1186/1471-2407-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trembley JH, Unger G, Korman VL, Wang G, Tobolt DK, Slaton J, Ahmed K. Molecular downregulation of CK2 in prostate cancer cells induces apoptosis and shrinks xenograft tumors [Abstract]. AACR:2009; Proceedings of the 100th Annual Meeting of the American Association for Cancer Research; 2009 Apr 18-22; Denver, CO. Philadelphia (PA). 2009. Abstract nr 3647. [Google Scholar]
- 79.Kren BT, Unger GM, Sjeklocha L, Trossen AA, Korman V, Diethelm-Okita BM, Reding MT, Steer CJ. Nanocapsule-delivered Sleeping Beauty mediates therapeutic Factor VIII expression in liver sinusoidal endothelial cells of hemophilia A mice. J Clin Invest. 2009;119:2086–2099. doi: 10.1172/JCI34332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown MS, Diallo OT, Hu M, Ehsanian R, Yang X, Arun P, Lu H, Korman V, Unger G, Ahmed K, Van Waes C, Chen Z. CK2 modulation of NF-kappaB, TP53, and the malignant phenotype in head and neck cancer by anti-CK2 oligonucleotides in vitro or in vivo via sub-50 nm nanocapsules. Clin Canc Res. 2010 doi: 10.1158/1078-0432.CCR-09-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven challenges for nanomedicine. Nature Nanotech. 2008;3:242–244. doi: 10.1038/nnano.2008.114. [DOI] [PubMed] [Google Scholar]
- 82.Dominguez I, Sonenshein GE, Seldin DC. CK2 and its role in Wnt and NF-kB signaling: Linking development and cancer. Cell Molec Life Sci. 2009;66:1850–1857. doi: 10.1007/s00018-009-9153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Waes C. Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clin Canc Res. 2007;13:1076–1082. doi: 10.1158/1078-0432.CCR-06-2221. [DOI] [PubMed] [Google Scholar]
- 84.Allen CT, Ricker JL, Chen Z, Van Waes C. Role of activated nuclear factorkappaB in the pathogenesis and therapy of squamous cell carcinoma of the head and neck. Head Neck. 2007;29:959–971. doi: 10.1002/hed.20615. [DOI] [PubMed] [Google Scholar]
- 85.Hazem IA, Lessard L, Mes-Masson AM, Saad F. Expression of NFκB in prostate cancer lymph node metastases. Prostate. 2004;58:308–313. doi: 10.1002/pros.10335. [DOI] [PubMed] [Google Scholar]
- 86.Brown M, Cohen J, Arun P, Chen Z, Van Waes C. NF-kappaB in carcinoma therapy and prevention. Expert Opin Ther Targets. 2008;12:1109–1122. doi: 10.1517/14728222.12.9.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shen J, Channavajhala P, Seldin DC, Sonenshein GE. Phosphorylation by the protein kinase CK2 promotes calpain-mediated degradation of IkappaBalpha. J Immunol. 2001;167:4919–4925. doi: 10.4049/jimmunol.167.9.4919. [DOI] [PubMed] [Google Scholar]
- 88.Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE. Protein kinase CK2 promotes aberrant activation of nuclear factor-kappaB, transformed phenotype, and survival of breast cancer cells. Canc Res. 2002;62:6770–6778. [PubMed] [Google Scholar]
- 89.Yu M, Yeh J, Van Waes C. Protein kinase CK2 mediates inhibitor-kappaB kinase and aberrant nuclear factor-kappaB activation by serum factor(s) in head and neck squamous cell carcinoma cells. Canc Res. 2006;66:6722–6731. doi: 10.1158/0008-5472.CAN-05-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang D, Westerheide SD, Hanson JL, Baldwin AS., Jr Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J Biol Chem. 2000;275:32592–32597. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 91.Lee TL, Yang XP, Yan B, Friedman J, Duggal P, Bagain L, Dong G, Yeh NT, Wang J, Zhou J, Elkahloun A, Van Waes C, Chen Z. A novel nuclear factor-kappaB gene signature is differentially expressed in head and neck squamous cell carcinomas in association with TP53 status. Clin Cancer Res. 2007;13:5680–5691. doi: 10.1158/1078-0432.CCR-07-0670. [DOI] [PubMed] [Google Scholar]
- 92.Friedman J, Nottingham L, Duggal P, Pernas FG, Yan B, Ping X, Yang P, Chen Z, Van Waes C. Deficient TP53 expression, function, and cisplatin sensitivity are restored by quinacrine in head and neck cancer. Clin Cancer Res. 2007;13:6568–6578. doi: 10.1158/1078-0432.CCR-07-1591. [DOI] [PubMed] [Google Scholar]
- 93.Keller DM, Zeng X, Wang Y, Zhang QH, Kapoor M, Shu H, Goodman R, Lozano G, Zhao Y, Lu H. A DNA damage-induced p53 serine 392 kinase complex contains CK2, hSpt16, and SSRP1. Mol Cell. 2001;7:283–292. doi: 10.1016/s1097-2765(01)00176-9. [DOI] [PubMed] [Google Scholar]
- 94.McKendrick L, Milne D, Meek D. Protein kinase CK2-dependent regulation of p53 function: evidence that the phosphorylation status of the serine 386 (CK2) site of p53 is constitutive and stable. Mol Cell Biochem. 1999;191:187–199. [PubMed] [Google Scholar]
- 95.Prowald A, Schuster N, Montenarh M. Regulation of the DNA binding of p53 by its interaction with protein kinase CK2. FEBS Lett. 1997;408:99–104. doi: 10.1016/s0014-5793(97)00399-2. [DOI] [PubMed] [Google Scholar]
- 96.Schuster N, Götz C, Faust M, Schneider E, Prowald A, Jungbluth A, Montenarh M. Wild-type p53 inhibits protein kinase CK2 activity. J Cell Biochem. 2001;81:172–183. doi: 10.1002/1097-4644(20010401)81:1<172::aid-jcb1033>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 97.Van Waes C, Kozarsky KF, Warren AB, Kidd L, Paugh D, Liebert M, Carey TE. The A9 antigen associated with aggressive human squamous carcinoma is structurally and functionally similar to the newly defined integrin alpha 6 beta 4. Cancer Res. 1991;51:2395–2402. [PubMed] [Google Scholar]
- 98.Van Waes C. Cell adhesion and regulatory molecules involved in tumor formation, hemostasis, and wound healing. Head and Neck. 1995;17:140–147. doi: 10.1002/hed.2880170212. [DOI] [PubMed] [Google Scholar]
- 99.Schneider CC, Hessenauer A, Montenarh M, Götz C. p53 is dispensable for the induction of apoptosis after inhibition of protein kinase CK2. Prostate. 2010;70:126–134. doi: 10.1002/pros.21044. [DOI] [PubMed] [Google Scholar]